Abstract
Background
Our objective was to identify potential avenues for resource allocation and patient advocacy to improve outcomes by evaluating the association between recipient sociodemographic and patient characteristics and medication nonadherence after lung transplantation.
Methods
States US adult, lung-only transplantations per the United Network for Organ Sharing database were analyzed from October 1996 through December 2006, based on the period during which nonadherence information was recorded. Generalized linear models were used to determine the association of demographic, disease, and transplantation center characteristics with early nonadherence (defined as within the first year after transplantation) as well as late nonadherence (years 2 to 4 after transplantation). Outcomes comparing adherent and nonadherent patients were also evaluated.
Results
Patients (n = 7,284) were included for analysis. Early and late nonadherence rates were 3.1% and 10.6%, respectively. Factors associated with early non-adherence were Medicaid insurance compared with private insurance (adjusted odds ratio [AOR] 2.45, 95% confidence interval [CI]: 1.16 to 5.15), and black race (AOR 2.38, 95% CI: 1.08 to 5.25). Medicaid insurance and black race were also associated with late nonadherence (AOR 2.38, 95% CI: 1.51 to 3.73 and OR 1.73, 95% CI: 1.04 to 2.89, respectively), as were age 18 to 20 years (AOR 3.41, 95% CI: 1.29 to 8.99) and grade school or lower education (AOR 1.88, 95% CI: 1.05 to 3.35). Early and late non-adherence were both associated with significantly shorter unadjusted survival (p < 0.001).
Conclusions
Identifying patients at risk of non-adherence may enable resource allocation and patient advocacy to improve outcomes.
In 2011, more than 1,800 lung transplantations were performed in the United States alone [1]. Despite improvements in outcomes after lung transplantation, the 1-year survival remains close to 80% and the median survival is only 5.5 years. Despite immunosuppressive drugs, 35% of adult recipients still have at least one episode of acute rejection within the first year [2].
Adherence to medical therapy has repeatedly been shown to be associated with improved outcomes among solid-organ transplant recipients [3–8]. Despite this, non-adherence is a persistent and costly problem in the overall transplantation population [6]. A 2007 meta-analysis found that the incidence of nonadherence was 19% to 25% per year [9]. Of the studies analyzed, only a few reported on lung transplant recipients [10–13]. More recent studies have suggested similar rates of nonadherence in patients who received a lung transplant compared with other solid-organ transplants [14–19]. Furthermore, nonadherence was more common among patients who experienced bronchiolitis obliterans syndrome [20, 21].
Despite this body of evidence regarding the importance of medication adherence in lung transplant recipients, a paucity of literature exists regarding risk factors for nonadherence in this patient population. Furthermore, medication nonadherence has been recognized as not only related to factors on an individual level but also results from failings of the health system related to health access, cost, and communication [22–25]. Our objective was to evaluate the association between recipient socio-demographic and disease characteristics and the incidence of nonadherence after lung transplantation using a cohort of all lung transplantations performed in the United States. A secondary objective was to report the survival implications of nonadherence after lung transplantation.
Patients and Methods
The Institutional Review Board at Duke University Medical Center approved this study.
Study Population
The Organ Procurement and Transplantation Network’s national computerized database as maintained by the United Network of Organ Sharing (UNOS) was used for this analysis [26]. This contains data regarding every organ donation and transplantation event occurring in the United States since October 1, 1987 [1]. The dataset used for the present study included lung transplantations performed through December 31, 2011, with follow-up through March 31, 2012. All adult (≥18 years) lung transplant recipients were included for analysis. Multiple organ, en block, lobar, and repeat transplantations were excluded. The study period included transplantations performed from October 1996 through December 2006 based on the time period during which nonadherence information was recorded in the UNOS database. To be included in the study, patients had to have a follow-up visit after transplantation documenting the presence or absence of evidence of noncompliance with immuno-suppressive medication during this follow-up period that compromised the patient’s recovery within the first 4 years after transplantation (this is a yes/no field on the Adult Thoracic Transplant Recipient Follow-Up Worksheet [Office of Management and Budget approved form number 0915-0157]). Patients with unknown or missing adherence information were excluded.
Variable Definitions
The UNOS database includes donor, recipient, and transplantation-related characteristics. To describe our study cohort, we included the following characteristics: age; sex; race; cause of lung failure; diabetes; hypertension; creatinine; body mass index, steroid use before transplantation that required life support at the time of transplantation; Lung Allocation Score (available after May 2005); days on the waitlist; insurance carrier; education level; smoking history; type of transplantation; human leukocyte antigen mismatch level; donor/recipient sex, race, and cytomegalovirus (CMV) mismatch; total ischemic time (hours); transplantation year; and transplantation center volume.
PREDICTOR VARIABLES
Predictor variables for analysis were determined a priori based on factors previously demonstrated in the literature to affect medication adherence [8, 16, 25, 27–29]. These variables included recipient age, sex, race, smoking history, insurance carrier, education level, cause of lung failure, comorbidities, year of transplantation, and transplantation center volume.
OUTCOME MEASURES AND FOLLOW-UP
The primary outcome variable was nonadherence with immunosuppressive medications. This was separately assessed for non-adherence within the first year (termed early non-adherence) and nonadherence during years 2 to 4 (termed late nonadherence). To be included in an analysis for a given postoperative period (early or late) a patient must have had definitive documentation of the presence or absence of nonadherence during that time. Overall survival for patients with nonadherence was also assessed in comparison with patients without nonadherence. Outcome data for each patient were ascertained from the date of transplantation until patient death, date of last follow-up, or the end of study period (March 31, 2012).
Study Design and Statistical Analysis
We performed a retrospective, observational cohort analysis of lung transplant recipients subject to inclusion/exclusion criteria as described in the sections above. Baseline characteristics were described for the overall study population, with medians and interquartile range (IQR) reported for continuous variables and proportions (frequency, percentage) for discrete variables.
Multivariable logistic regression was performed to assess the association between predictor variables defined in the section above and nonadherence with immunosuppressive medications. Logistic regression models were performed separately for early and late nonadherence. The late nonadherence models included transplantations before year 2003 to allow adequate follow-up time to assess late nonadherence. Continuous variables included in the model (recipient age, year of transplantation, and transplantation center volume) were assessed for linearity with respect to the outcome measures. Only recipient age demonstrated a nonlinear relation and was accordingly stratified by age 18 to 20 years, 21 to 50 years, and 51 years and older.
To assess survival implications of nonadherence, unadjusted patient survival rates for early and late non-adherence were estimated using the product-limit (Kaplan-Meier) method [30] and compared with control subjects using the log-rank test. Patients with early non-adherence were excluded from the analysis of late non-adherence to isolate the association of late nonadherence with survival.
Statistical analyses were performed using JMP Version 10.0 (SAS Institute Inc, Cary, NC) and R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria). For all comparisons, p values less than or equal to 0.05 were considered statistically significant, and all tests were two sided.
Results
Study Population and Baseline Characteristics
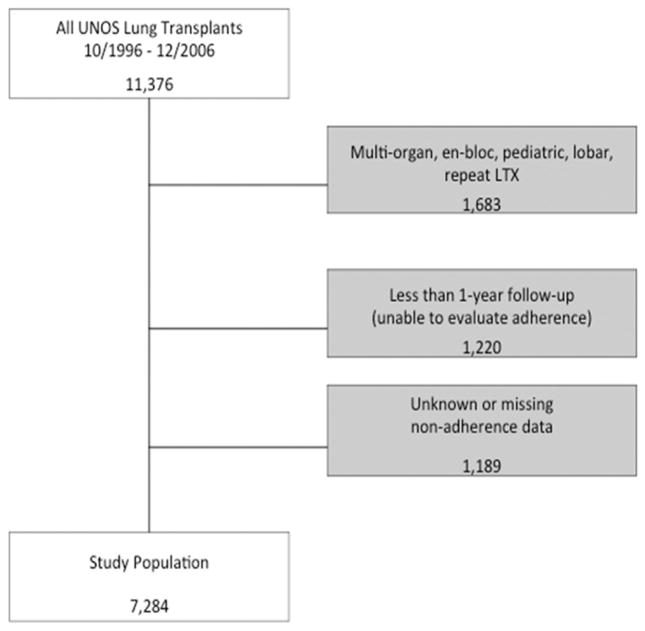
A total of 7,284 lung transplant recipients met the inclusion and exclusion criteria of this study (Fig 1). Median recipient age was 54 years (IQR, 44 to 60 years), whereas median donor age was 29 years (IQR, 20 to 44 years) (Table 1). Most of the lung transplant recipients were white (n = 6,472, 88.9%), whereas 490 (6.7%) recipients were black, 233 (3.2%) recipients were Hispanic. Private insurance/self-payment was the most frequent recipient insurance at time of transplantation (n = 4,556, 63.0) followed by Medicare (n = 1,789, 24.7%) and Medicaid (n = 529, 7.3%) (Table 2).
Fig 1.
Study inclusion algorithm. (LTX = lung transplantation; UNOS = United Network of Organ Sharing.)
Table 1.
Baseline Characteristics for the Entire Cohort (n = 7,284)
| Characteristic | Value |
|---|---|
| Donor characteristics | |
| Donor age, years | 29 (20–44) |
| Donor diabetes | 235 (3.2) |
| Donor smoking history (>20 pack-years ever) | 1,819 (25.2) |
| Donor cocaine use (ever) | 518/5,564 (9.3) |
| Terminal serum creatinine, mg/dL | 0.9 (0.7–1.2) |
| Donor BMI, kg/m2 | 23.8 (21.3–26.8) |
| PO2 on 100% inspired oxygen (n = 5,390) | 455 (383–514) |
| Recipient characteristics | |
| Age, years | 54 (44–60) |
| Age ≥ 60 years | 1,919 (26.4) |
| Female sex | 3,561 (48.9) |
| Race | |
| White | 6,472 (88.9) |
| Black | 490 (6.7) |
| Hispanic | 233 (3.2) |
| Asian | 54 (0.7) |
| Other/unknown | 35 (0.5) |
| Cause of lung failure | |
| Obstructive disease | 4,110 (56.8) |
| Restrictive disease | 1,836 (25.4) |
| CF or immunodeficiency | 1,023 (14.1) |
| Pulmonary vascular disease | 273 (3.8) |
| Comorbidities | |
| Diabetes | 631 (8.8) |
| Hypertension | 1,141 (16) |
| Cerebrovascular disease | 50 (0.7) |
| Creatinine at transplantation, mg/dL | 0.8 (0.7–1.0) |
| BMI at transplantation, kg/m2 | 23.7 (20.2–27.2) |
| Chronic steroid use before transplantation | 3,396/6,831 (49.7) |
| Status before transplantation | |
| Hospitalized | 293 (4) |
| Intensive care unit | 163 (2.2) |
| Requiring life support at transplantationa | 300 (4.1) |
| Ventilator dependent at transplantation | 124 (1.7) |
| Lung allocation score (n = 827) | 36.3 (33.4–42.0) |
| Pulmonary function and hemodynamics | |
| Oxygen requirement, L (n = 6,376) | 2 (2–3) |
| FEV1, % predicted (n = 6,695) | 25 (18–45) |
| FVC, % predicted (n = 6,671) | 49 (38–61) |
| FEV/FVC (n = 6,648) | 0.56 (0.39–0.98) |
| Mean PA pressure, mm Hg (n = 5,002) | 25 (20–30) |
| PVR, Wood units (n = 4,270) | 2.6 (1.8–3.6) |
| Cardiac index, L/min/m2 (n = 4,713) | 2.8 (2.4–3.4) |
| Days on waitlist | 312 (112–656) |
| Transplantation characteristics | |
| Bilateral transplantation | 3,358 (46.1) |
| HLA mismatch level 3+ | 5,655/5,890 (96) |
| Donor/recipient sex mismatch | 2,321 (31.9) |
| Donor/recipient race mismatch | 2,354 (32.3) |
| Donor/recipient CMV mismatch | 1,113/5,194 (21.4) |
| Ischemic time, hours (n = 6,369) | 4.5 (3.4–5.6) |
Includes ventilator, extracorporeal membrane oxygenation, intravenous inotropes, intra-aortic balloon pump, or inhaled nitric oxide.
Values are expressed as median (IQR), n (%), or n/N (%).
BMI = body mass index; CF = cystic fibrosis; CMV = cytomegalovirus; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; HLA = human leukocyte antigen; IQR = interquartile range; PA = pulmonary pressure; PO2 = partial pressure of oxygen; PVR = pulmonary vascular resistance.
Table 2.
Recipient Socioeconomic Characteristics (n = 7,284)
| Characteristic | Value |
|---|---|
| Recipient insurance at time of transplantation | |
| Private/self | 4,556 (63.0) |
| Medicaid | 529 (7.3) |
| Medicare | 1,789 (24.7) |
| Other | 360 (5.0) |
| Education level | |
| Grade school or lower | 190/6,112 (3.1) |
| High school or GED | 2,893/6,112 (47.3) |
| Attended college or technical school (no degree) | 1,578/6,112 (25.8) |
| College degree or higher | 1,451/6,112 (23.7) |
| Smoking history (>10 pack years) | 4,137/5,968 (69.3) |
Values are expressed as n (%) or n/N (%).
GED = General Educational Development.
Early and Late Nonadherence
Factors associated with early nonadherence were Medicaid insurance (adjusted odds ratio [AOR] compared with private insurance/self-pay 2.45, 95% confidence interval [CI]: 1.16 to 5.15, p = 0.019), and black race (AOR compared with white 2.38, 95% CI: 1.08 to 5.25, p = 0.031). Medicaid insurance and black race were also associated with late nonadherence (AOR 2.38, 95% CI: 1.51 to 3.73, p < 0.001 and AOR 1.73, 95% CI: 1.04 to 2.89, p = 0.035, respectively). Additional factors associated with late nonadherence included age 18 to 20 years (AOR compared with age 21 to 50 years 3.41, 95% CI: 1.29 to 8.99, p = 0.013) and grade school or lower education (AOR compared with high school or equivalent diploma 1.88, 95% CI: 1.05 to 3.35, p = 0.034; Table 3). Patients aged 51 years and older demonstrated a significantly decreased risk of early nonadherence (AOR compared with age 21 to 50 years 0.43, 95% confidence interval: 0.23 to 0.77, p = 0.005) and late nonadherence (AOR 0.57, 95% CI: 0.41 to 0.79, p < 0.001).
Table 3.
Multivariable Logistic Regression for Patient Nonadherence Based on Secondary Predictor Variables
| Demographic and Socioeconomic Characteristics | Early Nonadherence
|
Late Nonadherencea
|
||
|---|---|---|---|---|
| AOR (95% CI) | p Value | AOR (95% CI) | p Value | |
| Age (reference: 21–50 years [n = 2,596]) | ||||
| 18–20 years (n = 138) | 3.34 (0.93–11.94) | 0.064 | 3.41 (1.29–8.99) | 0.013 |
| ≥51 years (n = 4,550) | 0.43 (0.23–0.77) | 0.005 | 0.57 (0.41–0.79) | 0.001 |
| Female sex | 0.81 (0.49–1.34) | 0.410 | 0.84 (0.64–1.12) | 0.240 |
| Ethnicity (reference: white) | ||||
| Black | 2.38 (1.08–5.25) | 0.031 | 1.73 (1.04–2.89) | 0.035 |
| Hispanic/Asian/other/unknown | 1.26 (0.36–4.36) | 0.714 | 0.42 (0.13–1.37) | 0.151 |
| Cause of lung failure (reference: obstructive disease) | ||||
| Restrictive disease | 0.70 (0.35–1.39) | 0.302 | 0.84 (0.55–1.27) | 0.399 |
| CF or immunodeficiency | 0.87 (0.31–2.42) | 0.789 | 1.21 (0.60–2.46) | 0.592 |
| Pulmonary vascular disease | 0.35 (0.04–2.71) | 0.312 | 0.96 (0.39–2.36) | 0.926 |
| Chronic comorbidities (DM or HTN) | 1.34 (0.77–2.34) | 0.295 | 0.89 (0.62–1.28) | 0.524 |
| Smoking history (>10 pack years) | 1.41 (0.65–3.04) | 0.383 | 1.57 (0.93–2.65) | 0.088 |
| Insurance status (reference = private/self) | ||||
| Medicaid | 2.45 (1.16–5.15) | 0.019 | 2.38 (1.51–3.73) | 0.001 |
| Medicare | 1.41 (0.78–2.54) | 0.252 | 1.26 (0.90–1.75) | 0.180 |
| Other | 1.91 (0.71–5.11) | 0.198 | 0.56 (0.26–1.24) | 0.156 |
| Education level (reference: high school or equivalent diploma) | ||||
| Grade school or lower | 1.08 (0.31–3.81) | 0.904 | 1.88 (1.05–3.35) | 0.034 |
| Attended college or technical school | 1.15 (0.63–2.09) | 0.653 | 0.92 (0.65–1.32) | 0.660 |
| College degree or higher | 1.33 (0.69–2.59) | 0.394 | 1.29 (0.89–1.88) | 0.172 |
| Year of transplantation (AOR per year increase) | 0.80 (0.71–0.90) | <0.001 | 0.89 (0.80–0.98) | 0.016 |
| Center volume (AOR per increase of 10/year) | 1.01 (0.87–1.18) | 0.902 | 1.07 (0.98–1.16) | 0.129 |
Includes ventilator, extracorporeal membrane oxygenation, intravenous inotropes, intra-aortic balloon pump, or inhaled nitric oxide.
AOR = adjusted odds ratio; CF = cystic fibrosis; CI = confidence interval; DM = diabetes mellitus; HTN = hypertension.
Overall Survival
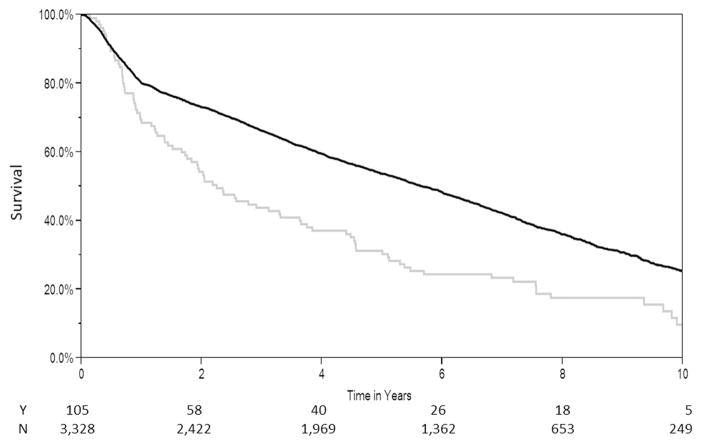
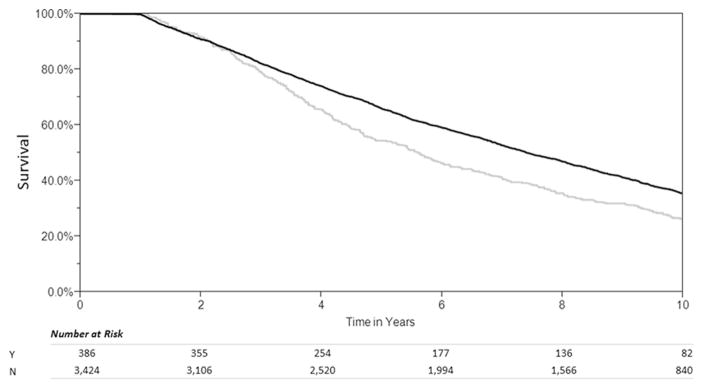
Survival of patients with early nonadherence was significantly shorter than control subjects (median survival, 2.25 versus 5.67 years, log-rank test: p < 0.001) (Fig 2). After excluding patients with early nonadherence, patients with late nonadherence also demonstrated a lower survival rate than control subjects (median survival, 5.6 versus 7.4 years, log-rank test: p < 0.001) (Fig 3).
Fig 2.
Unadjusted Kaplan-Meier survival curves. (N = no; Y = yes.)
Fig 3.
Late nonadherence (within years 2 to 4 after transplantation). (N = no; Y = yes.)
Comment
Adherence to medication regimens has long been recognized as an important component to length and quality of life not only for lung transplant recipients but also across the health care spectrum. There are clear implications for clinical outcomes, as well as added cost associated with avoidable hospitalizations and other preventable consequences of lapses in medical therapy [22, 23]. Data reported from all transplantation centers in the United States demonstrated that more than 10% of those patients struggle with taking their immunosuppressive medications, with early nonadherence being less prevalent than late nonadherence. Age between 18 and 20 years, black race, Medicaid insurance, and grade school or lower education were identified as independent risk factors for nonadherence. Early and late nonadherence was shown to be associated with shorter survival after lung transplantation than patients without reported nonadherence.
Barriers to adherence are frequently intertwined with intrinsic deficiencies of the health care system, including limited access to care, prohibitive cost, and failures in communication [22]. Given that the recipients’ abilities to address these barriers are limited, resources allocated to improving adherence should focus on high-risk populations who are the most likely to benefit from their use [24]. Furthermore, the international guidelines for selection of candidates for lung transplantation states that documented nonadherence or inability to follow through with medical therapy or office follow-up or both is an absolute contraindication for lung transplantation [31]. This further emphasizes the importance of better understanding factors contributing to nonadherence to ensure that patients are not denied this life-saving intervention due to failings of the health system.
Our data suggest that younger patients have an independent increased risk of nonadherence, which confirms earlier findings in lung transplant recipients [16]. Similar influences are likely contributing to nonadherence observed in the young adults included in our study population. Similarly to our findings, adolescent age, black race, and Medicaid insurance have shown an association with nonadherence in pediatric heart transplant recipients [32, 33]. In addition, the biology and human leukocyte antigen status of different racial groups [34–37] may lead to increased rates of graft dysfunction, rejection, or both being misinterpreted as nonadherence. It should also be acknowledged that those recording nonadherence may introduce a racial bias in determining whether a decline in clinical status for a given patient is assumed to be due to lack of medication adherence.
Low income as well as high-cost medication regimens (as is the situation with transplant recipients) have been demonstrated to correlate with medication nonadherence as well as potentially harmful strategies to cope with medication costs, such as underdosing, delaying refills, or skipping doses [22, 38, 39]. However, nonadherence has been demonstrated to occur despite full coverage of medication cost [40], reinforcing the multidimensional nature of nonadherence incorporating factors such as cognitive impairment, depression, lack of insight or asymptomatic disease or both, side effects, inadequate follow-up or discharge planning, and poor provider-patient communication [22].
The strong association with worse survival after transplantation in our study population underscores the importance of systematically addressing nonadherence. This is independent of whether patients showed early or late nonadherence. In the lung transplantation setting, it is well documented that reduced immunosuppressive drug levels after transplantation are associated with chronic allograft rejection, especially bronchiolitis obliterans syndrome [20]. The importance of adherence to immunosuppressive medications has also been documented in other solid-organ transplantations, with studies reporting up to a sevenfold increase in graft loss among nonadherent recipients [5]. Not only is non-adherence associated with potentially preventable deaths, but there are also implications for cost to the health care system and allocation of resources. In a review of adherence to medication by Osterberg and Blaschke [22], it was estimated that more than $100 billion are spent each year on avoidable hospitalizations from non-adherence, demonstrating that the problem does not only exist in the transplantation setting but throughout medicine.
Advances in information technology may hold promise for improvement in medication adherence by effectively monitoring, interfacing, and engaging patients with their regimens. Many interventions to help patients with medication adherence are currently under investigation, including hand-held computer-based interventions [38], text messaging [39], and electronic medication tracking devices [40]. Although results of these investigations are encouraging, it is increasingly perceived that interventions will have to be specifically tailored to individual needs in coordination with provider relationships, communication, and scheduled follow-up.
Limitations
In UNOS, the reporting of nonadherence is based on the discretion of each reporting facility and was not necessarily assessed in a formal and structured interview. As such, this may be an insensitive measure to adequately capture the full spectrum of nonadherent patients. Similarly, nonadherent patients without any adverse consequences are less likely to be detected. Still, the prevalence of more than 10% in the UNOS patient cohort is in line with other reports that range from 4.5% to 26% [9, 12, 15–17].
As a further limitation, information about non-adherence may be poorly reported, was only recorded until December 2006 (not allowing for a more recent analysis) and was not assessed based on severity of nonadherence. Different age cut-off values may have been used by other investigators in such analysis. Finally, by the retrospective nature of this analysis, inherent unmeasured potential confounders such as information on psychosocial characteristics, income, distance of living to medical facilities, and living in rural versus urban areas may exist.
Conclusions
In summary, we have found that age, race, insurance status, and education level were associated with non-adherence, which subsequently portends a decrease in survival after transplantation. This information should help identify patients at increased risk before transplantation to increase awareness and to put in place efficient strategies to assist patients in their efforts to remain adherent to medication regimens. We would advocate that the medical community should resume collecting information about adherence in a structured way because such an investment would likely pay dividends in increased survival and improved quality of life for lung transplant recipients.
Acknowledgments
By use of the UNOS database, this work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content herein is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) 2011 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration; 2012. p. 13. [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report–2011. J Heart Lung Transplant. 2011;30:1104–22. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Denhaerynck K, Dobbels F, Cleemput I, et al. Prevalence, consequences, and determinants of nonadherence in adult renal transplant patients: a literature review. Transpl Int. 2005;18:1121–33. doi: 10.1111/j.1432-2277.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 4.Denhaerynck K, Steiger J, Bock A, et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant. 2007;7:108–16. doi: 10.1111/j.1600-6143.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 5.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77:769–76. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 6.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant. 2009;9:2597–606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 7.Michelon TF, Piovesan F, Pozza R, et al. Noncompliance as a cause of renal graft loss. Transplant Proc. 2002 Nov;34:2768–70. doi: 10.1016/s0041-1345(02)03403-6. [DOI] [PubMed] [Google Scholar]
- 8.Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58:824–34. doi: 10.1007/s10620-012-2412-0. [DOI] [PubMed] [Google Scholar]
- 9.Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83:858–73. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 10.DeVito Dabbs A, Hoffman LA, Iacono AT, et al. Pattern and predictors of early rejection after lung transplantation. Am J Crit Care. 2003;12:497–507. [PubMed] [Google Scholar]
- 11.Evon DM, Burker EJ, Sedway JA, Cicale R, Davis K, Egan T. Tobacco and alcohol use in lung transplant candidates and recipients. Clin Transplant. 2005;19:207–14. doi: 10.1111/j.1399-0012.2005.00320.x. [DOI] [PubMed] [Google Scholar]
- 12.Matthees BJ, Anantachoti P, Kreitzer MJ, Savik K, Hertz MI, Gross CR. Use of complementary therapies, adherence, and quality of life in lung transplant recipients. Heart Lung. 2001;30:258–68. doi: 10.1067/mhl.2001.116135. [DOI] [PubMed] [Google Scholar]
- 13.Teichman BJ, Burker EJ, Weiner M, Egan TM. Factors associated with adherence to treatment regimens after lung transplantation. Prog Transplant. 2000;10:113–21. doi: 10.1177/152692480001000208. [DOI] [PubMed] [Google Scholar]
- 14.Dew MA, Dimartini AF, De Vito Dabbs A, et al. Adherence to the medical regimen during the first two years after lung transplantation. Transplantation. 2008;85:193–202. doi: 10.1097/TP.0b013e318160135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kugler C, Fischer S, Gottlieb J, et al. Symptom experience after lung transplantation: impact on quality of life and adherence. Clin Transplant. 2007;21:590–6. doi: 10.1111/j.1399-0012.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- 16.Bosma OH, Vermeulen KM, Verschuuren EA, Erasmus ME, van der Bij W. Adherence to immunosuppression in adult lung transplant recipients: prevalence and risk factors. J Heart Lung Transplant. 2011;30:1275–80. doi: 10.1016/j.healun.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 17.De Bleser L, Dobbels F, Berben L, et al. The spectrum of nonadherence with medication in heart, liver, and lung transplant patients assessed in various ways. Transpl Int. 2011;24:882–91. doi: 10.1111/j.1432-2277.2011.01296.x. [DOI] [PubMed] [Google Scholar]
- 18.Germani G, Lazzaro S, Gnoato F, et al. Nonadherent behaviors after solid organ transplantation. Transplant Proc. 2011;43:318–23. doi: 10.1016/j.transproceed.2010.09.103. [DOI] [PubMed] [Google Scholar]
- 19.Dobbels F, Vanhaecke J, Desmyttere A, Dupont L, Nevens F, De Geest S. Prevalence and correlates of self-reported pretransplant nonadherence with medication in heart, liver, and lung transplant candidates. Transplantation. 2005;79:1588–95. doi: 10.1097/01.tp.0000158430.06507.87. [DOI] [PubMed] [Google Scholar]
- 20.Husain AN, Siddiqui MT, Holmes EW, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 1999;159:829–33. doi: 10.1164/ajrccm.159.3.9607099. [DOI] [PubMed] [Google Scholar]
- 21.Kugler C, Fuehner T, Dierich M, et al. Effect of adherence to home spirometry on bronchiolitis obliterans and graft survival after lung transplantation. Transplantation. 2009;88:129–34. doi: 10.1097/TP.0b013e3181aad129. [DOI] [PubMed] [Google Scholar]
- 22.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 23.Cutler DM, Everett W. Thinking outside the pillbox–medication adherence as a priority for health care reform. N Engl J Med. 2010;362:1553–5. doi: 10.1056/NEJMp1002305. [DOI] [PubMed] [Google Scholar]
- 24.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19:638–45. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–35. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 26.Brown RS, Belton AM, Martin JM, Simmons DD, Taylor GJ, Willard E. Evolution of quality at the Organ Center of the Organ Procurement and Transplantation Network/United Network for Organ Sharing. Prog Transplant. 2009;19:221–6. doi: 10.1177/152692480901900306. [DOI] [PubMed] [Google Scholar]
- 27.Wille KM, Harrington KF, deAndrade JA, Vishin S, Oster RA, Kaslow RA. Disparities in lung transplantation before and after introduction of the lung allocation score. J Heart Lung Transplant. 2013;32:684–92. doi: 10.1016/j.healun.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quon BS, Psoter K, Mayer-Hamblett N, Aitken ML, Li CI, Goss CH. Disparities in access to lung transplantation for patients with cystic fibrosis by socioeconomic status. Am J Respir Crit Care Med. 2012;186:1008–13. doi: 10.1164/rccm.201205-0949OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quadros AS, Welter DI, Camozzatto FO, et al. Identifying patients at risk for premature discontinuation of thienopyridine after coronary stent implantation. Am J Cardiol. 2011;107:685–9. doi: 10.1016/j.amjcard.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 31.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update–a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–55. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer PE, Pfeffer JM, Hodson ME. The psychosocial and psychiatric side of cystic fibrosis in adolescents and adults. J Cyst Fibros. 2003;2:61–8. doi: 10.1016/S1569-1993(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 33.Oliva M, Singh TP, Gauvreau K, Vanderpluym CJ, Bastardi HJ, Almond CS. Impact of medication non-adherence on survival after pediatric heart transplantation in the U.S.A. J Heart Lung Transplant. 2013;32:881–8. doi: 10.1016/j.healun.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Maiers M, Gragert L, Klitz W. High-resolution HLA alleles and haplotypes in the United States population. Hum Immunol. 2007;68:779–88. doi: 10.1016/j.humimm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Bengle R, Sinnett S, Johnson T, Johnson MA, Brown A, Lee JS. Food insecurity is associated with cost-related medication non-adherence in community-dwelling, low-income older adults in Georgia. J Nutr Elder. 2010;29:170–91. doi: 10.1080/01639361003772400. [DOI] [PubMed] [Google Scholar]
- 36.Heisler M, Wagner TH, Piette JD. Patient strategies to cope with high prescription medication costs: who is cutting back on necessities, increasing debt, or underusing medications? J Behav Med. 2005;28:43–51. doi: 10.1007/s10865-005-2562-z. [DOI] [PubMed] [Google Scholar]
- 37.Doshi JA, Zhu J, Lee BY, Kimmel SE, Volpp KG. Impact of a prescription copayment increase on lipid-lowering medication adherence in veterans. Circulation. 2009;119:390–7. doi: 10.1161/CIRCULATIONAHA.108.783944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeVito Dabbs A, Dew MA, Myers B, et al. Evaluation of a hand-held, computer-based intervention to promote early self-care behaviors after lung transplant. Clin Transplant. 2009;23:537–45. doi: 10.1111/j.1399-0012.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miloh T, Annunziato R, Arnon R, et al. Improved adherence and outcomes for pediatric liver transplant recipients by using text messaging. Pediatrics. 2009;124:e844–50. doi: 10.1542/peds.2009-0415. [DOI] [PubMed] [Google Scholar]
- 40.Hayes TL, Hunt JM, Adami A, Kaye JA. An electronic pillbox for continuous monitoring of medication adherence. Conf Proc IEEE Eng Med Biol Soc. 2006;1:6400–3. doi: 10.1109/IEMBS.2006.260367. [DOI] [PMC free article] [PubMed] [Google Scholar]