Abstract
How do migratory birds, herding dogs, and navigating sea turtles do the amazing things that they do? For hundreds of years, scientists and philosophers have struggled over possible explanations. In time, one word came to dominate the discussion: instinct. It became the catch-all explanation for those adaptive and complex abilities that do not obviously result from learning or experience. Today, various animals are said to possess a survival instinct, migratory instinct, herding instinct, maternal instinct, or language instinct. But a closer look reveals that these and other “instincts” are not satisfactorily described as inborn, pre-programmed, hardwired, or genetically determined. Rather, research in this area teaches us that species-typical behaviors develop—and they do so in every individual under the guidance of species-typical experiences occurring within reliable ecological contexts.
Keywords: Species-typical behavior, ethology, epigenesis, developmental systems, imprinting, locomotion, developmental plasticity, developmental anomaly, vestibular system, righting response, inheritance
INTRODUCTION
Every complex behavior challenges us to identify its origins. How do birds know to migrate south for the winter? How do border collies know to herd sheep? How do sea turtles find their way back home to the beach on which they hatched? As a shorthand—as an aid to communication—we might talk about a migratory instinct, a herding instinct, or a homing instinct. Such labels may seem gratifying, but it is an illusory gratification. Scratch the surface of any complex, adaptive behavior and one is confronted with a seemingly endless array of hard questions spanning evolutionary and developmental time, the intricacies of ecological and social experience, and the machinations of the nervous system with its billions of neurons. The more we dive into these matters, the harder it is to settle on any clear notion of what an instinct actually is. As Patrick Bateson1 has pointed out, this conceptual confusion about instinct is reflected in the many meanings that are routinely ascribed to it, including:
present at birth,
not learned
developed before it is used
unchanged once developed
shared by all members of a species
adapted during evolution
served by a distinct module in the brain
attributable to genes
Scientists often unknowingly invoke more than one of these meanings at any given time, and may even unwittingly switch between meanings in a single article. This isn't just a matter of lazy thinking. The murkiness of the term reflects actual confusion about the subject. No one doubts the existence of species-typical behaviors, and we can all agree that any science of behavior must endeavor to make sense of them. But there is an unsettling gulf between widely accepted assumptions surrounding instinct and the actual science available to explain it.
THE ETHOLOGICAL APPROACH TO INSTINCT
The modern study of instinct began in the 1930s with the emergence of ethology. Ethology is a subdiscipline of zoology devoted to understanding behavior in its natural context. One of the founders of ethology, Konrad Lorenz, popularized this new discipline for the general public with his many famous images of “imprinted” ducklings walking behind the bearded Austrian as if he were their mother. In 1973, the young science of ethology received a significant vote of approval when three of its founders—Lorenz, Niko Tinbergen, and Karl von Frisch—received the Nobel Prize in Physiology or Medicine.
Lorenz aimed to do for behavior what Charles Darwin's evolutionary insights did for bones. Writing in Scientific American in 19582, Lorenz begins with a familiar discussion of the evolution of forelimbs: “A whale's flipper, a bat's wing and a man's arm are as different from one another in outward appearance as they are in the functions they serve. But the bones of these structures reveal an essential similarity of design. The zoologist concludes that whale, bat and man evolved from a common ancestor” (p. 119).
Lorenz then makes his critical transition from bones to behavior: “[I]s it not possible that beneath all the variations of individual behavior there lies an inner structure of inherited behavior which characterizes all the members of a given species, genus or larger taxonomic group—just as the skeleton of a primordial ancestor characterizes the form and structure of all mammals today” (p. 119)?
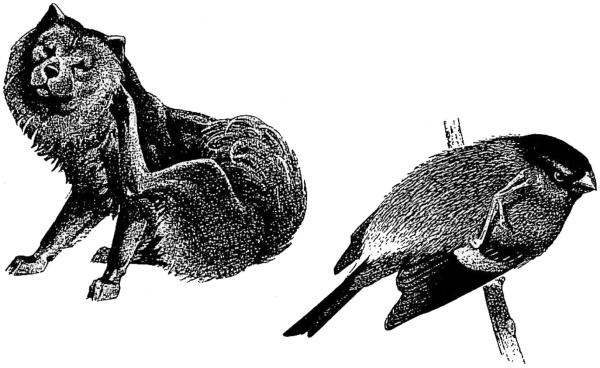
As his first example, he cites head-scratching in birds, which he observes to be perfectly consistent from bird to bird: produced by crossing a hindlimb over the wing so as to reach the head (Figure 1). Lorenz exclaims that most birds scratch using “precisely the same motion” (p. 120)! He then turns to other vertebrates, including mammals, and notes that they also scratch in the same way. For Lorenz, only one conclusion could be drawn from similar behaviors expressed by such different animals: “I do not see how to explain this clumsy action unless we admit that it is inborn. Before the bird can scratch, it must reconstruct the old spatial relationship of the limbs of the four-legged common ancestor which it shares with mammals” (p. 120). In other words, scratching in dogs, birds, and other animals is the ultimate instinct: ancient, pre-programmed, and immutable.
Figure 1.
Head-scratching in a dog and a European bullfinch. Konrad Lorenz used scratching in these two very different species to argue for the notion that behavior is shaped by evolution. With respect to head-scratching, he stated unequivocally that it “is part of their genetic heritage and is not shaped by training” (p. 119). From Lorenz, 19582.
THE RESPONSE TO LORENZ
Decades of subsequent research have since taught us to be skeptical of Lorenz's broad assertions about the origins of behavior. For one thing, head-scratching turns out to be more flexibly produced than Lorenz assumed. Burtt and Hailman3, for example, reported that small, young birds typically scratch their heads by moving a leg under a wing. Moreover, some adults will use the overwing method when perching and will switch to the underwing method in flight. Based on these and other observations, they suggested that a bird's method of scratching depends not on pre-programmed instructions but on the bird's posture, balance, and center of gravity at any given moment4. Terms such as hardwired and innate gloss over the fact that scratching depends on context—on multiple factors acting in real time. By changing context, we reveal how flexible a behavior can be.
Writing in Scientific American, in an article cunningly titled “How an instinct is learned,” Hailman5 challenged Lorenz's fundamental notion of instinct: “The term `instinct,' as it is often applied to animal and human behavior, refers to a fairly complex, stereotyped pattern of activity that is common to the species and is inherited and unlearned. Yet, braking an automobile and swinging a baseball bat are complex, stereotyped behavioral patterns that can be observed in many members of the human species, and these patterns certainly cannot be acquired without experience. Perhaps stereotyped behavior patterns of animals also require subtle forms of experience for development” (p. 241). Hailman meticulously demonstrated the influence of such subtle forms of experience through his investigations of pecking in newly hatched sea gulls.
Hailman's perspective is a forerunner to today's developmental systems approach to the origins of abilities, traits, and behaviors6. The striking observation that guides the developmental systems approach is that processes—sometimes obvious, sometimes subtle—give rise to the emergent properties of each individual's behavior. DNA plays a critical role in these processes, but does not by itself create traits. Accordingly, instincts are not preprogrammed, hardwired, or genetically determined; rather, they emerge each generation through a complex cascade of physical and biological influences7–9. (This process-oriented developmental perspective is has long been referred to as epigenesis. This term should not be confused with epigenetics, which refers specifically to the study of how non-genetic factors influence gene expression. See David Moore's article, Behavioral Epigenetics, in this collection.)
Lorenz's instinct concept did not adequately consider the roles that development and experience play in the emergence of species-typical behaviors and in the transmission of behavior across generations. Even Lorenz's explanation for the phenomenon that is most closely associated with him—visual imprinting in ducklings—has undergone significant modification over the years. Whereas Lorenz believed that hatchlings come into the world equipped with a single learning program that simply needs to be activated by an appropriate stimulus, subsequent research shows that imprinting comprises two independent processes10. The first process entails a predisposition for chicks to orient toward stimuli that resemble the head and neck region of a generic mother hen; under natural conditions, this predisposition typically results in the chick orienting toward its own mother. The second process entails the acquisition of detailed information about the stimulus; again, under natural conditions, this process typically results in the chick learning about its mother. Interestingly, this two-process model has been applied to the problem of how human infants develop their ability to recognize faces (for a recent review, see Johnson et al.11)
Gilbert Gottlieb spent much of his career investigating another form of imprinting— auditory imprinting—in which newly hatched chicks and ducklings are attracted to the mother's call8. Because the behavior of hatchlings seemed to be expressed without any obvious experience with the mother or her call, this adaptive behavior was thought to be an instinct. However, Gottlieb pursued this question in a way that no one else had before him by asking whether embryos obtain critical experiences while still in the egg. Amazingly, he found that they do: Embryos vocalize from within the egg, and these vocalizations shape the development of the auditory system in a way that is critical for their post-hatching attraction to the mother's call. Gottlieb also found that he could make a hatchling of one species prefer the maternal call of another species by manipulating its earlier embryonic experiences. Thus, even prenatal experiences shape the development of species-typical behavior, often in subtle and non-obvious ways.
GRAVITY AS AN INHERITANCE
Inheritance was once strictly defined as the passing on, upon one's death, of money, property, debts, and other earthly possessions. In contrast, within the biological sciences, inheritance has become synonymous with the transmission of DNA from one generation to the next. A developmental systems perspective, however, encourages a broader definition of inheritance to include all of the biological and environmental factors that influence individual development, especially those that are reliably transmitted. By this view, DNA is certainly part of our inheritance, but so are all the species-specific cytoplasmic factors in the egg that are passed from mother to daughter. And so are the numerous environmental factors in which every biological system develops, including (but not limited to) temperature, oxygen, carbon dioxide, atmospheric pressure, and gravity.
Consider gravity, which exerts its effects everywhere and continually. It shapes and orders life on our planet: A tree's trunk is rooted in the ground and its leaves point skyward, where birds fly with their bellies directed back toward the ground. Behavioral responses to gravity are universally expressed, being found in unicellular organisms and mammals. For example, as many pet owners can attest, a cat falling upside-down will gracefully flip itself over and land on its paws. This righting response is made possible by the vestibular system, which includes an apparatus in our inner ear that detects changes in linear and angular acceleration. As a cat falls, the system detects the changes in acceleration and activates muscles throughout the body to flip the cat right-side up before it hits the ground.
Rat pups exhibit the righting response at birth. In a variant of the cat-falling-to-the-ground test, experimenters release a pup upside-down in a tank of warm water. The typical behavior of a pup in this water immersion test is to flip over immediately and land right-side up, a demonstration of an already-functioning vestibular system. But is this system a hardwired and ancient instinctive response to a perennially reliable feature of life on our planet? For researchers, this was a particularly difficult question to answer, as it is not possible to simply turn gravity on and off at will.
To circumvent this problem, April Ronca, Jeffrey Alberts, and their colleagues flew pregnant rats on the NASA Space Shuttle during a period of gestation when the vestibular system is developing12. These pregnant rats returned to Earth two days before delivering their offspring, which were then compared to “ground controls” that were gestated normally on Earth. These researchers observed a variety of behavioral and neuroanatomical changes to the vestibular system resulting from gestation in microgravity. For example, whereas the ground-control pups exhibited normal responses in the water immersion test, the pups gestated in space often failed to even attempt to flip over, falling to the bottom of the tank on their backs (see Video 1).
Interestingly, after a week of experience in Earth's gravity the pups' righting responses were no longer impaired, which raises the question of whether isolation from Earth's gravity across the entire period of vestibular system development would lead to more lasting effects. Regardless, the lesson from this research is clear: As with Gottlieb's mallard ducklings, the presence of complex and adaptive behavior at birth tells us very little about the developmental importance of environmental factors to that behavior. Clearly, even the “simplest” instincts develop, and do so in response to numerous factors that we inherit from our parents, including the gravitational environment of our parents' home planet.
ANOMALOUS INDIVIDUALS AND DEVELOPMENTAL PLASTICITY
Ethology generally emphasizes species-typical behavior in natural settings. But focusing on the behavior of typically formed animals can also engender the illusion that behavioral development is a highly scripted and predetermined process. In contrast, the study of anomalous creatures—whether they arise through physical or genetic manipulation or alteration of the developmental environment—can provide key insights otherwise unavailable13. Critically, anomalous creatures also help us to better understand the processes the guide typical development.
For example, Johnny Eck was a performer best known for his role in the 1932 cult classic movie, Freaks. Born with a condition known as amelia, his legs were exceedingly short and functionless. Like other individuals with this condition, Eck learned to walk using his hands. As he demonstrates repeatedly in Freaks, Eck's locomotion was fluid and graceful. Eck could walk down steps and climb ladders (https://www.youtube.com/watch?v=z4aET2RGG5Q). He used his hands the way most humans use their feet.
Similarly, Faith is a dog that was born in Oklahoma City with short, functionless forelimbs (https://www.youtube.com/watch?v=oSB9aBMayxU). As has occasionally been documented in animals with this condition, Faith learned to walk upright on her hind limbs. But this is not merely a circus trick, as Faith's body grew in such a way to make upright walking possible, including a curved spine that shifted forward her center of mass. Thus, incredibly, Faith accomplished in one brief lifetime what has long been considered the crowning achievement of human evolution. Perhaps even more striking is Duncan, a boxer with malformed hind legs that walks and runs on his fore legs (https://www.youtube.com/watch?v=xaM-xXgl4Bs).
Johnny Eck, Faith, and Duncan force us to reconsider our standard ideas about normal and abnormal, typical and atypical, well formed and deformed. These individuals grew into their bodies and learned to use them in highly functional ways. In fact, the process by which they learned to move their bodies is no different from the process by which all animals do.
To see this, let's now return to the realm of typical development and consider the diverse patterns of locomotion in mammals: From quadrupedal walking, trotting, and galloping to bipedal walking and hopping. Across all rodent species, all of these locomotor patterns are observed and there is a clear relationship between the shape of an animal's body—its morphology—and the pattern of locomotion that it displays. In fact, at each stage of development, as an animal's morphology changes, its locomotor pattern changes as well.
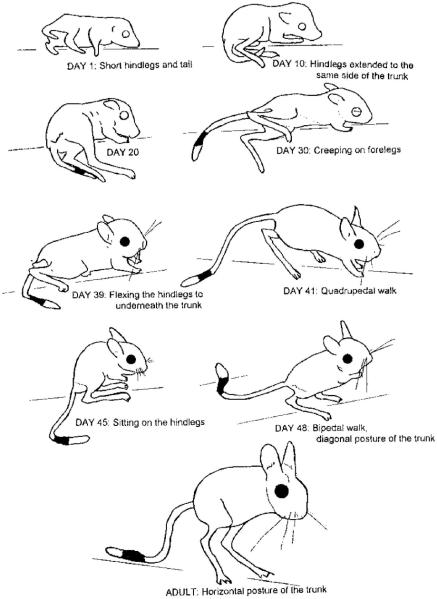
For example, jerboas are desert rodents that, as adults, have very long hind legs and exhibit bipedal walking and hopping gaits14. One might think that jerboas instinctively exhibit these gaits, but studies of the development of locomotion in this species tell a different story: As newborns, jerboas have similarly proportioned limbs as other rodents and they exhibit locomotor patterns that are identical to other newborn rodents with similar shapes (Figure 2). But as jerboas grow and their hind legs lengthen disproportionately, their locomotor patterns change accordingly. Specifically, as the hind legs grow longer than the fore legs, jerboas pass through an awkward stage where they struggle to accommodate their overly long legs. Later in development as their hind legs gain strength, they are able to lift themselves up and walk and hop about.
Figure 2.
The limbs of jerboas change dramatically across early development and their locotomor patterns change in lock-step. As newborns, these desert rodents look much like other rodents and they move around similarly as well. As their hind legs elongate, they crawl around very awkwardly. Finally, with gaining strength, they can walk and hop upright. From Eilam & Shefer14.
In other rodent species, such as rats and gerbils, we see similar patterns relating the shape and size of a body to the locomotor patterns expressed15. All rodent species examined thus far pass through a series of locomotor patterns that reflect their specific morphologies at each age. As bodies change and species-typical morphologies emerge, locomotor patterns diverge. As with head-scratching in birds, posture, balance, and center of mass—all intimately linked with morphology—determine how we move.
Demonstrating close correspondences between body morphology and behavior does not necessarily mean that behavior flows from morphology. A skeptic might respond by saying that evolution ensured that behavior and morphology develop in a synchronous way without actually influencing one another. But let's not forget Johnny Eck, Faith, and Duncan: the locomotor patterns in these individuals cannot be due to any preprogramming of behavior because their behavior reflects unique solutions to unique, species-atypical bodies. In other words, individual behaviors emerge from individual development. Whether typically or atypically formed, we all must learn through individual experience to use the bodies that we have—not the bodies that we were `supposed' to have.
CONCLUSIONS
History teaches us that we always learn important, critical details about a behavior by asking about its development. When Gottlieb saw that hatchlings are attracted to the maternal call, he could have stopped his investigation there and simply labeled the behavior an instinct. Instead, he asked the next question, revealed the developmental process that gives rise to the behavior, and ultimately taught us something general and profound about the nature of development and its often non-obvious causes.
Species-typical behaviors can begin as subtle predispositions in cognitive processing or behavior. They also develop under the guidance of species-typical experiences occurring within reliable ecological contexts. Those experiences and ecological contexts, together comprising what has been called an ontogenetic niche, are inherited along with parental genes16. Stated more succinctly, environments are inherited—a notion that shakes the nature-nurture dichotomy to its core. That core is shaken still further by studies demonstrating how even our most ancient and basic appetites, such as that for water, are learned17. Our natures are acquired.
None of this should be taken to mean that all behaviors are equally malleable. On the contrary, behaviors lie along a continuum from highly malleable or plastic to highly rigid or robust18 (See Patrick Bateson's article, Plasticity and robustness in development, in this collection). Our challenge, then, is to move beyond the age-old practice of applying dichotomous labels to behaviors19. Instead, we should focus more on understanding the developmental contexts and conditions in which a behavior is more or less malleable.
So the next time you see a marvelous and complex behavior—such as a border collie herding sheep or birds flying south for the winter—try to resist the temptation to label it as instinctive, hardwired, genetic, or innate. By foregoing a label and digging deeper, you will open yourself to consideration of the myriad of factors that shape who we are and why we behave the way we do.
Supplementary Material
ACKNOWLEDGMENTS
Preparation of this article was made possible in part by grants from the National Institutes of Health (R37-HD081168; R01-MH050701).
REFERENCES
- 1.Bateson P. The corpse of a wearisome debate. Science. 2002;297:2212. [Google Scholar]
- 2.Lorenz KZ. The evolution of behavior. Sci Am. 1958;199:67–74. doi: 10.1038/scientificamerican1258-67. passim. [DOI] [PubMed] [Google Scholar]
- 3.Burtt EH, Hailman JP. Head-Scratching among North-American Wood-Warblers (Parulidae) Ibis. 1978;120:153–170. [Google Scholar]
- 4.Burtt EH, Bitterbaum EJ, Hailman JP. Head-Scratching Method in Swallows Depends on Behavioral Context. Wilson Bulletin. 1988;100:679–682. [Google Scholar]
- 5.Hailman JP. How an instinct is learned. Scientific American. 1969;221:98–106. [Google Scholar]
- 6.Oyama S, Griffiths PE, Gray RD. Cycles of Contingency: Developmental Systems and Evolution. MIT Press; Cambridge: 2003. [Google Scholar]
- 7.Blumberg M. Basic Instinct: The Genesis of Behavior. Thunders Mouth Press; New York, New York: 2006. [Google Scholar]
- 8.Gottlieb G. Synthesizing Nature-Nurture: Prenatal Roots of Instinctive Behavior. Lawrence Erlbaum; Mahwah, NJ: 1997. [Google Scholar]
- 9.Johnston TD, Edwards L. Genes, interactions, and the development of behavior. Psychol Rev. 2002;109:26–34. doi: 10.1037/0033-295x.109.1.26. [DOI] [PubMed] [Google Scholar]
- 10.Bolhuis JJ, Honey RC. Imprinting, learning and development: from behaviour to brain and back. Trends Neurosci. 1998;21:306–311. doi: 10.1016/s0166-2236(98)01258-2. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MH, Senju A, Tomalski P. The two-process theory of face processing: modifications based on two decades of data from infants and adults. Neurosci Biobehav Rev. 2015;50:169–179. doi: 10.1016/j.neubiorev.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Ronca AE, Fritzsch B, Bruce LL, Alberts JR. Orbital spaceflight during pregnancy shapes function of mammalian vestibular system. Behav Neurosci. 2008;122:224–232. doi: 10.1037/0735-7044.122.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumberg M. Freaks of Nature: What Anomalies Tell Us About Development and Evolution. Oxford University Press; New York: 2009. [Google Scholar]
- 14.Eilam D, Shefer G. The developmental order of bipedal locomotion in the jerboa (Jaculus orientalis): pivoting, creeping, quadrupedalism, and bipedalism. Dev Psychobiol. 1997;31:137–142. doi: 10.1002/(sici)1098-2302(199709)31:2<137::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Eilam D. Postnatal development of body architecture and gait in several rodent species. J Exp Biol. 1997;200:1339–1350. doi: 10.1242/jeb.200.9.1339. [DOI] [PubMed] [Google Scholar]
- 16.West MJ, King AP. Settling Nature and Nurture into an Ontogenic Niche. Developmental Psychobiology. 1987;20:549–562. doi: 10.1002/dev.420200508. [DOI] [PubMed] [Google Scholar]
- 17.Hall WG, Arnold HM, Myers KP. The acquisition of an appetite. Psychological Science. 2000;11:101–105. doi: 10.1111/1467-9280.00223. [DOI] [PubMed] [Google Scholar]
- 18.Bateson P, Gluckman P. Plasticity, Robustness, Development and Evolution. Cambridge University Press; Cambridge: 2011. [Google Scholar]
- 19.Johnston TD. The Persistence of Dichotomies in the Study of Behavioral-Development. Developmental Review. 1987;7:149–182. [Google Scholar]
FURTHER READING
- Blumberg MS, Freeman JH, Robinson SR, editors. Oxford handbook of developmental behavioral neuroscience. Oxford University Press; New York: 2010. [Google Scholar]
- Lehrman DS. A critique of Konrad Lorenz's theory of instinctive behavior. Q Rev Biol. 1953;4:337–363. doi: 10.1086/399858. [DOI] [PubMed] [Google Scholar]
- Spencer J, Blumberg MS, McMurray R, Robinson S, Samuelson L, Tomblin J. Short arms and talking eggs: Why we should no longer abide the nativist-empiricist debate. Child Dev Perspect. 2009;3:79–87. doi: 10.1111/j.1750-8606.2009.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental plasticity and evolution. Oxford University Press; New York: 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.