Abstract
To evaluate the association between the Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) and the recently developed age-adjusted HCT-CI (HCT-CI/age) and transplant outcomes in the setting of CD34-selected allogeneic HCT, we analyzed a homogeneous population of patients undergoing allogeneic HCT with CD34-selected grafts for acute myeloid leukemia and myelodysplastic syndrome (n= 346). Median HCT-CI and HCT-CI/age scores were 2 (percentile 25–75: 1–4) and 3 (percentile 25–75: 1–5), respectively. Higher HCT-CI and HCT-CI/age scores were associated with higher non-relapse mortality (NRM) and lower overall survival (OS). The HCT-CI distinguished two risk groups (0–2 vs. ≥3) whereas, with the HCT-CI/age, there was a progressive increase in NRM and decrease in OS with increasing scores in all 4 groups (0 vs. 1–2 vs. 3–4 vs. ≥ 5). Higher scores in both models were associated with lower chronic graft-versus-host disease relapse-free survival (CRFS), but not with higher relapse. Both models showed a promising predictive accuracy for NRM (c- = 0.616 for HCT-CI and c- = 0.647 for HCT-CI/age). In conclusion, the HCT-CI and HCT-CI/age predict transplant outcomes in CD34-selected allo-HCT, including NRM, OS and CRFS, and may be used to select appropriate patients for this approach.
Keywords: HCT-CI, T-cell depletion, allogeneic hematopoietic cell transplantation, comorbidity
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is the only curative treatment for high risk acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS). However, this curative potential is complicated by the development of graft-versus-host disease (GVHD), which contributes significantly to transplant-related morbidity and mortality. Many approaches have attempted to address this shortcoming. One such approach is ex-vivo T-cell depletion of the allograft through CD34+ cell selection, which has been shown to significantly decrease the incidence of acute and chronic GVHD with acceptable long term relapse-free (RFS) and overall survival (OS) in diseases including AML and MDS.(1–9) Moreover, the absence of a requirement for post-transplant immunosuppressive therapy makes this approach attractive in patients with comorbidities that would complicate the use of calcineurin inhibitors. The use of CD34-selected grafts is currently being studied by the Blood and Marrow Transplantation Clinical Trials Network (BMT CTN) in a randomized phase 3 study (NCT02345850). The primary endpoint of the study is chronic GVHD-free, relapse-free survival (CRFS), a newly defined and increasingly utilized composite end-point developed in the HCT field to determine survival without ongoing morbidity associated with chronic GVHD.
Patient selection plays a key role in transplant success. The Hematopoietic Cell Transplantation Comorbidity Index (HCT-CI), initially proposed by Sorror and colleagues,(10) has become a widely validated tool to predict outcomes in many transplant settings. This includes children(11) and adults undergoing allo-HCT for various diseases,(12–15) as well as in patients receiving different conditioning regimens.(16, 17) This model was initially developed to predict non-relapse mortality (NRM), although it has also been proven useful to stratify patients for the risk of other outcomes including OS,(12) and GVHD.(18) Additionally, Sorror et al. have recently proposed a modification of the HCT-CI, named HCT-CI/age that adds an extra point for patients older than 40 years to the classic HCT-CI.(19)
The goal of this study was to determine the impact of comorbidities in the setting of T-cell depleted allo-HCT by validating the classical HCT-CI and HCT-CI/age scores in a homogeneous group of patients undergoing transplant with CD34-selected grafts for MDS or AML.
Patients and Methods
Patients
The analysis included consecutive patients aged 18 or older diagnosed with AML or MDS undergoing a T-cell depleted allo-HCT at Memorial Sloan Kettering Cancer Center (MSKCC) from January 2001 through June 2013. All patients included had the required data to perform complete scoring for either prognostic index. Patients received grafts from HLA-identical or single mismatched related or unrelated donors. Written informed consent for treatment was obtained from all patients and donors. Approval for this retrospective review was obtained from the Institutional Review and Privacy Board.
Transplant procedure and supportive care
All patients received myeloablative conditioning with either total body irradiation (TBI) or chemotherapy-based regimens. Either of two TBI-based regimens were used: TBI 1375 cGy given in 11 fractions followed by 2 daily doses of thiotepa (5 mg/kg/day) and, either 2 daily doses of cyclophosphamide (60 mg/kg/day) starting after thiotepa, or 5 daily doses of fludarabine (25 mg/m2/day) beginning on the first day of thiotepa.(1, 2) The chemotherapy-based preparative regimen consisted of intravenous busulfan (0.8 mg/kg/dose) every 6 hours for 10 or 12 doses, melphalan (70 mg/m2/day) for 2 doses and fludarabine (25 mg/m2/day) for 5 doses.(3) These 3 regimens were used continuously over the course of the study period, and two of the regimens (TBI/Thiotepa/cyclophosphamide and busulfan/melphalan/fludarabine) are included as options in the BMT CTN phase 3 trial mentioned above (NCT02345850).
T cell depletion of granulocyte colony stimulating factor (G-CSF) mobilized peripheral blood stem cells (PBSC) was performed as previously described.(1, 2, 20) Positive selection of CD34+ cells was performed by using Isolex 300i Magnetic Cell Separator (Baxter, Deerfield, IL) and subsequent sheep RBC rosette depletion,(2) or using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany).(8, 21) Equine or rabbit anti-thymocyte globulin (ATG) was used to promote engraftment in the majority of cases. Patients did not receive any other post-transplant immunosuppressive prophylaxis. HLA matching was established by DNA sequence-specific oligonucleotide typing for HLA-A, -B, C, DR-B1 and DQ-B1 loci. All patients received supportive care and prophylaxis against opportunistic infections according to standard guidelines.
Comorbidity assessment and scoring
Each patient’s electronic medical record was retrospectively reviewed. Definition of comorbidities followed the detailed recommendations on comorbidity assessment.(22) In brief, pre-transplant evaluation of comorbidities was assessed within the 30 to 10 days prior to the infusion of hematopoietic stem cells. Assessment of comorbidities and calculation of the HCT-CI was performed by a single investigator (RR) and thereafter independently reviewed by a second investigator (PB) in order to avoid inter-observer bias. A third investigator (MAP) reviewed all information and adjudicated any discrepancies.
The HCT-CI/age was calculated as originally defined by adding an extra point to the HCT-CI for patients ≥ 40 years of age.(19) Classification of patients into risk groups were the same as the original studies. For the HCT-CI, patients were classified into low (HCT-CI =0), intermediate (HCT-CI =1–2) or high (HCT-CI ≥ 3) risk groups. For the HCT-CI/age, patients were classified into low (HCT-CI/age =0), intermediate-low (HCT-CI =1–2), intermediate-high (HCT-CI =3–4) or high (HCT-CI ≥ 5) risk groups. For exploratory purposes we defined ‘very-high HCT-CI score’ and ‘very-high HCT-CI/age score’ categories for patients with scores ≥7 and ≥8, respectively.
Endpoints, definitions and statistical analysis
The primary endpoint of the study was NRM at 3 years. Secondary end-points included OS, CRFS and relapse. NRM was defined as death by any cause but relapse. CRFS was defined as follows: an event was defined as moderate to severe chronic GVHD according to NIH consensus criteria global score,(23) disease relapse, or death by any cause. Descriptive statistics were used to summarize patient characteristics. The cumulative incidence of relapse and NRM were calculated using the cumulative incidence method for competing risks, considering death without prior relapse and relapse as competing events, respectively. OS and CRFS were calculated using the Kaplan-Meier method. Univariate and multivariate Cox proportional hazards regression models were performed for overall survival, CRFS, and the cause specific hazard of NRM, in order to investigate the effect of potential prognostic factors including: age (>40 years), gender, diagnosis (AML vs. MDS), conditioning regimen (TBI-based vs. chemo-based), year of transplantation (continuous variable), HLA-match (vs. mismatched), donor (related vs. unrelated), C34+ cell dose infused (< vs. ≥ median [7.6×106/kg]), CD34+ selection method (CliniMACS® vs. Isolex/e-roseting), and scores from HCT-CI and HCT-CI/age. The c-index was used to evaluate the predicative ability of the HCT-CI and HCT-CI/age scores with respect to NRM over the first 2 years post transplant. Patients who relapsed, died, or were lost to followup greater than 2 years post transplant were considered censored at 2 years in calculating the c-index. Standard error estimates for the c-index were based on 1000 bootstrap samples. Patients were analyzed according to their status as of June 2015. All statistical analyses were performed using R version 3.1.2.
Results
Patients characteristics
A total of 346 patients fulfilled the inclusion criteria and constituted the study population. The main clinical characteristics are summarized in Table 1. In brief, the median age at HCT was 56 years (range 18–73). Underlying diseases included AML (n=244, 71%) and MDS (n=102, 29%). Patients with AML were in first (n=158, 65%), second (n= 83, 34%) or third complete remission (CR, n=3, 1%). Patients with MDS were distributed as follows: refractory anemia with excess of blasts (RAEB)-2= 31 (30%), RAEB-1= 23 (23%), refractory anemia= 19 (19%), refractory cytopenia with multilineage dysplasia= 17 (17%) and other= 12 (12%). The majority of the patients had an intermediate score according to the Disease Risk Index. The median follow-up for survivors was 57 months (range 12–164).
Table 1.
Patient characteristics and transplant outcomes
| Characteristics | Patients= 346 |
|---|---|
| Median age, years (range) | 56 (18–73) |
| Gender Male, n (%) | 186 (54) |
| Female donor to male recipient | 62 (18) |
| Underlying disease, n (%) | |
| AML | 244 (71) |
| MDS | 102 (29) |
| Disease status for acute leukemia*, n (%) | |
| CR1 | 158 (65) |
| CR2–3 | 86 (35) |
| Disease Risk Index**, n(%) | |
| Low | 16 (5%) |
| Intermediate | 285 (82%) |
| High | 43 (12%) |
| Very High | 1 (<1%) |
| Donor type, n (%) | |
| Related HLA identical | 125 (36) |
| Related non-HLA identical | 7 (2) |
| Matched unrelated | 129 (38) |
| Mismatched unrelated | 85 (25) |
| Conditioning regimen***, n (%) | |
| TBI-based | 118 (34) |
| Chemotherapy-based | 228 (66) |
| ATG, n (%) **** | |
| Rabbit 2.5–7.5 mg/kg | 268 (77) |
| Equine 30–45 mg/kg | 48 (14) |
| No ATG | 27 (8) |
|
Infused cell dose CD34+ ×106/kg, median (range) |
7.6 (1.5–28.8) |
| Previous auto-HCT, n (%) | 15 (4) |
| T-cell depletion method, n (%) | |
| Clinimacs® | 152 (44) |
| Isolex | 194 (56) |
| Patients comorbidities*****, n (%) | |
| Pulmonary severe / moderate | 45 (13) / 108 (31) |
| Psychiatric disturbance | 70 (20) |
| Hepatic moderate-severe / mild | 8 (2) / 54 (16) |
| Previous malignancy | 61 (18) |
| Obesity | 28 (8) |
| Cardiac | 30 (9) |
| Infection | 24 (7) |
| Diabetes | 21 (6) |
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; n, number; HLA, human leukocyte antigen; CR, complete remission; TBI, total body irradiation; HCT, hematopoietic cell transplantation
Percentages over total number of AML patients (n=244).
Since the DRI does not account for mixed phenotype acute leukemia, 1 patient could not be categorized.
For detailed information on conditioning regimen see Methods.
3 patients included in a clinical trial received rabbit ATG 2.5mg/kg in combination of equine ATG 15mg/kg
Only comorbidities present in ≥ 5% of the patients are shown.
HCT-CI and HCT-CI/age
Each of the 17 comorbidities included in the HCT-CI were present in at least one patient. The prevalence of the most frequent comorbidities (>5%) is shown in Table 1. The most frequent comorbidities included pulmonary impairment (moderate [n = 108, 31%], severe [n = 45, 13%],), psychiatric disorders (n= 70, 20%), hepatic impairment (mild [n = 54, 16%], moderate/severe [n = 8, 2%]) and prior malignancy (n = 61, 18%) (solid tumor [n = 47, 14%], hematological [n = 14, 4%]. Infrequent comorbiditites (<5%) not included in Table 1 were cerebrovascular disease (n=14, 4%), rheumatologic comorbidity (n=13, 4%), heart valve disease (n=12, 3%) arrhythmia (n=8, 2%), peptic ulcer (n=8, 2%), inflammatory bowel disease (n=2, 1%) and renal comorbidity (n=1, 0.3%).
Distribution of patients according to both models is shown in Table 2. The median HCT-CI score was 2 (percentile 25–75: 1–4). According to the HCT-CI, 75 patients (22%) had a score of 0, 110 (32%) a score of 1–2 and 161 (47%) had a score ≥ 3. Twenty-two patients (6%) had a HCT-CI score ≥ 7. According to the HCT-CI/age, the median score was 3 (percentile 25–75: 1–5) and distribution of patients was as follows: HCT-CI/age = 0 (n=15, 4%), HCT-CI/age = 1–2 (n=122, 35%), HCT-CI/age = 3–4 (n=118, 34%), HCT-CI/age ≥ 5 (n=91, 27%). Twenty-one patients (6%) had a HCT-CI/age score ≥ 8.
Table 2.
Patient distribution and outcome according to the HCT-CI and HCT-CI/age (n=346).
| MODEL | Patients n (%) |
Cum. Inc. NRM at 100 days (95%CI) |
Cum. Inc. NRM at 1 years (95%CI) |
Cum. Inc. NRM at 3 years (95%CI) |
Probability OS at 1 years (95%CI) |
Probability OS at 3 years (95%CI) |
CRFS at 1 year (95%CI) |
CRFS at 3 year (95%CI) |
|---|---|---|---|---|---|---|---|---|
| HCT-CI | ||||||||
| 0 | 75 (22) | 5.3 (1.7–12.1) | 9.3 (4.1–17.2) | 15.2 (8–24.6) | 82.7 (72–89.5) | 67 (54.8–76.6) | 74.7 (63.2–83) | 58.2 (46.1–68.5) |
| 1–2 | 110 (32) | 4.5 (1.7–9.6) | 11.8 (6.6–18.6) | 16.5 (10.2–24.1) | 82.7 (74.3–88.6) | 69.4 (59.7–77.2) | 74.5 (65.3–81.7) | 65.9 (56.1–74) |
| ≥3 | 161 (47) | 8.7 (5–13.7) | 19.9 (14.1–26.4) | 32.2 (25–39.6) | 68.3 (60.5–74.9) | 48.6 (40.5–56.2) | 61.5 (53.5– 68.5) | 46 (38.1–53.6) |
| HCT-CI/age | ||||||||
| 0 | 15 (4) | 0 | 0 | 7.3 (0.4–29.2) | 93.3 (61.3–99) | 85.6 (53.3–96.2) | 86.7 (56.4–96.5) | 66.7 (37.5–84.6) |
| 1–2 | 122 (35) | 4.9 (2–9.8) | 9.8 (5.4–15.9) | 14.3 (8.7–21.3) | 83.6 (75.8–89.1) | 68.8 (59.6–76.4) | 74.6 (65.9–81.4) | 63.4 (54.1–71.4) |
| 3–4 | 118 (34) | 3.4 (1.1–7.9) | 14.4 (8.8–21.4) | 25 (17.5–33.2) | 77.1 (68.4–83.7) | 56.4 (46.8–65) | 66.9 (57.3–74.4) | 53.5 (44–62.1) |
| ≥5 | 91 (26) | 14.3 (8–22.3) | 25.3 (16.8–34.6) | 36.7 (26.7–46.7) | 61.5 (50.7–70.6) | 45.4 (34.8–55.4) | 59.3 (48.5–68.6) | 43.5 (33.1–53.5) |
| Overall | 346 (100) | 6.6 (4.3–9.6) | 15 (11.5–19) | 23.5 (19.2–28.2) | 76 (71.2–80.2) | 59.2 (53.8–64.3) | 68.5 (63.3–73.1) | 55 (49.5–60.1) |
Abbreviations: Cum. Inc, cumulative incidence; NRM, non-relapse mortality; OS, overall survival; CRFS, chronic graft-versus-host disease-free, relapse-free survival.
Non-relapse mortality
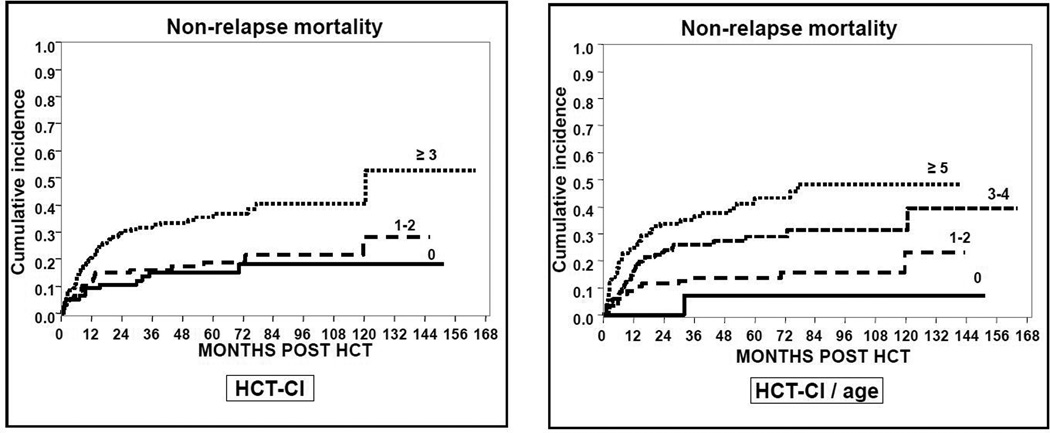
Ninety-two patients had NRM at last follow-up date for a cumulative incidence of NRM for the whole cohort at 100 days, 1 year and 3 years of 6.6% (95%CI 4.3–9.6), 15% (95%CI 11.5–19) and 23.5% (95%CI 19.2–28.2), respectively. When the classical HCT-CI was used, NRM was similar in patients with HCT-CI scores =0 and 1–2 while it was higher in patients with scores ≥ 3 at all time points (Figure 1). With the HCT-CI/age there was a progressive increase in the risk of NRM with rising scores in all risk groups. For instance, cumulative incidence of NRM at 3 years was 7%, 14%, 25% and 37% in patients scoring 0, 1–2, 3–4 and ≥5, respectively (Table 2). The predictive accuracy measured by the c-statistic for the HCT-CI and the HCT-CI/age was 0.616 (95%CI 0.558–0.674) and 0.647 (95%CI 0.587–0.708), respectively.
Figure 1.
Cumulative incidence of NRM according to the HCT-CI and HCT-CI/age scores.
In the univariate analysis, risk factors for a higher NRM included age ≥ 40 (HR 2.3 [95%CI 1.1–4.8], p= 0.02), HLA non-identical donor (HR 1.6 [95%CI 1–2.4], p=0.04), chemotherapy-based conditioning regimen (HR 1.9 [95%CI 1.1–3], p=0.01), HCT-CI 1–2 and ≥3 (HR 1.2 [95%CI 0.6–2.5] and HR 2.8 [95%CI 1.5–5.3], respectively, compared to reference HCT-CI = 0, p<0.001) and HCT-CI/age 1–2, 3–4 and ≥5 (HR 2.7 [95%CI 0.4–20.5], HR 5.8 [95%CI 0.8–42.3] and HR 10 [95%CI 1.4–72.7], respectively, compared to reference HCT-CI/age = 0, p<0.001) (Table 3). In the multivariate analysis, HCT-CI and HCT-CI/age were the only variables retaining significance: HCT-CI 1–2 and ≥3 (HR 1.3 [95%CI 0.6–2.6] and HR 2.5 [95%CI 1.4–4.8], respectively, compared to reference HCT-CI = 0, p=0.002) and HCT-CI/age 1–2, 3–4 and ≥5 (HR 2.4 [95%CI 0.3–18], HR 5.1 [95%CI 0.7–37.8] and HR 8.3 [95%CI 1.2–62.1], respectively, compared to reference HCT-CI/age = 0, p<0.001).
Table 3.
Univariate and multivariate analysis for NRM
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Patient and Graft Characteristics |
HR (95%CI) | p value | HR (95%CI) | p value |
| Sex | ||||
| Male | Ref. | 0.12 | - | |
| Female | 1.4 (0.9–2.1) | |||
| D/R gender combination | ||||
| Female to Male | Ref. | 0.14 | - | |
| Other | 1.6 (0.9–2.9) | |||
| Age (years) | ||||
| < 40 | Ref. | 0.02 | Ref. | 0.12 |
| ≥40 | 2.3 (1.1–4.8) | 1.8 (0.9–4) | ||
| Donor | ||||
| Related donor | Ref. | 0.06 | Ref. | 0.22 |
| Non-related donor | 1.5 (1–2.4) | 1.4 (0.8–2.2) | ||
| HLA compatibility | ||||
| Identical | Ref. | 0.04 | Ref. | 0.15 |
| Non-identical | 1.6 (1–2.4) | 1.4 (0.9–2.5) | ||
| Diagnosis | ||||
| Acute myeloid leukemia | Ref. | 0.47 | - | |
| Myelodisplastic syndrome | 1.2 (0.8–1.8) | |||
| Transplant year | Ref. 1.0 (0.9–1.0) |
0.2 | - | |
| HCT-CI | ||||
| 0 | Ref. | < 0.001 | Ref. | 0.002 |
| 1–2 | 1.2 (0.6–2.5) | 1.3 (0.6–2.6) | ||
| ≥3 | 2.8 (1.5–5.3) | 2.5 (1.4–4.8) | ||
| HCT-CI/age | ||||
| 0 | Ref. | < 0.001 | Ref. | < 0.001 |
| 1–2 | 2.7 (0.4–20.5) | 2.4 (0.3–18) | ||
| 3–4 | 5.8 (0.8–42.3) | 5.1 (0.7–37.8) | ||
| ≥5 | 10 (1.4–72.7) | 8.3 (1.1–62.1) | ||
| Conditioning regimen | ||||
| TBI based | Ref. | 0.01 | Ref. | 0.5 |
| Chemo based | 1.9 (1.1–3) | 0.8 (0.5–1.4) | ||
| CD34+ × 106/kg dose | ||||
| < 7.6×106/kg | Ref. | 0.27 | - | |
| ≥ 7.6×106/kg | 0.8 (0.5–1.2) | |||
| CD34+ selection method | ||||
| Clinimacs ® | Ref. | 0.29 | - | |
| OTHER | 1.3 (0.8–2) | |||
Abbreviations: NRM, non-relapse mortality; OS, overall survival, CRFS Chronic GVHD-Free, Relapse-Free Survival; D/R, donor/recipient; HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index.
Relapse
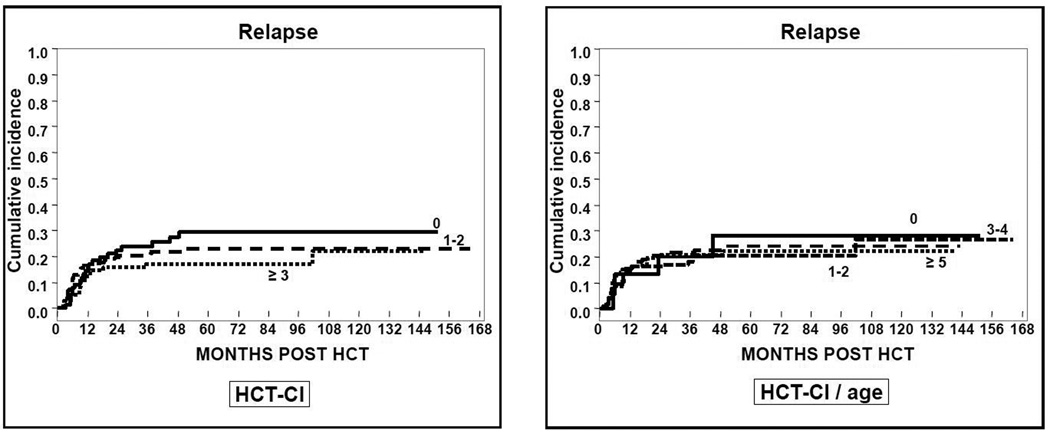
Seventy-six patients relapsed for a cumulative incidence for the whole cohort at 1 and 3 years of 15% (95%CI 12–19) and 20% (95%CI 16–25), respectively. Neither the HCT-CI nor the HCT-CI/age was associated with relapse incidence. At 3 years, relapse risk for patients with HCT-CI = 0, 1–2 and ≥ 3 were 24% (95%CI 15–34), 17% (95%CI 10–25) and 21% (95%CI 15–27), respectively (p= 0.3). Similar results were obtained after classifying the patients according to the HCT-CI/age (p> 0.9) (Table 2, Figure 2). In the univariate analysis, the only risk factor for a higher risk of relapse was year of transplantation (HR 1.1 [95%CI 1–1.2, p= 0.03).
Figure 2.
Cumulative incidence of relapse according to the HCT-CI and HCT-CI/age scores.
Causes of death
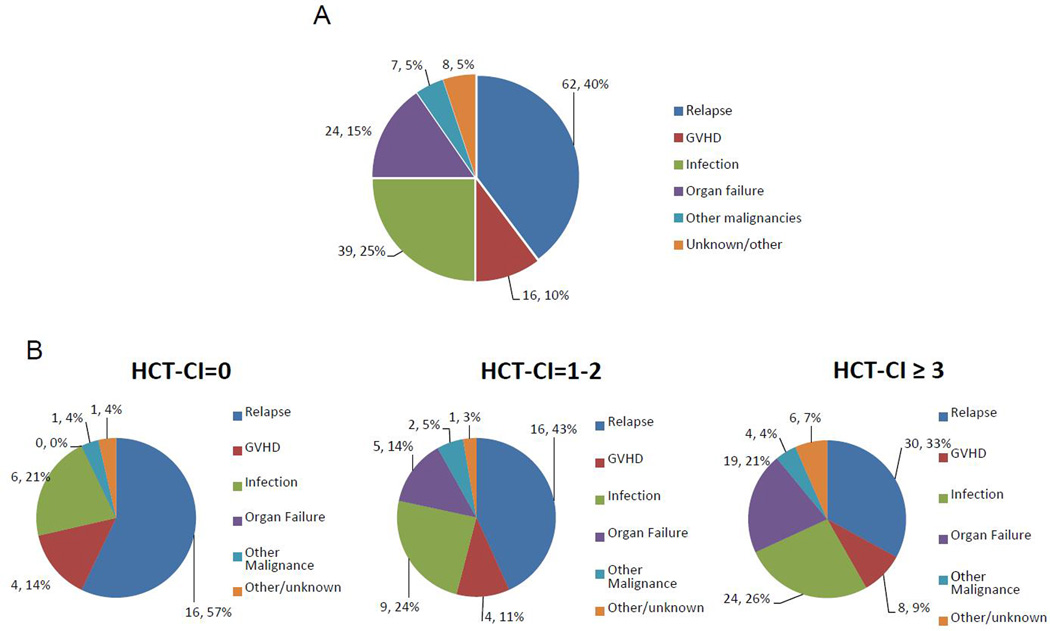
One hundred and fifty-six patients had died at the time of the analysis, 62 (40%) from disease relapse and 94 (60%) from other non-relapse related causes including infections (n=39, 25%), organ failure (n=24, 15%), GVHD (n= 16, 10%), other malignancies (n=7, 4%) and other / unknown causes (n=8, 5%). Patients with higher HCT-CI were more likely to die from organ failure than patients with no comorbidities (HCT-CI of 0: 0%; HCT-CI of 1–2: 14%; HCT-CI ≥ 3: 21%). Similar results were obtained by using the HCT-CI / age (death from organ failure in HCT-CI / age score of 0: 0%; 1–2: 5%; 3–4: 18%; ≥ 5: 21%). After analyzing the cause of death in patients with severe pulmonary and hepatic impairment, the attributed cause of death did not seem to be related to the same pretransplant organ comorbidity. Hence, only 1 of the 30 deaths occurring in patients with severe pulmonary comorbidity was attributed to direct pulmonary toxicity, whereas none of the 8 patients with severe hepatic impairment (5 deaths) died of direct liver toxicity. Details on causes of death for the entire population and according to the HCT-CI at transplant are summarized in Figure 3.
Figure 3.
Causes of death in the entire population (A), and according to the HCT-CI (B)
Overall Survival
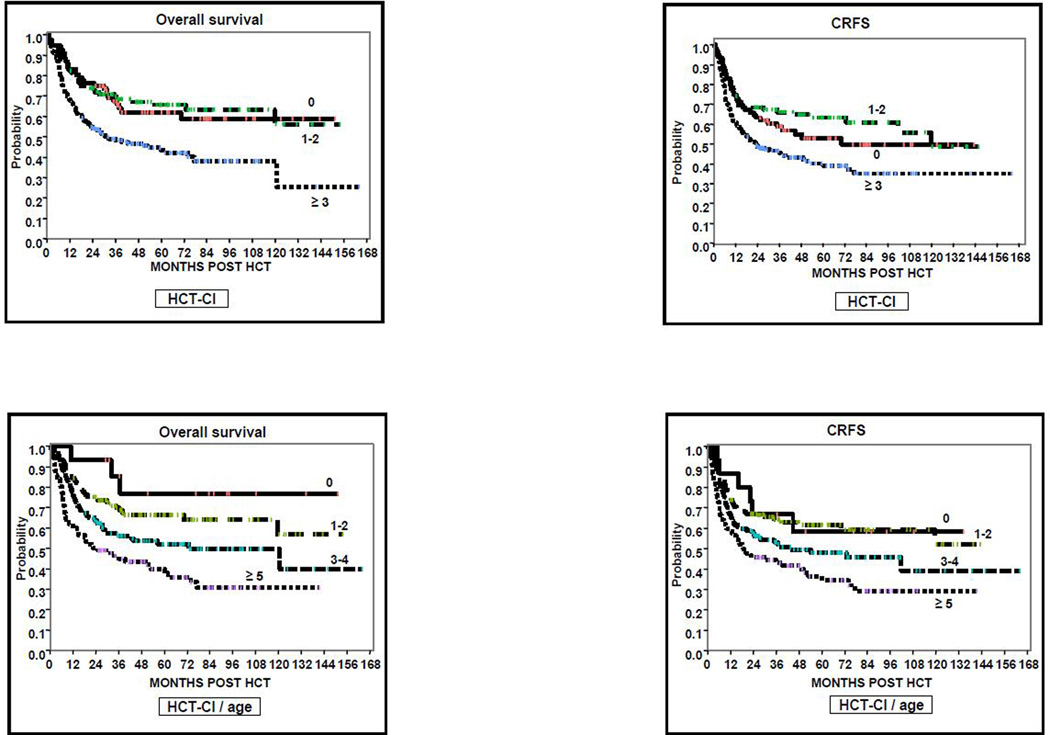
One hundred and ninety-patients were alive at last follow-up for a probability of OS at 1 and 3 years for the whole cohort of 76% (95%CI 71–80) and 59% (95%CI 54–64), respectively. When using the classical HCT-CI, OS was similar for patients scoring 0 and 1–2, while it was lower for patients with HCT-CI ≥3. According to the HCT-CI/age, the probability of OS steadily decreased in all 4 risk-groups, ranging from 86% to 45% at 3 years (p<0.001). Details of OS according to the HCT-CI and HCT-CI/age risk groups are summarized in Table 2 and Figure 4.
Figure 4.
Probability of OS, RFS, CRFS according to the HCT-CI and HCT-CI/age scores.
In the univariate analysis, the only risk factor for a lower OS was the HCT-CI ≥3 (HR 1.9 [95%CI 1.3–2.9], compared to reference HCT-CI = 0, overall p<0.001) and the HCT-CI/age = 1–2, 3–4 and ≥5 (reference, HR 2.1 [95%CI 0.6–6.6], HR 3.2 [95%CI 1–10.1] and HR 5 [95%CI 1.6–16.1], respectively, compared to reference HCT-CI/age = 0, overall p<0.001) (supplementary table).
Chronic GVHD-relapse free survival
CRFS at 1 and 3 years for all patients was 69% (95%CI 63–73) and 55% (95%CI 50–60), respectively. Patients with HCT-CI = 0, 1–2 and ≥ 3 had a CRFS at 1 year of 75% (95%CI 63–83), 75% (95%CI 65–82) and 62% (95%CI 54–69), respectively (p=0.001). By using the HCT-CI/age, patients with scores of 0, 1–2, 3–4 and ≥ 5 had a 1-year CRFS of 87% (95%CI 56–97), 75% (95%CI 66–81), 67% (95%CI 58–75), 59% (95%CI 49–69), respectively (p=0.001). Other details on CRFS are summarized in Table 2 and Figure 4.
In the univariate analysis, the only risk factor for a lower CRFS was the HCT-CI ≥3 (reference, HR 1.5 [95%CI 1.0–2.3], compared to reference HCT-CI=0, overall p=0.001) and the HCT-CI/age= 1–2, 3–4 and ≥5 (reference, HR 1.1 [95%CI 0.5–2.5], HR 1.6 [95%CI 0.7–3.6] and HR 2.3 [95%CI 1–5.2], respectively, compared to reference HCT-CI/age=0, overall p=0.001) (Supplementary table 1).
Outcome of patients with high comorbidity burden
The outcomes of 22 patients with a very high HCT-CI (scores ≥ 7, median follow-up for survivors of 43 months, range 26–98) were analyzed. Fifteen of these patients have died: 11 patients (50%) experienced NRM and 4 patients (18%) died due to disease relapse. Seven patients (32%) remained alive at last contact. Similarly, of 21 patients with a very high HCT-CI/age (scores ≥ 8, median follow-up for survivors of 57 months, range 26–98), fifteen have died (see causes of death above) and 6 remained alive at last contact.
Discussion
The HCT-CI has not been previously evaluated in a large cohort of patients receiving T-cell depleted grafts. Similarly, the predictive value of this model has not been validated for the CRFS. We now demonstrate that both the HCT-CI and HCT-CI/age are predictive of transplant outcomes, including NRM, OS and CRFS, after TCD allogeneic HCT in a cohort of nearly 350 patients. Patients with higher scores according to both models showed higher NRM and lower OS compared to patients with lower scores. Our results are consistent with and expand on those of a recently published small cohort of patients undergoing TCD HCT for several hematological diseases where the HCT-CI alone was studied.(24) In our study, patients with HCT-CI and HCT-CI/age scores of 0–2 had promising outcomes with a risk of NRM at 1 year of around 10%. Conversely, older patients with severe comorbidities (HCT-CI/age ≥ 5) showed much higher risk of NRM and a probability of long term OS < 45%. Interestingly, the HCT-CI appears to discriminate only two risk groups (0–2 and ≥ 3), while the HCT-CI/age was associated with a higher risk of NRM in all 4 risk groups. In fact, patients with HCT-CI/age score of 0 (younger patients <40 years without comorbidities) had an excellent outcome at one year with a NRM of 0% and an OS > 90%. It should be noted that this group had a low number of patients (4.3% of the whole cohort), and while promising, these results will require validation in a larger dataset. As shown in other studies, the HCT-CI had a limited capacity to predict disease relapse in our population.(15, 19)
Patients included in the study showed a frequency of comorbidities similar to what has been previously reported in the setting of myeloablative conditioning with unmodified grafts. As in several other studies,(10, 16, 25, 26) pulmonary impairment (44%) and hepatic disease (18%) were among most frequent comorbidities. Interestingly, psychiatric disturbances (20%) were more prevalent in our cohort than in the original HCT-CI manuscript.
Another notable observation in our study is the fact that the HCT-CI only identified two risk groups (0–2 and ≥ 3) after TCD HCT, whereas 3 groups were identified in series of unmodified HCT. While we cannot exclude that further discrimination would be detected in a significantly larger cohort, another potential interpretation is that patients with HCT-CI of 1–2 have less complications after TCD HCT as they are not exposed to post HCT methotrexate and calcineurin inhibitors. A generally accepted concept is that allogeneic HCT in patients with very high HCT-CI (e.g. HCT-CI ≥7) is associated with a prohibitive NRM, which may preclude the use of HCT in these patients. While only 22 patients had a HCT-CI ≥7 in our cohort, nearly a third of these high comorbid patients had a long term survival without relapse. Thus, the potential benefit of allo-HCT with a CD34 selected graft in the setting of a higher NRM should be carefully weighed against the risks of the underlying malignancy.
Besides NRM and OS, high scores in both comorbidity models were also associated with lower CRFS. This new end-point (the primary endpoint for BMT CTN 1301 trial, NCT02345850) determines survival without ongoing morbidity due to chronic GVHD and may be used as an indirect measure of quality of life after HCT. Again, the HCT-CI only classified patients into 2 groups for 1-year CRFS (0–2 vs ≥3) whereas the HCT-CI/age divided patients into 4 risk-groups with a 10% incremental decrease in CRFS in all 4 risk-groups at 1 year. In the era of patient self-reported outcomes and greater emphasis on quality of life including in the field of HCT, we believe that this is a key finding of this study and might contribute to improve discussions with patients, rather than just focusing on overall survival.
Limitations of this study include challenges related to retrospective data collection. Although specific recommendations for comorbidity assessment in the HCT population were strictly followed,(22) we cannot exclude the possibility that differences seen between categories in our analysis were accentuated by differences between the scoring method compared to those conducted by other investigators.
Ultimately, this analysis demonstrates the validity of both the HCT-CI and the HCT-CI/age in predicting outcomes in patients undergoing allogeneic transplant with CD34+ selected grafts, including CRFS. Critical information in this regard will be provided by the results of the ongoing randomized clinical trial focusing on calcineurin inhibitor-free GVHD prevention strategies including T-cell depleted grafts, which will prospectively address the role of HCT-CI in this transplant setting and determine the best approach for GVHD prevention among the 3 tested strategies.
Supplementary Material
Highlights.
CD34-selected alloHCT is associated with high chronic GVHD relapse-free survival in AML and MDS.
Higher HCT-CI and HCT-CI/age are associated with higher NRM and lower OS in CD34− selected alloHCT.
HCT-CI and HCT-CI/age may be used to select appropriate patients for CD34-selected alloHCT.
Acknowledgments
This research was supported in part by National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. PB was supported by a grant of the Red Tematica de Investigacion Cooperativa en Cancer (RTICC) cofunded by the European Regional Development Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–1090. [PubMed] [Google Scholar]
- 2.Jakubowski AA, Small TN, Young JW, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007;110:4552–4559. doi: 10.1182/blood-2007-06-093880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, et al. Transplantation in remission improves the disease-free survival of patients with advanced myelodysplastic syndromes treated with myeloablative T cell-depleted stem cell transplants from HLA-identical siblings. Biol Blood Marrow Transplant. 2008;14:458–468. doi: 10.1016/j.bbmt.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquini MC, Devine S, Mendizabal A, et al. Comparative outcomes of donor graft CD34+ selection and immune suppressive therapy as graft-versus-host disease prophylaxis for patients with acute myeloid leukemia in complete remission undergoing HLA-matched sibling allogeneic hematopoietic cell transplantation. J Clin Oncol. 2012;30:3194–3201. doi: 10.1200/JCO.2012.41.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg JD, Linker A, Kuk D, et al. T Cell-Depleted Stem Cell Transplantation for Adults with High-Risk Acute Lymphoblastic Leukemia: Long-Term Survival for Patients in First Complete Remission with a Decreased Risk of Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2013;19:208–213. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19:898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50:493–498. doi: 10.1038/bmt.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamari R, Chung SS, Papadopoulos EB, et al. CD34-Selected Hematopoietic Stem Cell Transplants Conditioned with Myeloablative Regimens and Antithymocyte Globulin for Advanced Myelodysplastic Syndrome: Limited Graft-versus-Host Disease without Increased Relapse. Biol Blood Marrow Transplant. 2015;21:2106–2114. doi: 10.1016/j.bbmt.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood. 2011;117:2728–2734. doi: 10.1182/blood-2010-08-303263. [DOI] [PubMed] [Google Scholar]
- 12.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 14.Pollack SM, Steinberg SM, Odom J, Dean RM, Fowler DH, Bishop MR. Assessment of the hematopoietic cell transplantation comorbidity index in non-Hodgkin lymphoma patients receiving reduced-intensity allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:223–230. doi: 10.1016/j.bbmt.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelis FV, Messner HA, Atenafu EG, et al. Patient age, remission status and HCT-CI in a combined score are prognostic for patients with AML undergoing allogeneic hematopoietic cell transplantation in CR1 and CR2. Bone Marrow Transplant. 2015;50:1405–1410. doi: 10.1038/bmt.2015.165. [DOI] [PubMed] [Google Scholar]
- 16.Barba P, Pinana JL, Martino R, et al. Comparison of two pretransplant predictive models and a flexible HCT-CI using different cut off points to determine low-, intermediate-, and high-risk groups: the flexible HCT-CI Is the best predictor of NRM and OS in a population of patients undergoing allo-RIC. Biol Blood Marrow Transplant. 2010;16:413–420. doi: 10.1016/j.bbmt.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Ponce DM, Sauter C, Devlin S, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor-derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19:799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorror ML, Martin PJ, Storb RF, et al. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014;124:287–295. doi: 10.1182/blood-2014-01-550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32:3249–3256. doi: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernan NA, Flomenberg N, Collins NH, O'Reilly RJ, Dupont B. Quantitation of T lymphocytes in human bone marrow by a limiting dilution assay. Transplantation. 1985;40:317–322. doi: 10.1097/00007890-198509000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Keever-Taylor CA, Devine SM, Soiffer RJ, et al. Characteristics of CliniMACS(R) System CD34− enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant. 2012;18:690–697. doi: 10.1016/j.bbmt.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013;121:2854–2863. doi: 10.1182/blood-2012-09-455063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401 e381. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le RQ, Tian X, Jain NA, et al. Clinical comorbidity predictive measures in ex vivo T-cell-depleted allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2015;50:1138–1140. doi: 10.1038/bmt.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farina L, Bruno B, Patriarca F, et al. The hematopoietic cell transplantation comorbidity index (HCT-CI) predicts clinical outcomes in lymphoma and myeloma patients after reduced-intensity or non-myeloablative allogeneic stem cell transplantation. Leukemia. 2009;23:1131–1138. doi: 10.1038/leu.2009.1. [DOI] [PubMed] [Google Scholar]
- 26.Majhail NS, Brunstein CG, McAvoy S, et al. Does the hematopoietic cell transplantation specific comorbidity index predict transplant outcomes? A validation study in a large cohort of umbilical cord blood and matched related donor transplants. Biol Blood Marrow Transplant. 2008;14:985–992. doi: 10.1016/j.bbmt.2008.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.