Abstract
Purpose
The King-Devick Test (KD) has been studied as a remove-from-play sideline test in college-age athletes and older; however, studies in younger athletes are limited. A cross-sectional study of the KD and other vision correlates was completed on school-aged athletes during pre-season physicals for a variety of sports to determine the repeatability of the KD. The study also evaluated how convergence, alignment, or pupil function contributed to a slower King-Devick baseline reading.
Methods
785 athletes underwent vision screenings in a hospital or school setting by trained/certified staff as part of pre-season physicals. 619 had KD testing completed per the manufacturer’s suggested protocol and repeated. Other baseline vision testing included visual acuity, Modified Thorington testing for alignment, convergence testing, and pupil function using the NeurOptics (NPI-200) NPi.
Results
The mean fastest, error-minimized KD time for all participants was 43.9 seconds(s) (sd±11.6, range 24–120). Median KD time got faster (+) with age (p <.0001). The inter-class correlation coefficient for all scores was 0.92. The absolute mean time difference for any two tests was 3.5s (sd±2.5, range 0–23). There was no association between the best KD time and reduced NPC (p=0.63), Modified Thorington measure of alignment (p = 0.55), or NPi pupil function (p=0.79). The Bland Altman repeated measure limits of agreement was ±6.5s for those in the 10th–12th grades, and ±10.2s for those in 6th–9th grades.
Conclusions
King-Devick score in junior high and high-school athletes is variable but gets faster and more repeatable with increasing age. The KD does not correlate significantly with reduced convergence, alignment, or pupil function. Based on grouped data, a slowing of 10 seconds for younger athletes and 6 seconds for older athletes on a second administration represents a true difference in testing speed. Within-player variability should be considered when removal-from-play decisions are influenced by KD results.
Keywords: King Devick, convergence, pupillometer, alignment, concussion, adolescent, sport
The Centers for Disease Control (CDC) estimates that approximately 250,000 children (19 years old and younger) were treated in U.S. emergency departments in 2009 for sports and recreation-related injuries that included a diagnosis of concussion or traumatic brain injury (TBI).1 The CDC also reported that from 2001–2009, the estimated number of sports and recreation-related TBI visits to emergency departments increased 62%.1 Therefore, the need for objective, reliable, and feasible testing of concussion in adolescents is becoming increasingly important. The 4th International Conference on Concussion in Sport published a consensus report that made suggestions for on-field and sideline evaluation of acute concussion which included using the SCAT3 and Standardized Assessment of Concussion (SAC).2 However, these tests are not considered screening tests and therefore may be considered time consuming to athletic trainers, coaches, or team physicians during game situations.
There have been several reports of eye movement and eye coordination difficulties following concussion. Master and Scheiman recently reported that among 11 to 17 year olds evaluated in a comprehensive concussion program, 69% had a vision disorder that included accommodative disorder, convergence insufficiency, or saccadic eye function.3 Others have reported eye conditions in concussion in as high as 30 to 47%.4–7 These reports are consistent with the suggestion that future studies should evaluate the value of incorporating vision-based testing into sideline diagnosis and long-term clinical assessments as a supplement to the medical diagnosis of concussion.8
The King-Devick test was developed in 1976 as tool to measure saccadic function.9 More recently, the KD has been studied as a remove-from-play sideline test in college-age athletes and older; however, studies in junior high and high school athletes are limited.10–11 If the test is to be used as part of sideline assessment, an understanding of the psychometric properties of the test under the conditions it is likely to be employed and its association with other vision function measures in this age group are needed. The purpose of this project was to assess the repeatability of the King Devick test in the setting of mass athletic screening in high school and junior high athletes. Since the underlying visual parameters related to King Devick performance are not well defined, we also explored the association of the King Devick test times to other measured visual functions including convergence, alignment, and objective pupil function.
METHODS
After approval by University of Alabama at Birmingham (UAB) Institutional Review Board, certification and training of screening personnel was completed, informed consent by athletes was waived with IRB approval, and health-screening data was retrieved in a de-identified manner. The research followed the tenets of the Declaration of Helsinki.
Procedures
A total of 785 athletes from 14 Birmingham, Alabama metropolitan areas schools were screened during a two-week period in April-May 2015. The State of Alabama requires pre-season physicals for all junior high and high school athletes as part of a mandatory clearance program for sports participation in the following academic year. The health screenings are frequently conducted en mass by school. In conjunction with its Sport Medicine Program, Children’s of Alabama (COA) has provided physicals to area schools for many years. The multi-disciplinary physicals are provided by sports medicine physicians, certified athletic trainers, and optometrists, as well as trainees in optometry, physical therapy and nursing. In 2015, testing took place at COA Sport Medicine clinic (students from 12 schools in a hospital setting) or a designated area within the high school (2 schools).
As part of the 2015 physicals, an enhanced vision test battery was performed. Prior to taking part in the vision testing, certified athletic trainers and optometry students were trained and certified by the lead authors in the conduct of procedures. Training consisted of written protocols for each test. Certification occurred when the lead authors determined that a vision tester had executed the protocol properly. Each athlete had testing done by the same individual screening personnel. An optometrist was on site at all times. The vision screening battery consisted of Snellen distance (10 feet) visual acuity with the participant’s habitual correction, the Modified Thorington (MT) test for alignment, King-Devick (KD) test, the near-point of convergence test (NPC), and automated reflex pupillometry (NeurOptics NPi-200). Type of school attended (private or public) was documented. History of concussion was obtained as well as sport(s) in which the athlete participated, as reported by athlete.
All athletes had KD testing completed per the manufacturer’s suggested protocol: testing was explained and the demonstration card with numbers and eye-movement guiding arrows was administered to each athlete once. The cumulative time required to read a series of numbers on the three KD test cards with the fewest errors was documented; this was repeated with the cumulative time documented. The faster of the two times with fewest errors was noted as baseline 1. This protocol was repeated immediately with two additional cumulative times documented, the fastest of the two with fewest errors noted as baseline 2. In all, there were four total cumulative readings of the three KD test cards and two baseline scores.
Snellen visual acuity was measured at 10 feet. The refractive correction of the participant was the participant’s habitual correction. If the participant was wearing glasses at the time of testing, it was documented that the participant was wearing his habitual glasses. If the participant was not wearing glasses at the time of testing, the reason for not wearing glasses was documented as “no glasses prescribed” or “glasses prescribed but not wearing.”
Alignment and convergence were tested, then analyzed with KD data, to determine if such factors might contribute to a slower KD test time. Additionally, given the near triad (miosis, accommodation, and convergence), an objective measure of pupil function was assessed to explore how pupillary reaction might affect KD test time. Modified Thorington testing for alignment was performed by placing a horizontal Maddox rod over the athlete’s right eye which allowed the athlete to view a red, vertically oriented line with his/her right eye. The athlete viewed a central white penlight with his/her left eye through a central hole in the Modified Thorington card (www.good-lite.com) at 16 inches. The Modified Thorington card is a hand-held card with numbers and letters radiating away from the central white light. Numbers radiate up and right and are spaced to represent one prism diopter of eso (with red lens lines oriented horizontally over right eye) per number. Letters radiate down and left and are spaced to represent one prism diopter of exo (with red lens lines oriented horizontally over right eye). The athlete was asked to keep the numbers and letters clear to control for accommodation while looking at the white light and noticing which number or letter the vertical red line was nearest. Near-point of convergence was measured using an accommodative fixation target (angle of full target subtending 100 minutes of arc at 40 cm and smaller detail on target subtending 5 to 25 minutes of arc) to the nearest half centimeter, asking the athlete to keep a target single for as long as possible as it approached closer to his/her eyes. The NPC break point was measured one time at the subjective report of sustained diplopia. In the absence of a subjective report of diplopia, the point at which there was an observed loss of fusion was considered the NPC break point.
Pupil function using the NeurOptics (NPI-200) pupillometer was tested and repeated on each eye. The NeurOptics pupillometer presents a light to the participant’s eye as the hand-held instrument rests on the participant’s cheek. Pupil reaction is viewed through the monitor on the instrument and is video recorded. Testing takes roughly 10 seconds per eye. The NPi (NeurOptics Pupillary Index) is displayed immediately after testing when the scan is good. The NPi is a proprietary index based on seven pupil functions (maximum diameter, minimum diameter, percent change in size, average constriction velocity, maximum constriction velocity, latency of constriction, and dilation velocity). The individual findings of the seven pupil functions are also displayed. The NPi was tested and documented for the right eye and left eye, and this was repeated for the right eye and left eye.
Data was collected on protocol-driven exam forms; school athletic trainers who did not perform vision screenings removed identifiable data and submitted the exam forms en mass to the research optometrists.
Analysis
Of the 785 athletes screened, the final cohort analyzed consisted of 619 athletes who had all four administrations of the KD test times documented. Only those with fewer than all four KD test times documented were excluded in the analyses. For these 619 athletes, all available data collected were used in the analyses. This included participants with incomplete demographic information and other data collection. Comparisons between included and excluded subjects were done using a general linear regression and chi-square as appropriate for continuous and categorical data respectively. Intraclass correlation coefficients (ICC) were calculated on KD times using a mixed random effects model. Calculations were performed for all four times, first two administrations only and between the better time of the first two tests and better of the third and fourth tests. Relative and absolute mean test differences were calculated per subject and as a grand mean. Relative values were calculated by subtracting later measures for each of six possible time combinations (time1–time2, time1–time3, time1–time4, time2–time3, time2–time4, time 3–time4) from earlier ones to give a mathematical difference, which would be positive for faster times and negative for slower times. Absolute differences were taken from the absolute value (distance) of the relative difference to give seconds of difference regardless of faster or slower. The Bland Altman coefficient of repeatability also known as the smallest real difference (SRD)12,13 was calculated using the same KD groupings that were done for ICCs. The SRD was calculated as the within group standard deviation (standard error of measurement) X ±1.96 X √2. 12,13 Within group error was calculated using a general linear model one way ANOVA.
Non-parametric techniques, Spearman correlation (corr) and Kruskal Wallis, were used to evaluate associations between baseline KD time (fastest overall), demographic and visual function measures. Grade in school was dichotomized to 10th grade and above and 9th and below. Race was categorized into three groups: African American, White and Others. Athletes’ responses about sports participation were categorized into 11 sports activities. The arithmetic total was used in calculations. Visual acuity was grouped into three categories: those with 20/30 or better in each eye, those with 20/30 or better in one eye and less than 20/30 in the other, and those with less than 20/30 in both eyes. A majority (more than half) of letters missed on the 20/30 line was considered less than 20/30. Results of the Modified Thorington test were linearized with negative numbers indicating esophoria and positive numbers exophoria. Additionally, absolute values were generated resulting in a scale of no phoria to higher phoria. Result of convergence was dichotomized as pass fail, with failing defined as a distance greater than or equal to 6 centimeters from the participant’s bridge of the nose.14,15 The NPi number was used in analysis. Per the manufacturer, values less than 3 are considered abnormal. A dichotomous pass-fail pupil category was created. The NPi value for both administrations in the same eye was averaged. If the average NPi value in either eye was less than 3 then the pupil test was considered a failure.
RESULTS
Demographic characteristics of the participants and those excluded are found in Table 1. Those who had incomplete KD data and were excluded were on average about one year older, one grade further in school, were more likely to be of African American or other race and more likely to be male. No differences in the groups were found for previous history of concussion, number of sports played, and the measured vision characteristics.
Table 1.
Demographic characteristics.
| Included Group (n=619) | Excluded* (n-166) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | sdev | N | % | Mean | sdev | N | % | p | |
| Age | 14.2 | 1.7 | 609 | 15.2 | 1.7 | 163 | <.0001 | ||
|
| |||||||||
| Grade | 8.8 | 1.5 | 611 | 9.8 | 1.9 | 166 | <.0001 | ||
| <10th | 354 | 57.9 | 66 | 39.8 | |||||
| >=10th | 257 | 42.1 | 100 | 60.2 | <.001 | ||||
|
| |||||||||
| Race | |||||||||
| African American | 346 | 48.9 | 135 | 88.8 | <.001 | ||||
| White | 175 | 24.8 | 2 | 1.3 | |||||
| Other | 34 | 4.8 | 15 | 9.9 | |||||
|
| |||||||||
| Gender | |||||||||
| Male | 457 | 74.8 | 157 | 95.8 | <.001 | ||||
| Female | 154 | 25.3 | 7 | 4.2 | |||||
|
| |||||||||
| No Concussion History | 480 | 93 | 107 | 96.4 | 0.28 | ||||
|
| |||||||||
| Number Sports Played | 1.7 | 1 | 610 | 1.7 | 1.1 | 166 | 0.24 | ||
|
| |||||||||
| Visual Acuity | |||||||||
| Better than 20/30 Each Eye | 509 | 85.3 | 141 | 84.9 | 0.75 | ||||
| One Eye Better/One Less | 39 | 6.5 | 9 | 5.4 | |||||
| Both Less than 20/30 | 49 | 8.2 | 16 | 9.6 | |||||
|
| |||||||||
| Modified Thoringtonǂ | 2.7 | 3.7 | 611 | 2.5 | 3.2 | 161 | 0.56 | ||
|
| |||||||||
| Convergence | |||||||||
| Pass | 502 | 81.8 | 134 | 81.2 | 0.87 | ||||
| Fail | 112 | 18,2 | 31 | 18.8 | |||||
| NPI | |||||||||
| >=3 Both | 515 | 97.7 | 158 | 96.8 | 0.57 | ||||
| < 3 in One or Both | 12 | 5 | |||||||
sdev-standard deviation of mean, N-number participating,
- positive number indicates exophoria
Excluded were those with fewer than all four KD test times
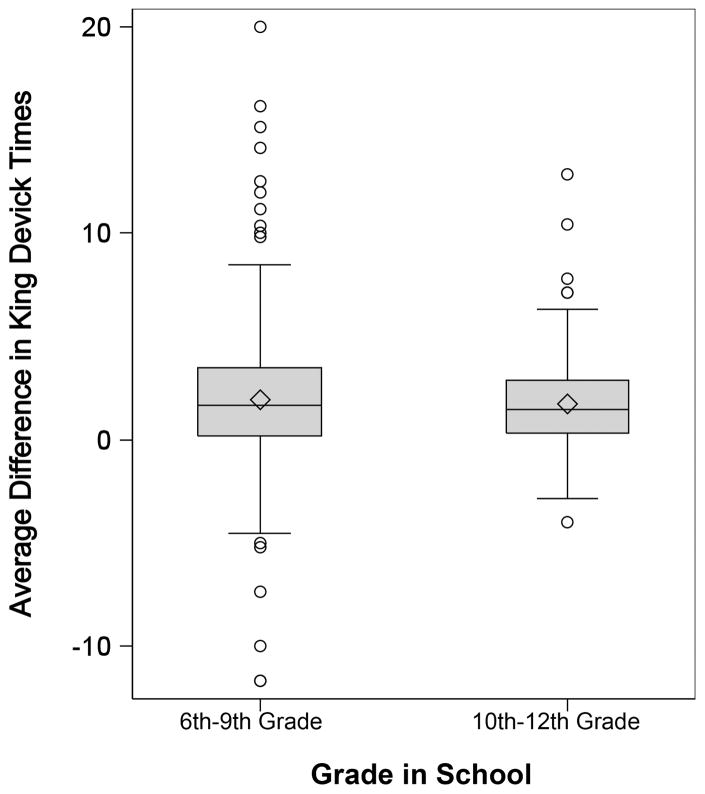
The mean error-minimized fastest KD time for all participants was 43.9 seconds(s) (sd±11.6, range 24–120), with the median time 42s (IQR=13.0). KD time decreased with increasing age (p <.0001) (Figure 1). Athletes’ median time in the 10th grade or higher was almost 4–6 seconds faster than that of athletes in the 9th or lower grades in completing the KD. (Table 2)
Figure 1.
Mean difference in King-Devick times by school grade.
Table 2.
Associations with faster of two baseline King Devick time.
| Median KD Time(IQR) | Spearman Corr | Spearman p | Kruskal Wallis p | |
|---|---|---|---|---|
| Overall | 42.0(13.0) | |||
|
| ||||
| Age | −0.35 | 0.0001 | ||
| 10 | 45.0(10.0) | |||
| 11 | 54.0(14.0) | |||
| 12 | 49.0(15.0) | |||
| 13 | 46.0(14.0) | |||
| 14 | 41.0(11.0) | |||
| 15 | 40.0(10.5) | |||
| 16 | 39.0(7.0) | |||
| 17 | 35.0(8.0) | |||
| 18 | 31.5(7.0) | |||
|
| ||||
| Grade | ||||
| <10th | 45.4(15.0) | 0.0001 | ||
| >=10th | 37.4(8.1) | |||
|
| ||||
| School | 0.0001 | |||
|
| ||||
| Race | ||||
| African American | 42.0(13.3) | |||
| White | 39.6(12.0) | 0.0001 | ||
| Other | 43.5(12.7) | |||
|
| ||||
| Gender | ||||
| Male | 42.0(13.0) | 0.63 | ||
| Female | 41.3(13.5) | |||
|
| ||||
| Concussion History | ||||
| None | 41.5(12.0 ) | 0.09 | ||
| Previous | 38.5(14.4 ) | |||
| Number Sports Played | 0.19 | 0.001 | ||
|
| ||||
| Visual Acuity | ||||
| Better than 20/30 Each Eye | 42.0(12.0) | |||
| One Eye Better20/30/One Less | 40.0(13.9) | |||
| Both Less than 20/30 | 46.6(16) | 0.005 | ||
|
| ||||
| Modified Thorington (absolute value) | −0.02 | 0.55 | ||
|
| ||||
| Convergence | ||||
| Pass | 42.0(13.0) | 0.63 | ||
| Fail | 42.0(13.0) | |||
|
| ||||
| NPI | ||||
| >3 Both | 39.7(12.2) | 0.79 | ||
| < 3 in One or Both | 42.0(13.1) | |||
IQR-Interquartile range; Corr-Correlation coefficient
The mean time difference between any two individual measures for the cohort was 1.9s (sd± 3.0, range −11.6 to +20). The absolute mean time difference (discounting faster or slower) for any two tests was 3.5 (sd±2.5, range 0–23). Mean time differences for 10th grader and higher were not significantly different than those 9th grade and lower (p=0.39) while absolute differences were (p=0.004).
In addition to age, race (p≤.0001) and school attended (p=0.0001) were also associated with the best of the two baseline KD times. Both race (p≤0.0001) and school attended (p0.0001) were also correlated with age. While not statistically significant (p=0.09), those with a history of prior concussion as a group did perform testing faster than those without a history of concussion. The KD time was also associated with the number sports activities (p=0.0001). This relationship is also confounded by the association of total sports participation to age (p=0.0001). As might be expected, older athletes tended to more narrowly focus more on fewer sports.
There was no association between the best KD time and reduced NPC (p=0.63) or the absolute magnitude of Modified Thorington’s measure of alignment (p = 0.55). Pupil function based on NPi also showed no association with KD time (p=0.79). Visual acuity less than 20/30 in both eyes was associated with slower KD time (p=0.005) compared to those with better than 20/30 in one or both eyes.
Correlation among KD times was excellent. The intraclass correlation coefficient (ICC) for all four KD times was 0.92, and for the best two among the first and second pair was 0.94. Consistent with a short-term learning effect in 412/619 (66.6%), the fastest of the error-minimized times was in the second pair of KD tests. The average improvement from baseline 1 to baseline 2 across all subjects was 1.6s (sd ±3.6). However, in 149/619 (24.1%) the second KD pair administration times were slower than the baseline value that would have been developed from the initial two tests. In the remainder, best test times were unchanged from the first to second pair. For any individual, testing resulted in the same baseline score in only 10% of participants.
To further illustrate individual variability between subjects, Table 2 shows the distribution being substantially wider in younger athletes with outliers both faster and slower more common. The Bland Altman repeated measure limits of agreement (smallest real difference) for all four tests in this environment and format was ±8.9s for the cohort, being lower for those in the 10th grade or higher at ±6.5s and slower for those in lower grades at ±10.2s. If the best time between the first pair and second pair are compared, the SRD becomes 7.2s, 5.4s, and 8.1s 10.4s respectively. (Table 3) Three subjects had no standard deviation as all four test times were identical, equating to a smallest real difference of 0 or any time change being significant. The largest single smallest real difference for a subject was 28.6 seconds (16 year old).
Table 3.
Smallest real differences in different King Devick Test formats by grade†
| All Four Administrations | First Two Only | Best First Pair/Best Second Pair | |
|---|---|---|---|
| Grade Level | |||
| 9th and below | 10.2 | 10.9 | 10.4 |
| 10th and above | 6.5 | 6.16 | 5.4 |
| Total | 8.9 | 8.9 | 7.2 |
Time in seconds
DISCUSSION
Through much of its history, the KD test has been used as a measure of eye movement ability in conjunction with reading assessment. More recently, the KD test has shown promise as a screener for concussion. It has been shown to be associated with medically diagnosed concussion, the ImPACT battery, and other markers of concussion.10,11,16–19 It has been used at the boxing ringside, on the sideline at football games, and the rugby pitch among other venues.10,11,16–19 Testing has also been accomplished by both parents and lay personnel.19 In this study, we were able to effectively administer the KD test to an adolescent population in the setting of a mass athlete screening. The mean time to completion, the ICCs, and standard deviations found from testing in this setting were similar to that which has been reported for other testing scenarios of athletes.20–22 This suggests that mass screening is also an appropriate target venue for assessment.
As has been seen in other studies, the best KD time in this cohort decreased with increasing age.16,18,20–21 As might be expected, the variability between test administrations also narrowed as age increased. Within this population, school attended was also associated with best KD time. The schools were a mixture of public and private, middle school and high school. The KD is often described as a number-reading task and may be reflective of more global reading level at various schools. Consistent with other studies, prior history of concussion was not associated with KD time beyond the immediate concussion and post-concussion period.23 Among this group, those with a history of concussion had faster median times.
Visual acuity less than 20/30 in both eyes was the only vision factor found to be associated with baseline KD. Athletes who had reduced acuity in both eyes less than 20/30 had median times 4–6 seconds slower compared to those with 20/30 or better distance acuity in at least one eye. This might have implications if an athlete were prescreened with best correction and chose not to participate in sports events with their correction. This finding bares further study. Basic binocular screening was not associated with KD time. Further studies of the basic oculomotor and neurological underpinnings of the KD test are needed.
One of our primary goals was to explore the repeatability of the KD test in younger athletes. A number of studies have reported ICC from KD testing among college and high school athletes to be good to excellent.21–23 We found similarly high ICC for a much younger cohort with a majority being 6th to 9th grade athletes. Intraclass correlation coefficients are widely used as a marker of repeatability in continuous measures. It is superior to Pearson correlation in that it accounts for both within subject and change in mean across testing.12 As Bland and Altman13 have pointed out, relying solely on intraclass correlation gives an incomplete picture of agreement. Being a unitless measure, ICC is more difficult to interpret beyond descriptors such as ‘good’ or ‘poor’. As an alternative, the SRD provides a value in the same unit as measurement beyond which a real change lies. One previous paper has reported directly on the minimum detectable change in high school football players aged 13–18.22 Alsalaheen and collegues reported 6.10 seconds as the minimum detectable change in a group of 9th–12th graders in Flint, Michigan. The smallest real difference in our complete cohort using all four tests was 8.9 seconds. This longer time is likely related to the larger number of younger subjects (6th–9th) in our cohort. The testing paradigm was also somewhat different from ours with the Alsalahee participants’ repeated measure being one hour after the initial test pair. However, when looking only at high school athletes the numbers are quite similar (6.5s) for all four and identical for best of each pair.
To date, almost all studies of the KD test have evaluated athletes who have been diagnosed with concussion by other methods; KD times have then been compared times pre- and post-event. Currently, the recommendations from the manufacturer are for any change from baseline to be considered as a criterion for removal from play. The KD time change that has been reported with concussion has varied from study to study. Galetta, et. al.18 in the original report using the KD as a concussion marker found greater than 5 seconds to be the minimum time that distinguished those with head injury from those not head injured in boxers and mixed martial arts fighters. In a separate report of youth and college athletes, Galleta, et. al.17 reported a mean change of 5.2 seconds in those with a concussion event. In looking at Seidman, et. al.’s23 report of nine concussed high school athletes, the smallest reported change in KD time associated with a medically diagnosed concussion was 6.5 seconds. In a study of junior rugby players (mean aged 10 years), greater than 3 seconds was associated with concussion with the average slowing over 7 seconds.11 Many of these studies show prolonged retest times well beyond any question of a true difference. The minimum times reported are, however, quite near the smallest real difference we note.
The focus of most concussion research has been high school and college athletes. Age 12–16 is a critical period of late brain maturation. Neuron growth and significant pruning occurs in this period. 24 Younger children have longer recovery time from concussion than older teens.25 Being able to reliably detect damage is critical in this group. This study is one of the largest studies of KD done to date and includes one of the largest cohorts of young athletes. The population is also unique with a majority of subjects African American. We did have a substantial number of potential candidates that did not complete all four administrations of the KD. The vast majority of these were among those in the 10th grade and beyond. The inclusion of more older athletes would likely have shifted the overall results towards shorter smallest real difference times.
Our methodology focused on short-term repeatability and is most applicable in baseline determination. Our data support the smallest real difference numbers of approximately 6 seconds for high school athletes and approximately 10 seconds for athletes in the 6–9th grade. We followed the KD protocol and repeated once so that in all, the three test cards were read four times by each athlete. We found that administering the KD four times was feasible in a mass screening of high school and junior high athletes, and doing so may better capture variability in any one individual athlete. Based on our results, the King-Devick test is repeatable within grouped data, but individual player norming may provide the best method for guiding future removal-from-play decisions.
Acknowledgments
This study was supported by the UAB Department of Optometry and the UAB Vision Science Research Center’s National Institutes of Health P30 EY003039 core grant.
References
- 1.Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report (MMWR) Nonfatal Traumatic Brain Injuries Related to Sports and Recreation Activities among Persons Aged ≤19 Years — United States, 2001–2009. [Accessed January 14, 2016];MMWR. 2011 60:1337–42. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6039a1.htm. [PubMed] [Google Scholar]
- 2.McCrory P, Meeuwisse W, Aubry M, Cantu B, Dvořák J, Echemendia R, Engebretsen L, Johnston K, Kutcher J, Raftery M, Sills A, Benson B, et al. Consensus statement on Concussion in Sport - The 4th International Conference on Concussion in Sport held in Zurich, November 2012. Phys Ther Sport. 2013;14:e1–e13. doi: 10.1016/j.ptsp.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Master CL, Scheiman M, Gallaway M, Goodman A, Robinson RL, Master SR, Grady MF. Vision diagnoses are common after concussion in adolescents. Clin Pediatr (Phila) 2016;55:260–7. doi: 10.1177/0009922815594367. [DOI] [PubMed] [Google Scholar]
- 4.Stelmack JA, Frith T, Van Koevering D, Rinne S, Stelmack TR. Visual function in patients followed at a Veterans Affairs polytrauma network site: an electronic medical record review. Optometry. 2009;80:419–24. doi: 10.1016/j.optm.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Brahm KD, Wilgenburg HM, Kirby J, Ingalla S, Chang CY, Goodrich GL. Visual impairment and dysfunction in combat-injured service members with traumatic brain injury. Optom Vis Sci. 2009;86:817–25. doi: 10.1097/OPX.0b013e3181adff2d. [DOI] [PubMed] [Google Scholar]
- 6.Goodrich GL, Kirby J, Cockerham G, Ingalla SP, Lew HL. Visual function in patients of a polytrauma rehabilitation center: A descriptive study. J Rehabil Res Dev. 2007;44:929–36. doi: 10.1682/jrrd.2007.01.0003. [DOI] [PubMed] [Google Scholar]
- 7.Suchoff IB, Kapoor N, Waxman R, Ference W. The occurrence of ocular and visual dysfunctions in an acquired brain-injured patient sample. J Am Optom Assoc. 1999;70:301–8. [PubMed] [Google Scholar]
- 8.Ventura RE, Jancuska JM, Balcer LJ, Galetta SL. Diagnostic tests for concussion: is vision part of the puzzle? J Neuroophthalmol. 2015;35:73–81. doi: 10.1097/WNO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 9.King Devick Test. [Accessed January 14, 2016];About King Devick Test. Available at http://www.kingdevicktest.com/about/
- 10.Galetta KM, Morganroth J, Moehringer N, Mueller B, Hasanaj L, Webb N, Civitano C, Cardone DA, Silverio A, Galetta SL, et al. Adding vision to concussion testing: a prospective study of sideline testing in youth and collegiate athletes. J Neuroophthalmol. 2015;35:235–41. doi: 10.1097/WNO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 11.King D, Hume P, Gissane C, Clark T. Use of the King-Devick test for sideline concussion screening in junior rugby league. J Neurol Sci. 2015;357:75–9. doi: 10.1016/j.jns.2015.06.069. [DOI] [PubMed] [Google Scholar]
- 12.Vaz S, Falkmer T, Passmore AE, Parsons R, Andreou P. The case for using the repeatability coefficient when calculating test-retest reliability. PLoS One. 2013;8:e73990. doi: 10.1371/journal.pone.0073990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 14.CITT-ART Investigator Group. Scheiman M, Mitchell GL, Cotter SA, Kulp M, Chase C, Borsting E, Arnold E, Denton C, Hertle R. Convergence Insufficiency Treatment Trial - Attention and Reading Trial (CITT-ART): design and methods. Vis Dev Rehabil. 2015;1:214–28. [PMC free article] [PubMed] [Google Scholar]
- 15.The convergence insufficiency treatment trial: design, methods, and baseline data. Convergence Insufficiency Treatment Trial (CITT) Study Group. Ophthalmic Epidemiol. 2008;15:24–36. doi: 10.1080/09286580701772037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tjarks BJ, Dorman JC, Valentine VD, Munce TA, Thompson PA, Kindt SL, Bergeron MF. Comparison and utility of King-Devick and ImPACT® composite scores in adolescent concussion patients. J Neurol Sci. 2013;334:148–53. doi: 10.1016/j.jns.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Galetta MS, Galetta KM, McCrossin J, Wilson JA, Moster S, Galetta SL, Balcer LJ, Dorshimer GW, Master CL. Saccades and memory: baseline associations of the King-Devick and SCAT2 SAC tests in professional ice hockey players. J Neurol Sci. 2013;328:28–31. doi: 10.1016/j.jns.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Galetta KM, Barrett J, Allen M, Madda F, Delicata D, Tennant AT, Branas CC, Maguire MG, Messner LV, Devick S, Galetta SL, Balcer LJ. The King-Devick test as a determinant of head trauma and concussion in boxers and MMA fighters. Neurology. 2011;76:1456–62. doi: 10.1212/WNL.0b013e31821184c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong DF, Balcer LJ, Galetta SL, Liu Z, Master CL. The King-Devick test as a concussion screening tool administered by sports parents. J Sports Med Phys Fitness. 2014;54:70–7. [PubMed] [Google Scholar]
- 20.Leong DF, Balcer LJ, Galetta SL, Evans G, Gimre M, Watt D. The King-Devick test for sideline concussion screening in collegiate football. J Optom. 2015;8:131–9. doi: 10.1016/j.optom.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vartiainen MV, Holm A, Peltonen K, Luoto TM, Iverson GL, Hokkanen L. King-Devick test normative reference values for professional male ice hockey players. Scand J Med Sci Sports. 2015;25:e327–30. doi: 10.1111/sms.12307. [DOI] [PubMed] [Google Scholar]
- 22.Alsalaheen B, Haines J, Yorke A, Diebold J. King-Devick Test reference values and associations with balance measures in high school American football players. Scand J Med Sci Sports. 2016;26:235–9. doi: 10.1111/sms.12628. [DOI] [PubMed] [Google Scholar]
- 23.Seidman DH, Burlingame J, Yousif LR. Evaluation of the King-Devick test as a concussion screening tool in high school football players. J Neurol Sci. 2015;356:97–101. doi: 10.1016/j.jns.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Campbell IG, Grimm KJ, de Bie E, Feinberg E. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc Natl Acad Sci USA. 2012;109:5740–3. doi: 10.1073/pnas.1120860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JH, Gill C, Kuhn EN, Rocque BG, Menendez JY, O’Neill JA, Agee BS, Brown ST, Crowther M, Davis RD, et al. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. J Neurosurg Pediatr. 2015;18:1–6. doi: 10.3171/2015.8.PEDS14332. [DOI] [PMC free article] [PubMed] [Google Scholar]