Summary
Whole cell cancer vaccines are a promising strategy for treating cancer, but the characteristics of a favorable immune response are not fully understood. New insights could enable development of better vaccines, discovery of new antigens, and identification of biomarkers of efficacy. Using glyco-antigen microarrays, we demonstrate that GVAX Pancreas induces large IgG and IgM responses to many antigens, including tumor-associated carbohydrates, blood group antigens, alpha-Gal, and bovine fetuin. Antibody responses to alpha-Gal, a glycan found in fetal bovine serum (FBS) used to produce the vaccine, correlated inversely with overall survival and appear to compete with productive responses to the vaccine. H1299 lysate vaccine, produced with FBS, also induced responses to alpha-Gal and fetuin but not K562-GM, which is produced in serum free media. Our results provide new potential biomarkers to evaluate productive/unproductive immune responses and suggest that removal/reduction of FBS could improve the efficacy of whole cell vaccines.
Keywords: whole-cell vaccine, glycan microarray, anti-glycan antibody, carbohydrate antigen, immunotherapy
Graphical abstract
eTOC
Glycan microarrays were used to profile anti-carbohydrate antibody responses induced by whole cell vaccines. Large IgG and IgM responses were detected, and certain responses correlated with survival. The results provide new indicators of vaccine efficacy and provide simple strategies to improve whole cell vaccines.
Introduction
Therapeutic cancer vaccines are designed to program a patient's own immune system to recognize and eliminate tumor cells. This approach can be highly effective, often with only minimal side effects/toxicity. To date, a number of therapeutic vaccines have progressed into late stage clinical trials (Guo, et al., 2013; Melero, et al., 2014), and one vaccine, Sipuleucel-T, has gained FDA approval (Cheever and Higano, 2011). One challenge for therapeutic cancer vaccines is that clinical responses often vary considerably from one patient to another. Some patients can have remarkable and durable responses while other patients have no apparent clinical benefit. Unfortunately, the immune response(s) that contribute to improved clinical outcomes are not fully understood. This problem is particularly challenging for whole cell-based vaccines, wherein patients’ immune systems are exposed to a complex mixture of undefined antigens displayed on tumor cells. Although this strategy offers many advantages, it can be difficult to identify clinically relevant immune responses from the plethora of potential responses (de Gruijl, et al., 2008; Keenan and Jaffee, 2012). Various genomic and proteomic approaches have been applied to cancer vaccines for antigen/biomarker discovery, but results from these studies do not fully explain variations in clinical outcomes (Kulasingam and Diamandis, 2008; Rifai, et al., 2006). Therefore, a more comprehensive understanding of immune responses induced by cancer vaccines is needed.
The vast majority of studies on immune responses to cancer vaccines have focused on human protein antigens, but there are many other antigens that could be relevant. Carbohydrates are abundantly expressed on cell surfaces in the forms of glycoproteins, proteoglycans, and glycolipids, and many carbohydrates are critical for biological processes relevant to cancer, such as cell-cell adhesion and cell signaling. A number of glycans, such as the Thomsen-Friedenreich antigen (TF antigen), Tn, Sialyl Tn, GD2, GD3, Globo H, and Lewis Y, are highly over-expressed on cancer cells (Hakomori, 1989; Zhang, et al., 1997; Zhang, et al., 1997) and immunogenic (Lucas, et al., 2005; Ravindranath, et al., 2005; Slovin, et al., 1999; Tai, et al., 1985). Moreover, prior studies have shown that whole-cell vaccines can induce antibodies to carbohydrate antigens (Livingston and Ragupathi, 1997; Ravindranath and Morton, 1997). While these studies highlighted the importance of studying immune responses to carbohydrate antigens, they were limited to a small number of glycans, primarily due to technical challenges. High-throughput approaches to evaluate potential immune responses to carbohydrates could uncover new and important responses.
Antigen microarrays provide a high-throughput technology to profile serum antibody populations (Davies, et al., 2005; Geissner, et al., 2014; Muthana and Gildersleeve, 2014; Rillahan and Paulson, 2011). Our group has developed a glyco-antigen microarray containing a diverse assortment of synthetic and commercially available carbohydrate antigens, glycopeptides, and glycoproteins. We have previously used this technology to evaluate serum antibodies in healthy individuals and to identify potential biomarkers for cancer vaccines (Campbell, et al., 2014; Campbell, et al., 2013; Muthana, et al., 2015; Oyelaran and Gildersleeve, 2010; Oyelaran, et al., 2009). Herein, we used our antigen microarray to profile antibody responses induced by whole-cell vaccines. GVAX Pancreas, a granulocyte macrophage colony-stimulating factor (GM-CSF)-modified whole-cell tumor vaccine (Jaffee, et al., 2001), has shown promising results in phase II clinical trials, inducing cancer regressions in some patients with metastatic pancreatic cancer who have failed chemotherapy (Laheru, et al., 2008; Le, et al., 2015; Lutz, et al., 2011). Recent studies have provided insight into the factors that influence efficacy. For example, T cell responses to mesothelin (2004) and antibody responses to Galectin-3, Annexin A2 and thyroglobulin (De Remigis, et al., 2015; Kouo, et al., 2015; Zheng, et al., 2011) have been shown to correlate with survival. In this study, we evaluated potential responses to glyco-antigens using our microarray and demonstrated that large and frequent responses to carbohydrates and glycoproteins were induced. Potential effects of those responses on patient clinical outcomes were also assessed.
Results
Antibody responses to glycans and glycoproteins were induced by GVAX Pancreas
Our glyco-antigen microarray was used to measure serum IgG and IgM antibodies of 28 pancreatic cancer patients from a Phase II clinical trial (Lutz, et al., 2011). The microarray contained 407 components, including a variety of N-linked and O-linked glycans, glycopeptides, glycoproteins, and glycan portions of glycolipids found in human cells, as well as a number of non-human glycans. Patient antibody profiles were measured prior to the first vaccination (week 8), 8 months after the first vaccination (week 40), and after receiving a total of 3 vaccinations (week 48).
Patients on the clinical trial underwent surgery prior to vaccination. Our initial serum profiles were obtained from samples collected 8 weeks after surgery and prior to the first vaccination. Given that the patients had cancer and had undergone surgery, we first compared initial serum profiles with healthy controls to assess their overall status. Pre-vaccination IgG and IgM profiles were compared with the average antibody signals from 220 healthy subjects previously profiled in our lab (Muthana and Gildersleeve, 2016). Overall, no major differences were observed between the pancreatic cancer patients in this study and healthy subjects, indicating that the cancer patients do not have any major abnormalities or deficiencies in their anti-glycan antibody repertoires (Figure S2).
Patients were also assessed at two additional time points. After receiving their first vaccination, patients then underwent chemoradiation from weeks 12-36. Our second set of serum samples were collected at week 40, four weeks after completing chemoradiation and just before patients received their second immunization. Overall, patients had very similar array profiles at weeks 8 and 40, indicating no general immunosuppression or stimulation. For example, scatter plots of signals at baseline versus week 40 yielded Pearson coefficients ranging from 0.90 to 0.98 for individual patients (for an example, see Figure S3). Thus, the repertoire of glycan binding antibodies in serum was either largely unaffected by chemoradiation or had fully recovered. In addition, we also assayed serum samples collected at week 48, after patients had received three vaccine doses in total. Again, overall array profiles were very similar to week 8 and 40.
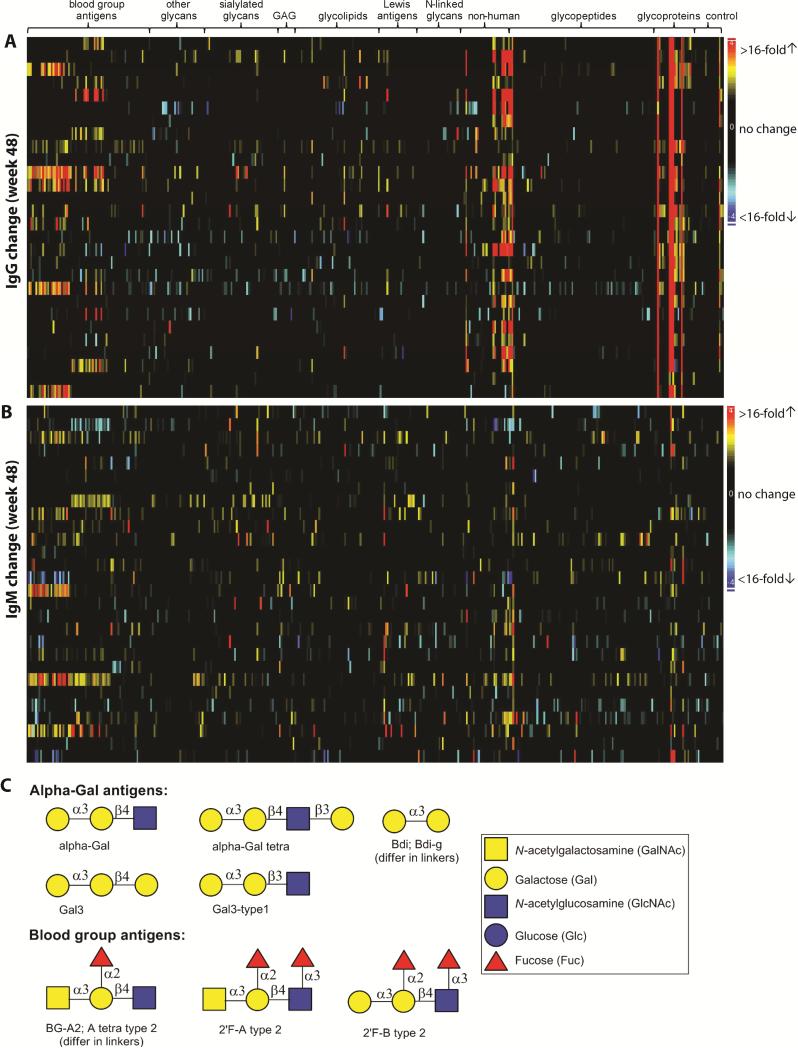
While the overall profiles at weeks 40 and 48 were mostly unchanged, sizeable responses to some individual array components were observed. Overall, the antibody changes were predominantly increased (increase/decrease = 6:1). More antibody changes were observed at week 48 (Figure 1A and 1B) compared to week 40 (Figure S4). In each patient at week 48, an average of 27 IgG and 19 IgM signals changed 4-fold or greater and, among them, 12 IgG and 4 IgM had signal increase of greater than 10-fold (Table 1, see selected antigen structures in Figure 1C).
Figure 1.
Summary of antibody changes at week 48. Heatmaps of IgG and IgM changes are shown in panels A and B, respectively. Each row contains data from the same patient and each column contains data from the same antigen. Antigens are grouped by families. The magnitude of antibody changes are denoted by different colors: black (no or low change), yellow-red (signal increase) and cyan-blue (signal decrease). Structures of a few targeted antigens are shown in panel C.
Table 1.
List of array components that had antibody changes in at least 25% of patients.
| Week 40 | Week 48 | |||||
|---|---|---|---|---|---|---|
| Family | Array component | Antibody Isotype | #pt with changesa | Average changeb,c | #pt with changes | Average change |
| Glyco-proteins | fetuin (bovine) | IgG | 23 | 44(22)** | 28 | 257(257)** |
| IgM | 3 | na | 12 | 11(3.7)** | ||
| fetuin (asialo) | IgG | 22 | 31(15)** | 27 | 233(198)** | |
| fetuin (ox) | IgG | 18 | 33(10)** | 28 | 165(165)** | |
| ovine submaxillary mucin | IgG | 0 | na | 10 | 6.3(2.8)** | |
| bovine submaxillary mucin | IgG | 1 | na | 8 | 6.9(2.6)* | |
| keyhole limpet hemocyanin | IgG | 0 | na | 8 | 5.0(2.2)** | |
| glycophorin A | IgG | 0 | na | 13 | 8.0 (3.3)** | |
| glycophorin (asialo) | IgG | 2 | na | 15 | 11(4.2)** | |
| alpha-fetoproteind | IgG | 11 | 11(3.4)** | 28 | 125(125)** | |
| gp120d | IgG | 7 | 15(3)** | 28 | 96(96)** | |
| IgM | 1 | na | 8 | 9.9** | ||
| mouse IgMd | IgG | 3 | na | 21 | 10(7.4)** | |
| Alpha-Gal antigens | alphaGal- 08 | IgG | 9 | 8.9(2.4)* | 20 | 16(9.2)** |
| alpha-Gal tetra - 17 | IgG | 6 | na | 16 | 18(7.7)** | |
| alpha-Gal tetra - 04 | IgG | 9 | 8.0(2.3)* | 25 | 22(17)** | |
| IgM | 1 | na | 17 | 8.9(4.3)** | ||
| alphaGal-6-deoxy - 11 | IgG | 6 | na | 20 | 20(11)** | |
| Gala3-type1 - 09 | IgG | 5 | na | 16 | 21(7.3)** | |
| IgM | 1 | na | 8 | 6.0(2.2)* | ||
| Gal3- 07 | IgG | 5 | na | 15 | 17(5.9)** | |
| Bdi -23 | IgG | 5 | na | 14 | 20(5.7)** | |
| Bdi-g - 16 | IgG | 4 | na | 14 | 16(5.8)** | |
| Bdi-g - 06 | IgG | 3 | na | 17 | 12(5.1)** | |
| Blood Group antigens | 2'F-B type 2-Sp - 15 | IgG | 0 | na | 9 | 9.4(1.9) |
| 2'F-B type 2-Sp - 07 | IgG | 0 | na | 10 | 9.4(2.0) | |
| 2'F-B type 2-Sp - 03 | IgG | 0 | na | 8 | 8.6(1.9) | |
| BG-A2-04 | IgG | 4 | na | 9 | 14(2.5) | |
| A tetra type 2-Sp - 07 | IgG | 2 | na | 7 | 11(2.0) | |
| A tetra type 2-Sp - 05 | IgG | 2 | na | 8 | 11(2.3) | |
| 2'F-A type 2-Sp - 05 | IgG | 3 | na | 8 | 2.6(15) | |
| Lewis | LeY -08 | IgM | 1 | na | 8 | 17(2.8)* |
| Glycolipid | GM3(Gc)-Sp - 14 | IgG | 1 | na | 9 | 7.4(2.1)** |
The number of patients are those who had at least a 4-fold change relative to baseline signals.
The average fold-change was calculated for all patients (in parenthesis) and for selected patients who had antibody changes (no parenthesis). If the change was observed in <7 patients, fold-change was not calculated and “na” was listed.
ANOVA was used to compare post-vaccination signals and baseline signals in all patients: p<0.05 (*), p≤0.001(**).
Responses to these array components were due to FBS contamination.
We were particularly interested in responses to glycans that are overexpressed on cancer cells, referred to as tumor-associated carbohydrate antigens. Approximately 100 components on our array contain glycan epitopes that have increased expression on cancer cells, and antibody responses to many of them were observed by week 48. The largest and most frequent IgG responses to known tumor-associated antigens were directed towards Sialyl LeX, the Neu5Gc variant of ganglioside GM3 [GM3(Gc)], and Sialyl Tn [as presented on ovine submaxillary mucin (OSM)]. For example, IgG responses to Sialyl LeX were observed in 6 patients, with one patient having an increase of 53-fold. The largest and most frequent IgM responses to known tumor-associated antigens were directed towards Lewis Y (8 patients with responses; largest increase was 114-fold). Large (~20-40 fold) but infrequent IgM and IgG responses were observed to MUC4 glycopeptides containing the TF antigen, MUC1 peptides containing the Tn antigen, GD3, 6’Neu5Gc-LacNAc, 3’Neu5Gc-LacNAc and Lewis X.
Beyond known tumor-associated carbohydrate antigens, large responses were detected to several other human glycans. For example, IgG responses to ABO blood group antigens were observed in about one third of the patients (Table 1). Responses to blood group A and blood group B ranged from 7 to 53-fold. These responses are likely due to differences in the patients’ blood types and blood group expression on the vaccine cells. IgG responses were also observed to hyaluronan oligosaccharides, 3’KDN-LacNAc, and NGA4. Expression levels of these glycans on tumor cells have not been well characterized.
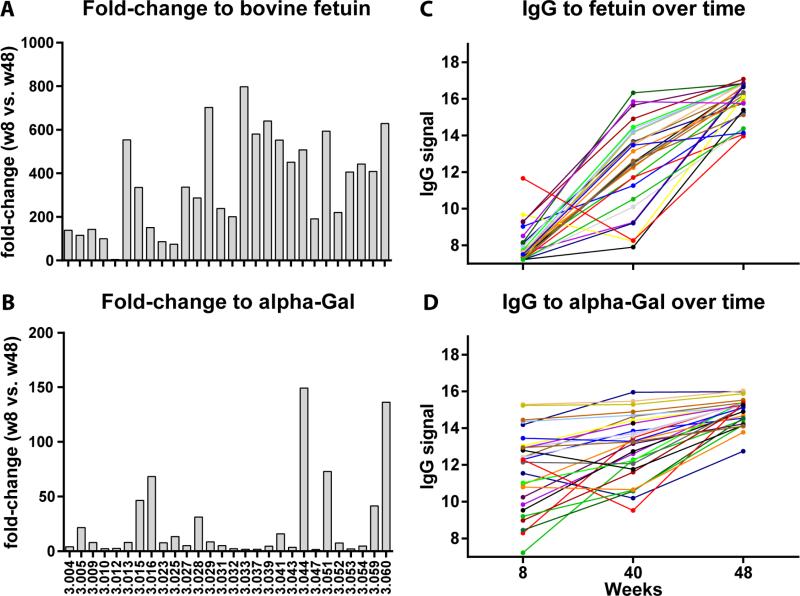
In addition to responses to tumor-associated carbohydrates and other human glycans, we also observed antibody responses to several non-human glycans and glycoproteins. Large and frequent responses were detected to bovine fetuin, a heavily glycosylated protein found at high concentrations in fetal bovine serum (Spiro, 1960). At week 40, IgG changes to bovine fetuin were observed in 82% of the patients (23/28), with an average signal increase to fetuin of 22-fold (SD=6.4) across all 28 patients. At week 48, all 28 patients had IgG responses to fetuin, with an average signal increase of 257-fold and a maximum increase of 798-fold (Figure 2A). In addition to fetuin, large and frequent responses were also observed to alpha-Gal (Figure 2B), a xenogenic terminal disaccharide epitope (Galα1-3Gal, see structure in Figure 1C) found on glycoproteins and glycolipids of non-human species (Macher and Galili, 2008). This terminal disaccharide sequence can be found on a variety of glycan carrier chains, and the carrier glycan can influence recognition by antibodies. Our array has 10 structurally similar array components with a terminal Galα1-3Gal. IgG changes were detected for 9 alpha-Gal-related array components (see alpha-Gal antigens in Table 1 and Figure 1C), although the magnitude of the change varied depending on the specific component. At week 40, 20−30% of the patients had IgG changes to alpha-Gal antigens (Table 1). At week 48, 50-90% of patients had responses to alpha-Gal, with the largest response being 150-fold. Large responses were also observed to 3 other glycoproteins (alpha-fetoprotein, mouse IgM, and gp120); however, these responses are likely due to impurities (i.e. bovine fetuin) in the samples used for the array (see below).
Figure 2.
IgG responses to bovine fetuin and alpha-Gal antigens. The fold-changes to bovine fetuin and alpha-Gal at week 48 (relative to baseline) in individual patients were shown in panels A and B, respectively. Panels C and D represented IgG responses overtime. Each line traces a patient at three time points: week 8 (baseline), week 40 (after 1 vaccine) and week 48 (after 3 vaccines). IgG signals are in Log2 scale.
Antibody responses are due to vaccination
Several lines of evidence indicate that the observed responses were due to vaccination and not due to natural/random variation over time or other treatments. First, there were significantly more increased signals than decreased signals (6:1), indicative of real antibody response rather than random fluctuations of antibody levels. Second, many of the increases in antibody signals (e.g., 150-fold for alpha-Gal and 798-fold for bovine fetuin) were far larger than any changes we have measured previously in other patients, including healthy subjects over time (Oyelaran, et al., 2009), cancer patients treated with Quadramet chemotherapy (Campbell, et al., 2014), and cancer patients treated with the cancer vaccine PROSTVAC-VF (Campbell, et al., 2014). Third, certain changes were observed in a large proportion of patients, whereas natural fluctuations to a particular antigen in healthy subjects were rarely observed in more than 10% of subjects (Muthana and Gildersleeve, 2016). Fourth, the changes for many of the antigens increased in magnitude with successive vaccinations (Figure 2C and 2D). For instance, no patients had IgG responses to Sialyl Lewis X at week 40, whereas 6 had responses at week 48. As another example, only 1 patient had an IgM response to Lewis Y at week 40, whereas 8 had responses at week 48. Finally, the measured IgG values at week 48 for Sialyl Lewis X, GM3(Gc), OSM, fetuin, and alpha-Gal were well above the normal range found in healthy subjects (at least 5 standard deviations higher). For example, average values for fetuin and alpha-Gal after the third vaccination (15.0±0.6 and 16.0±1.0,respectively, on a log base 2 scale) were well above the normal range [fetuin (7.8±1.1) and alpha-Gal (9.8±2.0) for 220 healthy subjects (Muthana and Gildersleeve, 2016)]. Taken together, the results indicate that the observed antibody responses were specifically induced by vaccine treatment with GVAX Pancreas.
Antibody responses to non-human antigens target components used for vaccine production
GVAX Pancreas is composed of two human cell lines, Panc 10.05 and Panc 6.05 (Jaffee, et al., 2001), grown in 15% fetal bovine serum (FBS). Since FBS contains high concentrations of bovine fetuin and glycoproteins glycosylated with the alpha-Gal antigen (Macher and Galili, 2008), we postulated that immune responses to alpha-Gal and fetuin were due to the presence of FBS in the vaccine formulation. To test this possibility, sera from two patients were incubated with FBS (20% v/v) and then profiled on the array to evaluate potential inhibition of IgG signals. In each case, FBS significantly inhibited IgG binding to bovine fetuin glycoproteins (up to 99% decrease) and alpha-Gal antigens (up to 83% decrease, Figure S5). The resulting IgG signal intensities for both antigens decreased to the level of baseline signals in individual patients. The only other signals that decreased significantly were alpha-fetoprotein, gp120 and mouse IgM (Figure S6). All three of these glycoproteins were produced in cell culture and likely contain small amounts of FBS glycoproteins as impurities (Figure S7). Finally, we also incubated patient sera with bovine fetuin, human fetuin A, or human fetuin B. While bovine fetuin inhibited IgG binding, neither human protein had any effect on signals to bovine fetuin or any other array components (data not shown). Therefore, the antibody responses are directed specifically to bovine fetuin and do not cross-react with human fetuin.
Evaluation of potential correlations with survival
We next evaluated whether any of the observed antibody responses correlated with patient clinical outcomes. We note that these analyses are retrospective and that the clinical trial was not powered to evaluate correlations of our data with survival. Nevertheless, assessment of potential relationships with survival is useful for discovery purposes. We only considered array components where at least 25% of the patients had a significant antibody response. While this conservative criterion does not allow for detection of infrequent but important responses, it minimizes the number of false positives in the survival analysis. The 34 IgG and 5 IgM antibody changes that met the criteria (see Table 1) were assessed for potential correlations with survival using Cox regression analysis. Hazard ratios (HR) and p-values for all antibody changes are listed in Table 2. While none of the IgM changes correlated with survival, several of the IgG changes correlated inversely with survival (HR>1). In particular, IgG responses to apha-Gal-08 and alpha-Gal tetra-04 showed correlations with survival at both week 40 and week 48 [apha-Gal-08: HR=1.73, p=0.003 (week 40), HR=1.27, p=0.049 (week 48); alpha-Gal tetra-04: HR=1.64, p=0.007 (week 40), HR=1.38, p=0.031 (week 48)]. To assess the probability of obtaining this result by chance, we randomized survival data 200 times and analyzed each permutation by Cox regression. The frequency of getting four hits with similar p-values was less than 5%. Moreover, the likelihood of getting four hits with similar p-values all from the alpha-Gal family was less than 2%. If one only includes cases where all four hits have consistent hazard ratios (either all four hits have HR > 1.0 or all four have HR < 1.0), the frequency is even lower. Finally, the likelihood that either alpha-Gal-08 or alpha-Gal tetra-04 correlated with survival at both week 40 and week 48 was less than 2%. Taken together, the permutation test indicates that our results occur infrequently by chance. Four other array components with a terminal Galα1-3Gal epitope showed similar trends with survival, with p-values in the range of 0.05−0.102 (alpha-Gal tetra-17, Bdi-g-06, Bdi-g-16, Gal3-07). Beyond alpha-Gal, IgG responses to bovine fetuin, glycophorin A, glycolipid GM3(Gc), and Sialyl LeX showed a trend toward a correlation with survival, and continued evaluation of these responses in the future may be worthwhile. IgG responses to blood group antigens, and IgM responses to LeY were not correlated to survival. Responses to other tumor-associated antigens, such as MUC4 glycopeptides containing the TF antigen, MUC1 peptides containing the Tn antigen, GD3, 6’Neu5Gc-LacNAc, 3’Neu5Gc-LacNAc and Lewis X, were too infrequent to assess relationships with clinical outcomes.
Table 2.
Summary of Cox regression results for selected IgG responses.
| Array componenta | Time point | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|---|
| alphaGal- 08 | week 40 | 1.73 | 1.20-2.50 | 0.003* |
| alphaGal- 08 | week 48 | 1.27 | 1.00-1.62 | 0.049* |
| alpha-Gal tetra - 04 | week 40 | 1.64 | 1.14-2.34 | 0.007* |
| alpha-Gal tetra - 04 | week 48 | 1.38 | 1.03-1.84 | 0.031* |
| alpha-Gal tetra - 17 | week 48 | 1.24 | 0.97-1.56 | 0.089 |
| alphaGal-6-deoxy - 11 | week 48 | 1.10 | 0.89-1.37 | 0.375 |
| Bdi-g - 06 | week 48 | 1.24 | 0.96-1.62 | 0.102 |
| Bdi-g - 16 | week 48 | 1.24 | 1.00-1.55 | 0.050 |
| Bdi -23 | week 48 | 1.04 | 0.84-1.28 | 0.730 |
| Gal3- 07 | week 48 | 1.20 | 0.98-1.48 | 0.079 |
| Gala3-type1 - 09 | week 48 | 1.13 | 0.94-1.35 | 0.195 |
| GM3(Gc)-Sp - 14 | week 48 | 1.25 | 0.96-1.63 | 0.105 |
| fetuin (bovine) | week 48 | 1.48 | 0.94-2.34 | 0.091 |
| fetuin (asialo) | week 48 | 1.34 | 0.94-1.92 | 0.107 |
| Fetuin (ox) | week 48 | 1.32 | 0.89-1.96 | 0.161 |
| Glycophorin A | week 48 | 1.33 | 0.93-1.91 | 0.118 |
Responses to other components listed in Table 1 were not correlated with survival and had p-values >0.05.
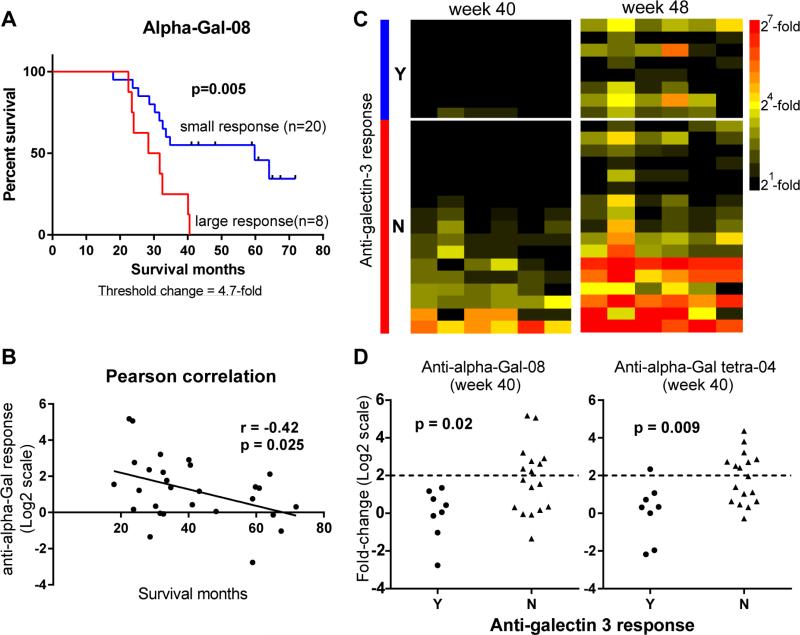
To further evaluate the results from Cox regression, the four alpha-Gal hits were assessed using Kaplan-Meier analysis. For each antigen, patients were stratified into two groups based on the magnitude of antibody response: large response group vs. low/no response group. The threshold for stratifying patients was systematically varied to evaluate consistency. After completing the analysis, multiple cutoffs resulted in a p-value of less than 0.05 for both alpha-Gal-08 and alpha-Gal tetra-04 at both week 40 and 48 (Table S2). Taking alpha-Gal-08 at week 40 as an example, 16 out of 27 stratifications resulted in a p-value less than 0.05. Using the optimal threshold to stratify patients, the median survival for patients who had high anti-alpha-Gal response (≥4.7-fold) was 30 months, while the patients with little or no response (<4.7-fold) lived especially long (median survival = 60 months; p=0.005, Figure 3A). In agreement with the survival analysis, a strong association between anti-alpha Gal response and patient survival time was also observed by Pearson correlation analysis (r = -0.42, p = 0.025, Figure 3B). While the change in alpha-Gal-specific IgG correlated with survival, the pre-vaccination level was not associated with clinical outcomes (Data not shown).
Figure 3.
Relationships between alpha-Gal responses, survival, and Galectin-3 responses. A) Kaplan-Meier estimate for alpha-Gal-08-specific IgG response at week 40. Eight patients had changes greater than 4.7-fold (red line, median survival=30 months) and 20 patients had changes 4.7-fold or less (blue line, median survival=60 months, Log-rank p = 0.005). B) The magnitude of anti-alpha-Gal-08 response (Log2 scale) is inversely associated with patient survival. Pearson correlation r = −0.42 (p = 0.025). C) Heatmap of anti-alpha-Gal responses and anti-galectin-3 response at both week 40 and week 48. Data for each patient are displayed in rows. Patients are grouped by their responses to galectin-3 (blue: positive response (Y), red: negative response (N), separated by a white line). Columns represent 6 array components from the alpha-Gal antigen family. The magnitude of anti-alpha-Gal response is denoted by different colors: black (no/low response), yellow (medium response) and red (large response). D) Dot plots of anti-alpha-Gal response and anti-galectin-3 response at week 40. The dotted line indicates a cutoff of 4-fold change. p-values were calculated using Mann-Whitney test.
Responses to alpha-Gal correlate inversely with antibody responses to Galectin-3
One potential mechanistic basis for the inverse correlation is antigen competition: responses to non-human components compete with responses to the tumor antigens on the vaccine. Jaffee and coworkers recently reported that antibody responses to Galectin-3 are induced by the vaccine and correlate positively with patient long-term survival (Kouo, et al., 2015). These antibodies are thought to bind and neutralize Galectin-3, thus relieving Galectin-3 mediated immunosuppression of T cells. The Galectin-3 study was performed using the same patients used in this study. To test our mechanistic hypothesis, we evaluated the potential correlation between the reported anti-Galectin-3 responses and the non-human antigen responses observed in our study. Consistent with our hypothesis, anti-Galectin 3 responses primarily occur in patients that do not produce anti-alpha-Gal responses (Figure 3C). At week 40, both alpha-Gal antigens (alpha-Gal-08 and alpha-Gal tetra-04) demonstrated statistically significant inverse correlations with Galectin-3 responses (Mann-Whitney test: p = 0.02 for alpha-Gal-08, p = 0.009 for alpha-Gal tetra-04; Figure 3D). The correlation was strongest at week 40 but was consistent at both week 40 and week 48. Taken together, these results support the hypothesis that responses to alpha-Gal compete with responses to vaccine antigens. However, additional studies are required to more fully evaluate this hypothesis.
Responses to non-human antigens occur in other whole-cell cancer vaccines
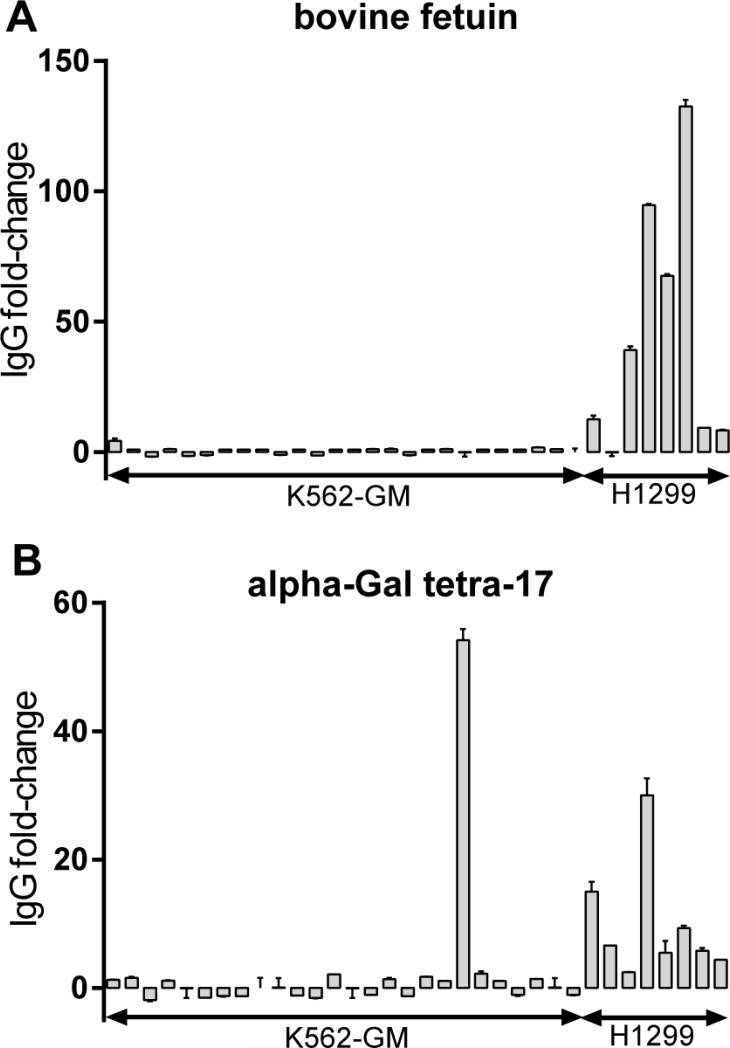
Responses to FBS components have been observed for other whole-cell vaccines (Livingston, et al., 1982; Sakamoto, et al., 2007), but responses to fetuin and alpha-Gal have not been evaluated. We next profiled responses to alpha-Gal and fetuin induced by two human cell vaccines grown under different conditions. One was derived from the K562-GM melanoma cell line grown in serum-free media lacking FBS. Like GVAX Pancreas, this vaccine is also modified to produce GM-CSF cytokine. The other vaccine was derived from the H1299 non-small cell lung carcinoma cell line grown in FBS-supplemented media. The results are summarized in Figure 4. IgG responses to both bovine fetuin and alpha-Gal antigens were observed in 7/8 patients treated with the H1299 vaccine which was grown in FBS-containing media. The average change was 45-fold to bovine fetuin and 10-fold to alpha-Gal antigens. It is worth mentioning that H1299 vaccine was washed four times to remove culture media components prior to vaccination. The magnitudes of the observed responses were lower than those observed for GVAX Pancreas patients at week 48 (fetuin: 257-fold, alpha-Gal-08:10-fold). By comparison, 25 out of 26 patients treated with the K562-GM vaccine, produced in serum-free media, had no anti-fetuin response (one patient had a modest 4-fold increase), and 25 of 26 patients also did not have anti-alpha-Gal responses. Taken together, these results indicate that the responses to non-human antigens from cell culture material are not unique to GVAX Pancreas. The effect of non-human responses to clinical outcomes could not be evaluated for these two vaccines as survival data were not available for these patients.
Figure 4.
IgG responses to bovine fetuin (A) and alpha-Gal (B) in patients treated with K562-GM and H1299 vaccines. IgG signals were measured at baseline and 7 months post-vaccination in 26 patients receiving the K562-GM vaccine and 8 patients receiving the H1299 vaccine. The fold-changes in individual patients were defined as the ratio of IgG signals at 7 month vs. baseline. Error bars represent SEM (n=2).
Discussion
Aberrant glycosylation is a hallmark of cancer, and numerous tumor-associated carbohydrate antigens are known (Hakomori, 1989; Zhang, et al., 1997; Zhang, et al., 1997). While abnormal glycans are abundantly expressed on the surface of cancer cells and could serve as potential antigens, carbohydrates are generally thought to have poor immunogenicity. Previous studies have shown that whole-cell cancer vaccines induce antibody responses to carbohydrates in humans, but the reported responses have been small in magnitude, primarily of the IgM isotype, and directed at only a handful of specific carbohydrate targets (Livingston and Ragupathi, 1997; Ravindranath and Morton, 1997).
In this study, we used a glyco-antigen microarray to evaluate human antibody responses induced by a whole-cell cancer vaccine, GVAX Pancreas. The microarray allowed us to evaluate potential responses to a much wider range of glyco-antigens than previously examined, including many gangliosides, N-linked glycans, O-linked glycans, glycopeptides, blood-group antigens, and Lewis antigens. Our results reveal much larger and more diverse responses to carbohydrate antigens than previously reported for whole-cell cancer vaccines. For example, large IgG and IgM responses to Sialyl LeX, the Neu5Gc variant of ganglioside GM3, Sialyl Tn, and Lewis Y were detected. Responses were also observed to ABO blood-group antigens, MUC4 glycopeptides containing the TF antigen, MUC1 peptides containing the Tn antigen, GD3, 6’Neu5Gc-LacNAc, 3’Neu5Gc-LacNAc and Lewis X. The responses to these antigens are likely induced by glycans found on the vaccine cell lines. The responses appear to be a direct result of vaccination, as the changes were much larger in magnitude than changes observed over time in non-vaccinated individuals, increased with successive vaccinations, and resulted in final antibody levels well above the normal range for healthy subjects.
In addition to responses to tumor-associated carbohydrate antigens and blood-group antigens, frequent responses were also observed to bovine fetuin and the alpha-Gal antigen, with increases as high as 798-fold and 150-fold, respectively. Antibody responses to bovine fetuin and the alpha-Gal antigen are likely due to the presence of minor amounts of FBS in the vaccine formulation. These antigens are not found in humans or human cell lines, but they are present in FBS. In support of this hypothesis, we observed responses to alpha-Gal and fetuin in patients treated with another whole-cell vaccine grown in FBS (H1299) but not in patients treated with the K562-GM whole-cell vaccine grown in serum free media. Moreover, FBS and bovine fetuin could inhibit binding of the serum antibodies to bovine fetuin and alpha-Gal antigens on the array. Responses to FBS components have been observed in other studies involving human cell lines. For example, antibody responses to bovine apolipoprotein B-100, another FBS component, have been observed in patients treated with 3 different types of cell-based therapies, all derived from cell culture (Sakamoto, et al., 2007). In another study, patients vaccinated with cultured melanoma cells produced antibody responses to components in fetal bovine serum, but the targeted antigens were not specified (Livingston, et al., 1982). Although antibody responses to FBS components have been reported previously, relationships between response and vaccine efficacy in patients have not been well studied.
Using a variety of statistical approaches, potential correlations with patient survival on GVAX Pancreas were evaluated. Antibody responses to the alpha-Gal antigen demonstrated the most consistent relationships with survival. For example, at the best threshold (≥4.7-fold) in the Kaplan-Meier analysis, patients with low or no anti-alpha-Gal response after one vaccine dose had remarkably long survival (median = 60 months), as compared to patients with large antibody responses (median = 30 months, difference in median survival = 30 months; p = 0.005). This inverse correlation was consistent when we varied the threshold used to assign patients to strata in the Kaplan-Meier analysis. It is important to mention that the median survival of patients with alpha-Gal responses (30 months) was on par with the average survival of non-vaccinated pancreas cancer patients treated with surgery and adjuvant chemoradiation in another study, suggesting that a response to alpha-Gal does not decrease survival. We note that these analyses were retrospective and involved a limited number of patients. Therefore, further confirmation with additional patients and prospective analysis are needed to fully understanding relationships with survival. Nevertheless, our results provide new insights into the possible roles of these responses and offer new potential biomarkers for evaluating productive/unproductive immune responses.
One likely mechanism for the inverse correlation is antigen competition. When multiple antigens are present, the immune system often focuses on a subset. For instance, antigen competition can lead to the suppression of responses to certain antigens within a multi-component vaccine even when all components are immunogenic on their own (Sun, et al., 2006). As another example, when a peptide immunogen contains small impurities due to epimerization of a C-α stereocenter, antibody responses can primarily target the immunodominant unnatural peptide, even when it is only a minor component (Pollack, et al., 1989). GVAX Pancreas contains human cells and FBS materials, both of which can be targeted by the immune system. Although FBS materials are very minor components in the vaccine formulation, they are more immunogenic than the tumor antigens present on the vaccine cells. In support of this hypothesis, we found that there was an inverse correlation between the anti-alpha-Gal responses and the anti-galectin-3 response, a favorable response associated with improved survival on GVAX Pancreas (Kouo, et al., 2015). One interesting implication of this hypothesis is that vaccine efficacy might be improved by reducing or eliminating competition with FBS, either through growth in serum free media or via more extensive washing of the cells.
Immune responses to fetuin and alpha-Gal could be relevant to many other vaccines and cell based therapies. A number of FDA-approved vaccines are manufactured from cells grown in FBS-supplement media, and FBS is listed as a minor component of these vaccines (Hamborsky, 2015). Many human cell-based vaccines and therapies currently in clinical trials are also produced using media supplemented with FBS, although some studies favor the use of serum-free media (Mannello and Tonti, 2007). Adsorption of xeno-antigens on human cell lines has been previously reported in in vitro and animal studies (Heiskanen, et al., 2007; Kerbel and Blakeslee, 1976; Toldbod, et al., 2003). Washing of cells can reduce the amount of FBS, but some components can be retained after washing. In support of this point, the H1299 cells in our study had been washed four times prior to immunization in patients. Although anti-fetuin and anti-alpha-Gal responses were observed, the magnitudes were lower. Based on the mechanistic hypothesis, we would anticipate that the presence of FBS would be most relevant for vaccines with low inherent immunogenicity, such as human tumor cells. It is important to note that the presence of FBS in a vaccine formulation does not preclude clinical use, as vaccines containing FBS have been FDA approved. Also, some vaccines that have minor amounts of FBS during pre-clinical and clinical trials may be produced without FBS when preparing clinical grade material. In these cases, potential antibody responses to FBS would be most relevant for the design and analysis of pre-clinical and clinical trials.
In broader terms, our current studies on GVAX Pancreas, the H1299 vaccine, and the K562-GM vaccine combined with our previous study on PROSTVAC-VF (Campbell, et al., 2014) highlight several key points. First, antibody responses to glyco-antigens were found to be large and potentially correlated with survival for both vaccines where survival analysis was possible. Therefore, continued and expanded studies on responses to glyco-antigens could provide further insight for vaccine development and biomarker discovery. Second, analysis of immune responses to carbohydrates can be useful even when the vaccine is not designed to stimulate a response to glycans. For example, PROSTVAC-VF is designed to stimulate a T-cell response to prostate specific antigen; however, an antibody response to a glycan (Forssman antigen) on the poxvirus surface was found to consistently correlate with survival (Campbell, et al., 2014). Third, immune responses can be extremely difficult to predict; therefore, it is advantageous to evaluate potential responses to a broad spectrum of antigens, even ones that are not anticipated to be relevant. Fourth, some key anti-carbohydrate responses for each of the vaccines were related to the production method. Thus, analysis of immune responses to glyco-antigens may be helpful for optimizing vaccine production. Finally, the results highlight the utility of glyco-antigen microarrays as chemical tools to probe biological systems.
Significance
Whole cell cancer vaccines can produce long lasting remissions and several have progressed into late stage clinical trials. While promising, clinical responses vary substantially from patient to patient. Advances in this area have been limited by an incomplete understanding of what constitutes a favorable immune response, what factors lead to a favorable response, and why responses vary among patients. Although whole cell vaccines display a diverse assortment of glycans to the immune system, little is known about responses to carbohydrates and their contributions. Herein, we used a glyco-antigen microarray to profile IgG and IgM responses induced by GVAX Pancreas, a whole cell cancer vaccine in late stage clinical trials for pancreas cancer. The microarray revealed much larger and more diverse responses to carbohydrate antigens than previously reported for whole-cell cancer vaccines. In particular, both IgG and IgM antibody responses were induced to a variety of tumor-associated carbohydrate antigens, blood group antigens, and other glycans. Unexpectedly, large responses to two non-human antigens, bovine fetuin and alpha-Gal, were also observed. Responses to these glycans were found to be derived from fetal bovine serum (FBS) used in the growth media for the cells. Responses to alpha-Gal antigens correlated inversely with survival and appear to compete with productive responses to the vaccine. Large IgG responses to alpha-Gal were also detected in patients treated with an H1299 lysate vaccine, which is produced with FBS, but not in patients treated with a K562-GM vaccine, which is produced in serum-free media. Taken together, the results highlight the utility of glyco-antigen microarrays for evaluating immune responses to vaccines and suggest that removal/reduction of FBS could potentially be a simple strategy to improve vaccine efficacy.
Experimental Procedures
Serum samples
Serum samples for GVAX Pancreas (n=28) were generously provided by Dr. Elizabeth Jaffee (Johns Hopkins University) under Institutional Review Board approval. They were collected from a previously reported Phase II clinical trial (NCT00084383) (Lutz, et al., 2011). Patients who were disease-free and eligible for receiving at least three vaccine doses were used in this study. Serum samples for K562-GM whole-cell vaccine (n=26) and H1299 tumor lysate vaccine (n=8) were obtained from the National Cancer Institute from two phase I/II clinical trials (NCT01313429 and NCT02054104, respectively). They were collected at baseline and one month following completion of six monthly vaccinations. The reference serum was pooled from 10 healthy subjects (Valley Biomedical Products and Services, Winchester, VA). All sera were aliquoted and stored at −80 °C until use. All clinical and survival data were blinded during data acquisition and processing.
Glyco-antigen microarray fabrication, binding assay, and data processing
Detailed procedures for fabricating the microarray, carrying out the assay, and processing the data have been reported previously (Campbell, et al., 2010; Xia and Gildersleeve, 2015). Briefly, glycans and glycopeptides used for microarrays were chemically conjugated to either bovine serum albumin (BSA) or human serum albumin (HSA) to produce neoglycoproteins prior to printing. Some were conjugated at different densities by varying the number of glycans per BSA or HSA, and the average number of glycans per molecule of albumin is indicated by the number following the abbreviation of the component names. A total of 407 array components were printed in duplicate on epoxy-coated slides. Each slide contained 16 identical arrays. Validation of the array platform (Manimala, et al., 2006; Manimala, et al., 2007) and analysis of reproducibility (Oyelaran, et al., 2009) have been published previously. Quality of this batch of slides was assessed by microarray scanner to detect printing defects (for a representative image, see Figure S1) and testing with a standard set of 3 lectins (ConA, WGA, and HPA) on representative slides. All serum samples were profiled in duplicate at 1:50 dilution. Antibody binding were detected by fluorescently labeled secondary anti-human antibodies (DyLight 549 anti-Human IgG and DyLight 649 anti-human IgM from Jackson ImmunoResearch) and quantified using microarray scanner (GenePix 4000B). The fold-change of an antibody signal was defined as the ratio of post-vaccination signal vs. prevaccination signal. We previously found that serum anti-glycan antibody levels fluctuate naturally over time, but changes of 4-fold or larger occur only rarely, even in prostate cancer patients treated with docetaxel and prostate cancer patients in the control arm of a clinical trial on PROSTVAC-VF (Campbell, et al., 2014; Oyelaran, et al., 2009). Therefore, a 4-fold change threshold was used to identify antibody responses induced by GVAX Pancreas. Additional details can be found in the Supplemental Information. The microarray data have been deposited in National Center for Biotechnology Information's (NCBI) Gene Expression Omnibus (GEO) (Edgar, et al., 2002) and are accessible through GEO Series accession number GSE83087.
Statistical methods: Cox regression and Kaplan-Meier analysis
All statistical analyses were performed using Partek Genomics Suite 6.6, GraphPad Prism 6 or Excel. Array components with at least 4-fold change in antibody signals in more than 25% patients (≥7 patients) were selected for survival analysis. The candidates that met these criteria were first analyzed by Cox regression with 95% confidence interval (CI) to identify potential hits that correlated with survival. The false positive rate for each candidate was estimated with Cox regression analysis from 200 permutations with randomized survivals. Hits identified from Cox regression were further assessed individually using Kaplan-Meier analysis. For each hit, Kaplan-Meier analysis was used to estimate the differences in median survival between patients having large antibody response and those with lower or no antibody response. To evaluate consistency, we systematically varied the number of patients in each stratum and estimated survival for all possible stratifications. The number of stratifications with Log-rank p-values less than 0.05 were listed in Table S2.
Supplementary Material
Highlights.
- antibody responses to a whole-cell vaccine were profiled with a glycan microarray
-large IgG and IgM responses to FBS antigens and tumor antigens were observed
-responses to non-human antigens correlated inversely with patient survival
-responses to non-human antigens out-compete productive response to the vaccine
Acknowledgements
We thank the Consortium for Functional Glycomics (GM62116; The Scripps Research Institute), Professors Tom Tolbert (University of Kansas), Lai-Xi Wang (University of Maryland), Xuefei Huang (Michigan State University), Todd Lowary (University of Alberta) and Dr. Joseph Barchi (National Cancer Institute) for contributing glycans for the array. We thank Dr. Elizabeth Jaffee (Johns Hopkins University) for providing serum samples for this study and Dr. Eric Sterner for assistance in the permutation test. This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
L.X. and J.C.G designed the research. L.X. performed the experiment, analyzed data and wrote the paper. D.S.S. collected serum samples for H1299 and K562-GM vaccines, analyzed data, and edited the manuscript.
Note: this manuscript includes data from subjects involved in three clinical trials: Registry numbers NCT00084383 and NCT01313429, and NCT02054104.
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- Campbell CT, Gulley JL, Oyelaran O, Hodge JW, Schlom J, Gildersleeve JC. Humoral response to a viral glycan correlates with survival on PROSTVAC-VF. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E1749–1758. doi: 10.1073/pnas.1314722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CT, Gulley JL, Oyelaran O, Hodge JW, Schlom J, Gildersleeve JC. Serum antibodies to blood group A predict survival on PROSTVAC-VF. Clin. Cancer. Res. 2013;19:1290–1299. doi: 10.1158/1078-0432.CCR-12-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CT, Zhang Y, Gildersleeve JC. Construction and use of glycan microarrays. Curr. Protoc. Chem. Biol. 2010;2:37–53. doi: 10.1002/9780470559277.ch090228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer. Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- Davies DH, Liang XW, Hernandez JE, Randall A, Hirst S, Mu YX, Romero KM, Nguyen TT, Kalantari-Dehaghi M, Crotty S, et al. Profiling the humoral immune response to infection by using proteome microarrays: High-throughput vaccine and diagnostic antigen discovery. Proc. Natl. Acad. Sci. U. S. A. 2005;102:547–552. doi: 10.1073/pnas.0408782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gruijl TD, van den Eertwegh AJM, Pinedo HM, Scheper RJ. Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol. Immunother. 2008;57:1569–1577. doi: 10.1007/s00262-008-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Remigis A, de Gruijl TD, Uram JN, Tzou SC, Iwama S, Talor MV, Armstrong TD, Santegoets SJ, Slovin SF, Zheng L, et al. Development of thyroglobulin antibodies after GVAX immunotherapy is associated with prolonged survival. Int. J. Cancer. 2015;136:127–137. doi: 10.1002/ijc.28973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissner A, Anish C, Seeberger PH. Glycan arrays as tools for infectious disease research. Curr. Opin. Chem. Biol. 2014;18:38–45. doi: 10.1016/j.cbpa.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Adv. Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989;52:257–331. doi: 10.1016/s0065-230x(08)60215-8. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . In: Epidemiology and Prevention of Vaccine-Preventable Diseases. 13 Edition Hamborsky J, Kroger A, Wolfe S, editors. Public Health Foundation; Washington D.C.: 2015. [Google Scholar]
- Heiskanen A, Satomaa T, Tiitinen S, Laitinen A, Mannelin S, Impola U, Mikkola M, Olsson C, Miller-Podraza H, Blomqvist M, et al. N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells. 2007;25:197–202. doi: 10.1634/stemcells.2006-0444. [DOI] [PubMed] [Google Scholar]
- Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J. Clin. Oncol. 2001;19:145–156. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- Keenan BP, Jaffee EM. Whole Cell Vaccines-Past Progress and Future Strategies. Semin. Oncol. 2012;39:276–286. doi: 10.1053/j.seminoncol.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS, Blakeslee D. Rapid adsorption of a foetal calf serum component by mammalian cells in culture. A potential source of artifacts in studies of antisera to cell-specific antigens. Immunology. 1976;31:881–891. [PMC free article] [PubMed] [Google Scholar]
- Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015;3:412–423. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat. Clin. Pract. Oncol. 2008;5:588–599. doi: 10.1038/ncponc1187. [DOI] [PubMed] [Google Scholar]
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin. Cancer. Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, Morse M, Zeh H, Cohen D, Fine RL, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston PO, Ragupathi G. Carbohydrate vaccines that induce antibodies against cancer .2. Previous experience and future plans. Cancer Immunol. Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston PO, Watanabe T, Shiku H, Houghton AN, Albino A, Takahashi T, Resnick LA, Michitsch R, Pinsky CM, Oettgen HF, et al. Serological response of melanoma patients receiving melanoma cell vaccines. I. Autologous cultured melanoma cells. Int. J. Cancer. 1982;30:413–422. doi: 10.1002/ijc.2910300406. [DOI] [PubMed] [Google Scholar]
- Lucas AH, Apicella MA, Taylor CE. Carbohydrate moieties as vaccine candidates. Clin. Infect. Dis. 2005;41:705–712. doi: 10.1086/432582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann. Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim. Biophys. Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-throughput carbohydrate microarray analysis of 24 lectins. Angew. Chem. 2006;45:3607–3610. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology. 2007;17:17C–23C. doi: 10.1093/glycob/cwm047. [DOI] [PubMed] [Google Scholar]
- Mannello F, Tonti GA. Concise review: No breakthroughs for human mesenchymal and embryonic stem cell culture: Conditioned medium, feeder layer, or feeder-free; Medium with fetal calf serum, human serum, or enriched plasma; Serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells. 2007;25:1603–1609. doi: 10.1634/stemcells.2007-0127. [DOI] [PubMed] [Google Scholar]
- Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- Muthana SM, Gildersleeve JC. Factors Affecting Anti-Glycan IgG and IgM Repertoires in Human Serum. Sci. Rep. 2016;6:19509. doi: 10.1038/srep19509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthana SM, Gildersleeve JC. Glycan microarrays: powerful tools for biomarker discovery. Cancer biomarkers : section A of Disease markers. 2014;14:29–41. doi: 10.3233/CBM-130383. [DOI] [PubMed] [Google Scholar]
- Muthana SM, Xia L, Campbell CT, Zhang YL, Gildersleeve JC. Competition between Serum IgG, IgM, and IgA Anti-Glycan Antibodies. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelaran O, Gildersleeve JC. Evaluation of human antibody responses to keyhole limpet hemocyanin on a carbohydrate microarray. Proteomics Clin. Appl. 2010;4:285–294. doi: 10.1002/prca.200900130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyelaran O, McShane LM, Dodd L, Gildersleeve JC. Profiling human serum antibodies with a carbohydrate antigen microarray. J. Proteome Res. 2009;8:4301–4310. doi: 10.1021/pr900515y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack SJ, Hsiun P, Schultz PG. Stereospecific Hydrolysis of Alkyl Esters by Antibodies. J. Am. Chem. Soc. 1989;111:5961–5962. [Google Scholar]
- Ravindranath MH, Morton DL. Immunogenicity of membrane-bound gangliosides in viable whole-cell vaccines. Cancer Invest. 1997;15:491–499. doi: 10.3109/07357909709047588. [DOI] [PubMed] [Google Scholar]
- Ravindranath MH, Muthugounder S, Presser N, Ye X, Brosman S, Morton DL. Endogenous immune response to gangliosides in patients with confined prostate cancer. Int. J. Cancer. 2005;116:368–377. doi: 10.1002/ijc.21023. [DOI] [PubMed] [Google Scholar]
- Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotechnol. 2006;24:971–983. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- Rillahan CD, Paulson JC. Glycan microarrays for decoding the glycome. Annu. Rev. Biochem. 2011;80:797–823. doi: 10.1146/annurev-biochem-061809-152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto N, Tsuji K, Muul LM, Lawler AM, Petricoin EF, Candotti F, Metcalf JA, Tavel JA, Lane HC, Urba WJ, et al. Bovine apolipoprotein B-100 is a dominant immunogen in therapeutic cell populations cultured in fetal calf serum in mice and humans. Blood. 2007;110:501–508. doi: 10.1182/blood-2007-01-066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro RG. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J. Biol. Chem. 1960;235:2860–2869. [PubMed] [Google Scholar]
- Sun W, Nisalak A, Gettayacamin M, Eckels KH, Putnak JR, Vaughn DW, Innis BL, Thomas SJ, Endy TP. Protection of Rhesus monkeys against dengue virus challenge after tetravalent live attenuated dengue virus vaccination. J. Infect. Dis. 2006;193:1658–1665. doi: 10.1086/503372. [DOI] [PubMed] [Google Scholar]
- Tai T, Cahan LD, Tsuchida T, Saxton RE, Irie RF, Morton DL. Immunogenicity of melanoma-associated gangliosides in cancer patients. Int. J. Cancer. 1985;35:607–612. doi: 10.1002/ijc.2910350507. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH, Jaffee EM. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J. Exp. Med. 2004;200:297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toldbod HE, Agger R, Bolund L, Hokland M. Potent influence of bovine serum proteins in experimental dendritic cell-based vaccination protocols. Scand. J. Immunol. 2003;58:43–50. doi: 10.1046/j.1365-3083.2003.01267.x. [DOI] [PubMed] [Google Scholar]
- Xia L, Gildersleeve JC. The Glycan Array Platform as a Tool to Identify Carbohydrate Antigens. Methods Mol. Biol. 2015;1331:27–40. doi: 10.1007/978-1-4939-2874-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, CordonCardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, Lloyd KO, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry .1. Focus on gangliosides. Int. J. Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Zhang HS, CordonCardo C, Reuter VE, Singhal AK, Lloyd KO, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry .2. Blood group-related antigens. Int. J. Cancer. 1997;73:50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT, et al. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011;6:e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.