Abstract
Waldenström macroglobulinemia/lymphoplasmacytic lymphoma (WM/LPL) is characterized by lymphoplasmacytic proliferation, lymph node and spleen enlargement, bone marrow involvement, and immunoglobulin M production. Treatment varies based on the extent and biology of disease. In some patients, the use of allogeneic hematopoietic cell transplantation (alloHCT) may have curative potential. We evaluated long-term outcomes of 144 patients that received adult alloHCT for WM/LPL. Data was obtained from the Center for International Blood and Marrow Transplant Research database (2001-2013). Patients received myeloablative (n=67) or reduced intensity conditioning (RIC; n=67). Median age at alloHCT was 53 years, and median time from diagnosis to transplantation was 41 months. Thirteen percent (n=18) failed prior autologous hematopoietic cell transplantation. About half (n=82, 57%) had chemo-sensitive disease at the time of transplantation, while 22% had progressive disease. Progression free survival, overall survival, rate of relapse, and non-relapse mortality at 5-years were 46%, 52%, 24%, and 30% respectively. Patients with chemo-sensitive disease and better pre-transplant disease status experienced significantly superior overall survival. There were no significant differences in progression-free survival based on conditioning (myeloablative 50% vs. RIC 41%) or graft source. Conditioning intensity did not impact treatment-related mortality or relapse. The most common causes of death were primary disease and graft-versus-host disease (GVHD). AlloHCT yielded durable survival in select patients with WM/LPL. Strategies to reduce mortality from GVHD and post-transplant relapse are necessary to improve this approach.
Keywords: relapsed lymphoma, allogeneic stem cell transplant
Introduction
Waldenström macroglobulinemia (WM) is a rare B-cell lymphoproliferative malignancy characterized by clonal paraprotein production of immunoglobulin (Ig)M and bone marrow involvement by lymphoplasmacytic lymphoma (LPL).1 WM/LPL accounts for 5% of non-Hodgkin lymphomas (NHL).2,3 WM/LPL is considered to be incurable, with a median survival of about 6 years.4 Patients with high-risk disease have a median survival of less than 3 years and are candidates for more aggressive therapeutic approaches, including hematopoietic cell transplantation (HCT).5-7
As with other indolent forms of NHL, allogeneic hematopoietic cell transplant (alloHCT) offers an immune based graft-versus-lymphoma response and may be curative.8 Given the rarity of WM/LPL, single institution series on outcomes after HCT are limited by low patient number.9-11 It has been over a decade since the Center for International Blood and Marrow Transplant Research (CIBMTR) reported data from 26 alloHCT recipients between 1986 and 2002.12 Since this study there have been major advances in peri-transplant management, including reduced intensity conditioning (RIC), improved supportive care, and allele-based HLA matching. AlloHCT is now feasible for a larger proportion of patients, including those that are older and those with co-morbidities.13
Significant progress for the treatment of WM/LPL, including routine use of rituximab, ibrutinib, bortezomib, bendamustine, and immunomodulatory agents, have changed the therapeutic landscape. However, these therapies are not curative and chemoresistance leads to eventual relapse in most patients.14 There is a paucity of data on outcomes from the use of alloHCT in the current era. Here, we present long-term outcomes of 144 alloHCT recipients for WM/LPL and examined prognostic factors associated with survival. All patients had multiple relapses and received a transplant after 2001. This study includes the largest series of patients treated with alloHCT in the era of modern treatment modalities.
Patients and Methods
Data Source
The CIBMTR® is a prospectively managed database collecting data from over 500 transplant centers worldwide. Participating centers are required to report all transplantations consecutively, compliance is monitored by on-site audits, and patients are followed longitudinally. Data quality is ensured by computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers. Studies conducted by the CIBMTR are performed in compliance with all federal regulations pertaining to the protection of human research participants. Protected health information used in the performance of such research is collected and maintained by CIBMTR as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule.
Two levels of data are collected: registration and research. Registration data include age, gender, disease type, date of diagnosis, conditioning regimen, cause of death, and post-transplant progression and survival. Research level data, with more detailed clinical data, is collected from a subgroup of registered patients through a weighted randomization scheme. Both levels of data are collected pre-transplant, at 100 days and 6 months post-transplant, and annually until death or last follow-up. Observational studies conducted by the CIBMTR are performed with approval of the institutional review boards of the National Marrow Donor Program and the Medical College of Wisconsin.
Patients
This study included patients registered with the CIBMTR who underwent alloHCT for WM/LPL between 2001 and 2013. Hematopoietic cells were obtained from a related HLA-matched donor or from an unrelated HLA-matched or mismatched donor. Umbilical cord blood stem cell grafts were excluded. All patients had relapsed WM/LPL after one or more lines of prior therapy. Data accuracy was verified by physician review and any discrepant data were resolved when present. Centers were contacted to provide additional data when necessary.
Definitions
Disease responses were defined according to the Third International Workshop on Waldenström's Macroglobulinemia.15 Patients with LPL were also evaluated with imaging per Cheson criteria.16 Complete response (CR) was defined as the absence of monoclonal IgM in serum and urine (assessed by immunofixation), resolution of organomegaly/adenopathy (if present at baseline), the absence of clinical features attributable to WM/LPL, and the absence of malignant cells in a bone marrow biopsy. Partial response (PR) was defined as ≥50% reduction in serum monoclonal IgM concentration (assessed by electrophoresis) and a ≥50% improvement in organomegaly/adenopathy (assessed by imaging) if present at baseline. Progressive disease (PD) was defined as a >25% increase in serum monoclonal IgM level from the lowest response value (assessed by electrophoresis) or the development of new disease-related symptoms. Patients that achieve PR or CR during salvage chemotherapy prior to transplantation were considered chemo-sensitive while those that did not respond were defined as chemoresistance. Conditioning intensity (myeloablative conditioning (MAC) or reduced intensity conditioning (RIC)) was defined per standard criteria.17
Outcomes
The primary outcomes were overall survival (OS), progression free survival (PFS), relapse, and non-relapse mortality (NRM). OS was defined as time from transplantation to death from any cause and censored at time of last follow-up. PFS was defined as time from transplantation to progressive disease, relapse, or death. NRM was defined as time to death without relapse or progression.
Statistical Analysis
The primary objective in this study was to describe the outcomes of adults with WM/LPL following alloHCT. Other variables considered included age, gender, Karnofsky score (<80% vs. 80-100%), year of alloHCT (2001-2005 vs. 2006-2013), prior autoHCT, time from diagnosis to transplant (<12 mo. vs. 12-36 mo. vs. >36 mo.), donor graft source, graft versus host disease (GVHD) prophylaxis, and conditioning intensity (RIC vs. MAC). Patient characteristics were summarized using descriptive statistics. Categorical variables were compared using Fisher's exact test or the chi-square test (where appropriate), and continuous variables were compared with the Kruskal-Wallis test. The probabilities of PFS and OS were estimated using the Kaplan-Meier estimator, with the variance estimated by Greenwood's formula. The cumulative incidences of NRM, relapse, and secondary malignancy were estimated by the Aalen-Johansen estimator to accommodate the competing risks.18 Variables to be considered in multivariable analyses are detailed above. A stepwise selection approach was used for model building. All covariates that reached significance level of 5% were included in the final models. The proportional hazards assumption was tested for each covariate in the final models; none were found to violate this assumption. All computations were made using the statistical package SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
Patient, Disease and Transplant Characteristics
We examined data on 144 patients with WM/LPL who received alloHCT from 2001-2013 (Table 1). MAC was used in 47% (n=67) and RIC in 47% (n=67). Conditioning was unknown in ten patients. Median age of patients was 53 years (range: 21-76 years). Median time from diagnosis to alloHCT was 41 months, and 36% of patients were transplanted within 2 years from their diagnosis. Data on stage of disease were not available. Approximately half of patients (n=82, 57%) had chemo-sensitive disease at time of transplantation, and 22% had progressive disease prior to transplantation. Thirteen percent (n=18) of patients had failed a prior autoHCT. The median time from autoHCT to alloHCT was 23 months (range: 2-80 months). Only 10% percent (n=15) of patients had low performance status (KPS <80%). The donor was an HLA-matched sibling, non-sibling relative, or unrelated donor in 52% (n=75), 5% (n=7) and 40% (n=57) of cases, respectively. Only 3% (n=5) of the cases involved donors from HLA-mismatched relatives. Tacrolimus (50%) or cyclosporine (46%) were predominantly used for prophylactic treatment of acute GVHD. The characteristics of patients who received MAC and RIC conditioning are described in Table 1. Patients that received MAC were younger (<50 years old) compare to those that received RIC (51% vs 27%). Previously autologous marrow grafts were more common in patients that received MAC conditioning (18% vs. 1%). The median follow-up for survivors from alloHCT was 70 months (range: 4-144 months).
Table 1.
Characteristics of patients with WM/LPL who underwent alloHCT reported to the CIBMTR
| Variable | All1 | MAC | RIC | p-value (MAC vs. RIC) |
|---|---|---|---|---|
| Number of Patients | 1442 | 67 | 67 | |
| Number of Centers | 84 | 39 | 45 | |
| Age at Transplant, years | 0.02 | |||
| Median Age | 53 (21-76) | 50 (21-66) | 53(34-76) | 0.008 |
| 21-49 | 55 (38) | 34 (51) | 18 (27) | |
| 50-59 | 63 (44) | 23 (34) | 35 (52) | |
| ≥ 60 | 26 (18) | 10 (15) | 14 (21) | |
| Gender: Male | 98 (68) | 44 (66) | 46(69) | 0.71 |
| Karnofsky score: <80% | 15 (10) | 6 (9) | 7 (10) | 0.74 |
| Region | ||||
| US | 109 (76) | 50 (75) | 50 (75) | 0.006 |
| Canada | 22 (15) | 15 (22) | 6 (9) | |
| Other2 | 13 (9) | 2 (2) | 11 (16) | |
| Graft Type | ||||
| Bone Marrow | 14 (10) | 12 (18) | 1 (1) | 0.001 |
| Peripheral Blood | 130 (90) | 55 (82) | 66 (99) | |
| Donor Type | 0.97 | |||
| HLA-identical sibling | 75 (52) | 36 (54) | 37(54) | |
| HLA-matched relative | 7 (5) | 3 (4) | 3 (4) | |
| HLA-matched non-relative | 57 (40) | 2 (3) | 2 (3) | |
| HLA-mismatched relative | 5 (3) | 26 (39) | 25 (37) | |
| Transplant-related | ||||
| Year of Transplant | 0.60 | |||
| 2001-2005 | 61 (42) | 27 (40) | 30 (45) | |
| 2006-2013 | 83 (58) | 40 (60) | 37 (55) | |
| Time from diagnosis to transplant | 0.66 | |||
| Median months (range) | 41 (<1-204) | 38 (<1-204) | 47 (<1-198) | 0.34 |
| <24 months | 52 (36) | 26 (39) | 21 (31) | |
| 24-60 months | 43 (30) | 20 (30) | 22 (33) | |
| >60 months | 49 (34) | 21 (31) | 24 (36) | |
| Disease Status prior to AlloHCT | 0.58 | |||
| Complete Response/CRu3 | 20 (14) | 11 (16) | 8 (12) | |
| Partial Response | 62 (43) | 28 (42) | 33 (49) | |
| Stable Disease | 7 (5) | 2 (3) | 5 (8) | |
| Progression/Relapse | 32 (22) | 16 (24) | 15 (22) | |
| Never Treated | 1 (11) | 1 (1) | 0 | |
| Unknown | 22 (15) | 9 (13) | 6 (9) | |
| Sensitivity to Chemotherapy | 0.56 | |||
| Sensitive | 82 (57) | 39 (58) | 41 (61) | |
| Resistant | 39 (27) | 18 (27) | 20 (30) | |
| Unknown | 23 (16) | 10 (15) | 6 (9) | |
| Prior AutoHCT | 18 (13) | 6 (9) | 6 (9) | 1 |
| Conditioning Regimen Intensity4 | ||||
| Myeloablative | 67 (47) | 67 (100) | 0 | |
| Reduced Intensity | 67 (47) | 0 | 67 (100) | |
| Unknown | 10 (7) | NA | NA | |
| Anti-thymocyte Globulin | 0.27 | |||
| No | 119 (81) | 57 (85) | 52 (78) | |
| Yes | 25 (19) | 10 (15) | 15 (22) | |
| GVHD prophylaxis | <0.001 | |||
| Cyclosporin-based | 37 (26) | 5 (8) | 28 (42) | |
| Methotrexate + Cyclosporin | 29 (20) | 20 (30) | 8 (12) | |
| Tacrolimus-based | 72 (50) | 37 (55) | 30 (45) | |
| Unknown | 6 (4) | 5 (8) | 1 (1) | |
| Medium Follow-up from Allo-HCT, months (range) | 70 (4-144) | 97 (4-143) | 35 (12-144) | 0.56 |
| Disease-related (research form only) | ||||
| Number of Patients | 36 | 18 | 18 | |
| Prior Lines of Chemotherapy | ||||
| 1-3 | 20 (56) | 8 (44) | 12 (67) | |
| >3 | 16 (44) | 10 (56) | 6 (33) | |
| Pre-alloHCT chemotherapy5 | ||||
| Cladribine/Fludurabine (+/− Chlorambucil) | 15 (42) | 8 (44) | 7 (39) | |
| Adriamycin-based | 14 (39) | 7 (39) | 7 (39) | |
| Bortezomib-based | 3 (8) | 1 (6) | 2 (11) | |
| Others3 | 4 (11) | 2 (11) | 2 (11) | |
| Pre-alloHCT Rituximab | 21 (58) | 12 (67) | 9 (50) | |
Data is displayed as the number of patient with the percent of patients shown in parentheses, unless otherwise noted.
Includes patients with unknown conditioning (n=10).
Central/South America (n=4), Australia/New Zealand (n=3), Asian (n=3), Mideast/Africa (3).
Unconfirmed complete response (CRu), not applicable (NA), not done (ND)
Conditioning regimens: Cytarabine (Cy) + Total body irradiation (TBI) (n=21), Fludarabine (Flu) + TBI (n=23), TBI + others (n=12), Busulfan (Bu) + Cy (n=10), Flu + Cy +/− TBI (n=19), Flu + Melphalan (Mel) (n=16), Flu + Bu (n=22), Mel (n=9), Carmustine, Etoposide, Cy, MEL (BEAM) (n=4), Total lymphoid irradiation + anti-thymocyte globulin (ATG) (n=5), Cy (n=1), ATG + Cy + Pentostatin (n=1), Bu + Rituximab + Pentostatin (n=1)
6. Other chemotherapies: Etoposide, Alemtuzumab, Imatinib, Procarabazine
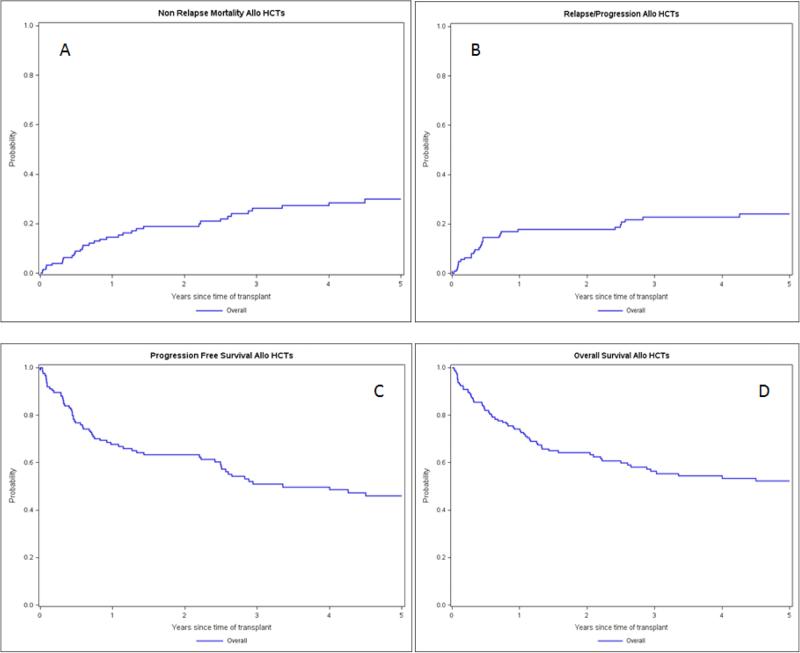
Non-relapse mortality
NRM was 15% at 1-year and 30% at 5-years post-alloHCT (Figure 1A). Among MAC recipients, NRM was 12% (95% CI 6-24%) and 24% (95% CI 12-36%) at 1 and 5 years, respectively. This was statistically similar to RIC recipients, where NRM was 15% (95% CI 9-27%) and 39% (95% 24-51%) at 1 and 5 years, respectively (Table 2). NRM was comparable in patients that received related HLA-matched and unrelated donor transplants within RIC and MAC cohorts (log-rank, p=0.4725). Causes of death are listed in Table 3. In multivariate analysis (MVA), we could not identify any factors that predicted significantly increased NRM.
Figure 1.
Outcomes of patients with WM/LPL treated with alloHCT as reported to CIBMTR between 2002-2013. A. Non relapse mortality B. Relapse or progression C. Progression free survival D. Overall survival
Table 2.
Patient outcomes based on conditioning intensity
| Myeloablative (N=67) | Reduced intensity (N=67) | |||
|---|---|---|---|---|
| Outcomes | N Eval | Prob (95% CI) | N Eval | Prob (95% CI) |
| NRM | 61 | 56 | ||
| 1-year | 12 (6-24)% | 15 (9-27)% | ||
| 3-year | 21 (12-33)% | 33 (21-48)% | ||
| 5-year | 24 (12-36)% | 39 (24-51)% | ||
| Relapse/Prog | 61 | 56 | ||
| 1-year | 18 (9-30)% | 15 (9-27)% | ||
| 3-year | 24 (15-36)% | 21 (12-33)% | ||
| 5-year | 27 (15-39)% | 21 (12-33)% | ||
| PFS | 61 | 56 | ||
| 1-year | 69 (56-80)% | 68 (55-79)% | ||
| 3-year | 55 (42-68)% | 46 (32-60)% | ||
| 5-year | 50 (36-63)% | 41 (27-55)% | ||
| OS | 67 | 67 | ||
| 1-year | 73 (62-83)% | 73 (62-83)% | ||
| 3-year | 60 (47-71)% | 50 (37-62)% | ||
| 5-year | 55 (43-68)% | 45 (33-58)% | ||
Overall survival (OS); progression (Prog); progression free survival (PFS); non-relapse mortality (NRM)
Table 3.
Causes of death by conditioning intensity
| Conditioning Regimen intensity |
||
|---|---|---|
| Variable | MAC | RIC |
| Number of patients | 67 | 67 |
| Number deceased | 30 | 35 |
| Cause of death | ||
| Primary disease | 8 (27) | 7 (20) |
| GVHD | 6 (20) | 12 (34) |
| Infection | 7 (23) | 5 (14) |
| Organ failure | 4 (13) | 6 (17) |
| Secondary malignancy | 0 | 1 (3) |
| Others | 2 (7) | 1 (3) |
| Unknown | 3 (10) | 3 (9) |
Graft-versus-host disease (GVHD)
Progression and Relapse post-alloHCT
At one-year post-alloHCT, 18% of patients experienced progression or relapse. Cumulative incidence of relapse was 24% at 5-years (Figure 1B). The median time to relapse or progression for patients with prior autoHCT was 30 months and was not reached in the non-autoHCT group. Relapse or progression occurred in 18% (95% CI 9-30%) and 15% (95% CI 9-27%) at 1 year and 27% (95% CI 15-39%) and 21% (95% CI 12-33%) at 5 years in patients that received MAC and RIC, respectively (Table 2). MVA revealed that prior autoHCT significantly increased relapse/progression risk (HR 3.94, 95% CI 1.68-9.27, p=0.002), although this subgroup only contained 18 patients.
Progression free survival
In the combined patient population, PFS was 68% after 1-year and 46% after 5 years (Figure 1C). There were no significant differences in PFS based on conditioning (p=0.27) or donor types (p=0.46). PFS was 69% (95% CI 56-80%) versus 68% (95% CI 55-79%) at 1-year and 50% (95% CI 36-63%) versus 41% (95% CI 27-55%) at 5-years in MAC and RIC patient populations, respectively (Table 2). Pre-treatment disease status (≥ PR vs. <PR) was associated with a higher rate of PFS at 5 years (58% (95% CI 41-74%) vs. 38% (95% CI 21-56%), however, this difference was not statistically significant. In MVA there were no variables identified impacting PFS.
Overall survival
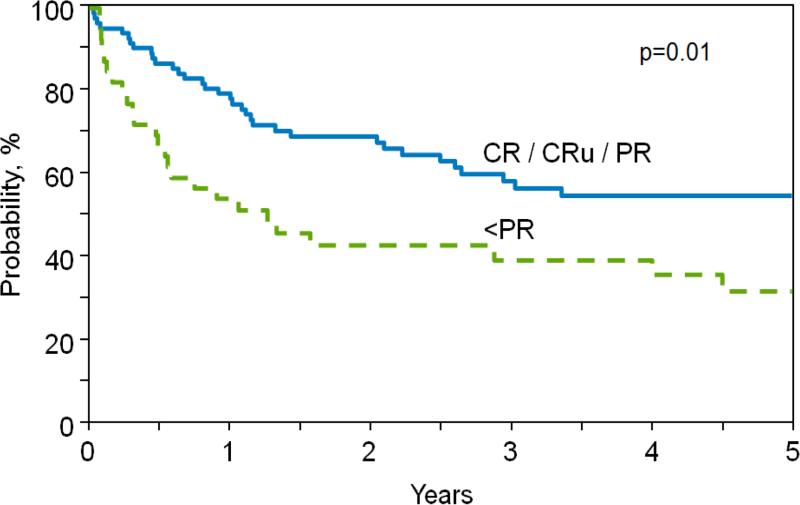
OS rates were 74% at 1-year and 52% at 5-years, with the majority of deaths occurring within 2-3 years post-alloHCT (Figure 1D). Patients with chemo-sensitive disease and pre-transplant disease status ≥PR experienced significantly superior OS compared with patients with chemo-resistant disease and poor disease status (i.e., <PR) (1-year, 79% (95% CI 69-88%) vs. 54% (95% CI 30-70%) and 5 year, 55% (95% CI 42-67%) vs. 32 (95% CI 17-49%); p=0.01; Figure 2). Neither conditioning intensity (p=0.27) nor donor type (p=0.46) impacted OS. In MVA there were no variables identified impacting OS.
Figure 2.
Adjusted probability of overall survival according to pre-alloHCT disease status
Discussion
The goal of this study was to evaluate the outcomes of alloHCT in patients with WM/LPL. The therapeutic landscape has changed with the availability of modern treatments, such as ibrutinib, rituximab, bortezomib and bendamustine. Emerging data suggest that idelalisib, carfilzomib and ofatumumab may be effective for relapsed WM, yet none of these treatments are curative. Most patients with WM/LPL have an indolent course of disease, for which alloHCT is not appropriate. However, alloHCT continues to have a role in select cases of high-risk aggressive WM/LPL.14,19-24 This analysis of 144 patients is the largest series published to date on the use of alloHCT for WM/LPL and provides important insight into the anticipated outcomes for this therapeutic strategy.
Our analysis revealed no discernible difference between RIC and MAC preparative regimens. It must be noted, that the RIC and MAC cohorts differ significantly. For example, peripheral blood grafts were used almost exclusively in RIC (99% compared to 82% in MAC). The presence of mature T cells in these grafts may have increased the risk of GVHD and NRM in the RIC cohort. Nevertheless, RIC can be used in older patients or those with higher co-morbidities for whom MAC is not feasible. This is particularly applicable to WM/LPL, since the median age at diagnosis is 63 years.25 While patients in this study were, on average, a decade younger than the median age of patients with WM/LPL, it did not detract from this.
Our data demonstrate that alloHCT for WM/LPL in the current era yields durable survival in 52% of patients 5 years post-transplant. The NRM was 15% at 1-year, 26% at 3-years, and 30% at 5-years. The CIBMTR previously reported a 40% NRM rate at 3-years (95% CI 23-59%) in 26 WM/LPL patients treated with alloHCT from 1986-2002.12 Similarly, Tournilhac et al. reported a 40% TRM rate following alloHCT in 10 patients WM/LPL.10 However, in line with our findings, the European Group for Blood and Marrow Transplantation Group (EBMT) reported a 3-year NRM of 27% (95% CI 19-38%) from series of 86 patients from 1986-2005.13
Despite the heavily pre-treated nature of the study population, there were relatively few relapses after 3 years post-alloHCT, suggesting extended remission and relapse-free potential. This is evidenced by the plateau in relapse/progression curve and applies to both RIC and MAC conditioning regimens. This suggests that there is a sustained disease control benefit with alloHCT regardless of regimen intensity. The aforementioned EBMT study also demonstrated durable remissions and 5-year OS rates of 62% and 64% in patients conditioned with MAC and RIC, respectively.14 Other studies, while limited by small numbers, have also demonstrated durable remission.10,12,26,27
We investigated the association between a number of clinical variables and favorable outcome following alloHCT. Our data demonstrated that chemo-resistant disease and poor disease status were associated with reduced OS. We would expect worse outcomes in patients that had undergone a prior autoHCT; however, the sample size of this patient subset (n=18) was likely inadequate to address this question. As with other types of indolent lymphoma, both autologous and allogeneic transplants are used for relapse WM/LPL, and the timing of transplant is critical to avoid chemoresistant disease.27,28 In WM, the coordinated use of autoHCT and alloHCT is often determined by risk assessment (International Prognostic Scoring System for WM; IPSSWM), response to prior therapies, pace of disease, and duration of first remission.5 Notably, 66% of current cohort underwent alloHCT within 5 years of diagnosis, suggesting poor early disease control. While autoHCT has been associated with disease control and improved PFS, comparisons of autoHCT to alloHCT are challenging due to differences in these highly selected patient populations.28,29
Due to the retrospective nature of the study and the time period under analysis, it was not possible to obtain independent hematopathology confirmation of diagnosis or to evaluate MYD88 and CXCR mutation status. This study was also limited by the lack of detailed GVHD data, which may provide insight into the impact of graft vs. tumor effect in these patients. Lower relapse rates have been reported in patients developing chronic GVHD after alloHCT.13 Data on the effect of donor lymphocyte infusions, which can result in additional responses post-alloHCT were also not available. While every attempt was made to obtain complete patient information, missing data was present regarding chemosensitivity, prior therapies, and details to calculate risk by IPSSWM. Due to these limitations, we were unable to perform a more refined alloHCT risk factor analysis.
In conclusion, we report promising relapse free and overall survival outcomes in WM/LPL. Based on these results, it is clear that alloHCT for WM/LPL can yield long-term remissions and survival in select patients. Further improvement in survival after alloHCT will require the judicious and early identification of patients with high-risk disease prior to the development of chemoresistance. Modern therapies such as ibrutinib could potentially serve as a bridge to alloHCT or be studied in the post-alloHCT setting to reduce relapse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cornell RF, Burns LJ, Bachanova V. Why Waldenström macroglobulinemia is not just another indolent lymphoma. International Journal of Hematologic Oncology. 2014;3:95–98. [Google Scholar]
- 2.Herrinton LJ, Weiss NS. Incidence of Waldenstrom's macroglobulinemia. Blood. 1993;82:3148–50. [PubMed] [Google Scholar]
- 3.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–76. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boise LH, Kaufman JL, Heffner LT, et al. Changing Epidemiology and Improved Survival In Patients With Waldenstrom Macroglobulinemia: Review Of Surveillance, Epidemiology, and End Results (SEER) Data. Blood. 2013;122:3135–3135. [Google Scholar]
- 5.Kastritis E, Kyrtsonis MC, Hadjiharissi E, et al. Validation of the International Prognostic Scoring System (IPSS) for Waldenstrom's macroglobulinemia (WM) and the importance of serum lactate dehydrogenase (LDH). Leuk Res. 2010;34:1340–3. doi: 10.1016/j.leukres.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009;113:4163–70. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos MA, Kastritis E, Delimpassi S, et al. The International Prognostic Scoring System for Waldenström's macroglobulinemia is applicable in patients treated with rituximab-based regimens. Haematologica. 2008;93:1420–1422. doi: 10.3324/haematol.12846. [DOI] [PubMed] [Google Scholar]
- 8.Fenske TS, Hamadani M, Cohen JB, et al. Allogeneic Hematopoietic Cell Transplantation as Curative Therapy for Patients with Non-Hodgkin Lymphoma: Increasingly Successful Application to Older Patients. Biology of Blood and Marrow Transplant. doi: 10.1016/j.bbmt.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meniane JC, El-Cheikh J, Faucher C, et al. Long-term graft-versus-Waldenstrom macroglobulinemia effect following reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;40:175–7. doi: 10.1038/sj.bmt.1705702. [DOI] [PubMed] [Google Scholar]
- 10.Tournilhac O, Leblond V, Tabrizi R, et al. Transplantation in Waldenstrom's macroglobulinemia--the French experience. Semin Oncol. 2003;30:291–6. doi: 10.1053/sonc.2003.50048. [DOI] [PubMed] [Google Scholar]
- 11.Martino R, Shah A, Romero P, et al. Allogeneic bone marrow transplantation for advanced Waldenstrom's macroglobulinemia. Bone Marrow Transplant. 1999;23:747–9. doi: 10.1038/sj.bmt.1701633. [DOI] [PubMed] [Google Scholar]
- 12.Anagnostopoulos A, Hari PN, Perez WS, et al. Autologous or allogeneic stem cell transplantation in patients with Waldenstrom's macroglobulinemia. Biol Blood Marrow Transplant. 2006;12:845–54. doi: 10.1016/j.bbmt.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Kyriakou C, Canals C, Cornelissen JJ, et al. Allogeneic stem-cell transplantation in patients with Waldenstrom macroglobulinemia: report from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2010;28:4926–34. doi: 10.1200/JCO.2009.27.3607. [DOI] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Kastritis E, Owen RG, et al. Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood. 2014;124:1404–1411. doi: 10.1182/blood-2014-03-565135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimby E, Treon SP, Anagnostopoulos A, et al. Update on recommendations for assessing response from the Third International Workshop on Waldenstrom's Macroglobulinemia. Clin Lymphoma Myeloma. 2006;6:380–3. doi: 10.3816/CLM.2006.n.013. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox DR. Regression Models and Life-Tables. Journal of the Royal Statistical Society Series B (Methodological). Journal of the Royal Statistical Society. 1972;34:187–220. [Google Scholar]
- 19.de Rooij MFM, Kuil A, Kraan W, et al. Ibrutinib and idelalisib target B cell receptor-but not CXCL12/CXCR4-controlled integrin-mediated adhesion in Waldenström macroglobulinemia. Haematologica. 2016;101:e111–e115. doi: 10.3324/haematol.2015.137265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimopoulos MA, Kastritis E, Ghobrial IM. Waldenstrom's macroglobulinemia: a clinical perspective in the era of novel therapeutics. Ann Oncol. 2016;27:233–40. doi: 10.1093/annonc/mdv572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom's macroglobulinemia. N Engl J Med. 2015;372:1430–40. doi: 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 22.Gertz MA. Waldenstrom macroglobulinemia: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90:346–54. doi: 10.1002/ajh.23922. [DOI] [PubMed] [Google Scholar]
- 23.Treon SP, Tripsas CK, Meid K, et al. Carfilzomib, rituximab, and dexamethasone (CaRD) treatment offers a neuropathy-sparing approach for treating Waldenstrom's macroglobulinemia. Blood. 2014;124:503–10. doi: 10.1182/blood-2014-03-566273. [DOI] [PubMed] [Google Scholar]
- 24.Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of Waldenstrom macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol. 2009;27:3830–5. doi: 10.1200/JCO.2008.20.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimopoulos MA, Panayiotidis P, Moulopoulos LA, et al. Waldenstrom's macroglobulinemia: clinical features, complications, and management. J Clin Oncol. 2000;18:214–26. doi: 10.1200/JCO.2000.18.1.214. [DOI] [PubMed] [Google Scholar]
- 26.Garnier A, Robin M, Larosa F, et al. Allogeneic hematopoietic stem cell transplantation allows long-term complete remission and curability in high-risk Waldenstrom's macroglobulinemia. Results of a retrospective analysis of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Haematologica. 2010;95:950–5. doi: 10.3324/haematol.2009.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson LD, Sandmaier BM, Maris MB, et al. Nonmyeloablative Allogeneic Hematopoietic Cell Transplantation (HCT) for Refractory Waldenstrom's Macroglobulinemia (WM): Evidence for a Graft-Versus-WM Effect. Blood. 2006;108:3034–3034. [Google Scholar]
- 28.Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol. 2016 doi: 10.1111/bjh.14046. n/a n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S-W, Tanimoto TE, Hirabayashi N, et al. Myeloablative allogeneic hematopoietic stem cell transplantation for non-Hodgkin lymphoma: a nationwide survey in Japan. Blood. 2006;108:382–389. doi: 10.1182/blood-2005-02-0596. [DOI] [PubMed] [Google Scholar]