Abstract
The increasing numbers of hematopoietic cell transplantations (HCTs) performed each year, the changing demographics of HCT recipients, the introduction of new transplantation strategies, incremental improvement in survival, and the growing population of HCT survivors demand a comprehensive approach to examining the health and well-being of patients throughout life after HCT. This report summarizes strategies for the conduct of research on late effects after transplantation, including consideration of the study design and analytic approaches; methodologic challenges in handling complex phenotype data; an appreciation of the changing trends in the practice of transplantation; and the availability of biospecimens to support laboratory-based research. It is hoped that these concepts will promote continued research and facilitate the development of new approaches to address fundamental questions in transplantation outcomes.
Keywords: National Institutes of Health, consensus, Late effects, Hematopoietic cell, transplantation
INTRODUCTION
Hematopoietic cell transplantation (HCT) is used with curative intent for malignant and nonmalignant conditions. In 2014, over 20,000 HCTs were performed in the United States, and the annual number of HCTs is increasing at the rate of ~5% per year (Center for International Blood and Marrow Transplant Registry [CIBMTR] estimates). Advances in transplantation strategies have yielded steady improvements in survival. Although 5-year survival rates now exceed 70% for patients who survive the first 2 years, HCT recipients are especially vulnerable to serious health problems, such as subsequent neoplasms, heart failure, and pulmonary toxicity, developing several years after transplantation. These complications are directly related to treatment (pre-HCT and HCT-related chemotherapy/radiation) and post-HCT chronic graft-versus-host disease (GVHD). Finally, the risk of these complications is likely modified by comorbidities [1–8]. In this report, we provide general recommendations for establishment of new cohorts or expansion/embellishment of existing cohorts to study late effects after HCT (Insert Box). We also provide priorities for data and biospecimen collection.
METHODOLOGICAL CHALLENGES UNIQUE TO SURVIVORSHIP AFTER HCT
HCT survivors are uniquely vulnerable to long-term morbidity for the reasons detailed below.
Therapeutic Exposures
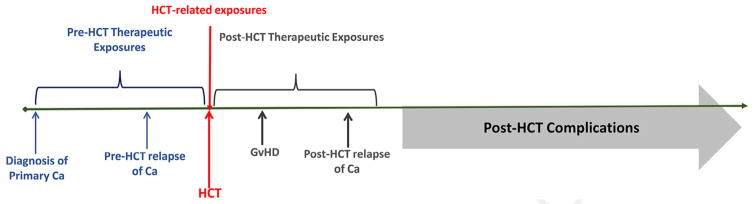
As shown in Figure 1, HCT recipients are exposed to chemotherapy and radiation before HCT (for management of primary cancer), at HCT (for the transplantation procedure), and after HCT (for management of GVHD and possibly relapse of primary cancer). Thus, unlike cancer patients treated in a nontransplantation setting with conventional doses of chemotherapy/ radiation, HCT survivors have typically received higher exposures to chemotherapy and radiation—both with respect to intensity as well as cumulative lifetime exposures. This cumulative exposure places them at a muchc higher risk of long-term morbidity [9]. In addition, the immunosuppressive therapy for management of GVHD increases risks for a variety of chronic health problems, such as chronic kidney disease, metabolic syndrome, osteonecrosis, and subsequent malignancies. Patients are frequently referred to dedicated HCT centers after treatment by physicians who do not provide this highly specialized type of treatment. This arrangement makes it difficult for HCT study teams to gather detailed information regarding therapeutic exposures that occurred before referral for HCT and after post-HCT relapse. For this reason, most previous studies have focused solely on therapeutic exposures at HCT (ignoring the prereferral exposures) when examining determinants of long-term morbidity. As a result, post-HCT complications have been attributed to HCT-related exposures alone, even though prereferral exposures have likely contributed to etiology.
Figure 1.
Therapeutic exposures associated with risk of late complications developing after HCT.
Post-Transplantation Follow-Up
After transplantation, most patients are discharged from the transplantation center and referred back to their primary oncologists or primary care providers. This arrangement makes it difficult for study teams to ensure complete long-term follow-up. Very often, post-HCT complications have a long latency (Table 1). Incomplete follow-up at the transplantation center can bias estimated frequencies of late effects, depending on the reasons for loss to follow-up (discharged/lost to follow-up because they live far away from the center, loss of health insurance/job, or inability to afford follow-up care, or good health precluding perceived need to be followed by the transplantation center).
Table 1.
Frequent Complications after HCT: Onset, Latency, and Course
| Post-HCT Complication | Earliest Onset | Median Latency | Plateau? |
|---|---|---|---|
| Gonadal failure | <1 Yr | 0–6 Mo | Yes |
| Infertility | <1 Yr | 0–6 Mo | Yes |
| Cardiac dysfunction | <1 Yr | 3–4 Yr | No |
| Coronary artery disease | 3–4 Yr | 10–15 Yr | No |
| Pulmonary dysfunction | <1 Yr | 0–12 Mo | No |
| Therapy-related leukemia | <6 Mo | 3–4 Yr | Yes - 15 Yr |
| Solid malignancies | 2–3 Yr | 10–15 Yr | No |
| Endocrine complications | <1 Yr | 2–3 Yr | No |
Technological solutions to address these problems of incomplete follow-up and data reporting burden are urgently needed. These should aim to reduce duplicate data entry and facilitate data transfer between databases. The electronic health record (EHR) is ideally positioned to facilitate patient-centered data collection, as data will theoretically continue to be collected through the EHR regardless of the patient’s location years after transplantation. Transplantation professionals and clinical informaticians should engage with existing vendors to build transplantation-specific data collection modules that will standardize the timing and data variables that are important for late effects research. Such systems must be designed to use standardized terminology, such as those developed by the National Cancer Institutes (NCI) common data elements initiative (https://wiki.nci.nih.gov/display/caDSR/CTEP+Common+Data+Elements#CTEPCommonDataElements-OverviewoftheCDEProject). In an ideal state, data entered once within the EHR would be transferred directly to research organizations without requiring duplicate entry by data professionals, while remaining compliant with consent requirements and protections for privacy and confidentiality.
Assessment of Health Status and Health-Related Complications
Ideally, a comprehensive health assessment of HCT survivors (in a clinic setting) would optimize accurate characterization of long-term survivor health. Although some institutions follow American Society for Blood and Marrow Transplantation guidelines [10] and conduct a clinical assessment of their survivors (albeit for varying lengths of time), others are unable to do so for a variety of reasons (insurance, distance, resources, etc.). In fact, the resources needed for comprehensive clinical assessment in large, geographically dispersed cohorts are often prohibitive. As a more practical approach, large cohort studies (for example, Women’s Health Initiative, Nurses’ Health Study, and Childhood Cancer Survivor Study [CCSS]) have relied upon self report of health outcomes.
The CCSS and the Bone Marrow Transplant Survivor Study (BMTSS) include survey questions regarding diagnosis by a health care provider of physical health conditions (endocrinopathies, central nervous system compromise, cardiopulmonary dysfunction, gastrointestinal and hepatic sequelae, musculoskeletal complications, and subsequent neoplasms) with age at diagnosis. These studies have shown that HCT survivors can report major health complications with acceptable levels of accuracy [11]. In studies focusing on late effects, strong consideration should be given to the inclusion of patient-centered outcomes (symptoms, functional status, financial toxicity, behavioral and lifestyle factors). These outcomes can be measured with patient-reported outcomes (PRO) or with a performance-based measure (eg, 6-minute walk) or sensor actigraphy. Although these methods allow assessment and capture of outcomes that are clinically overt (eg, fracture), they represent an underestimation of clinically asymptomatic disease (such as osteoporosis) and this limitation needs to be acknowledged when conducting these large studies.
Other modes of data collection can be very helpful to complement and augment clinician-reported outcomes and PRO. Comparatively few reports use performance-based measures. Additional methodologic work is needed to evaluate validity and responsiveness in HCT survivors. Explicit standards for the collection, analysis, and interpretation of patient-centered outcomes have been articulated by several professional groups and provide important guidance in the use of these endpoints.
The inclusion of health status assessments gathered using self-reported, performance-based measures or sensors can be optimized by procuring informed consent before HCT, covering the following elements: (1) consent to abstract relevant clinical information from the medical records at the transplantation center; (2) consent to contact other health care providers or facilities following the patient for relevant clinical information; (3) consent to contact patients in the future for new research initiatives; and (4) consent to bank biospecimens (in compliance with the latest National Institutes of Health guidelines for biospecimen research). Often, the patients are asked for the preferred option to contact and offered the ability to be contacted by mail, phone, email, or social media. Contact information of the patient and at least 1 other relative or friend should be obtained to minimize loss to follow-up. For patients younger than 18 years of age at HCT, reconsenting is required when they reach the age of majority.
Another approach is obtaining consent from the patient for direct contact through a centralized body (for example, CIBMTR), which could allow data to flow from the patient to their CIBMTR transplantation record and then back to the transplantation center. This model may be particularly of interest to smaller transplantation centers or those lacking the infrastructure to collect long-term data themselves. The EHR could be used as direct patient-contact portals for data exchange. For example, the EHR could be used to send reminders directly to patients to schedule specific follow-up tests at certain time points, and they could enable patients to report the results or other data (eg, clinical, medication or PROs) that could then be linked to their transplantation record. The collection of behavioral and lifestyle factors (eg, current use of tobacco, alcohol consumption, physical activity, diet quality, stress, and depression) can also leverage data elements that are being gathered increasingly as part of general health histories using electronic portals that link to the EHR. The EHR could also be used to communicate survivor-ship care plans directly to the patient and other providers. Such a centralized data collection model may also be possible outside of the EHR (in countries where the EHR is not yet prevalent, for example) using other free online data entry portals such as REDCap (https://redcap.vanderbilt.edu/) or patientslikeme (https://www.patientslikeme.com/).
TRENDS IN TRANSPLANTATION STRATEGIES DURING THE PAST FOUR DECADES
Numerous studies have shown associations between patient demographic, disease, and transplantation characteristics and the risk of specific long-term complications after HCT. These characteristics have changed over the past 4 decades; thus, an awareness of the specific nature of these changes is critical to understanding trends in long-term morbidity over time.
We used CIBMTR data to examine international trends in transplantation strategies over the past 4 decades. CIBMTR has been collecting HCT outcomes data worldwide for more than 40 years, resulting in a research database with information on more than 425,000 patients. Table 2 shows select data for registered recipients of first allogeneic or autologous transplantation for any disease during each of the 4 decades between 1980 and 2014 (first decade: 1980 to 1989; second decade: 1990 to 1999; third decade: 2000 to 2009; fourth decade: 2010 to 2014).
Table 2.
International Trends in HCT Strategies during the Past Four Decades
| 1980–1989 | 1990–1999 | 2000–2009 | 2010–2014 | |
|---|---|---|---|---|
| Autologous HCT | ||||
| No. of patients | 1551 | 57423 | 71999 | 49697 |
| Age at transplantation, median (range), yr | 34 (<1–66) | 45 (<1–79) | 53 (<1–86) | 58 (<1–84) |
| Allogeneic HCT | ||||
| No. of patients | 14138 | 57829 | 80019 | 44223 |
| Age at transplantation, median (range), yr | 24 (<1–72) | 31 (<1–79) | 37 (<1–83) | 47 (<1–84) |
| Primary diagnosis by stem cell source (age ≥18 at HCT) | ||||
| Autologous HCT | ||||
| No. of patients | 1324 | 53250 | 66620 | 46846 |
| Primary diagnosis | ||||
| AML | 190 (14) | 2873 (5) | 2986 (4) | 466 (<1) |
| ALL | 46 (3) | 546 (1) | 309 (<1) | 49 (<1) |
| NHL | 366 (28) | 14262 (27) | 21534 (32) | 13702 (29) |
| HL | 333 (25) | 5345 (10) | 8370 (13) | 4424 (9) |
| MM | 29 (2) | 5698 (11) | 29137 (44) | 26986 (58) |
| Other | 360 (27%) | 24526 (46%) | 4284 (6%) | 1219 (2.6%) |
| Stem cell source | ||||
| Bone marrow | 1103 (83) | 10432 (20) | 919 (1) | 107 (<1) |
| Peripheral blood | 221 (17) | 42816 (80) | 65698 (99) | 46681 |
| Umbilical cord | 0 | 2 (<1) | 3 (<1) | 58 (<1) |
| Allogeneic HLA identical sibling HCT | ||||
| No. of patients | 7683 | 28364 | 30864 | 12850 |
| Primary diagnosis | ||||
| AML | 2326 (30) | 7756 (27) | 10319 (33) | 5127 (40) |
| ALL | 1332 (17) | 3325 (12) | 3846 (12) | 2036 (16) |
| CML | 2382 (31) | 8262 (29) | 3971 (13) | 590 (5) |
| MDS | 354 (5) | 2238 (8) | 3431 (11) | 2136 (17) |
| NHL | 262 (3) | 2381 (8) | 3893 (13) | 1244 (10) |
| SAA | 701 (9) | 1489 (5) | 1630 (5) | 670 (5) |
| Other | 326 (4%) | 2913 (10%) | 3774 (12%) | 1047 (8%) |
| Stem cell source | ||||
| Bone marrow | 7682 (99) | 22143 (78) | 6915 (22) | 1737 (14) |
| Peripheral blood | 1 (<1) | 6221 (22) | 23949 (78) | 11113 (86) |
| Allogeneic other related HCT | ||||
| No. of patients | 882 | 2844 | 3787 | 2617 |
| Primary diagnosis | ||||
| AML | 259 (29) | 781 (27) | 1362 (36) | 1115 (43) |
| ALL | 151 (17) | 371 (13) | 504 (13) | 328 (13) |
| CML | 277 (31) | 839 (30) | 325 (9) | 113 (4) |
| MDS | 39 (4) | 227 (8) | 443 (12) | 383 (15) |
| NHL | 37 (4) | 251 (9) | 518 (14) | 289 (11) |
| SAA | 74 (8) | 77 (3) | 128 (3) | 92 (4) |
| Other | 45 (5) | 298 (10) | 507 (13) | 297 (11) |
| Stem cell source | ||||
| Bone marrow | 882 (100) | 2082 (73) | 796 (21) | 842 (32) |
| Peripheral blood | 0 | 762 (27) | 2991 (79) | 1775 (68) |
| Allogeneic unrelated HCT | ||||
| No. of patients | 402 | 9162 | 22339 | 17582 |
| Primary diagnosis | ||||
| AML | 66 (16) | 1998 (22) | 8529 (38) | 7653 (44) |
| ALL | 38 (9) | 1243 (14) | 3320 (15) | 2340 (13) |
| CML | 244 (61) | 4143 (45) | 2515 (11) | 705 (4) |
| MDS | 20 (5) | 852 (9) | 3361 (15) | 3433 (20) |
| NHL | 1 (<1) | 315 (3) | 1965 (9) | 1430 (8) |
| Stem cell source | ||||
| Bone marrow | 402 | 8866 (97) | 7780 (35) | 2709 (15) |
| Peripheral blood | 0 | 296 (3) | 14559 (65) | 14873 (85) |
| Cord blood (age ≥18 at HCT) | ||||
| No. of patients | N/A | 233 | 2075 | 2282 |
| Primary diagnosis | ||||
| AML | 65 (28) | 853 (41) | 1117 (49) | |
| ALL | 42 (18) | 398 (19) | 434 (19) | |
| CML | 75 (32) | 154 (7) | 103 (5) | |
| MDS | 15 (6) | 216 (10) | 263 (12) | |
| NHL | 12 (5) | 207 (10) | 174 (8) | |
| Primary diagnosis by stem cell source (age <18 at HCT) | ||||
| Autologous HCT | ||||
| No. of patients | 227 | 4173 | 5379 | 2851 |
| Primary diagnosis | ||||
| AML | 56 (25) | 589 (14) | 224 (4) | 29 (1) |
| ALL | 25 (11) | 280 (7) | 51 (<1) | 0 |
| NHL | 38 (17) | 353 (8) | 328 (6) | 114 (4) |
| HL | 21 (9) | 386 (9) | 648 (12) | 342 (12) |
| Other malignancies | 83 (37) | 2450 (59) | 4045 (75) | 2316 (81) |
| Stem cell source | ||||
| Bone marrow | 216 (95) | 2057 (49) | 482 (9) | 76 (3) |
| Peripheral blood | 11 (5) | 2115 (51) | 4887 (91) | 2770 (97) |
| Umbilical cord | 0 | 1 (<1) | 10 (<1) | 5 (<1) |
| Allogeneic HLA-identical sibling donor | ||||
| No. of patients | 3653 | 8671 | 7877 | 2943 |
| Primary diagnosis | ||||
| AML | 739 (20) | 1708 (20) | 1580 (20) | 414 (14) |
| ALL | 1207 (33) | 2456 (28) | 1880 (24) | 621 (21) |
| SAA | 570 (16) | 1134 (13) | 1142 (14) | 405 (14) |
| Other | 1137 (31%) | 3373 (39%) | 3275 (42%) | 1503 (51%) |
| Stem cell source | ||||
| Bone marrow | 3653 | 8105 (93) | 5817 (74) | 2469 (84) |
| Peripheral blood | 0 | 566 (7) | 2060 (26) | 474 (16) |
| Allogeneic other related donor | ||||
| No. of patients | 987 | 2110 | 1881 | 812 |
| Primary diagnosis | ||||
| AML | 133 (13) | 340 (16) | 349 (19) | 126 (16) |
| ALL | 262 (27) | 603 (29) | 451 (24) | 144 (18) |
| Other | ||||
| Stem cell source | ||||
| Bone marrow | 987 | 1718 (81) | 921 (49) | 499 (61) |
| Peripheral blood | 0 | 392 (19) | 960 (51) | 313 (39) |
| Allogeneic unrelated donor | ||||
| No. of patients | 187 | 4207 | 5685 | 3029 |
| Primary diagnosis | ||||
| AML | 17 (9) | 650 (15) | 1104 (19) | 571 (19) |
| ALL | 53 (28) | 1561 (37) | 1794 (32) | 678 (22) |
| SAA | 24 (13) | 298 (7) | 425 (7) | 304 (10) |
| Other | 93 (50) | 1698 (40) | 2362 (42) | 1476 (49) |
| Stem cell source | ||||
| Bone marrow | 187 | 4111 (98) | 4128 (73) | 2228 (74) |
| Peripheral blood | 0 | 96 (2) | 1557 (27) | 801 (26) |
| Cord blood | ||||
| No. of patients | 2 | 917 | 4288 | 2105 |
| Primary diagnosis | ||||
| AML | 0 | 174 (19) | 845 (20) | 406 (19) |
| ALL | 0 | 274 (30) | 1204 (28) | 514 (24) |
| Other | 2 (100) | 469 (51) | 2239 (52) | 1185 (56) |
AML indicates acute myeloid leukemia; ALL, acute lymphoid leukemia, NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; MM, multiple myeloma; CML, chronic myeloid leukemia; MDS, myelodysplastic leukemia; SAA, severe aplastic anemia.
Patient Characteristics
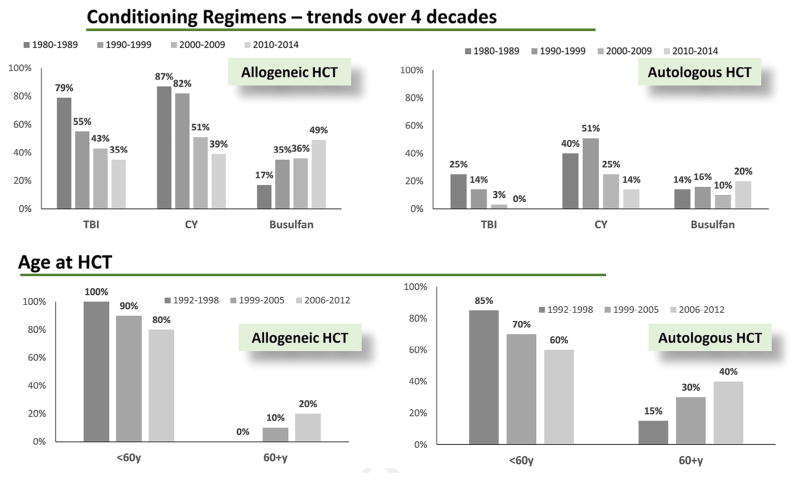
The median age at HCT has increased steadily during the 4 decades for both autologous HCT (34 years to 58 years) and allogeneic HCT (24 years to 47 years) (Figure 2). Furthermore, the upper age at HCT has also increased for autologous HCT (66 years to 84 years) and allogeneic HCT (72 years to 84 years).
Figure 2.
International trends in conditioning regimens and age.
Disease and Transplantation Characteristics
Conditioning regimens for allogeneic HCT have changed significantly during the past 4 decades (Figure 2). In the earliest decades, pretransplantation conditioning was always given with myeloablative intent. Since the 1990s, the intensity of conditioning regimens has decreased. Reduced-intensity conditioning regimens were used for 26% of patients in the 2000s and for approximately 40% of transplantations since 2010. High-dose total body irradiation remains a part of the conditioning regimen in >50% of children treated for malignant diseases, but the use of total body irradiation in adults has decreased to <50% in both the myeloablative and reduced-intensity conditioning setting [12].
Myeloma has become the most common indication for autologous HCT in adults, accounting for >50% of all autologous transplantations since 2010, compared with 11% in the 1990s [9,13]. In the 1980s, nearly one-third of the pediatric autologous transplantations were performed for treatment of hematological malignancies such as acute leukemia [14]. Nearly all pediatric autologous transplantations are now performed for treatment of nonhematological malignancies.
Allogeneic HCT has been performed predominantly for acute leukemia in patients of all ages. In adults, chronic myeloid leukemia was the most frequent indication for al-logeneic HCT in the 1980s [15]. This indication now represents <5% of cases, whereas myelodysplastic syndromes are now the most common indications for allogeneic HCT. In children, nonmalignant disease indications, such as severe aplastic anemia, have remained a focus for allogeneic HCT across all 4 decades.
Donor and Stem Cell Source
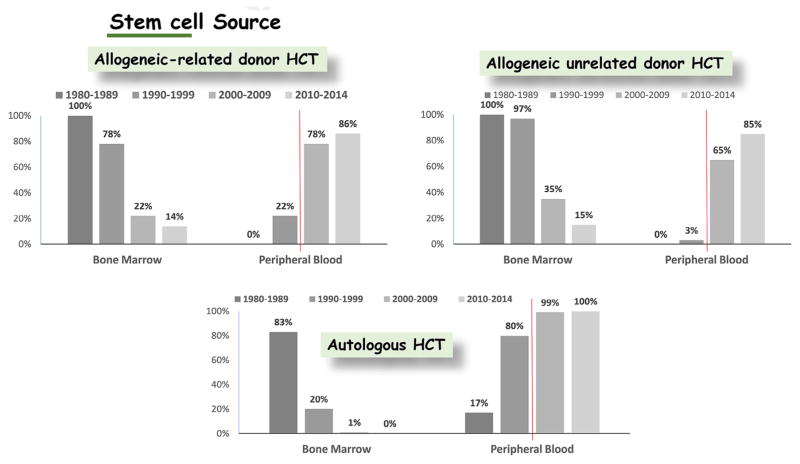
[16–20] HLA-identical siblings were the predominant source of stem cells in the early years of HCT, but grafts now come more frequently from unrelated than from related donors. HLA-haploidentical family donors were used with variable success before 2000. Since then, improvements with this approach have been dramatic. Successful HLA-haploidentical transplantation protocols have used innovative strategies to obtain large numbers of donor cells, together with in vitro or in vivo depletion of T cells or post-transplantation cyclophosphamide to decrease the risks of graft rejection and severe GVHD. The use of umbilical cord blood as a stem cell source was introduced in the 1980s. In the early 2000s, cord blood transplantation became widely accepted for both adults and children.
In the 1980s, aspirated marrow was used as the source of stem cells for nearly all allogeneic and autologous transplantation recipients. Transplantation of mobilized peripheral blood stem cells (PBSCs) was introduced in the 1990s. Mobilized PBSCs now represent the stem cell source of choice for autologous HCT for both adults and children. Likewise, mobilized PBSCs now represent the predominant stem cell source used for allogeneic HCT in adults (Figure 3). The use of marrow has predominated for pediatric HCT from the beginning to the present. Medications used for mobilization have changed over time as well. Granulocyte colony–stimulating factor has been used since the 1990s. Survival rates are comparable after HCT with granulocyte colony–stimulating factor–mobilized blood cells versus bone marrow, but the risk of chronic GVHD after allogeneic HCT is increased among those receiving mobilized blood cells [21]. Within the past 10 years, plerixafor has also been used to mobilize cells for autologous HCT. The long-term effects of using plerixafor for mobilization have not yet been assessed [22].
Figure 3.
International trends in stem cell source.
Characteristics of HCT Survivors
We used data from BMTSS to summarize the demographic and clinical characteristics of individuals who received HCT at 2 large transplantation centers (City of Hope and University of Minnesota) and survived 2 or more years (Table 3). These characteristics reflect the changes in transplantation strategies over the past 4 decades.
Table 3.
Demographic and Clinical Characteristics of Two-Year Survivors Who Underwent Transplantation between 1974 and 2010 in the United States, Regardless of Current Vital Status
| Table | BMTSS-2 n = 11,465 |
|---|---|
| Type of HCT | |
| Related | 3240 (28%) |
| Unrelated | 1573 (14%) |
| Autologous | 6652 (58%) |
| Age at HCT | |
| <18 Yr | 1161 (10%) |
| 18 to 45 Yr | 5116 (45%) |
| 46 to 60 Yr | 3469 (30%) |
| >60 Yr | 1719 (15%) |
| Race/ethnicity | |
| Whites | 9276 (81%) |
| Hispanics | 1215 (11%) |
| Blacks | 512 (5%) |
| Asians | 462 (4%) |
| Diagnosis | |
| AML/ALL | 4149 (36%) |
| CML | 1272 (11%) |
| NHL/HL | 4295 (38%) |
| Other | 1749 (15%) |
| Intensity of conditioning | |
| RIC | 1011 (9%) |
| Stem cell source | |
| Marrow | 3498 (31%) |
| PBSC | 7485 (65%) |
| Cord | 482 (4%) |
| Vital status | |
| Deceased | 3673 (32%) |
| Length of follow-up | |
| >10 Yr | 4190 (37%) |
RIC indicates reduced-intensity conditioning.
INFRASTRUCTURE TO UNDERSTAND THE MOLECULAR UNDERPINNINGS OF POST-TRANSPLANTATION MORBIDITY
Studies using biologic specimens represent a mainstay for understanding the pathogenesis of post-transplantation complications. The utility of such biospecimens for hypothesis-driven studies is greatly enhanced by the availability of well-annotated data summarizing the clinical history of study subjects. Although no existing biorepositories have been historically designed to collect biological specimens specifically for research related to post-HCT late complications, several currently available resources could possibly be utilized. Table 4 summarizes the strengths and limitations of these existing sources of biospecimens.
Table 4.
Current Sources of Biospecimens for Studies of Late Effects after HCT: Sample Types, Strengths and Weaknesses
| Biorepository | Sample Types | Strengths | Weaknesses |
|---|---|---|---|
| CIBMTR |
|
|
|
| BMT CTN 1202 |
|
|
|
| BMT CTN trials |
|
|
|
| CALGB/ACTION, SWOG, ECOG, COG, other cooperative group trials |
|
|
|
| Individual transplantation centers |
|
|
|
BMT indicates; CIBMTR, ; BMT, ; CTN, ; CALGB/ACTION, ; SWOG ECOG, ; COG,
Several considerations should be applied for prospective collection of biospecimens to study late effects after HCT. First, the nature of biospecimens and the timing of collection should be driven by clearly stated questions that address specific hypotheses and the specific assays needed to address the questions. For example, a DNA sample collected from the patient before HCT can be used to investigate how variation in the patient’s germline might affect the patient’s ability to handle toxicity stemming from therapies received before HCT and those related to the conditioning regimen. On the other hand, post-HCT DNA can be used to understand how chimeric donor-derived cells affect the risks of late complications after HCT. Distinctions should be made for predictive hypotheses requiring samples from before HCT or early after HCT to predict future events versus pathogenic hypotheses or diagnostic evaluations requiring samples from the time of onset of the late effect. Hypothesis-driven sample collection permits the biorepository and the end user to develop the optimal technical protocols to ensure that the specimens will meet the specific needs of the research. This hypothesis-driven approach has several limitations: specimens will take time to accrue, previously collected specimens might not be optimal for new research questions, and specimens originally collected for other specific studies might be limited in their scope and utility to the original hypothesis.
Another consideration for the collection of biospecimens to study HCT late effects is the feasibility of prospective collection of specimens from every patient at serial prespecified time points before and after autologous or allogeneic HCT. This would serve as an ambitious and expensive undertaking, requiring significant coordination with the treating physician and patient, and would be limited by the attrition of those who die at the intermediate time points. However, this approach in a subset of patients could enable an understanding of the intermediate events (eg, subclinical cardiotoxicity) before development of the overt complication (eg, heart failure).
A third consideration pertains to the need for collaborative multidisciplinary efforts to conduct research in late effects after transplantation. Coordinated efforts across laboratories and research teams to capture data and retain the history of testing for each specimen will enrich and enhance the entire research effort. A centralized database with details regarding the specific assays performed on each specimen would prevent duplication of effort and could expedite discovery. The resource requirements to assemble and maintain such a database, as well as an effective data governance framework, are required to ensure the availability, usability, integrity, and security of data.
A fourth consideration is the availability of detailed clinical annotation for patients with and without late effects. The use of existing biorepositories is an efficient and practical methodological approach, but the clinical annotation may not exist or be sufficiently detailed, thus requiring additional data collection efforts. Development of a comprehensive infrastructure consisting of clinically annotated biospecimens and accompanying bioinformatic support to address a multitude of hypotheses in the future, while keeping pace with technological advances is a critical initiative necessary for the study of HCT late effects. It is here that PROs could play a role in efficiently and validly capturing specific aspects of the phenotype (eg, comorbid conditions, depression, social support, socioeconomic status, body composition, and health behaviors [tobacco use, alcohol consumption, diet quality, sun exposure, and physical activity]) that are known to mediate or moderate the relationship between exposures and late complications of HCT. Consensus within the research community will be needed to determine the essential constructs required for this deep characterization, and to define a core measure set to capture these data. Resources will also be needed for electronic data capture and follow-up to ensure data completeness.
Lastly, the technical requirements and best practices for biorepositories will always be a moving target, as technological platforms become more sophisticated. Publicly available resources, such as the NCI Biospecimen Research Database [23], NCI Recommended Best Practices for Biospecimen Resources [24], and NCI Biospecimen Research Network [25], can provide peer-reviewed expert guidance on these considerations.
MAJOR STUDY DESIGNS AND ANALYTIC APPROACHES
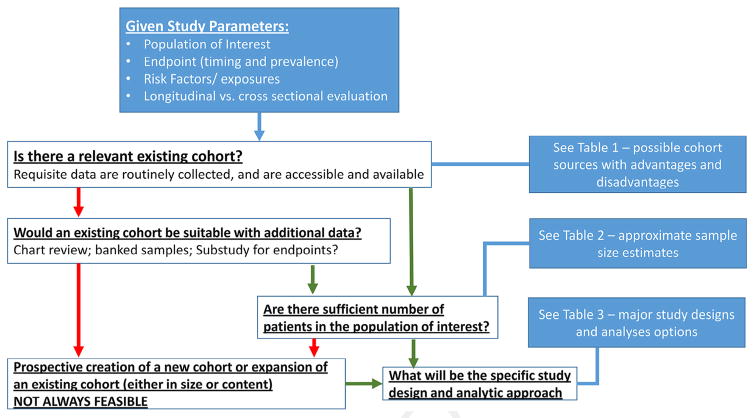
In this section, we describe methodological approaches used to conduct HCT survivorship research. Fundamentally, the study designs and analytic approaches that are relevant to research on late effects after HCT are not different from those applicable to any other medical condition. The primary factors affecting the design and analytic approach are defined by the particular study question and the data that are available to address it. In Table 5 we provide a summary of the strengths and limitations of the various study designs. Finally, we provide an integrated schematic overview in Figure 4, describing some of the key steps and decision points involved in planning and designing a study focused on long-term complications after transplantation. These points are elaborated below.
Table 5.
Designs Used for Studies of Long-Term Morbidity after HCT: Definitions, Strengths and Limitations
| Study Design | Definition | Strengths | Limitations |
|---|---|---|---|
| Cross-sectional | Examines relationship between exposure and outcome prevalence in a defined population at a single point in time |
|
|
| Case-control | Examines multiple exposures in relation to an outcome; subjects are defined as cases and controls, and exposure histories are compared |
|
|
| Prospective, longitudinal, cohort studies |
|
|
|
| Retrospective Cohort Studies |
|
|
|
| Nested case-control studies |
|
|
|
Figure 4.
Integrated schematic overview of design of a study focusing on long-term complications after HCT.
1. What are the study parameters as defined by the study question?
For example, what is the population of interest—is it defined by type of transplantation, by disease, or by outcome? What is the endpoint of interest; when does it occur (ie, latency); how frequently does it occur; is it a single irreversible event (eg, renal failure, subsequent malignant neoplasm, stroke, osteonecrosis, cataracts, etc.) or is it reversible and recurrent (eg, infection)? Is it a clinical endpoint derived from specific diagnostic or laboratory studies, a clinician-reported outcome (eg, chronic GVHD grading), or a PRO? What are the risk factors or exposures that are of interest; when do they occur; and how frequently do they occur? Does the research question require longitudinal data, derived at different points in time, or does it require cross-sectional data, from a single point in time. Is it a time-varying variable (eg, tobacco use, or comorbid conditions) or is it a fixed variables (ie, age at HCT, transplantation type etc.)?
2. Does a relevant transplantation cohort exist, with the requisite data routinely collected, available and accessible?
This could be a single-institution cohort, a multi-institutional cohort, a registry cohort (eg, CIBMTR), or a clinical trial cohort. Table 6 summarizes the strengths and limitations of the different types of cohorts. A major limitation of many existing cohorts is a lack of data pertaining to non-GVHD late effects or lack of data pertaining to pre-HCT exposures and risk factors. Some institutional cohorts may have excellent follow-up covering particular late effects of long-standing interest but no data on other late effects. Registries may collect data on some pre-HCT risk factors in the immediate pre-HCT period (eg, the HCT comorbidity index), but detailed summaries of prior exposures are typically not collected. Cohorts assembled from 1 or more clinical trials may provide some of the data lacking in other traditional cohorts, although the strengths and limitations will reflect the entity sponsoring the trial (single institution versus cooperative group) and the goals of the study with respect to late effects, which may be merely incidental to the main purpose or may, in fact, help to define the trial. For the most part, clinical trials are not geared to collect morbidities developing 10 or more years after HCT in a comprehensive fashion.
Table 6.
Relative Strengths and Limitations in Studies of Late Effects after HCT, According to Cohort Type
| Strength or Limitation | Single Institution | Multi-Institution Consortium | Registry (eg, CIBMTR) |
|---|---|---|---|
| Patient numbers | + | +++ | +++++ |
| Rare outcomes | + | ++ | +++++ |
| Outcomes with long latency | +++ | +++ | +++ |
| Control/matching | +++ | +++ | +++ |
| Center bias | +++++ | +++ | + |
| Multiple risk factors | +++ | +++ | +++++ |
| Long term follow up (intensive) | +++++ | ++++ | +++ |
| Pre-HCT exposures | +++++ | ++++ | ++ |
| Consistency in data points over time | +++++ | +++ | ++ |
| Associated biospecimens | +++++ | ++++ | ++ |
| Expense | ++ | ++++ | +++++ |
Although CIBMTR now captures data on every allogeneic transplantation performed in the United States, this practice was not previously the case. The collection of data items has changed over the years. For example, the HCT comorbidity index was added to the report forms in 2007. In addition, financial constraints allow for specific data related to late effects and their risk factors to be collected only in a minority of patients (those on the comprehensive research form track). The intermittent nature of the data is such that temporal associations of events cannot always be gauged, and detail of changes over time are lacking.
3. If existing cohorts are deficient as they stand, can they be adapted with reasonable effort to provide the requisite data?
For example, if pretransplantation characteristics of interest have not been systematically collected, could they be reliably acquired through retrospective chart review? Are banked samples available, even if they were originally collected for a different purpose? Could a special sub-study be implemented to ascertain the cross-sectional prevalence of the late effect of interest?
4. If an existing or modifiable cohort is available, are sufficient numbers of patients available in the population of interest to perform a study with reasonable power and precision?
The answer to this question will depend on many factors, including the study design and analytic methods, as well as the usual parameters of effect size, variability, type I and type II error rates, among other considerations. Table 7 provides estimates of sample size for a common scenario evaluating the impact of a risk factor on the incidence or prevalence of a late effect. The range of sample sizes required for standard specifications of power and type I error rates varies by more than an order of magnitude. Sample size is particularly sensitive to effect size (the difference in risk or prevalence) and it is important to consider effects that are both plausible and clinically relevant. Other important considerations for planning sample size include the number of factors under study—ie, is it a study of the association between a single risk factor and a single late effect, or an “omics” study of a large number of single nucleotide polymorphisms or other markers? In the latter case, one must consider issues of multiple comparisons, as well as an overall strategy for discovery and replication. Even in the former case, accounting for complex phenotypes or exposures in the presence of patient heterogeneity may require many more patients, if only to permit stratification and subset analysis. These issues emphasize the need for statistical expertise when planning the size and power for a study.
Table 7.
Sample Size Estimates*
| Incidence /Prevalence of Late Effect | Risk Difference | Proportion with Risk Factor
|
||||
|---|---|---|---|---|---|---|
| 10% | 20% | 30% | 40% | 50% | ||
| .05 | .05 | 1980 | 1070 | 780 | 650 | 600 |
| .05 | .10 | 560 | 300 | 210 | 170 | — |
| .10 | .10 | 920 | 500 | 370 | 310 | 280 |
| .10 | .20 | 250 | 140 | 100 | 80 | — |
| .20 | .10 | 1500 | 830 | 620 | 540 | 500 |
| .20 | .20 | 390 | 220 | 160 | 130 | 120 |
| .30 | .10 | 1900 | 1060 | 800 | 690 | 660 |
| .30 | .20 | 480 | 270 | 200 | 170 | 160 |
| *Sample size required for 80% power at a 2-sided .05 level of significance to detect specified difference in risk | ||||||
These estimates represent the best case scenario where only a single late effect/risk factor is being assessed.
5. How can appropriate controls be selected?
Assessment of the magnitude of risk of an adverse event in any population necessitates inclusion of a reference population or a control group. Selection of an appropriate control group is dependent on the hypothesis which is being tested. The selected control population should be as similar as possible to the experimental group, so that the outcome difference between the two groups can be attributed to the exposure of interest. However, there are inherent problems in obtaining a valid concurrent control group for patients undergoing HCT. Ideally, a control group for HCT patients should consist of cancer patients identical in all respects (demographics, clinical characteristics) but randomized to conventional chemotherapy without HCT. However, such a situation occurs rarely in the setting of randomized clinical trials—where the limited sample size precludes assessment of rare late effects. A real-life control group consisting of patients who have cancer but are not undergoing HCT (ie, a cancer control group) will generally include patients with more favorable stages of disease, and with lower cumulative exposures to chemotherapy and radiation.
An alternative (or concurrent) approach would be to obtain a healthy control group matched for age at study participation and gender. Recruitment of a representative control population can be challenging. Typically, controls are selected from 1 of the following sources: general population, spouse, friend(s), or sibling(s) of the experimental group. Control populations can also be obtained from large nontransplantation registries such as Surveillance, Epidemiology and End Results (SEER) and the SEER linkage databases and payer databases, such as Anthem, GroupHealth, and Kaiser. These sources serves as a good resource for providing expected age and sex-specific rates. Table 8 summarizes the advantages and limitations associated with the use of each of these groups. Siblings provide the following: (1) the ability to make direct comparisons with survivors, (2) data on outcomes in general population that are not available from other sources (eg, vital statistics, NHIS, etc.), and (3) an additional comparison group to determine consistency of findings between data sources (ie, SEER, NHIS). Siblings in other survivor studies (such as CCSS and BMTSS) have proven to be an effective comparison group, associated with high participation rates, ease of access, and general uniformity of socioeconomic status and level of health awareness. We recognize that siblings may not be representative of an unaffected population for psychosocial distress and quality of life, and we do not recommend the use of siblings for these comparisons.
Table 8.
Sources of Control Populations—Advantages and Limitations
|
|
Healthy Controls
|
Cancer Controls
|
||||
|---|---|---|---|---|---|---|
| Consideration | General Population | Spouse | Friends | Siblings | Non-HCT Controls | HCT Controls |
| Age and gender | Age, gender are frequency- matched to case pool | Age might be comparable; opposite gender | Age, gender frequency- matched to case pool | Age and gender frequency- match may or may not possible | Age, gender are frequency- matched to case pool | Age, gender are frequency matched to case pool |
| Availability | Random digit dialing; linkage with administrative databases, registries | Dependent on availability of a spouse | Dependent on availability of friend(s) | Dependent on availability of sibling(s) | Dependent on size of patient population | Dependent on size of patient population |
| Willingness | Participation rates very low; difficult to recruit/retain for serial tests; less motivated; participation bias; significant costs | Motivated to participate | Better than general control; highly motivated to participate. | If geographically distant, on- site testing could be challenging | Participation rates generally similar to those of HCT cases | Participation rates generally similar to the case population |
| Socio-economic status | May not be comparable to case | Similar | Similar | Similar | Similar | Similar |
| Genetic factors | Different | Different | Different | Similar profile | Different | Different |
| Overmatching? | No | Living environment; stress | Probably not | Genetic/early environmental | Probably not | Dependent on study question |
| Pool | Dependent on participation rate | One per case | Dependent on no. of friends | Dependent on family size | Dependent on patient pool at hospital | Dependent on patient pool at hospital |
6. What is the specific study design and analytic approach?
Study design and analytic methods can affect the choice of study population and sample size. Table 9 briefly summarizes some typical analytic approaches for common types of research questions. The list is by no means comprehensive, nor does it mandate that a particular analytic method be used for a particular type of study. Choosing the study design and analysis plan should involve input from statistical and epidemiological collaborators.
Table 9.
Major Types of Designs and Analytic Approaches in Studies of Late Effects after HCT
Prospective and retrospective cohort study—Risk factors for incidence of late effects
|
| Examples |
| Rizzo et al. Solid cancers after allogeneic hematopoietic cell transplantation—evaluated risk factors for solid cancers in a multi-institutional cohort of allogeneic HCT recipients [8]. |
| Sun et al. Chronic health conditions after hematopoietic cell transplantation—examined the magnitude of risk of chronic health conditions in a cohort of 2 + year survivors of HCT.26 |
Cross-sectional study—Risk factors for prevalent late effects
|
| Example |
| Bhatia et al. Bone mineral density in patients undergoing bone marrow transplantation for myeloid malignancies—bone mineral density measured at a single time point in patients who had undergone HCT to determine the prevalence of osteopenia/osteoporosis.27 |
Prospective longitudinal study—Risk factors for late effects
|
| Examples |
| Syrjala et al. Prospective neurocognitive function over 5 years after allogeneic hematopoietic cell transplantation for cancer survivors compared with matched controls at 5 years. This study prospectively examined the trajectory and extent of long-term cognitive dysfunction, with a focus on 1 to 5 years after treatment.28 |
| Wong et al. Long-term recovery after hematopoietic cell transplantation: predictors of quality of life concerns—This prospective longitudinal study examined the QOL after HCT and identified risk factors for poor QOL.29 |
Case-control sampling
|
| Example |
| Chakraborty et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. A prospective longitudinal study formed the sampling frame for a nested case-control study to compare changes in telomere length in serial blood samples from patients who developed t-MDS/AML with matched controls who did not develop this outcome.30 |
GEE indicates; QOL, quality of life; t-MDS.
A few issues are worth noting regarding analysis of late effects in general. By definition, these arise in the surviving members of the transplantation cohort, which in the present context requires survival for at least 1 year. The method of analysis has to be chosen so that the quantities estimated are interpretable relative to the population of interest. For example, risk factors related to incidence of late effects in the transplantation cohort as a whole must take into account censoring and the competing risk of death. These are best handled by using time-to-event cohort methods. On the other hand, if the interest is in risk factors for late effects constrained to a population of survivors at a defined point in time, then case-control methods may be used. The risk estimates from cohort studies and case-control studies are not interpretable interchangeably, particularly when the risk factor is also related to survival.
As noted above, studies of incidence of late effects (or of outcomes subsequent to late effects) may need to account for the risk of competing endpoints. Death is an obligatory competing risk, since it precludes the development of any future endpoint. Recurrence of disease or disease progression, although not usually precluding subsequent late effects, could be treated as a competing risk (if interest in the late effect is focused on patients for whom disease control is not the primary medical issue) or as an exposure of interest, if additional treatment of disease relapse is hypothesized to cause of late effects. Similarly, other late effects themselves might be considered as competing risks. For example, chronic GVHD might be treated as a competing risk for studies of non–GVHD-related infection or lung disease.
More generally, late effects often do not occur in isolation. Patients frequently experience multiple late effects or multiple instances of the same late effect, and it may be of interest to explore the relationship among different late effects. Some late effects could become baseline covariates or time-dependent covariates for the primary effect of interest. Alternatively, the number of late effects, of a single type or of multiple types, may be modeled as a multistate counting process. Cox regression models can be flexibly generalized to handle many of these situations, as can Poisson regression models. These methods focus primarily on the underlying hazard rate for the late effect, but other methods are possible, such as competing risks regression that directly models the incidence of a late effect.
Missing data in late effects studies are as problematic as in any other setting. In some cases, they represent only a minor annoyance; in other cases, they can compromise the ability of the study to draw valid conclusions. The key considerations are the proportion of data that are missing and whether the data are missing at random or are informatively associated with the late effect itself or with covariates of interest. For example, in longitudinal studies of quality-of-life endpoints with repeated assessments, one should carefully consider whether a missing assessment is truly random or reflects some information about the endpoint of interest or other factors. Randomly missing data are far more easily accommodated, perhaps using complete case series (if the amount of missing data is small), linear mixed models, or GEE models. Options may be limited if the missing data is thought to be nonrandom. One can evaluate the endpoint under different patterns of missing data or perhaps employ sensitivity analysis to evaluate the impact of different assumptions about the missing data. A full accounting for nonrandom missing data can only be accomplished through joint modeling of the endpoints of interest and the mechanism producing the missing data. This may be difficult or impossible and will likely rest on untestable assumptions about the reasons for missing data.
The above considerations reinforce the message that studies of late effects of HCT may be potentially difficult to design and analyze. Collaboration with statisticians and epidemiologists with relevant experience and expertise is essential to ensure that such studies are as informative as possible.
CONCLUSIONS
The key to achieving success in this challenging and rapidly growing field is a multidisciplinary approach. Key stakeholders include HCT recipients, healthcare providers, outcomes researchers, registries, molecular epidemiologists, statisticians, clinical informaticians and bioinformaticians, health economists, and policy makers as well as funding agencies. Critical pieces for establishing a long-term infrastructure include a core set of clearly defined validated outcomes, a strategic collection of clinically annotated biospecimens, mechanisms to follow patients for the outcomes long-term, and an ability to capture key exposures. PROs should be a key component of measuring the burden of morbidity in HCT survivors. Findings from these studies should set the stage for identifying patients at highest risk and developing targeted interventions.
To ensure that we are able to perform appropriate studies in the future, we call for funding initiatives for logistical support to improve data capture (short- and long-term) and reduce redundancy, and to improve biospecimen collection and biobanking. An immediate need is for data transfer initiatives to leverage sharing between existing data and samples sources, including registries, clinical trials, biorepositories, and single-center efforts, to perform the outcome analyses now which will inform the questions to be studied in the future.
Insert Box Recommendations.
| General recommendations for establishment of new cohorts or expansion/embellishment of existing cohorts to study late effects after hematopoietic cell transplantation | ||
| Comprehensive and complete follow-up of transplantation recipients | ||
| Capture of pre-HCT therapeutic exposures, conditioning regimens, post-HCT therapeutic and immunosuppressive therapy, extent and severity of chronic GVHD, sociodemographic data, PROs, and health care costs | ||
| Develop a biorepository of biospecimens before HCTs | ||
| Priority for data collection | ||
High priority
|
Examples of outcomes
|
Examples of exposures
|
| Recommendations for data collection | ||
Data collection should include the following data elements (at minimum)
| ||
| Priority for specimen collection | ||
High priority
|
Examples of outcomes Outcomes associated with therapeutic exposures
|
Examples of platforms (currently available)
|
Recommendations for sample collection
| ||
General recommendations for use of existing cohorts/resources
| ||
TBI indicates total body irradiation; SES, socioeconomic status
Acknowledgments
We thanks Drs Daniel Weisdorf, Corey Cutler and Gerard Socie for their insightful and helpful revisions and comments. We thank Heather Millard from CIBMTR for providing the trends data. We acknowledge the efforts of the NIH Late Effects Initiative Steering Committee: Minoo Battiwalla, Shahrukh Hashmi, Navneet Majhail, Steven Pavletic, Bipin Savani and Nonniekaye Shelburne.
Footnotes
Financial disclosure: This initiative was sponsored jointly by the National Heart, Lung and Blood Institute (NHLBI) and the National Cancer Institute (NCI).
Conflict of interest statement: The opinions expressed here are those of the authors and do not represent the official position of the NIH or the United States Government.
References
- 1.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 6.Gurney JG, Ness KK, Rosenthal J, Forman SJ, Bhatia S, Baker KS. Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood: results from the Bone Marrow Transplant Survivor study. Cancer. 2006;106:1402–1408. doi: 10.1002/cncr.21752. [DOI] [PubMed] [Google Scholar]
- 7.Baker KS, Gurney JG, Ness KK, et al. Late effects in survivors of chronic myeloid leukemia treated with hematopoietic cell transplantation: results from the Bone Marrow Transplant Survivor Study. Blood. 2004;104:1898–1906. doi: 10.1182/blood-2004-03-1010. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy PL, Jr, Hahn T, Hassebroek A, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995–2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19:1116–1123. doi: 10.1016/j.bbmt.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- 12.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014;124:344–353. doi: 10.1182/blood-2014-02-514778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa LJ, Zhang MJ, Zhong X, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19:1615–1624. doi: 10.1016/j.bbmt.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51:786–792. doi: 10.1038/bmt.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn T, McCarthy PL, Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31:2437–2449. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelbaum FR. Alternative donor transplantation for adults with acute leukemia. Best Pract Res Clin Haematol. 2014;27:272–277. doi: 10.1016/j.beha.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kekre N, Antin JH. Hematopoietic stem cell transplantation donor sources in the 21st century: choosing the ideal donor when a perfect match does not exist. Blood. 2014;124:334–343. doi: 10.1182/blood-2014-02-514760. [DOI] [PubMed] [Google Scholar]
- 19.Munoz J, Shah N, Rezvani K, et al. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cells Transl Med. 2014;3:1435–1443. doi: 10.5966/sctm.2014-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reisner Y, Aversa F, Martelli MF. Haploidentical hematopoietic stem cell transplantation: state of art. Bone Marrow Transplant. 2015;50(suppl 2):S1–S5. doi: 10.1038/bmt.2015.86. [DOI] [PubMed] [Google Scholar]
- 21.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemoli RM. New strategies for stem cell mobilization. Mediterr J Hematol Infect Dis. 2012;4:e2012066. doi: 10.4084/MJHID.2012.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamilton BK, Law AD, Rybicki L, et al. Prognostic significance of pre-transplant quality of life in allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transplant. 2015;50:1235–1240. doi: 10.1038/bmt.2015.122. [DOI] [PubMed] [Google Scholar]
- 24.Pidala J, Anasetti C, Jim H. Health-related quality of life following haematopoietic cell transplantation: patient education, evaluation and intervention. Br J Haematol. 2010;148:373–385. doi: 10.1111/j.1365-2141.2009.07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute Biospecimen Research Network.