Abstract
Purpose
To evaluate changes in objective measures of disparity vergence after office-based vision therapy (OBVT) for concussion-related convergence insufficiency (CI), and determine the feasibility of using this objective assessment as an outcome measure in a clinical trial.
Methods
This was a prospective, observational trial. All participants were treated with weekly OBVT with home reinforcement. Participants included two adolescents and three young adults with concussion-related, symptomatic CI. The primary outcome measure was average peak velocity for 4-degree symmetrical convergence steps. Other objective outcome measures of disparity vergence included time to peak velocity, latency, accuracy, settling time, and main sequence. We also evaluated saccadic eye movements using the same outcome measures. Changes in clinical measures (near point of convergence, positive fusional vergence at near, Convergence Insufficiency Symptom Survey (CISS) score) were evaluated.
Results
There were statistically significant and clinically meaningful changes in all clinical measures for convergence. Four of the five subjects met clinical success criteria. For the objective measures, we found a statistically significant increase in peak velocity, response accuracy to 4° symmetrical convergence and divergence step stimuli and the main sequence ratio for convergence step stimuli. Objective saccadic eye movements (5° and 10°) appeared normal pre-OBVT, and did not show any significant change after treatment.
Conclusions
This is the first report of the use of objective measures of disparity vergence as outcome measures for concussion-related convergence insufficiency. These measures provide additional information that is not accessible with clinical tests about underlying physiological mechanisms leading to changes in clinical findings and symptoms. The study results also demonstrate that patients with concussion can tolerate the visual demands (over 200 vergence and versional eye movements) during the 25-minute testing time and suggest that these measures could be used in a large-scale randomized clinical trial of concussion-related CI as outcome measures.
Keywords: convergence insufficiency, vision therapy, concussion, vision rehabilitation
Convergence insufficiency (CI) is a common binocular vision disorder that often results in visual symptoms including headaches, eyestrain, blurred vision, loss of place while reading, and diplopia during near visual activities.1,2 These symptoms may interfere with reading performance and other near-related activities.3 The prevalence of CI in the general population has been estimated to be about 2.25% to 8.3%.4–6 However, the prevalence of CI after mild traumatic brain injury (mTBI) has been reported to be considerably higher. Studies of US military personnel indicate a prevalence between 28% to 42%.7–10 Suchoff11, Ciuffreda12, and Alvarez13 have found similar prevalence rates in the civilian, adult population (23% to 42%). A recent study of the prevalence of concussion-related vision problems in children and adolescents found that 49% of the sample had CI.14
This high prevalence of CI in both the general and the mTBI populations has led to an interest in studying the effectiveness of treatment for CI. Three recently completed randomized clinical trials completed by the Convergence Insufficiency Treatment Trial Investigator Group (CITT) demonstrated that office-based vergence/accommodative vision therapy combined with home reinforcement is the most effective treatment for symptomatic CI in people 9 to 30 years of age.3,15–17 The CITT studies used traditional clinical measures of vergence and accommodation, along with a validated symptom questionnaire score, as outcome measures. However, both the symptom questionnaire and clinical measures are subjective measurements that depend on the patient’s ability to accurately report what he/she is experiencing and seeing. The use of objective measures of oculomotor function may provide additional valuable information about the underlying mechanisms leading to a decrease in symptoms after office-based vision therapy (OBVT). In addition, objective measures may be useful in clinical trials because they are not prone to bias by either participants or examiners.
There is a paucity of studies using objective eye movement recordings of vergence as outcome measures after treatment for CI.18–20 Alvarez, et al., 20 studied 13 adult control participants and 4 adult participants with symptomatic CI and no history of brain injury. They used traditional clinical measures for assessing CI, objective measures of convergence and divergence step responses, and fMRI to study the hemodynamic response of the neural substrates used to mediate a vergence response. Pre-OBVT, the convergence average peak velocities to symmetrical 4 degree step stimuli were significantly slower (p= 0.016) in CI participants compared with controls, while significant differences in average peak velocities were not observed for divergence step responses (p=0.30). The CI participants received 18 hours of vision therapy (1 hour per session) and the results showed that a reduction in symptoms was associated with the following: 1) a decrease in the near point of convergence, 2) an increase in positive fusional amplitude, 3) a reduction in the amount of exophoria at near, 3) an increase in convergence peak velocity, and 5) an increase in the percent signal change of functional activity in the frontal eye fields, posterior parietal cortex and the cerebellar vermis. In a more recent study in the same laboratory, investigators studied the same disparity vergence parameters after vergence therapy in participants with normal binocular vision. They found a significant decrease in response latency, time to peak velocity, settling time, and an increase in response accuracy after 12 hours of vergence therapy.21
Bucci et al 19 studied four children (ages 8–16 years) with CI, and administered a 12-session, vergence orthoptic program. In addition to marked improvement in clinical signs and reduction of symptoms after the therapy, the objective measures of vergence revealed somewhat faster responses: peak velocity increased and duration decreased. In another study of 8 children (ages 9–16 years) with clinical vergence deficits, Jainta et al 18 had the children perform a simple oculomotor task in which they executed 80 convergence and 80 divergence responses to midline targets at distances of 25, 68, and 153 cm in a single session. Immediately following this task, there were small but significant improvements in vergence dynamics with a decrease in duration and an increase in peak velocity for both convergence and divergence. Latency was normal and remained constant in all cases. Scheiman et al22 recently published a case report of a 10 year old child with CI that documented improvements in laboratory-based objective, dynamic measures of both accommodation and vergence following conventional office-based optometric vision therapy for CI.
In the only study to date on patients with mild traumatic brain injury (mTBI), Thiagarajan, Ciuffreda, et al.,23 used a strong cross-over, placebo-controlled design to investigate the effectiveness of oculomotor therapy in 12 adult participants with mild traumatic brain injury and vergence dysfunction. They found that after oculomotor training, the peak velocity for both convergence and divergence increased significantly. Increased peak velocity was significantly correlated with increased clinically-based vergence prism flipper rate. Steady-state response variability for convergence reduced significantly following training. None of the measures was found to change significantly following the placebo training. This study did not specifically target CI as a diagnostic condition, but did demonstrate that the use of laboratory-based therapy clearly led to objective improvements in vergence function. To our knowledge, the Thiagarajan et al., report is the only study in the literature to date that used objective measures of vergence and version eye movements as outcome measures after vision therapy in a sample of patients with mTBI. However, we were unable to find any study specifically using objective eye movement recording with patients with concussion-related, symptomatic CI that used traditional clinical vision therapy procedures
Thus, there is a need for additional research to evaluate the use of objective measures of vergence and saccadic eye movements as an outcome measure for the treatment of CI after concussion. The primary objective of this study was to determine whether OBVT affects various objectives vergence parameters (i.e., response latency, time to peak velocity, settling time, response amplitude, peak velocity, and main sequence ratio). Secondary objectives were to gather preliminary information about the feasibility of using these measures as outcome measures in a large-scale, randomized clinical trial studying concussion-related CI, and to produce pilot data about effect size and variability that can be used in future studies for sample size estimation. In addition, this study was designed as an effectiveness study using traditional clinical treatment, rather than an efficacy study using laboratory-based therapy.24
METHODS
The tenets of the Declaration of Helsinki were followed throughout the study. The institutional review board of Salus University approved the protocol and written informed consent and assent as well as Health Insurance Portability and Accountability Act (HIPAA) authorization were obtained before participation.
Patient Selection and Definition of CI
To be eligible, individuals had to be between 9 to 35 years old, have a medically-documented diagnosis of concussion and symptomatic CI. Symptomatic CI was defined as (1) a score of 16 or higher for children and 21 or higher for adults; (2) exophoria at near at least 4 prism diopters (Δ) greater than at distance; (3) a receded near point of convergence (NPC) of ≥ 6 cm break, and (4) insufficient positive fusional vergence (i.e., failing Sheard’s criterion or positive fusional vergence < 15Δ base-out) at near. Participants had to have 20/25 visual acuity or better with best correction. Participants with a previous history of vision therapy were excluded. The participants also had to have stable general health, intact cognitive function, and no other neurological conditions.
Eligibility Exam/Baseline Clinical CI Testing for Enrollment
After obtaining written consent/assent, a vision examination was performed to determine if the patient was eligible for the study. Eligibility testing included administration of the CI Symptom Survey (CISS) to identify whether or not the patient was symptomatic.25,26 Other eligibility tests included best-corrected visual acuity at distance and near, a sensorimotor examination (cover test at distance and near, NPC, positive and negative fusional vergence at near, vergence facility at distance and near, near stereoacuity, monocular accommodative amplitude, monocular accommodative facility), cycloplegic refraction, and an ocular health evaluation. This test battery is identical to that used in previous randomized clinical trials of symptomatic CI.3,15,27 Eligibility criteria are listed in Table 1.
Table 1.
Eligibility and exclusion criteria.
| Eligibility Criteria |
| Medical diagnosis of concussion of at least 2–4 weeks ( +/−1 week) duration |
| Age 11 to <35 years |
| CI Symptom Survey score ≥16 for ages <18 years old |
| CI Symptom Survey score ≥21 for ages >18 years old |
| Exophoria at near at least 4Δ greater than at far |
| Receded near point of convergence (NPC) of ≥ 6 cm break |
| Insufficient positive fusional vergence (PFV) at near (i.e., failing Sheard’s criterion or PFV ≤15Δ base-out break) |
| Best-corrected distance visual acuity of 20/25 or better in each eye |
| Random dot stereopsis appreciation of 500 seconds of arc or better |
| Willing to wear refractive correction for any of the following uncorrected refractive errors (based on cycloplegic refraction within prior 6 months). (Correction must be worn for at least 2 weeks): |
| Myopia ≥ −0.75 D spherical equivalent in either eye |
| Hyperopia ≥ +1.50 D spherical equivalent in either eye |
| Anisometropia ≥1.00 D spherical equivalent or ≥1.50 D in any meridian |
| Astigmatism ≥ 1.00 D in either eye |
| Willing to discontinue BI prism or plus add at near for duration of study |
| Normal pupils |
| Comitant deviation |
|
|
| Exclusion Criteria |
| Any strabismus at distance |
| Constant strabismus at near |
| Esophoria of ≥2Δ at distance |
| Vertical heterophoria ≥2Δ at distance or near |
| ≥2 line interocular difference in best-corrected visual acuity |
| Manifest or latent nystagmus |
| History of strabismus surgery or refractive surgery |
| Previous history of CI before concussion |
| Diseases known to affect accommodation, vergence, or ocular motility |
Objective Outcome Measures of Disparity Vergence: Instrumentation
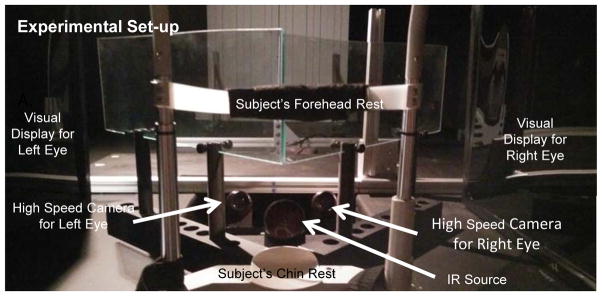
The experimental set-up is illustrated in Figure 1. For this study, the ISCAN RK-826PCI binocular tracking system (Burlington, MA, USA) objectively recorded horizontal vergence eye movements. This system utilizes an infrared emitter and camera (receiver) to capture the eye movement data at 240 frames per second (fps). The infrared source emits infrared light at a wavelength of 950 nm with a power of 1.2 mW/cm2, which is considerably lower than the ANSI Z136 specification safety limit of 10 mW/cm2. The manufacturer reported accuracy is 0.3° over a ± 20° horizontal and vertical range.
Figure 1.
Experimental set-up of haploscope used to record disparity vergence eye movements.
VisualEyes Software, Calibration, Stimuli Presentation, and Data Collection
A custom LabVIEWTM (National Instruments, Austin, TX, USA) program named VisualEyes controlled the stimuli presentation and data collection from the instrumentation.28,29 This software was designed to independently generate visual stimuli to the left and right eye with the use of two monitors and two partially (50%) reflecting mirrors. The VisualEyes program produces a stimulus in each monitor that is transposed onto reflective mirrors. A vertically oriented ‘difference of Gaussians’ (DOG) target was used, that only contains low spatial frequencies and yet provides effective vergence information when viewed binocularly. This target was designed to minimize blur cues and feedback to produce virtually accommodation ‘open loop’ conditions.30
The distance from each eye to the monitors was 40 cm, the same distance used clinically as ‘near’. Upon proper set-up, the mirrors reflect the stimulus to each eye, simulating a disparity vergence stimulus along the subject’s midline. A 12-bit digital acquisition (DAQ) card (National Instruments 6024 E series, Austin, TX, USA) digitized the eye movement data recorded from each eye from the ISCAN instrumentation using a range of ±5 volts.
Selection of Experimental Design Parameters
Three Separate Experiments
Three experiments were necessary to achieve the objectives of this study. These include presentation of disparity vergence stimuli as well as presentation of saccadic stimuli. CI participants are known to have more difficulty with convergence at close compared to far distances. Thus, the use of both far and near disparity vergence stimuli allowed an objective determination of this expected pattern. Stimuli were presented at binocular vergence angles from 6° to 12° (referred to as “near” stimuli) and binocular vergence angles from 2° to 8° (referred to as “far” stimuli). The three experiments were sequenced to begin with far disparity vergence, which is expected to be easier for CI participants. This was followed by near vergence and finally the presentation of saccadic stimuli. The closest vergence angle displayed to the subject was 12º. This vergence angle was chosen because it is the average NPC plus two standard deviations away from the average NPC recorded from the randomized clinical trial CITT.
Disparity Vergence Symmetrical Step Stimuli
The use of 4° and 6° symmetrical disparity steps were chosen to maximize the ability to gather quality data for each observation. A number of previous studies have used these parameters and found that these values minimize the likelihood of loss of data because of an inability to fuse the targets.20,31–35 Based on previous studies, 4° disparity vergence stimuli provide the highest likelihood of obtaining meaningful data from CI participants.
The disparity vergence stimuli remained visible to the participant for 3–5 seconds to allow enough time for the participant to attempt to fuse the symmetrical binocular visual stimuli presented along the subject’s midline. Prior research shows a binocularly normal control fuses typically well within 2 seconds, typically within the first half of the first second.21 Hence, for this study, we approximately doubled the amount of stimulus presentation time for CI patients to allow adequate time for them to fuse the symmetrical binocular vergence stimuli. The variability in duration of the presentation was designed to try to prevent anticipatory responses that are known to affect vergence responses.36–38
Number of Observation for Various Stimuli
Because we hope to use this protocol in a randomized clinical trial to study children, a decision was made to try to limit the total experimental time to 30 minutes or less. This meant limiting the number of observations presented to the participants. Given the results from previous studies, demonstrating that a 4° disparity vergence stimulus is most useful for CI participants, and the time constraints, a protocol with twice as many 4° stimuli was designed. This sequence required about 23 minutes if the participant did not require any breaks.
Prior to the experimental session, the stimuli presented on the computer screens and partially reflecting mirrors were adjusted to calibrate the visual stimuli with real targets located at measured distances from the subject’s midline. An inter-pupillary distance of 6 cm was assumed. Although this assumption had an effect on the absolute amount of vergence, since the outcome was a measure of change in vergence parameters, it did not affect the study results. The subject’s head was restrained using a chin/head rest to try to minimize head movement and influence from the vestibular system. A midline adjustment procedure was performed to insure proper positioning within the chinrest. All experiments are conducted in the dark to reduce influence from proximal vergence and the ”DOG” pattern visual stimulus displayed on the haploscope was used to reduce accommodative vergence.
Two separate calibrations were performed for each of the three experiments (vergence at far, vergence at near, and saccades). Six calibrations in total. Calibration for far vergence step responses consisted of a six-point, monocular calibration (1°, 3°, and 5° monocular, corresponding to 2°, 6°, 10° binocular vergence angle demand). Calibration for near vergence step responses consisted of a six-point, monocular calibration (4°, 5°, and 6° monocular, corresponding to 8°, 10°, 12° binocular vergence angle demand). Calibration for saccade responses consisted of a four-point, monocular calibration (5°, and 10° monocular into the left and right visual fields). These calibrations were performed before and after completion of each experimental group.
After the initial calibration was complete the experiment began, and 12 disparity vergence stimuli were presented (combination of convergence and divergence), followed by an opportunity to rest. This was repeated 6 times for a total of 72 pseudo-random observations for the far disparity vergence experiment, 72 pseudo-random observations for the near disparity vergence, and 40 observations each of 5° and 10° pseudo-random into the left and right visual fields.
Eye Movement Analyses
Eye movement data were processed and analyzed with a custom MATLAB program (Waltham, MA, USA). For all individual left and right eye movement responses, inward convergent rotation was plotted as positive to facilitate direct comparison between left and right eye movement responses. Blinks were easily identified based on manual inspection of the left and right eye movement responses. Responses with blinks within the transient portion of the movement were omitted because the peak velocity cannot be measured due to the loss of signal. However, blinks within the steady state portion of the response were analyzed. The eye movements were filtered with a 4th low pass order Butterworth, with a cut off frequency of 40Hz to eliminate instrumentation noise that is probably not physiological in nature.
Peak velocity was the primary outcome measure in this study. Velocity was computed by taking the derivative of the position response using a two-point central difference algorithm.39 Each individual left-eye and right-eye convergence movement response was manually inspected for the presence of a blink or a saccade during the transient portion of the vergence eye movement. Saccades were easily identified because saccade dynamics are an order of magnitude greater than vergence. Only when saccades obstruct the convergence peak velocity was the response omitted from the peak velocity analysis because several studies support that saccades facilitate the maximum velocity of vergence.33,34,40 The peak velocity of the left-eye, right-eye, and combined vergence response were quantified as the maximum value within the transient portion of the vergence movement.
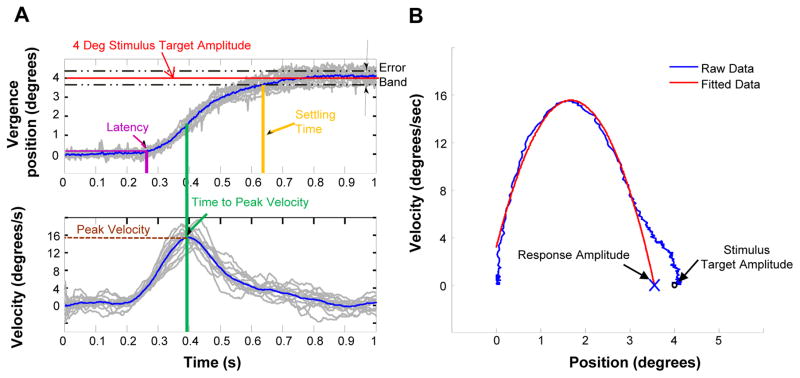
Other objective eye movement parameters assessed included time to peak velocity, latency, response amplitude, settling time. These parameters are illustrated in Figure 2A. Peak velocity was defined as the maximum value within the transient portion of the vergence movement. Time to peak velocity was defined as the time when the movement reaches its peak velocity within the transient portion of the response (green line in 2A). Latency was defined as the time at which the average positional data deviates 5% from the stimulus amplitude. Hence, for our study this threshold is at 0.2º away from the initial response position. An example of the average response latency is illustrated by the purple arrow and lines in Figure 2A. The settling time was defined as the time when the response was within the 5% error band (black dashed lines Figure 2A) of the stimulus target amplitude (red line Figure 2A). Figure 2B is the phase plane where velocity is plotted as a function of position. This plot allows a detailed analysis of the first order dynamics of an eye movement response. We fit the transient portion of the subject’s eye movement data (blue line Figure 2B) with a second order polynomial (red line Figure 2B). The roots of the polynomial were then calculated to determine which amplitude the response would attain when the response returns to zero º/sec velocity depicted as an ‘X’ in Figure 2B. This point represents the response amplitude when the vergence system was presented with a 4º symmetrical vergence step along midline. This analysis has been used in several other studies.41,42 Accuracy was defined as the difference between the stimulus and response (Figure 2B). The last analysis is a main sequence analysis. The main sequence is an assessment of the first order dynamics of eye movement responses.43
Figure 2.
Data analysis of objective eye movements. (A) The temporal properties where the position is plotted as a function of time (upper) and the velocity is plotted as a function of time (lower). (B) The phase plane, which is a plot of velocity as a function of position to calculate the accuracy of the movement.
Treatment
Office-based Vision Therapy with Home Reinforcement (OBVT)
OBVT was administered by a trained therapist (residency-trained optometrists) weekly, for 60-minute office visits with 45 minutes of therapy time, combined with procedures to practice at home (15 minutes, 5 times per week). This treatment sequence is a well-accepted approach for treatment of CI44 and has been successfully implemented in four previous studies.3,15,45 The number of office-based visits ranged from 12 to 20 with the endpoint dependent on the participants’ ability to reach pre-determined endpoint criteria for each prescribed therapy procedure. This variation in amount of therapy required is not unusual with the concussion population because some of these patients are so symptomatic that therapists typically have to slow the pace of the treatment. Fifteen minutes of home-based therapy was prescribed to be performed 5 days per week, and compliance with home-based therapy was monitored at each visit using a home-based therapy log that was completed by the participant.
Follow-up Visit
All participants were re-examined after completion of OBVT. Both the clinical and objective testing performed at enrollment were repeated at the outcome examination.
Determination of Outcome
To evaluate improvement in clinical measures and symptoms, we used the following criteria used in the CITT studies. A “successful” outcome was a score of <16 on the CI Symptom Survey for children or <21 for adults, a normal NPC (i.e., less than 6 cm), and normal PFV (i.e., greater than 15Δ and passing Sheard’s criterion). “Improved” was defined as a score of <16 (children) or <21(adults) or a 10 point decrease in the CI Symptom Survey score, and at least one of the following: normal NPC, an improvement in NPC of more than 4 cm, normal PFV or an increase in PFV of more than 10Δ. Patients who did not meet the criteria for “successful” or “improved” were considered “non-responders.”
Statistical Analysis
All analyses will be performed using SAS Version 9.3 with an alpha level of 0.05 used to determine statistical significance. Statistical significance of the improvement in clinical findings after OBVT was tested using a two-tailed paired t-test. For the main sequence analysis, a group level analysis was accomplished by averaging the 4 and 6 deg convergence movements recorded from near and far space for each subject and plotting the peak velocity as a function of response amplitude. This analysis was repeated for divergence movements. A main sequence analysis using the 5 and 10 deg saccadic eye movement responses was also calculated. To determine whether significant differences were observed within the main sequence plots the ratio of peak velocity divided by response amplitude was computed for each type of movement (convergence, divergence and saccade) and significance was assessed using a two tailed paired t-test.
RESULTS
Three of the participants were adults and the mean age of the five participants was 22.2 years old with a range from 13 to 28 years old. The number of visits varied from 12–20 (mean 13.6). The number of visits was related to the subject’s ability to complete all the assigned therapy tasks. The causes of the concussion were sports-related (2) automobile accident (3). The mean time between the concussion and the first vision examination was 11.6 months with a range from 2 months to 24 months.
Clinical Measures
The clinical measures before and after treatment are illustrated in Table 2. The mean CISS score was 33 before treatment and decreased significantly to 14 after OBVT (t=4.5, p=0.011). All four adults had CISS scores <21 after treatment and the child’s score was <16 after OBVT. A paired t-test revealed a significant difference comparing the pre-OBVT and post-OBVT parameters for NPC break (t=4.8, p=0.008), positive fusional vergence (t=5.2, p=0.006), and vergence facility (t=3.9, p=0.017). The near phoria decreased in 4/5 participants, but the amount of change was not clinically significant (2.2Δ) although it approached statistical significance (t=2.7, p=0.051).
Table 2.
Mean for each clinical outcome measure, before and after OBVT.
| Test/Time | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Mean |
|---|---|---|---|---|---|---|
| 17 years old | 13 years old | 31 years old | 28 years old | 22 years old | ||
| Near Phoria (Δ) | ||||||
| Pre-OBVT | 6 | 8 | 10 | 16 | 6 | 9.2 |
| Post-OBVT | 5 | 4 | 8 | 16 | 2 | 7.0 |
| Total Change | 1 | 4 | 2 | 0 | 4 | 2.2 |
|
| ||||||
| CISS score | ||||||
| Pre-OBVT | 25 | 32 | 24 | 40 | 43 | 32.8 |
| Post-OBVT | 16 | 13 | 12 | 20 | 10 | 14.2 |
| Total Change | −9 | −19 | −12 | −20 | −33 | −18.6 |
|
| ||||||
| Near point of convergence break (cm) | ||||||
| Pre-OBVT | 8 | 22 | 20 | 12 | 24 | 17.2 |
| Post-OBVT | 4 | 5 | 3.5 | 3 | 8 | 4.7 |
| Total Change | −4 | −17 | −16.5 | −9 | 16 | −6.1 |
|
| ||||||
| Positive fusional vergence blur or break (Δ) | ||||||
| Pre-OBVT | 10 | 2 | 6 | 8 | 6 | 6.4 |
| Post-OBVT | 30 | 20 | 25 | 50 | 40 | 33.0 |
| Total Change | 20 | 18 | 19 | 42 | 34 | 26.6 |
|
| ||||||
| Vergence Facility at Near (Flips/minute) | ||||||
| Pre-OBVT | 34 | 0 | 0 | 0 | 0 | 6.8 |
| Post-OBVT | 34 | 32 | 32 | 31 | 33 | 32.4 |
| Total Change | 0 | 32 | 32 | 31 | 33 | 25.6 |
|
| ||||||
| Near Point of Accommodation (cm) | ||||||
| Pre-OBVT | 10 | 19 | 20 | 13 | 24 | 17.2 |
| Post-OBVT | 10 | 13 | 13 | 12 | 11 | 11.8 |
| Total Change | 0 | −6 | −7 | −1 | −13 | −5.4 |
|
| ||||||
| Accommodative Facility (Flips/minute) | ||||||
| Pre-OBVT | 32 | 20 | 20 | 22 | 19 | 22.6 |
| Post-OBVT | 30 | 31 | 28 | 38 | 31 | 31.6 |
| Total Change | 2 | 11 | 8 | 16 | 12 | 9.0 |
Four of the five participants were categorized as “successful” based on the pre-determined composite criterion (CISS, NPC, PFV). Subject 5 had a normal CISS score and positive fusional vergence, but his NPC was still >6cm after treatment. Thus, he was considered “improved” but not “successful”.
Objective Measures - Convergence
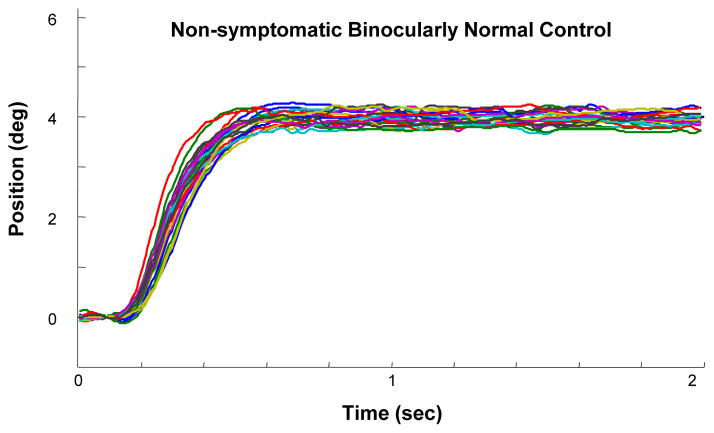
Both 4º and 6º convergence and divergence movements were evaluated in our study protocol. We only report the results for the 4º data because 3 of the 5 participants had difficulty responding to the 6º stimuli at baseline. Figure 3 illustrates an ensemble plot of multiple, 4º symmetrical convergence movements in a subject with normal binocular vision. The plot shows that there is very little variance in the responses and the response amplitude closely matches the 4º symmetrical binocular visual stimulus.
Figure 3.
Each colored trace is an individual convergence eye movement response to a 4º symmetrical binocular disparity vergence step stimulus from a non-symptomatic binocularly normal control subject.
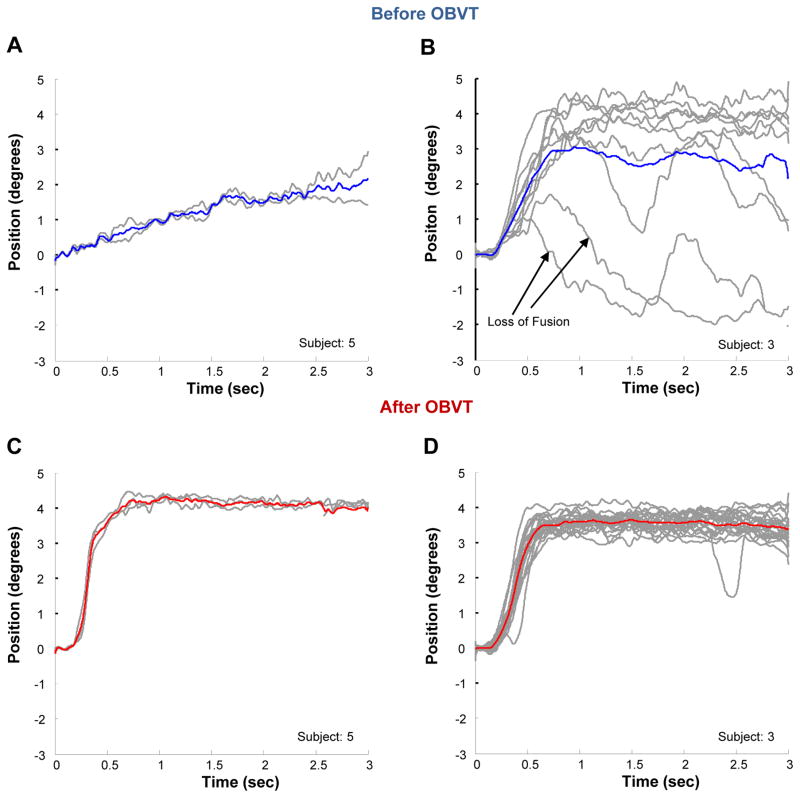
In contrast, all of the concussion patients studied here have impaired convergence responses before therapy. Figure 4 shows the subject who exhibited the greatest impairment of convergence (Figure 4A) and a typical subject (Figure 4B). Figure 4 represents an ensemble plot showing significant changes in the positional variance of 4º symmetrical, convergence eye movements before (Figures 4A and 4B) compared to after therapy (Figures 4C and 4D) for these two participants. In Figure 4A, the convergence response within the steady state reaches only 2º on average and it takes almost 3 seconds for the convergence response to reach that level. The second subject illustrated in Figure 4B demonstrates a high degree of variance in the convergence responses. While the average response amplitude within the steady state is about 3º degrees, it is apparent that some responses were greater than 4.5º and others less than 2º. In addition, the two lower movements indicate that the subject attempted to converge, could not fuse, and then tried to converge again over the 3 second period of time. After therapy (Figures 4C and 4D), the convergence response amplitudes for both participants are more accurate and the maximum response is achieved in less than 1 second. The responses after OBVT look more similar to the subject with normal binocular vision (Figure 3).
Figure 4.
Each gray line is an individual eye movement response from a 4º symmetrical binocular disparity vergence step stimulus. The blue traces show the average position before OBVT for subject 5 (A) and subject 3 (B). The red traces show the average position after OBVT for subject 5 (C) and subject 3 (D).
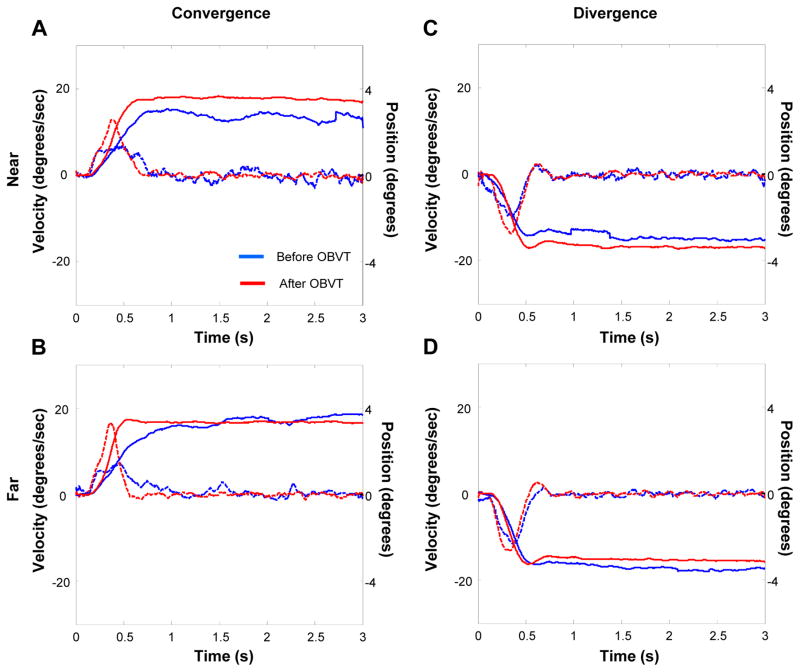
Figures 5A–B represents data from one subject with less impaired convergence before and after OBVT. In these figures, we combined information about eye position (solid lines) during the 3-second recording, as well as velocity (dotted lines) on the same graph. The blue, solid and dotted lines show the mean pre-therapy response and the red, solid and dotted lines, show the mean post-therapy response at the “near” (Figure 5A) and the “far” (Figure 5B) distances. In both cases, one can observe changes in a number of response parameters such as peak velocity, time to peak velocity, response accuracy. The divergence responses for this same subject are shown in Figure 5C (near space) and 5D (far space). We included these figures to demonstrate that while there are some changes after therapy for divergence, the magnitude of change is less than that of the change observed in convergence eye movement responses.
Figure 5.
Typical CI subject’s eye movement responses before and after OBVT. Position (solid) and velocity (dotted) as a function of time before (blue) and after (red) OBVT for convergence in near space (A), convergence in far space (B), divergence in near space (C) and divergence in far space (D).
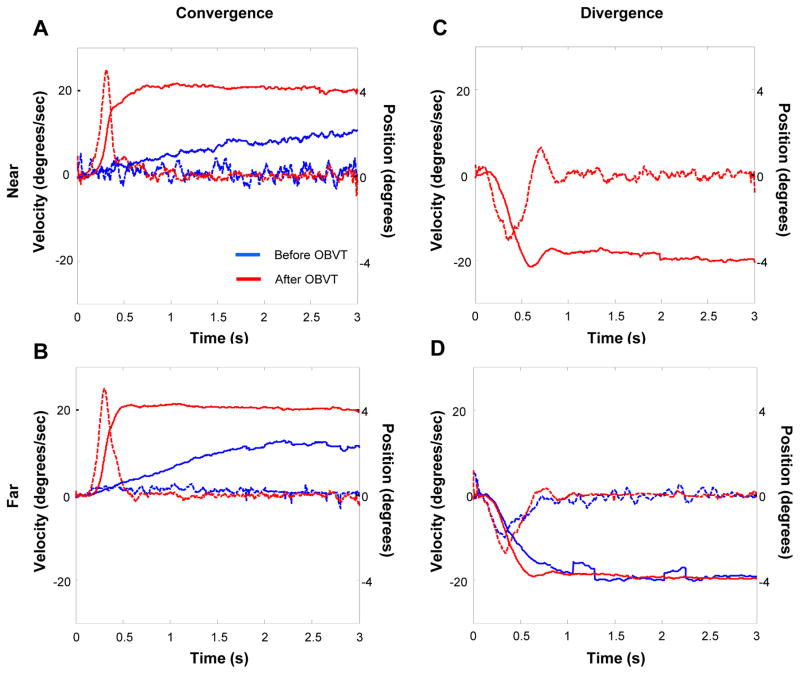
Figures 6A–D show similar data from the subject who exhibited the greatest changes in objective measures after OBVT. One can see very significant changes in peak velocity and position in both “near” and far” convergence responses to 4º symmetrical, step stimuli (Figures 6A and 6B). This subject was unable to make any measurable divergence responses to “near” stimuli before therapy (Figure 6C), but could do so after OBVT (Figure 6D).
Figure 6.
Eye movement responses before and after OBVT from the CI subject who exhibited the greatest change in position and velocity eye recording traces. Position (solid) and velocity (dotted) as a function of time before (blue) and after (red) OBVT for convergence in near space (A), convergence in far space (B), divergence in near space (C) and divergence in far space (D).
Tables 3 and 4 show the mean values and mean change for all five objective measures of disparity vergence for “far” and “near” stimuli before and after OBVT. There were statistically significant changes in peak velocity (p=.004) and accuracy (p=.011) for “far” 4° convergence movements as well as the peak velocity (p=.011), and accuracy (p=.032), for “near” convergence movements. For 4° symmetrical divergence steps there were statistically significant changes in peak velocity (p=.009) and accuracy (p=.033 as well as the peak velocity (p=.013), for “near” divergence movements (data not shown).
Table 3.
Mean for each objective outcome measure, before and after OBVT for 4 degree symmetrical convergence (far).
| Objective | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | |
|---|---|---|---|---|---|---|
| Measure/Time | 17 years old CMB | 13 years old FEM | 31 years old MXC | 28 years old PDJ | 22 years old SKH | Mean |
| Peak Velocity (°/sec) | ||||||
| Pre-OBVT | 20.1 | 12.4 | 5.7 | 15 | 20.9 | 14.8 |
| Post-OBVT | 31.4 | 20.8 | 27.2 | 27.7 | 32.9 | 28.0 |
| Total Change | 11.3 | 8.4 | 21.5 | 12.7 | 12 | 13.2 |
|
| ||||||
| Time to Peak Velocity (sec) | ||||||
| Pre-OBVT | 0.4 | 0.5 | 0.6 | 0.5 | 0.3 | 0.5 |
| Post-OBVT | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 |
| Total Change | 0 | −0.1 | −0.3 | −0.2 | 0 | 0.12 |
|
| ||||||
| Accuracy (°) (Stimulus –Response) | ||||||
| Pre-OBVT | −0.3 | 1.1 | 1.0 | 1.1 | 2.6 | 1.0 |
| Post-OBVT | −1.1 | 0.2 | −0.5 | −0.3 | −0.1 | −0.5 |
| Total Change | −0.8 | 0.9 | 0.5 | 0.8 | 2.5 | 0.5 |
|
| ||||||
| Latency (sec) | ||||||
| Pre-OBVT | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | 0.22 |
| Post-OBVT | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.20 |
| Total Change | 0 | 0.1 | 0 | 0 | 0 | −0.02 |
|
| ||||||
| Settling Time (sec) | ||||||
| Pre-OBVT | 2.5 | 2.6 | 2.7 | 3.0 | 2.6 | 2.7 |
| Post-OBVT | 3.0 | 2.9 | 2.2 | 2.9 | 1.7 | 2.5 |
| Total Change | 0.5 | 0.3 | −0.5 | −0.1 | −0.9 | −0.3 |
Table 4.
Mean for each objective outcome measure, before and after OBVT for 4 degree symmetrical convergence (near).
| Objective | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | |
|---|---|---|---|---|---|---|
| Measure/Time | 17 years old CMB | 13 years old FEM | 31 years old MXC | 28 years old PDJ | 22 years old SKH | Mean |
| Peak Velocity (°/sec) | ||||||
| Pre-OBVT | 18.9 | 13.7 | 6.0 | 13.4 | 14.8 | 13.4 |
| Post-OBVT | 26.2 | 18.4 | 25.2 | 29.0 | 30.6 | 25.9 |
| Total Change | 7.3 | 4.7 | 19.2 | 15.6 | 15.8 | 12,5 |
|
| ||||||
| Time to Peak Velocity (sec) | ||||||
| Pre-OBVT | 0.3 | O.5 | 0.5 | 0.6 | 0.4 | 0.5 |
| Post-OBVT | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 |
| Total Change | 0.1 | −0.1 | −0.2 | −0.3 | −0.1 | −0.2 |
|
| ||||||
| Accuracy (°) (Stimulus –Response) | ||||||
| Pre-OBVT | 3.6 | 2.9 | 0.8 | 2.7 | 2.8 | 2.6 |
| Post-OBVT | 3.8 | 3.8 | 3.5 | 4.3 | 4.5 | 4.0 |
| Total Change | 0.2 | 0.9 | 2.7 | 1.6 | 1.7 | 1.4 |
|
| ||||||
| Latency (sec) | ||||||
| Pre-OBVT | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Post-OBVT | 0.3 | 0.2 | 0.2 | 0.2 | 02 | 0.2 |
| Total Change | 0.1 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Settling Time (sec) | ||||||
| Pre-OBVT | 2.8 | 2.7 | 2.8 | 2.9 | 2.5 | 2.7 |
| Post-OBVT | 3.0 | 2.9 | 2.5 | 2.9 | 2.5 | 2.8 |
| Total Change | 0.2 | 0.2 | −0.3 | 0 | 0 | 0.1 |
Objective Measures - Saccades
Unlike convergence movements, which were markedly abnormal before treatment, the saccadic eye movements closely resembled eye movements from participants with normal binocular vision. Statistical analysis indicated no significant changes were observed in any of the five parameters for the saccadic eye movement after OBVT compared to baseline (p>0.05).
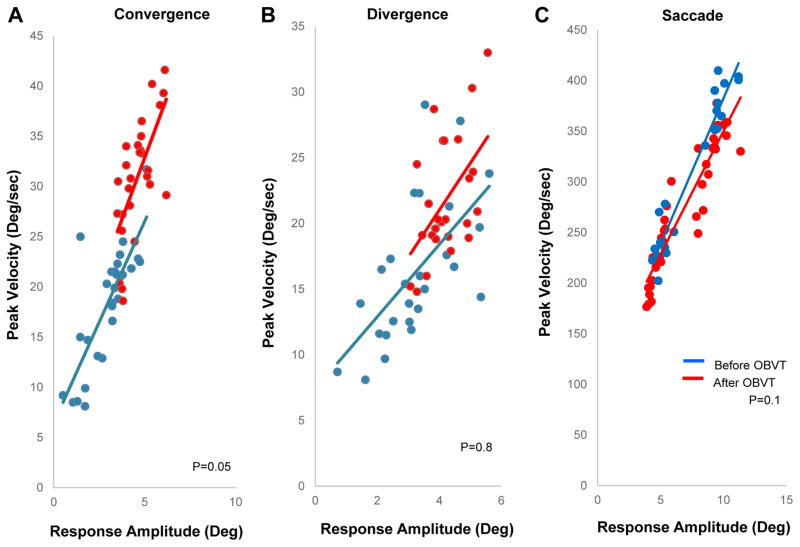
The main sequence plots for convergence, divergence and saccades are illustrated in Figures 7A–9C respectively. We plotted the peak velocity as a function of the response amplitude for all five participants for all types of movements. To perform a group level analysis, each type of eye movement was averaged as a ratio of the peak velocity divided by the response amplitude. The following types of eye movements ratios were calculated for each subject, 4º and 6º convergence at near and at far (Figure 7A), 4º and 6º divergence at near and far (Figure 7B), as well as 5 º and 10 º saccades (Figure 9C). A two tailed paired t-test showed that a significant change in the main sequence ratio was observed for convergence after OBVT compared to baseline measurements (p<0.05). The average main sequence ratio was 5.9 deg/sec2 ± 0.33 before and changed to 6.9 ± 0.98 deg/sec2 after OBVT for convergence eye movements. Conversely, divergence and saccades did not exhibit significant changes in the main sequence ratio after OBVT compared to the baseline measurements. Before OBVT, the divergence main sequence ratio was 5.2 deg/sec2 ± 0.79 and after OBVT the main sequence ratio was 5.1 deg/sec2 ± 0.78 (p=0.8). For saccades, the main sequence ratio was 41.5 deg/sec2 ± 1.5 before OBVT and 43.9 deg/sec2 ± 2.6 after OBVT (p=0.1).
Figure 7.
Main sequence analysis of (A) convergence, (B) divergence, and (C) saccades
DISCUSSION
Clinical and Objective Findings
In this pilot study, we found significant changes in symptoms and both clinical and objective measures of disparity vergence after completion of OBVT in a small group of participants with concussion-related symptomatic CI. To our knowledge, this is the first report on the treatment of concussion-related CI in which both clinical and objective eye movement measurements have been used to evaluate the results of treatment.
The changes observed after OBVT for concussion-related CI are comparable to those reported in previous randomized clinical trials with participants with symptomatic CI, unrelated to concussion. For example, in the CITT study the mean CISS score at outcome was 15.1 (change of 14.8 from baseline), the mean NPC at outcome was 4 cm (change of 11.7 cm from baseline), and the mean PFV at outcome was 30.5Δ (change of 19.7Δ from baseline). The findings were very similar for this sample with a final CISS of 14 (change = 14), NPC 5 cm (change=12 cm), and PFV 33Δ (change=26.6 Δ). Although the sample size is small in this study, these findings are encouraging and suggest that even though the underlying etiology of the CI is likely different in concussion-related CI, OBVT seems to be an effective treatment approach.
Objective Measures
The unique aspect of this study was the addition of objective measures of temporal (response latency, time of peak velocity, settling time) and accuracy measurements (peak velocity, response accuracy) of disparity vergence as well as an analysis of the main sequence of convergence, divergence, and saccades. The use of objective recording of vergence and versional eye movements allows the investigator to access additional, more subtle information about the physiology of these eye movements that is not available with traditional clinical testing. We found statistically significant improvements in both peak velocity and response accuracy after OBVT for 4º convergence steps with “far” and “near” targets. This finding is consistent with the limited previous literature. There were also comparable changes in 4º divergence for “far” targets. There is only one previous report of the use of objective measures of disparity vergence in participants who have had binocular vision problems associated with mild traumatic head injury/concussion. In this previous report, the authors23 found a significant increase in convergence peak velocity. In a study of four participants with CI without a history of concussion, Alvarez, et al.20 found a significant increase in peak velocity. Specifically, the average peak velocity before vision therapy was 10.7 ± 4.3 deg/s which increased to 14.3 ± 4.6 deg/s after vision therapy where a two-tailed paired t-test showed the change was significant (p=0.015).46
Although our main objective was to study disparity vergence, we included an assessment of 5º and 10º saccadic eye movements into the left and right visual field from an initial midline position to demonstrate that our calibration protocol was effective and to determine whether significant changes would occur within saccadic responses after OBVT compared to the baseline measures. In participants with CI and no history of concussion, objective measures of saccades resemble those from participants with normal binocular vision. 47 Specifically, if vergence responses were abnormal, while saccadic responses were normal it would demonstrate our ability to gather valid data from a subject. This is precisely what we found with our five participants. In all 5 participants with significant abnormalities in disparity vergence, the saccadic data appeared normal. It is also interesting to note that there were no significant changes in objective measures of saccadic function after OBVT (p>0.5). Previous studies have reported significant abnormalities in saccadic function using both clinically- and laboratory-based testing48 in patients soon after the concussion occurs. The patients in this study all had sustained a concussion at least 2 months before participating in this study.
Main Sequence and the Dynamic Neural Control of Disparity Vergence: Dual Mode Theory
We included an analysis of the main sequence for convergence, divergence, and saccades in an attempt to decipher the underlying physiological mechanism that may lead to improved outcomes after OBVT. Disparity vergence can be described using a two-component model. The first experimental behavioral support for this concept was reported by Jones who showed when two non-fusable lines (horizontal and vertical) were abruptly moved there was a fusion-initiating component to begin a vergence response but the response was not maintained. Hence, he described vergence as a system containing two components – the fusion-initiating component (FIC) and the fusion-sustaining component (FSC).49 A few years later Semmlow and colleagues used a disappearing step stimulus and confirmed that when two vertical lines were abruptly moved and disappeared within 200 ms, the disparity vergence system initiated a vergence response but did not maintain it.50 Neurophysiological evidence also support there are burst and tonic cells observed within the midbrain of primates through the study of local field potentials.51 We interpret the burst and tonic cells to encode for the preprogrammed and feedback controlled components, respectively. In addition, patients with pontine lesions52 as well as different set of patients with cerebellar lesions53 have demonstrated impaired ramp (feedback controlled) vergence but normal responses to step vergence responses (initially preprogrammed controlled). Such clinical data further support two components are present within the neural control of vergence.
These experimental and clinical findings lead to the Dual Mode Theory, which states when the disparity vergence system is stimulated to change gaze in 3D space (near to far space or vice versa) then two components are activated. The fusion-initiating component (FIC) is preprogrammed and is mainly active within the transient portion of the vergence response. It allows the eyes to quickly rotate inward or outward to the new visual target but does not always yield accurate movements. The second component is the fusion-sustaining component (FSC) which is feedback controlled. A feedback controlled system samples the error by computing the difference between where the eyes are currently located in space and where the desired target is located. The FSC provides high accuracy but requires time to guide the eyes to the desired target. When the FIC is optimized meaning it brings the eyes closer to the intended visual stimulus then less adjustment is needed by the FSC to have the eyes fuse on the final vergence angular demand. Our laboratory has provided substantial experimental and modeling research to support the Dual Model Theory of vergence.54–56
The main sequence quantitatively analyzes the transient portion of the movement when the FIC dominates the response.42,43,57 Our results support a significant change in the main sequence ratio of convergence after OBVT compared to the baseline measurements (p<0.05). Conversely, the main sequence ratio of divergence and saccades did not significantly change (p>0.1) after OBVT compared to the baseline measurements. These results support that one of the potential underlying mechanisms by which OBVT is leading to a sustained reduction in visual symptoms may be through the change in the neural control of the FIC. When the FIC is optimized meaning the eyes are brought close to the intended visual stimulus, then less modification is required from the FSC, which may be a factor leading to the sustained reduction of visual symptoms. Our laboratory has published other investigations showing optimization of the FIC in binocular normal controls after vergence therapy21 and in those with CI.58 A randomized clinical trial is needed to further confirm whether it is the FIC of vergence that is being optimized by OBVT in patients with CI and concussion.
Study Limitations and Future Direction
One limitation of this study is the small sample size. It is possible that because of the small sample size, we may have missed a significant effect of OBVT on other objective measures and may see a larger effect size on the parameters that did show a significant change. We are planning to use this objective assessment protocol with a larger sample size in a randomized clinical trial that is actively being planned. The pilot data from this study is sufficient to help us plan sample size and validate that patients with concussion and symptomatic CI can complete the 25–30 minute testing protocol without excessive discomfort. Another potential issue is that the time from injury to the start of therapy varied from 2 to 24 months. A criticism might be that the natural recovery process for PCS-CI may have confounded the results. However, as Pearce et al, state “Little is known about the spontaneous recovery and functional adaptation of CI after a concussion. Therefore, researchers should examine NPC across different post-injury time intervals including acute, subacute, and chronic.” 59 Given the lack of data, we think that the wide range of time elapsed from injury to treatment in this study provides useful information. The fact that some participants were still suffering from the symptoms related t0 PCS-CI as long as 2 years post-concussion argues against the natural healing process.
CONCLUSIONS
This is the first report of the use of objective measures of disparity vergence as outcome measures for concussion-related CI. These measures provide additional information about underlying physiological mechanisms leading to changes in clinical findings and symptoms. The study results also demonstrate that patients with concussion–related CI can tolerate the visual demands (over 200 vergence and versional eye movements) during the 25-minute testing time and suggest that these measures could be used in a large-scale randomized clinical trial of concussion-related CI as outcome measures. Finally, the data from this study can be used in future large scale clinical trials to develop sample size estimates.
Acknowledgments
This research was supported in part by NSF MRI CBET 1428425 to TLA and NIH 1R01EY023261 to TLA and MS.
References
- 1.Borsting E, Rouse MW, Deland PN, Hovett S, Kimura D, Park M, Stephens B. Association of symptoms and convergence and accommodative insufficiency in school-age children. Optometry. 2003;74:25–34. [PubMed] [Google Scholar]
- 2.Barnhardt C, Cotter SA, Mitchell GL, Scheiman M, Kulp MT. Symptoms in children with convergence insufficiency: before and after treatment. Convergence Insufficiency Treatment Trial (CITT) study group. Optom Vis Sci. 2012;89:1512–20. doi: 10.1097/OPX.0b013e318269c8f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Convergence Insufficiency Treatment Trial Investigator Group. Randomized clinical trial of treatments for symptomatic convergence insufficiency in children. Arch Ophthalmol. 2008;126:1336–49. doi: 10.1001/archopht.126.10.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letourneau JE, Ducic S. Prevalence of convergence insufficiency among elementary school children. Can J Optom. 1988;50:194–97. [Google Scholar]
- 5.Porcar E, Martinez-Palomera A. Prevalence of general binocular dysfunctions in a population of university students. Optom Vis Sci. 1997;74:111–13. doi: 10.1097/00006324-199702000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, Scheiman M, Gallaway M, De Land PN. Frequency of convergence insufficiency among fifth and sixth graders. The Convergence Insufficiency and Reading Study (CIRS) group. Optom Vis Sci. 1999;76:643–9. doi: 10.1097/00006324-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Goodrich GL, Flyg HM, Kirby JE, Chang CY, Martinsen GL. Mechanisms of TBI and visual consequences in military and veteran populations. Optom Vis Sci. 2013;90:105–12. doi: 10.1097/OPX.0b013e31827f15a1. [DOI] [PubMed] [Google Scholar]
- 8.Brahm KD, Wilgenburg HM, Kirby J, Ingalla S, Chang CY, Goodrich GL. Visual impairment and dysfunction in combat-injured servicemembers with traumatic brain injury. Optom Vis Sci. 2009;86:817–25. doi: 10.1097/OPX.0b013e3181adff2d. [DOI] [PubMed] [Google Scholar]
- 9.Stelmack JA, Frith T, Van Koevering D, Rinne S, Stelmack TR. Visual function in patients followed at a Veterans Affairs polytrauma network site: an electronic medical record review. Optometry. 2009;80:419–24. doi: 10.1016/j.optm.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Capo-Aponte JE, Urosevich TG, Temme LA, Tarbett AK, Sanghera NK. Visual dysfunctions and symptoms during the subacute stage of blast-induced mild traumatic brain injury. Mil Med. 2012;177:804–13. doi: 10.7205/milmed-d-12-00061. [DOI] [PubMed] [Google Scholar]
- 11.Suchoff IB, Kapoor N, Waxman R, Ference W. The occurrence of ocular and visual dysfunctions in an acquired brain-injured patient sample. J Am Optom Assoc. 1999;70:301–8. [PubMed] [Google Scholar]
- 12.Ciuffreda KJ, Kapoor N, Rutner D, Suchoff IB, Han ME, Craig S. Occurrence of oculomotor dysfunctions in acquired brain injury: a retrospective analysis. Optometry. 2007;78:155–61. doi: 10.1016/j.optm.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez TL, Kim EH, Vicci VR, Dhar SK, Biswal BB, Barrett AM. Concurrent vision dysfunctions in convergence insufficiency with traumatic brain injury. Optom Vis Sci. 2012;89:1740–51. doi: 10.1097/OPX.0b013e3182772dce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Master CL, Scheiman M, Gallaway M, Goodman A, Robinson RL, Master SR, Grady MF. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) 2016;55:260–7. doi: 10.1177/0009922815594367. [DOI] [PubMed] [Google Scholar]
- 15.Scheiman M, Mitchell GL, Cotter S, Cooper J, Kulp M, Rouse M, Borsting E, London R, Wensveen J. A randomized clinical trial of treatments for convergence insufficiency in children. Convergence Insufficiency Treatment Trial Study Group. Arch Ophthalmol. 2005;123:14–24. doi: 10.1001/archopht.123.1.14. [DOI] [PubMed] [Google Scholar]
- 16.Scheiman M, Mitchell GL, Cotter S, Kulp MT, Cooper J, Rouse M, Borsting E, London R, Wensveen J. A randomized clinical trial of vision therapy/orthoptics versus pencil pushups for the treatment of convergence insufficiency in young adults. Optom Vis Sci. 2005;82:583–95. doi: 10.1097/01.opx.0000171331.36871.2f. [DOI] [PubMed] [Google Scholar]
- 17.Scheiman M, Gwiazda J, Li T. Non-surgical interventions for convergence insufficiency. Cochrane Database Syst Rev. 2011:CD006768. doi: 10.1002/14651858.CD006768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jainta S, Bucci MP, Wiener-Vacher S, Kapoula Z. Changes in vergence dynamics due to repetition. Vision Res. 2011;51:1845–52. doi: 10.1016/j.visres.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Bucci MP, Kapoula Z, Yang Q, Bremond-Gignac D, Wiener-Vacher S. Speed-accuracy of saccades, vergence and combined eye movements in children with vertigo. Exp Brain Res. 2004;157:286–95. doi: 10.1007/s00221-004-1842-0. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez TL, Vicci VR, Alkan Y, Kim EH, Gohel S, Barrett AM, Chiaravalloti N, Biswal BB. Vision therapy in adults with convergence insufficiency: clinical and functional magnetic resonance imaging measures. Optom Vis Sci. 2010;87:985–1002. doi: 10.1097/OPX.0b013e3181fef1aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talasan H, Scheiman M, Li X, Alvarez TL. Disparity vergence responses before versus after repetitive vergence therapy in binocularly normal controls. J Vis. 2016;16:1–7. doi: 10.1167/16.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheiman M, Ciuffreda KJ, Thiagarajan P, Tannen B, Ludlam DP. Objective assessment of vergence and accommodation after vision therapy for convergence insufficiency in a child: a case report. Optom Vis Perf. 2014;2:10–5. [Google Scholar]
- 23.Thiagarajan P, Ciuffreda K. Effect of oculomotor rehabilitation on vergence responsivity in mild traumatic brain injury (mTBI) J Rehabil Res Dev. 2013;50:1223–40. doi: 10.1682/JRRD.2012.12.0235. [DOI] [PubMed] [Google Scholar]
- 24.Godwin M, Ruhland L, Casson I, MacDonald S, Delva D, Birtwhistle R, Lam M, Seguin R. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rouse M, Borsting E, Mitchell GL, Cotter SA, Kulp M, Scheiman M, Barnhardt C, Bade A, Yamada T. Validity of the convergence insufficiency symptom survey: a confirmatory study. Convergence Insufficiency Treatment Trial (CITT) Investigator Study Group. Optom Vis Sci. 2009;86:357–63. doi: 10.1097/OPX.0b013e3181989252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borsting EJ, Rouse MW, Mitchell GL, Scheiman M, Cotter S, Cooper J, Kulp MT, London R. Validity and reliability of the revised convergence insufficiency symptom survey in children aged 9–18 years. Optom Vis Sci. 2003;80:832–8. doi: 10.1097/00006324-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Scheiman M, Cotter S, Rouse M, Mitchell GL, Kulp M, Cooper J, Borsting E. Randomised clinical trial of the effectiveness of base-in prism reading glasses versus placebo reading glasses for symptomatic convergence insufficiency in children. Br J Ophthalmol. 2005;89:1318–23. doi: 10.1136/bjo.2005.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y, Kim EH, Alvarez TL. VisualEyes: a modular software system for oculomotor experimentation. J Vis Exp. 2011:49. doi: 10.3791/2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han SJ, Guo Y, Granger-Donetti B, Vicci VR, Alvarez TL. Quantification of heterophoria and phoria adaptation using an automated objective system compared to clinical methods. Ophthalmic Physiol Opt. 2010;30:95–107. doi: 10.1111/j.1475-1313.2009.00681.x. [DOI] [PubMed] [Google Scholar]
- 30.Kotulak JC, Schor CM. The effects of optical vergence, contrast, and luminance on the accommodative response to spatially bandpass filtered targets. Vision Res. 1987;27:1797–806. doi: 10.1016/0042-6989(87)90108-8. [DOI] [PubMed] [Google Scholar]
- 31.Kim EH, Granger-Donetti B, Vicci VR, Alvarez TL. The relationship between phoria and the ratio of convergence peak velocity to divergence peak velocity. Invest Ophthalmol Vis Sci. 2010;51:4017–27. doi: 10.1167/iovs.09-4560. [DOI] [PubMed] [Google Scholar]
- 32.Kim E, Alvarez TL. The frequency of saccades correlates to peak velocity in symmetrical disparity vergence. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:1664–7. doi: 10.1109/IEMBS.2011.6090479. [DOI] [PubMed] [Google Scholar]
- 33.Kim EH, Alvarez TL. The frequency of horizontal saccades in near and far symmetrical disparity vergence. Vision Res. 2012;63:9–19. doi: 10.1016/j.visres.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez TL, Kim EH. Analysis of saccades and peak velocity to symmetrical convergence stimuli: binocularly normal controls compared to convergence insufficiency patients. Invest Ophthalmol Vis Sci. 2013;54:4122–35. doi: 10.1167/iovs.13-11797. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez TL, Jaswal R, Gohel S, Biswal BB. Functional activity within the frontal eye fields, posterior parietal cortex, and cerebellar vermis significantly correlates to symmetrical vergence peak velocity: an ROI-based, fMRI study of vergence training. Front Integr Neurosci. 2014;8:50. doi: 10.3389/fnint.2014.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez TL, Bhavsar M, Semmlow JL, Bergen MT, Pedrono C. Short-term predictive changes in the dynamics of disparity vergence eye movements. J Vis. 2005;5:640–9. doi: 10.1167/5.7.4. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez TL, Semmlow JL, Yuan W, Munoz P. Comparison of disparity vergence system responses to predictable and nonpredictable stimulations. Curr Psychol Cogn. 2002;21:243–61. [Google Scholar]
- 38.Kim EH, Alvarez TL. The changes in phoria and convergence to divergence peak velocity ratio are correlated. Curr Eye Res. 2012;37:1054–65. doi: 10.3109/02713683.2012.694551. [DOI] [PubMed] [Google Scholar]
- 39.Bahill AT, Kallman JS, Lieberman JE. Frequency limitations of the two-point central difference differentiation algorithm. Biol Cybern. 1982;45:1–4. doi: 10.1007/BF00387207. [DOI] [PubMed] [Google Scholar]
- 40.Zee DS, Fitzgibbon EJ, Optican LM. Saccade-vergence interactions in humans. J Neurophysiol. 1992;68:1624–41. doi: 10.1152/jn.1992.68.5.1624. [DOI] [PubMed] [Google Scholar]
- 41.Alvarez TL, Semmlow JL, Yuan W, Munoz P. Disparity vergence double responses processed by internal error. Vision Res. 2000;40:341–7. doi: 10.1016/s0042-6989(99)00175-3. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez TL, Semmlow JL, Yuan W. Closely spaced, fast dynamic movements in disparity vergence. J Neurophysiol. 1998;79:37–44. doi: 10.1152/jn.1998.79.1.37. [DOI] [PubMed] [Google Scholar]
- 43.Hsu FK, Bahill AT, Stark L. Parametric sensitivity analysis of a homeomorphic model for saccadic and vergence eye movements. Comput Programs Biomed. 1976;6:108–16. doi: 10.1016/0010-468x(76)90032-5. [DOI] [PubMed] [Google Scholar]
- 44.Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative and Eye Movement Disorders. 4. Philadelphia: Lippincott, Williams and Wilkins; 2014. [Google Scholar]
- 45.Scheiman M, Chase C, Mitchell GL, Borsting E, Kulp MT, Cotter S CITT-RS Study Group. The effect of successful treatment of symptomatic convergence insufficiency on reading performance in school-aged children. Invest Ophthalmol Vis Sci. 2011;53 E-Abstract 6370. [Google Scholar]
- 46.Alvarez TL, Vicci VR, Alkan Y, Kim EH, Gohel S, Barrett AM, Chiaravalloti N, Biswal BB. Vision therapy in adults with convergence insufficiency: clinical and functional magnetic resonance imaging measures. Optom Vis Sci. 2010;87:985–1002. doi: 10.1097/OPX.0b013e3181fef1aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leigh RJ, Zee DS. The Neurology of Eye Movement. 5. New York: Oxford University Press; 2015. [Google Scholar]
- 48.Heitger MH, Jones RD, Macleod AD, Snell DL, Frampton CM, Anderson TJ. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain. 2009;132:2850–70. doi: 10.1093/brain/awp181. [DOI] [PubMed] [Google Scholar]
- 49.Jones R. Fusional vergence: sustained and transient components. Am J Optom Physiol Opt. 1980;57:640–4. [PubMed] [Google Scholar]
- 50.Semmlow JL, Hung GK, Horng JL, Ciuffreda K. Initial control component in disparity vergence eye movements. Ophthalmic Physiol Opt. 1993;13:48–55. doi: 10.1111/j.1475-1313.1993.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 51.Mays LE. Neural control of vergence eye movements: convergence and divergence neurons in midbrain. J Neurophysiol. 1984;51:1091–108. doi: 10.1152/jn.1984.51.5.1091. [DOI] [PubMed] [Google Scholar]
- 52.Rambold H, Neumann G, Sander T, Helmchen C. Pontine lesions may cause selective deficits of "slow" vergence eye movements. Ann N Y Acad Sci. 2005;1039:567–70. doi: 10.1196/annals.1325.069. [DOI] [PubMed] [Google Scholar]
- 53.Sander T, Sprenger A, Neumann G, Machner B, Gottschalk S, Rambold H, Helmchen C. Vergence deficits in patients with cerebellar lesions. Brain. 2009;132:103–15. doi: 10.1093/brain/awn306. [DOI] [PubMed] [Google Scholar]
- 54.Yuan W, Semmlow JL, Alvarez TL, Munoz P. Dynamics of the disparity vergence step response: a model-based analysis. IEEE Trans Biomed Eng. 1999;46:1191–8. doi: 10.1109/10.790495. [DOI] [PubMed] [Google Scholar]
- 55.Chen YF, Lee YY, Chen TS, Semmlow JL, Alvarez TL. Behaviors, models, and clinical applications of vergence eye movements. J Med Biol Eng. 2010;30:1–15. [Google Scholar]
- 56.Lee YY, Semmlow JL, Alvarez TL. Assessment of dual-mode and switched-channel models with experimental vergence responses. J Eye Movement Res. 2012;5:1–14. [Google Scholar]
- 57.Alvarez TL, Semmlow JL, Yuan W, Munoz P. Dynamic details of disparity convergence eye movements. Ann Biomed Eng. 1999;27:380–90. doi: 10.1114/1.162. [DOI] [PubMed] [Google Scholar]
- 58.Alvarez TL. A pilot study of disparity vergence and near dissociated phoria in convergence insufficiency patients before vs after vergence therapy. Front Hum Neurosci. 2015;9:419. doi: 10.3389/fnhum.2015.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pearce KL, Sufrinko A, Lau BC, Henry L, Collins MW, Kontos AP. Near point of convergence after a sport-related concussion: measurement reliability and relationship to neurocognitive impairment and symptoms. Am J Sports Med. 2015;43:3055–61. doi: 10.1177/0363546515606430. [DOI] [PMC free article] [PubMed] [Google Scholar]