Abstract
Objective
This study examines the reciprocal associations between sleep macrostructure and levels of cellular inflammation in rheumatoid arthritis (RA) patients and controls.
Methods
RA patients (n=24) and matched controls (n=48) underwent all-night polysomnography (PSG), along with assessment of spontaneous- and Toll-like receptor (TLR)-4 stimulated monocytic production of tumor necrosis factor-α (TNF) and interleukin (IL)-6 at 23:00 and 8:00.
Results
As compared to controls, RA patients showed lower levels of sleep efficiency (mean ± SD, 88.1 ±6.1 vs. 83.8 ± 7.0), a higher percentage Stage 3 sleep (9.3 ± 6.4 vs. 13.1 ± 6.9), and higher levels of percentage of monocytes either spontaneously expressing TNF at 23:00 (log transformed, 1.07 ± 0.28 vs. 1.22 ± 0.17), and higher TLR4 stimulated production of IL6 at 8:00 (log transformed, 3.45 ± 0.80 vs. 3.83 ± 0.39). Higher levels of stimulated production of TNF at 23:00 were associated with higher sleep efficiency (.74). In turn, sleep efficiency had a countervailing relationship on TNF production at 8:00 (. 64). Higher levels of spontaneous and stimulated production of IL6 at 23:00 were associated with more Stage 3 (.39), 4 (.43), and slow wave sleep (.49), with evidence that Stage 4 had a countervailing relationship on IL6 production at 8:00 (.60).
Conclusion
RA patients show evidence of sleep fragmentation, greater sleep depth, and higher levels of cellular inflammation. Sleep maintenance and sleep depth show countervailing relationships with evening- and morning levels of monocytic production of TNF and IL-6, respectively, which support the hypothesis of a feedback loop between sleep maintenance, slow wave sleep and cellular inflammation that is cytokine specific.
Keywords: rheumatoid arthritis, polysomnography, sleep macrostructure, cytokines, inflammation
INTRODUCTION
Impaired sleep quality and related daytime dysfunction are consistently reported in rheumatoid arthritis (RA) populations. Clinically significant sleep disturbance is found in over 60% of RA patients (1,2), which is thought to have adverse impact on systemic inflammation, synovitis, joint function, and quality-of-life, as well as medical co-morbidity and all-cause mortality (3–8). Despite the prevalence of sleep problems in RA, few studies have objectively examined sleep using polysomnography (PSG), and this prior work is limited by small sample size (9–13), lack of control group (12,13) and inadequate consideration of confounding factors (14). PSG provides a laboratory-based assessment of disturbances of sleep continuity and sleep architecture. Sleep continuity includes total sleep time, sleep latency, sleep efficiency, and wake after sleep onset. Sleep architecture is characterized by non-rapid eye movement (NREM) sleep including amounts of Stages 1–2 sleep and Stages 3–4 sleep (slow wave sleep, SWS); SWS or deep sleep is thought to be important in the restorative function of sleep as compared to Stages 1–2 sleep. Rapid-eye-movement (REM) sleep includes measures of REM sleep amount, REM latency, REM density, and REM duration.
Animal models, cell culture data, and the use of anti-inflammatory cytokine antagonist treatments provide converging evidence that dysregulation of the pro-inflammatory cytokine network underlies synovial inflammation in patients with RA (15). Pro-inflammatory cytokines play a key role in the progression of RA (15). Moreover, pro-inflammatory cytokines show potent additive effects; tumor necrosis factor-α (TNF) strongly induces production of interleukin (IL)-1β and IL-6, whereas TNF blockade potently antagonizes IL-6 and TNF production.
In RA patients who show increases in levels of pro-inflammatory cytokine activity, sleep deprivation induces an exaggerated increase in self-reported pain as compared to responses in controls (16). Sleep disturbance is also associated with increases in circulating levels of IL-6 and TNF, but not IL-1, as well as increases in transcriptional expression of IL-6 and TNF (17,18). To test the causal role of sleep in regulating inflammation, sleep deprivation has been found to induce increases in the spontaneous or constitutive monocytic expression of IL-6 and TNF. Monocytes make up about 5% of circulating leukocytes and are a major contributor to pro-inflammatory cytokine production in peripheral blood. Additionally, sleep loss induces increases in lipopolysaccharide (LPS) stimulated monocytic production of IL-6 and TNF (3,17–19). LPS acts by stimulating the Toll-like receptor (TLR)-4. In turn, increases in TLR-4 stimulated production of IL-6 and TNF correlate with symptoms of fatigue (20,21); elevated TNF levels also occur in association with fatigue and daytime sleepiness in patients with chronic fatigue syndrome, insomnia, or obstructive sleep apnea (22).
Alternatively, animal models suggest that inflammation can have reciprocal effects on sleep (23), with evidence that cytokines have both somnogenic and inhibitory effects on sleep depending on the cytokine, plasma level, and circadian phase (24). For example, TNF and IL-1β influence regulation of sleep macrostructure, under both physiological and pathological conditions, in which central or systemic injections of TNF induce dose-dependent increases of duration and intensity of NREM sleep; however, when dosage of cytokine is increased, both NREM and rapid-eye-movement (REM) sleep are inhibited (22). Opposite effects on NREM sleep are exerted by inhibitors of these cytokines (22). Finally, central administration of IL-6 modulates NREM sleep and induces sleep fragmentation in rats (25). In humans, we have found that a TNF receptor antagonist, which decreases bioactivity of TNF as well as IL-6, decreases amounts of REM sleep (26).
Less is known about the reciprocal relationship between inflammation and sleep in humans, with no available data in RA patients. Based on previous findings from animal and human experimental studies, we hypothesized that sleep fragmentation would be associated with increases in monocytic expression of TNF and IL-6 in the morning. In contrast, given the somnogenic effects of pro-inflammatory cytokines, we further hypothesized that a countervailing relationship between evening levels of cellular inflammation and sleep continuity, in which increases in monocytic expression of TNF and IL-6 in the evening would be associated with sleep maintenance, as opposed to sleep fragmentation. Further, given compelling animal data that pro-inflammatory cytokines induce NREM or slow wave sleep, we hypothesized that increases in monocytic expression of pro-inflammatory cytokines in the evening would be associated with increases in slow wave sleep. Moreover, because SWS is thought to have a restorative effect on the immune system, increases in slow wave would then have countervailing effects on cellular inflammation and be associated with decreases in morning expression of pro-inflammatory cytokines. Finally, we hypothesized that RA patients will show increases in cellular inflammation, greater amounts of NREM sleep and specifically greater amounts of Stages 3–4 sleep or SWS as compared to controls. Hence, the overarching objectives of this study were (1) to determine the nature and severity of disordered sleep using PSG in RA patients vs. controls, and (2) to examine the reciprocal associations between sleep macrostructure and evening- and morning levels of constitutive or spontaneous inflammation and of stimulated monocytic production of pro-inflammatory cytokines. Levels of cellular inflammation were obtained before and after the nocturnal sleep period to evaluate the direction of the reciprocal relationship between inflammation, sleep continuity, and slow wave sleep. The cellular sources of pro-inflammatory cytokine activity, as opposed to circulating levels of pro-inflammatory cytokines, was assessed by measuring the production of pro-inflammatory cytokines by monocytes following ligation of the Toll-like 4 receptor (TLR-4) with lipopolysaccharide (LPS) in monocytes. In addition to assay of TLR-4 stimulated production of IL-6 and TNF, we evaluated the resting or constitutive expression of pro-inflammatory cytokines by monocytes, i.e., spontaneous monocytic production of IL-6 and TNF.
MATERIALS AND METHODS
Design overview
The current study was part of an experimental study that evaluated the effects of partial night sleep deprivation on pain symptoms in RA patients (16). In this report, only PSG data from the baseline night, prior to sleep deprivation, were used for cross-sectional comparison of RA patients (n = 24) and age-, sex- and body mass index-matched comparison controls (n = 48). Data were collected between November 2006 and July 2015. Study procedures were approved by the UCLA Institutional Review Board (IRB), and all participants provided informed consent.
Participants
Details about recruitment, eligibility screening and characteristics of subjects have previously been described (16). For both RA patients and controls, the following exclusion criteria were applied as ascertained by medical history and physical examination: active, uncontrolled medical disorders; chronic infections; comorbid pain disorders; current psychiatric disorders; steroid use exceeding 10 mg of prednisone/day; multiple daily doses of opioids; use of psychotropic or hypnotic medications; substance use; body mass index (BMI) > 35 kg/m2; and pregnancy. Screening lab tests (complete blood count and metabolic panel) confirmed reference range results for all participants. Briefly, RA patients fulfilled the 1987 American College of Rheumatology revised criteria for RA according to diagnostic evaluation performed by a study rheumatologist and had a stable disease course without flares/were stable on a disease-modifying antirheumatic drug (DMARD) regimen ≥ 3 months prior to study entry. The RA patients reported taking following medications: DMARDs (71%), TNF inhibitors (42%), non-steroidal anti-inflammatory drugs (NSAIDs) (54%), steroids (13%), beta blockers (8%), other anti-hypertensive medications (29%), statins (13%) and levothyroxine (13%). Only one RA patient reported use of an opioid (not regular intake); one patient was taking an antidepressant (bupropion); and one patient was prescribed hormonal replacement therapy. Overall, a low number of comparison controls were taking any type of medication. None of the controls were taking NSAIDs, steroids, opioids or beta blockers; statins were the most commonly prescribed medication (14%).
Regular bedtimes and rising times (between 22:30 and 07:30) were confirmed for all participants via administration of two-week sleep diaries prior to the PSG assessment. For both groups, primary sleep disorders were ruled out through a comprehensive medical history in conjunction with PSG screening as described below. Diagnoses of psychopathologies and/or insomnia disorder were determined through structured clinical interviews for Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV).
Procedures
Sleep assessment
Prior to assessment of sleep, subjects underwent a night of adaptation to acclimate subjects to the PSG protocol and to exclude those with primary sleep disorders such as sleep apnea (apnea-hypopnea index (AHI) > 15) or nocturnal myoclonus (periodic limb movements during sleep (PLMS) index > 10). PSG data obtained during the night after adaptation were used for analyses. Details about the sleep protocol, and electroencephalogram (EEG) sleep methods have previously been described (27). A standard PSG montage was used, including six EEG channels (C4, F4, O2 referenced to M1; C3, F3, O1 referenced to M2), bilateral electrooculogram (EOG), bipolar electromyogram (EMG), pulse oximetry, abdominal respiratory effort, and electrocardiogram (ECG). Data from each 30-second epoch were entered into a computer program that tallied the summary statistics for each subject. All sleep records were visually scored in accordance with Rechtschaffen and Kales criteria (28) using methods detailed (27,29). Sleep onset was defined as the first minute of stage 2 or REM sleep followed by at least eight minutes of sleep in the next nine minutes. A REM period was defined by not less than three consecutive minutes of REM sleep. Sleep efficiency was the ratio of total sleep time to the time in bed, multiplied by 100. Sleep architecture was defined as the duration of time spent asleep in NREM sleep, Stages 1 through 4. REM density was an estimate of the number of eye movements per minute of REM sleep, scored on a scale of 0 to 4 per 30-second epoch but expressed on a scale of 0 to 8 per minute. Certified sleep research technicians were regularly tested on scoring reliability and high standards were maintained: sleep latency (r=0.96), REM latency (r=0.99), REM density (r=0.91), amounts of stages 3 and 4 (r=0.85), and total sleep time (r=0.99).
During the study days, both groups followed identical rest/activity schedules: no strenuous activity; lights out at 23:00, uninterrupted sleep, good morning time at 07:00. Caffeine was not allowed after 13:00, and no eating or drinking (except for water and medications) was allowed after 20:00. Prior to entry into the sleep protocol, subjects underwent urine toxicology to rule out current substance use.
Questionnaires
To assess subjective measures of sleep, pain and health-related function, the following validated questionnaires were administered: Pittsburgh sleep quality index (PSQI) (range: 0–2, >5 indicates clinical sleep impairment; Cronbach’s alpha=0.83); Epworth sleepiness scale (ESS) (range: 0–24; Cronbach’s alpha=0.74 to 0.88), Beck depression inventory (BDI-II) (range: 0–63; 0–13, minimal depression; 14–19, mild depression; 20–28, moderate depression; 29–63, severe depression; Cronbach’s alpha=0.91), fatigue symptom inventory (FSI) interference scale (range: 0–70; Cronbach’s alpha = 0.93 to 0.95), short form McGill pain questionnaire (sf-MPQ) (range: 0–15, Cronbach’s alpha>0.75), and the short form (36) health survey (SF-36)(SF-36 Physical Component Scale, PCS, and Mental Component Scale, MCS, normative mean = 50 ± 10; PCS Cronbach’s alpha = .94; MCS Cronbach’s alpha= .89.)
RA disease activity
For all RA patients a structured clinical comprehensive joint count assessment was completed; disease activity score (DAS) 28 – C-reactive protein (CRP)/erythrocyte sedimentation rate (ESR) were calculated (30).
Systemic inflammation and cellular production of pro-inflammatory cytokines
CRP was measured using high-sensitivity immunoassay on a BN-II System (Dade-Behring, Newark, DE). Samples were automatically diluted 1:20 with N Diluent. This technique has a limit of detection of 0.175 mg/L and intra- and inter-assay coefficients of variation of <4%, and all samples had detectable levels of CRP. ESR was estimated by a modified Westergren method(Seditainer;BectonDickinson, Oxford, UK).
Monocyte intracellular production of TNF and IL-6 at rest (i.e., spontaneous) and in response to lipopolysaccharide (LPS) (i.e., TLR-4) stimulation of whole-blood leukocytes was assessed by flow cytometry (as described previously (31)); measures were obtained at 23:00 (before lights out), and at 8:00 in the following morning. Briefly, heparin-treated blood was mixed without (spontaneous) and with 00 pg/mL lipopolysaccharide (Sigma, St Louis, Mo) and 10 μg/mL brefeldin A (Sigma) and incubated for 4 hours at 37°C. After cells were permeabilized in fluorescence-activated cell sorting permeabilizing buffer (BD Biosciences, San Jose, Calif ) and fluorescence-conjugated antibodies were added, about 12 000 CD14_events were counted to determine the net unstimulated or spontaneous and stimulated percentage of cytokine-secreting monocytes, with quadrant coordinates set based on unstimulated cells. Intra- and inter-assay coefficienta of variation is <7%.
Statistics
Prior to analyses, all data were explored; normality was determined graphically by inspection of Q-Q plots and histograms, and numerically through the Kolmogorov-Smirnov test. Measures observed to be skewed were natural log transformed. Comparisons of continuous demographic, clinical and PSG data between the RA and control groups were conducted through two-tailed t-tests; chi-squared analyses were utilized for categorical variables. Relationships between inflammatory markers and sleep variables were examined using multiple regression while controlling for the group factor, depressive symptoms and physical health functioning, with inclusion of inflammatory markers assessed at 23:00 and 8:00 to determine whether inflammation prior to sleep (23:00) was associated with SWS, for example, or whether SWS amounts were associated with inflammation in the morning (8:00). Data entry and analyses were conducted utilizing SPSS version 22 (IBM, 2013). Means are reported ± SD.
RESULTS
Demographic and clinical characteristics
Demographic characteristics for the RA and control groups are described in Table 1. Age, sex, ethnicity, body mass index, educational level, marital status and employment status were similar for both groups. Although there were more non-white subjects in the RA group compared to controls, the difference was not significant (χ2=3.57, p=0.059). Within the RA group, mean DAS28-ESR and DAS28-CRP were 3.7 ± 1.7 and 3.3 ± 1.0 respectively, indicative of moderate disease activity.
Table 1.
Demographic characteristics of the rheumatoid arthritis and control groups.
| Rheumatoid arthritis (n = 24) | Controls (n = 48) | p | |
|---|---|---|---|
| Age (years) | 60.4 ± 11.5 | 61.8 ± 10.2 | 0.60 |
| Gender (% female) | 87.5 | 81.3 | 0.74 |
| Race (% Caucasian) | 41.7 | 66.7 | 0.059 |
| Ethnicity (% Hispanic) | 16.7 | 8.9 | 0.43 |
| BMI (kg/m2) | 27.3 ± 4.8 | 25.8 ± 4.7 | 0.20 |
| Education (years) | 15.6 ± 4.5 | 16.5 ± 3.5 | 0.37 |
| Marital status (% married) | 41.7 | 37.0 | 0.80 |
| Employment status (% employed) | 29.2 | 40 | 0.44 |
Results are presented as means ± SD or % as indicated.
Abbreviations: BMI = body mass index.
Subjective sleep measures, self-reported pain, and health functioning
Subjective reports of sleep showed significant differences between RA patients and controls, in which 67% of RA patients had a PSQI score exceeding 5, indicating poor sleep quality, compared to 22% in the control group (Table 2). In addition, 33% of the RA subjects met criteria for current persistent insomnia disorder, as compared to 11% of the controls (χ2=4.8, p=0.028). RA patients also reported more depressive symptoms, fatigue, impairments in physical health functioning, and increase in self-reported pain symptoms prior to- and in the morning after sleep (Table 2). However, none of the measures of sleep were correlated with self-reported pain symptoms (data not shown).
Table 2.
Clinical characteristics of the rheumatoid arthritis and control groups.
| Rheumatoid arthritis (n = 24) | Controls (n = 48) | p | |
|---|---|---|---|
| PSQI global score | 7.1 ± 3.9 | 3.3 ± 2.5 | <0.001 |
| Epworth sleepiness scale | 6.7 ± 3.0 | 5.0 ± 3.4 | 0.088 |
| Beck depression inventory | 5.6 ± 4.5 | 2.4 ± 4.0 | 0.004 |
| FSI interference score | 18.9 ± 20.4 | 5.3 ± 8.0 | .007 |
| SF-36 | |||
| PCS | 39.5 ± 11.1 | 53.5 ± 7.4 | <0.001 |
| MCS | 52.0 ± 8.8 | 55.4 ± 10.0 | 0.18 |
| sf-MPQ | |||
| Bedtime prior to PSG | |||
| Present pain intensity | 1.0 ± 0.7 | 0.1 ± 0.3 | <0.001 |
| Pain descriptor score | 3.7 ± 5.6 | 0.4 ± 1.3 | 0.010 |
| Morning following PSG | |||
| Present pain intensity | 1.0 ± 0.8 | 0.2 ± 0.4 | <0.001 |
| Pain descriptor score | 2.8 ± 2.8 | 0.4 ± 1.3 | 0.001 |
Results are presented as means ± SD.
Abbreviations: FSI = fatigue symptom inventory, MCS = mental component summary, PCS = physical component summary, PSG = polysomnography, PSQI = Pittsburgh sleep quality index, SF-36 = short form (36) health survey, sf-MPQ = short form McGill pain questionnaire.
Sleep macrostructure
Macrostructure sleep parameters for the RA and control groups are described in Table 3. Compared to controls, the RA patients exhibited statistically significant alterations of sleep continuity measures (total sleep time, wake after sleep onset, sleep efficiency) indicative of shorter sleep duration and sleep fragmentation.
Table 3.
Macrostructure sleep parameters for the rheumatoid arthritis and control groups.
| Rheumatoid arthritis (n = 24) | Controls (n = 48) | p | |
|---|---|---|---|
| Sleep continuity | |||
| Total sleep time (min) | 378.2 ± 33.5 | 399.6 ± 32.2 | 0.011 |
| Sleep onset latency (min) | 25.1 ± 18.0 | 21.4 ± 20.1 | 0.45 |
| Wake after sleep onset (min) | 73.3 ± 32.3 | 52.4 ± 28.3 | 0.006 |
| Number of awakenings | 9.9 ± 4.9 | 9.0 ± 4.9 | 0.47 |
| Sleep efficiency (%) | 83.8 ± 7.0 | 88.1 ± 6.1 | 0.009 |
| Sleep architecture | |||
| NREM sleep | |||
| Stage 1 (min) | 17.2 ± 4.9 | 20.8 ± 15.1 | 0.13 |
| Stage 1 (%) | 4.6 ± 1.4 | 5.3 ± 4.1 | 0.25 |
| Stage 2 (min) | 197.4 ± 47.4 | 229.0 ± 46.6 | 0.009 |
| Stage 2 (%) | 52.2 ± 11.8 | 57.5 ± 11.3 | 0.070 |
| Stage 3 (min) | 49.5 ± 26.5 | 38.0 ± 25.5 | 0.078 |
| Stage 3 (%) | 13.1 ± 6.9 | 9.3 ± 6.4 | 0.024 |
| Stage 4 (min) | 20.1 ± 25.9 | 18.3 ± 24.6 | 0.78 |
| Stage 4 (%) | 5.2 ± 6.7 | 4.5 ± 6.0 | 0.66 |
| SWS (min) | 62.1 ± 38.4 | 53.4 ± 38.9 | 0.39 |
| SWS (%) | 16.4 ± 9.7 | 13.2 ± 9.7 | 0.21 |
| REM measures | |||
| REM (min) | 94.0 ± 21.4 | 93.5 ± 30.0 | 0.94 |
| REM (%) | 24.9 ± 5.3 | 23.3 ± 7.1 | 0.35 |
| REM latency, corrected (min) | 63.2 ± 31.4 | 69.2 ± 42.9 | 0.55 |
| REM duration 1st period (min) | 22.5 ± 12.8 | 21.2 ± 10.5 | 0.64 |
| REM density | 1.7 ± 1.0 | 1.1 ± 0.7 | 0.009 |
Results are presented as means ± SD.
Abbreviations: NREM = non-rapid-eye-movement, REM = rapid-eye-movement, SWS = slow wave sleep.
Sleep architecture measures also differed significantly between groups. In addition to lower levels of absolute amounts of Stage 2 sleep, RA patients had higher levels of relative amounts of Stage 3 sleep, and elevated levels of REM density compared to controls. Within the RA patients, no sleep continuity or architecture measures differed by DMARD or NSAID use. However, those taking biologics had higher TST (397.4 ± 17.0 vs. 363.1 ± 37.1; p=0.018) and REM Density (2.31 ± 0.94 vs. 1.33 ± 0.84; p=0.021). Thus RA patients not treated with biologics had even worse TST compared to controls (363.1 ± 37.1 vs. 398.9 ± 32.2; p=0.001) but similar REM Density compared to controls (1.33 ± 0.84 vs. 1.11 ± 0.67; p=0.35) (compare to Table 3).
Inflammatory activity
As expected, RA patients had higher levels of high-sensitivity CRP (hs-CRP), with a trend for higher levels of ESR, compared to controls (Table 4).
Table 4.
Systemic markers of inflammation, monocytic intracellular production of TNF and IL-6.
| Rheumatoid arthritis (n = 24) | Controls (n = 48) | P | |
|---|---|---|---|
| ESR (mm/hr) | 22.7 ± 23.3 | 9.0 ± 9.2 | 0.059 |
| hs-CRP (mg/L) | 3.7 ± 6.5 | 1.5 ± 1.8 | 0.025 |
| 23:00 | |||
| Spontaneous TNF† | 1.22 ± 0.17 | 1.07 ± 0.28 | 0.043 |
| Spontaneous IL-6† | 1.17 ± 0.22 | 1.04 ± 0.33 | 0.13 |
| 8:00 | |||
| Spontaneous TNF† | 1.31 ± 0.34 | 1.26 ± 0.38 | 0.34 |
| Spontaneous IL-6† | 1.32 ± 0.25 | 1.18 ± 0.35 | 0.13 |
| 23:00 | |||
| Stimulated TNF† | 3.70 ± 0.52 | 3.44 ± 0.64 | 0.14 |
| Stimulated IL-6† | 3.32 ± 0.63 | 3.14 ± 0.78 | 0.41 |
| 8:00 | |||
| Stimulated TNF† | 3.48 ± 0.63 | 3.49 ± 0.59 | 0.96 |
| Stimulated IL-6† | 3.83 ± 0.39 | 3.45 ± 0.80 | 0.017 |
Results are presented as means ± SD.
Abbreviations: ESR = erythrocyte sedimentation rate; hs-CRP = high-sensitivity C-reactive protein, natural log; IL-6 = interleukin 6; TNF = tumor necrosis factor.
For spontaneous and stimulated production of IL-6 and TNF, results are presented as % of total number of monocytes expressing TNF or IL-6, respectively, natural log-transformed.
Spontaneous (unstimulated) monocyte intracellular production of TNF at 23:00 was significantly higher in the RA group compared to controls (Table 4). TLR-4 stimulated production of IL-6 at 8:00 was higher in RA patients compared to controls (Table 4). Within the RA group, none of the inflammatory markers differed by the use of DMARD, biologic, or NSAID medications.
Association between inflammatory activity and sleep macrostructure
High-sensitivity CRP or ESR, taken only in the morning after sleep, did not show any significant associations to any of the PSG sleep measures.
To explore the reciprocal relationship between cellular inflammation and objective sleep measures, we tested the associations between sleep measures and spontaneous- and TLR-4 stimulated production of IL-6 and TNF taken at 23:00 prior to sleep, and at 8:00 after a night of sleep, taking into account group, depressive symptoms, and physical functioning. Depressive symptoms and physical functioning differed between the groups and are known to influence sleep.
Table 5 shows the associations between spontaneous production of IL-6 and TNF and PSG measures of sleep. For the sleep continuity measures, level of spontaneous IL-6 expression at 23:00, but not TNF, was negatively associated with sleep onset latency. In contrast, sleep onset latency showed a countervailing relationship with spontaneous IL-6 expression at 8:00, in which sleep onset latency was positively associated with spontaneous IL-6 expression at 8:00. Together, these data suggest a reciprocal and countervailing link between sleep onset latency and constitutive IL-6 expression.
Table 5.
Associations between PSG sleep measures and evening- and morning spontaneous levels of monocytic expression of TNF and IL-6.
| Group | BDI | PCS | TNF 23:00 |
IL-6 23:00 |
TNF 8:00 |
IL-6 8:00 |
|
|---|---|---|---|---|---|---|---|
| Sleep continuity | |||||||
| Total sleep time (min) | −.33 | .12 | −.13 | −.08 | .27 | −.11 | −.05 |
| Sleep onset latency (min) | .05 | −.33+ | −.19 | .06 | −.49* | .06 | .40* |
| Wake after sleep onset (min) | .37+ | .23 | .32 | .12 | −.04 | .16 | −.22 |
| Number of awakenings | .20 | .25 | .42+ | .29 | −.11 | .22 | −.37+ |
| Sleep efficiency (%) | −.38+ | −.20 | −.31 | −.10 | .09 | −.17 | .18 |
| Sleep architecture | |||||||
| NREM sleep | |||||||
| Stage 1 (%) | −.25 | .25 | −.06 | −.04 | .13 | −.03 | −.21 |
| Stage 2 (%) | −.19 | .11 | −.06 | .36 | −.57** | −.10 | .02 |
| Stage 3 (%) | .15 | −.08 | −.03 | −.13 | .39+ | −.09 | .08 |
| Stage 4 (%) | .03 | −.14 | −.02 | −.26 | .43* | .23 | −.09 |
| SWS (%) | .12 | −.13 | −.03 | −.22 | .49* | .06 | .01 |
| REM (%) | .28 | −.12 | .18 | −.26 | .15 | .11 | .06 |
Abbreviations: BDI = Beck depression inventory II, NREM = non-rapid-eye-movement, PCS = physical component summary, PSG = polysomnographic, REM = rapid-eye-movement, SF-36 = short form (36) health survey, SWS = slow wave sleep.
Numbers are standardized betas.
p<.10,
p<.05,
p<.01
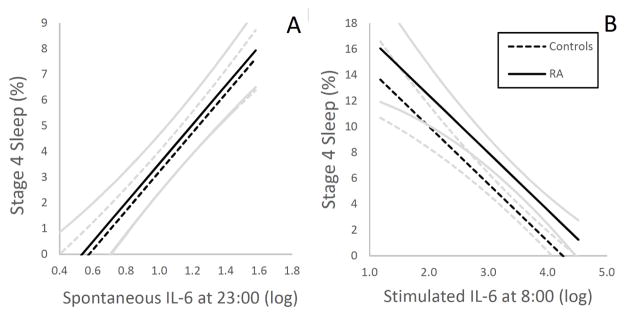
For the sleep architecture measures, level of spontaneous IL-6 expression at 23:00, but not TNF, was negatively associated with Stage 2 sleep and positively associated with Stage 3-, Stage 4 (Figure 2 A), and SWS. However, none of the sleep architecture measures were associated with levels of spontaneous IL-6 or TNF expression at 8:00. There was no relationship between the inflammatory markers and relative amounts of REM sleep or any of the REM measures (data not shown).
Figure 2.
Associations between percentage of Stage 4 sleep and evening- (A) spontaneous levels of monocytic expression of IL-6, and morning (B) levels of stimulated monocytic expression of IL-6 in rheumatoid arthritis (RA) patients and controls, as illustrated by estimated regression lines by group, controlling for depressive symptoms and physical health functioning. Curvilinear brackets indicate residual standard error. For spontaneous and stimulated production of IL-6, results are presented as % of total number of monocytes expressing IL-6, respectively, natural log-transformed.
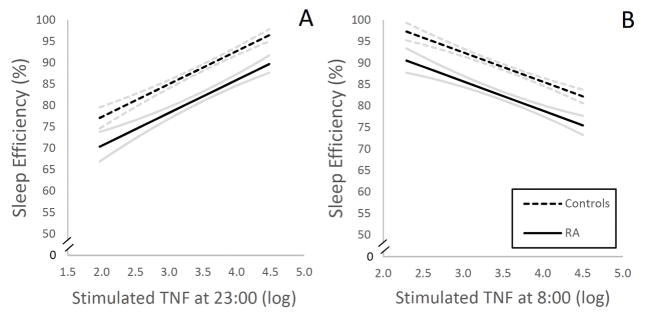
Table 6 shows the associations between TLR-4 stimulated production of IL-6 and TNF and PSG measures of sleep. For the sleep continuity measures, level of stimulated TNF production, but not IL-6, at 23:00 was positively associated with sleep latency, negatively associated with wake after sleep onset and number of awakenings, and positively associated with sleep efficiency (Figure 1 A), indicating that higher level of TNF production at 23:00 correlated with sleep maintenance. In contrast, measures of sleep maintenance (i.e., wake after sleep onset, number of awakenings and sleep efficiency) showed countervailing associations with TNF production at 8:00 (Figure 1 B).
Table 6.
Associations between PSG sleep measures and evening- and morning levels of stimulated monocytic expression of TNF and IL-6.
| Group | BDI | PCS | TNF 23:00 |
IL-6 23:00 |
TNF 8:00 |
IL-6 8:00 |
|
|---|---|---|---|---|---|---|---|
| Sleep continuity | |||||||
| Total sleep time (min) | −.31 | .02 | −.07 | .15 | −.00 | −.28 | .01 |
| Sleep onset latency (min) | −.08 | −.29 | −.18 | .82* | −.04 | −.27 | −.21 |
| Wake after sleep onset (min) | .50* | .19 | .26 | −.81* | −.02 | .66* | .03 |
| Number of awakenings | .44* | .19 | .37+ | −.85* | −.08 | .87** | −.19 |
| Sleep efficiency (%) | −.49 | −.17 | −.25 | .74* | .05 | −.64* | −.05 |
| Sleep architecture | |||||||
| NREM sleep | |||||||
| Stage 1 (%) | −.14 | .21 | −.07 | −.71* | −.08 | .42 | .11 |
| Stage 2 (%) | −.27 | .11 | −.12 | .19 | −.17 | −.21 | .04 |
| Stage 3 (%) | .22 | .00 | .04 | .02 | .41 | .13 | −.22 |
| Stage 4 (%) | .21 | −.15 | .03 | −.08 | .33 | .45 | −.60* |
| SWS (%) | .26 | −.07 | .05 | −.03 | .45+ | .33 | −.46 |
| REM (%) | .14 | −.17 | .16 | .11 | −.33 | −.35 | .52+ |
Abbreviations: BDI = Beck depression inventory II, NREM = non-rapid-eye-movement, PCS = physical component summary, PSG = polysomnographic, REM = rapid-eye-movement, SF-36 = short form (36) health survey, SWS = slow wave sleep.
Numbers are standardized betas.
p<.10,
p<.05,
p<.01
Figure 1.
Associations between sleep efficiency and evening- (A) and morning (B) levels of stimulated monocytic expression of TNF in rheumatoid arthritis (RA) patients and controls, as illustrated by estimated regression lines by group, controlling for depressive symptoms and physical health functioning. Curvilinear brackets indicate residual standard error. For stimulated production of TNF, results are presented as % of total number of monocytes expressing TNF, respectively, natural log-transformed.
For the sleep architecture measures, level of IL-6 production at 23:00 was positively associated with SWS as a trend. In contrast, Stage 4 sleep showed a countervailing negative association with IL-6 production at 8:00 (Figure 2 B). In addition, level of TNF production at 23:00, but not IL-6, was negatively associated with Stage 1 sleep.
There was no relationship between the TLR-4 stimulated production of inflammatory cytokines and relative amounts of REM sleep or any of the REM measures (data not shown). There were no interactions between group and cellular markers of inflammation on sleep measures (IL6: F(1, 45)=1.92, p=.18; TNFa: F(1, 45)=1.317, p=.26), indicating that the identified relationships between the inflammatory markers and sleep measures were similar in the two groups. In other words, the relationships between cellular inflammation and sleep measures were not stronger in the RA patients as compared to the controls.
DISCUSSION
Basic research on neural-immune signaling has shown that peripheral pro-inflammatory cytokines exert potent effects on neural processes which lead to a constellation of behavior changes including abnormal sleep (32). In this study, RA patients showed disturbances in sleep continuity, increases in sleep depth, and increases in systemic and cellular markers of inflammation as compared to controls. In addition, in both groups of RA patients and controls, the associations between cellular inflammation and sleep showed countervailing effects dependent on time of assessment of inflammation, cytokine, and sleep measures. For example, level of stimulated production of TNF prior to sleep was associated with improvements in sleep maintenance, whereas disturbance of sleep maintenance correlated with increases in the production of TNF in the morning. Although these correlational data do not test a causal pathway, it is possible that sleep fragmentation leads to increases in morning inflammation, which persists over the day (i.e., 8:00 and 23:00 levels of pro-inflammatory cytokines were highly correlated) and then such inflammation has a reciprocal influence to improve sleep maintenance. The association between TNF, but not IL-6, and sleep maintenance is striking, as increases in both of these pro-inflammatory cytokines have separately been associated with disturbances of sleep continuity. However, the vast majority of research has not examined the relative contribution of TNF vs. IL-6 on sleep; hence, these data provide novel insight into the potential unique role of TNF in regulation of sleep maintenance.
A similar reciprocal loop between IL-6 and measures of sleep depth is also suggested by these correlative data. Consistent with experimental animal data, constitutive expression of IL-6 prior to sleep was associated with increases in Stage 3-, Stage 4, and slow wave sleep, with evidence that stimulated production of IL-6 prior to sleep was also associated with increases in slow wave sleep. In turn, increases in Stage 4 correlated with decreases in the stimulated production of IL-6 in the morning, consistent with the notion that amounts of slow wave sleep might have countervailing effects on inflammation. Given the restorative effects of slow wave sleep to reduce sympathetic activity, this may be a mechanism to account for the association between Stage 4 sleep and stimulated IL-6 production in the morning. Indeed, sympathetic activation is known to induce increases in inflammatory signaling and cellular inflammation (33), and blockade of β-adrenergic tone abrogates stress-induced inflammation.
Prior studies in humans that have examined the association between inflammation and sleep have relied almost exclusively on circulating measures of cytokines (e.g., IL-6) with mixed results. For example, Burgos et al. (34) found a negative correlation between nocturnal IL-6 levels and slow wave sleep in a small sample (n = 11) of insomniacs, whereas Hong et al. (35) found no association between circulating levels of IL-6 and slow wave sleep; similar to the negative results reported here between CRP and sleep measures. Furthermore, experimental studies that have tested whether cytokines or cytokine antagonists modulate sleep structure have also yielded mixed results. Whereas pharmacologic increases in interferon-alpha, a pro-inflammatory cytokine, administered over 12 weeks, induced decreases in slow wave sleep in patients with hepatitis C (36), pharmacologic blockade with infliximab, an anti TNF monoclonal antibody, had no effect on sleep architecture (37). We have previously found that etanercept, a TNF antagonist, produces robust decreases of REM sleep in abstinent alcohol dependent subjects, but had no effect on slow wave sleep (26).
A number of other mechanisms are thought to contribute to sleep disturbance in RA patients. For example, pain is often cited as a factor that leads to disturbances in sleep (38,39). Indeed, sleep disturbance has been found to alter conditioned pain modulation (40) and to increase pain perception in RA patients (16). Additionally, a limited number of studies, with partially overlapping patient cohorts, have demonstrated significant associations between RA disease activity (i.e., pain symptoms) and sleep macrostructure parameters (11,41,42), although other studies have found no associations (9,13). Nevertheless, this study did not find any association between self-reported pain and subjective or objective measures of sleep. Other factors that might adversely affect sleep homeostasis in RA patients include altered melatonin secretion (43), comorbidities (4), primary sleep disorders (44), analgesics (45) and disease modifying medications (46,47), although use of RA specific medications did not alter the sleep measures with the exception of increases in total sleep time in those taking biologic medications.
Fragmentation of sleep with reduced sleep efficiency was found in the RA patients, consistent with four previous PSG studies (9–12). Nevertheless, a relatively large ambulatory PSG study revealed normative sleep continuity measures in an outpatient RA sample (42), suggesting that differences in clinical characteristics and disease activity of the RA patients, or other methodological factors might have led to these mixed findings (14).
Whereas our study holds several advantages compared to previous controlled PSG studies in RA patients, a few limitations deserve consideration. First, although the present work constitutes, to date, one of the largest controlled sleep laboratory studies of RA patients, the sample was relatively small. Second, whereas the groups were matched for important demographic factors, such as age, sex, BMI, race, ethnicity and education level, physical activity was not considered. Third, we did not include a multiple sleep latency test (MSLT) to objectively evaluate abnormal daytime sleepiness. Previous MSLT results in RA patients have been mixed (9,10,12), and future studies should ideally implement this measure. Fourth, EEG power spectral analysis might have added further detail to the findings, although these analyses are beyond the scope of this report.
The neurobiological mechanisms that contribute to disordered sleep in RA and other chronic inflammatory disorders are not well known. Indeed, the present study is the first to incorporate objective sleep assessment in conjunction with examination of cellular inflammation before and after a sleep period, and to provide evidence that cellular inflammation has a reciprocal relationship with sleep maintenance and sleep depth. Dependent on the time of day, countervailing associations are found between TNF production and measures of sleep maintenance, and between IL-6 expression and slow wave sleep, to suggest the possibility of a homeostatic relationship between sleep maintenance, slow wave sleep and cellular inflammation that is cytokine specific.
Acknowledgments
Source of Funding: R01-AG034588, R01-AG026364, R01-CA160245, R01-DA032922, R01-HL095799, R01-CA119159, P30-AG028748; UL RR 033176; the Cousins Center for Psychoneuroimmunology, and UCLA Claude D. Pepper Older Americans Independence Center (Dr. Irwin), Stiftelsen Olle Engkvist Byggmästare (Dr. Bjurström).
Acronyms
- BMI
body mass index
- CRP
C-reactive protein
- DAS
disease activity score
- DMARD
disease-modifying antirheumatic drug
- ESR
erythrocyte sedimentation rate
- ESS
Epworth sleepiness scale
- IL
interleukin
- NREM
non-rapid-eye-movement
- NSAID
non-steroidal anti-inflammatory drug
- PSG
polysomnography
- PSQI
Pittsburgh sleep quality index
- RA
rheumatoid arthritis
- REM
rapid-eye-movement
- SF-36
short form (36) health survey
- sf-MPQ
short form McGill pain questionnaire
- TLR-4
Toll-like receptor-4
- TNF
tumor necrosis factor-α
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- 1.Sariyildiz MA, Batmaz I, Bozkurt M, Bez Y, Cetincakmak MG, Yazmalar L, Ucar D, Celepkolu T. Sleep quality in rheumatoid arthritis: relationship between the disease severity, depression, functional status and the quality of life. J Clin Med Res. 2014;6:44–52. doi: 10.4021/jocmr1648w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loppenthin K, Esbensen BA, Jennum P, Ostergaard M, Tolver A, Thomsen T, Midtgaard J. Sleep quality and correlates of poor sleep in patients with rheumatoid arthritis. Clin Rheumatol. 2015;34:2029–2039. doi: 10.1007/s10067-015-2875-4. [DOI] [PubMed] [Google Scholar]
- 3.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66:143–172. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 5.Sivertsen B, Lallukka T, Salo P, Pallesen S, Hysing M, Krokstad S, Simon O. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT Study in Norway. J Sleep Res. 2014;23:124–132. doi: 10.1111/jsr.12102. [DOI] [PubMed] [Google Scholar]
- 6.Smagula SF, Stone KL, Redline S, Ancoli-Israel S, Barrett-Connor E, Lane NE, Orwoll ES, Cauley JA. Actigraphy- and Polysomnography-Measured Sleep Disturbances, Inflammation, and Mortality Among Older Men. Psychosom Med. 2016 doi: 10.1097/PSY.0000000000000312. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marron MM, Anderson SJ, Garrity J, Reynolds CF, 3rd, Lotrich FE. Association of Baseline Sleep Quality With Trajectories of Depressive Symptoms in Patients Undergoing Interferon Treatment. Psychosom Med. 2015;77:911–920. doi: 10.1097/PSY.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tell D, Mathews HL, Janusek LW. Day-to-day dynamics of associations between sleep, napping, fatigue, and the cortisol diurnal rhythm in women diagnosed as having breast cancer. Psychosom Med. 2014;76:519–528. doi: 10.1097/PSY.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch M, Carlander B, Verge M, Tafti M, Anaya JM, Billiard M, Sany J. Objective and subjective sleep disturbances in patients with rheumatoid arthritis. A reappraisal. Arthritis Rheum. 1994;37:41–49. doi: 10.1002/art.1780370107. [DOI] [PubMed] [Google Scholar]
- 10.Roehrs T, Diederichs C, Gillis M, Burger AJ, Stout RA, Lumley MA, Roth T. Nocturnal sleep, daytime sleepiness and fatigue in fibromyalgia patients compared to rheumatoid arthritis patients and healthy controls: a preliminary study. Sleep Med. 2013;14:109–115. doi: 10.1016/j.sleep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Crosby LJ. Factors which contribute to fatigue associated with rheumatoid arthritis. J Adv Nurs. 1991;16:974–981. doi: 10.1111/j.1365-2648.1991.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 12.Mahowald MW, Mahowald ML, Bundlie SR, Ytterberg SR. Sleep fragmentation in rheumatoid arthritis. Arthritis Rheum. 1989;32:974–983. doi: 10.1002/anr.1780320806. [DOI] [PubMed] [Google Scholar]
- 13.Lavie P, Nahir M, Lorber M, Scharf Y. Nonsteroidal antiinflammatory drug therapy in rheumatoid arthritis patients. Lack of association between clinical improvement and effects on sleep. Arthritis Rheum. 1991;34:655–659. doi: 10.1002/art.1780340605. [DOI] [PubMed] [Google Scholar]
- 14.Bjurstrom MF, Irwin MR. Polysomnographic characteristics in nonmalignant chronic pain populations: A review of controlled studies. Sleep Med Rev. 2016;26:74–86. doi: 10.1016/j.smrv.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 16.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Fitzgerald JD, Ranganath VK, Nicassio PM. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35:537–543. doi: 10.5665/sleep.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin MR, Olmstead R, Carroll JE. Sleep Disturbance, Sleep Duration, and Inflammation: A Systematic Review and Meta-Analysis of Cohort Studies and Experimental Sleep Deprivation. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.05.014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 19.Irwin MR, Witarama T, Caudill M, Olmstead R, Breen EC. Sleep loss activates cellular inflammation and signal transducer and activator of transcription (STAT) family proteins in humans. Brain Behav Immun. 2015;47:86–92. doi: 10.1016/j.bbi.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 21.Louati K, Berenbaum F. Fatigue in chronic inflammation - a link to pain pathways. Arthritis Res Ther. 2015;17:254. doi: 10.1186/s13075-015-0784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis CJ, Krueger JM. Sleep and Cytokines. Sleep Med Clin. 2012;7:517–527. doi: 10.1016/j.jsmc.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan D, Morrow JD, Smith EM, Opp MR. Interleukin-6 alters sleep of rats. J Neuroimmunol. 2003;137:59–66. doi: 10.1016/s0165-5728(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 26.Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol Psychiatry. 2009;66:191–195. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin M, Gillin JC, Dang J, Weissman J, Phillips E, Ehlers CL. Sleep deprivation as a probe of homeostatic sleep regulation in primary alcoholics. Biol Psychiatry. 2002;51:632–641. doi: 10.1016/s0006-3223(01)01304-x. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A. A Manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda: N inst Neurl Dis & Blindness; 1968. [DOI] [PubMed] [Google Scholar]
- 29.Thompson PM, Gillin JC, Golshan S, Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biol Psychiatry. 1995;38:831–836. doi: 10.1016/0006-3223(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 30.Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, Aletaha D, van Riel PL. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–960. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin MR, Olmos L, Wang M, Valladares EM, Motivala SJ, Fong T, Newton T, Butch A, Olmstead R, Cole SW. Cocaine dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: autonomic mechanisms. J Pharmacol Exp Ther. 2007;320:507–515. doi: 10.1124/jpet.106.112797. [DOI] [PubMed] [Google Scholar]
- 32.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–253. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 2005;19:165–172. doi: 10.1016/j.bbi.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, Miller AH. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberger JF, Raison CL, Rye DB, Montague AR, Woolwine BJ, Felger JC, Haroon E, Miller AH. Inhibition of tumor necrosis factor improves sleep continuity in patients with treatment resistant depression and high inflammation. Brain Behav Immun. 2015;47:193–200. doi: 10.1016/j.bbi.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicassio PM, Ormseth SR, Kay M, Custodio M, Irwin MR, Olmstead R, Weisman MH. The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain. 2012;153:107–112. doi: 10.1016/j.pain.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552. doi: 10.1016/j.jpain.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YC, Lu B, Edwards RR, Wasan AD, Nassikas NJ, Clauw DJ, Solomon DH, Karlson EW. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum. 2013;65:59–68. doi: 10.1002/art.37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drewes AM, Nielsen KD, Hansen B, Taagholt SJ, Bjerregard K, Svendsen L. A longitudinal study of clinical symptoms and sleep parameters in rheumatoid arthritis. Rheumatology (Oxford) 2000;39:1287–1289. doi: 10.1093/rheumatology/39.11.1287. [DOI] [PubMed] [Google Scholar]
- 42.Drewes AM, Svendsen L, Taagholt SJ, Bjerregard K, Nielsen KD, Hansen B. Sleep in rheumatoid arthritis: a comparison with healthy subjects and studies of sleep/wake interactions. Br J Rheumatol. 1998;37:71–81. doi: 10.1093/rheumatology/37.1.71. [DOI] [PubMed] [Google Scholar]
- 43.Sulli A, Maestroni GJ, Villaggio B, Hertens E, Craviotto C, Pizzorni C, Briata M, Seriolo B, Cutolo M. Melatonin serum levels in rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:276–283. doi: 10.1111/j.1749-6632.2002.tb04227.x. [DOI] [PubMed] [Google Scholar]
- 44.Abad VC, Sarinas PS, Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev. 2008;12:211–228. doi: 10.1016/j.smrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 45.Bohra M, Kaushik C, Temple D, Chung S, Shapiro C. Weighing the balance: how analgesics used in chronic pain influence sleep? British Journal of Pain. 2014;8:107–118. doi: 10.1177/2049463714525355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ethgen O, de Lemos Esteves F, Bruyere O, Reginster JY. What do we know about the safety of corticosteroids in rheumatoid arthritis? Curr Med Res Opin. 2013;29:1147–1160. doi: 10.1185/03007995.2013.818531. [DOI] [PubMed] [Google Scholar]
- 47.Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, Zink A, Buttgereit F. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68:1119–1124. doi: 10.1136/ard.2008.092163. [DOI] [PubMed] [Google Scholar]