Abstract
This study investigates the potential application of polymeric nanospheres (known as TyroSpheres) as a formulation carrier for topical delivery of cholecalciferol (i.e., Vitamin D3, VD3) with the goal to improve the skin delivery and stability of VD3. High drug loading and binding efficiencies were obtained for VD3 when loaded in TyroSpheres. VD3 was released from TyroSpheres in a sustained manner and was delivered across the stratum corneum, which occurred independent of the initial drug loading. An ex vivo skin distribution study showed that TyroSphere formulations delivered 3–10 μg of active into the epidermis which was significantly higher than that delivered from Transcutol® (the control vehicle). In addition, an in vitro cytotoxicity assay using keratinocytes confirmed that VD3 encapsulation in the nanoparticles did not alter the drug activity. Photodegradation of VD3 followed zero-order kinetics. TyroSpheres were able to protect the active against hydrolysis and photodegradation, significantly enhancing the stability of VD3 in the topical formulation.
Keywords: Vitamin D3, TyroSpheres, nanoparticles, skin permeation, photostability
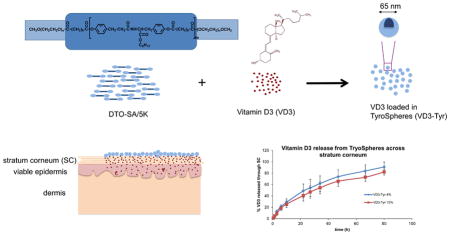
Graphical Abstract
Introduction
Vitamin D3 (VD3) or cholecalciferol is a steroid hormone generated in the skin by UVB radiation of 7-dehydrocholesterol or it is obtained from dietary sources. The active form of cholecalciferol, 1,25-dihydroxycholecalciferol (calcitriol) plays an important role in the regulation of calcium homeostasis and mineralization of bones (Picciano, 2010). VD3 and its analogues have also been associated with other functions in the body. For example, they can influence keratinocyte differentiation and are therefore used in treatment of several skin disorders including psoriasis (Barker et al., 1999). Vitamin D3 and its analogues are also involved in the control of multiple intracellular pathways responsible for the melanin synthesis and melanocyte survival. They can control the activation, proliferation, and migration of melanocytes and therefore induce skin pigmentation, which is potentially useful in treatment of vitiligo (Birlea et al., 2009). Maxacalcitol, one of the active analogues of VD3, has proven to be effective in treatment of comedones (Hayhoe et al., 2010),(Nieves et al., 2010) and there are a few studies reporting antineoplastic activity of calcitriol (Krishnan and Feldman, 2011). The anti-psoriatic properties of VD3 analogues are suggested from their effects in decreasing proliferation and promoting differentiation of keratinocytes, as well as immunomodulatory actions (Nagpal et al., 2005). Even though the exact mechanism behind these effects is not completely understood, it is well-known that VD3 anti-psoriatic effects are partially genomic and mediated via the vitamin D receptor (Lehmann, 2005). As far back as the 1930s, VD3 was used as an oral therapy for psoriasis but eventually fell out of favor due to hypercalcemic side effects. After the discovery of VD3 receptors on keratinocytes and fibroblasts, interest in topical therapy with VD3 analogues resurfaced (DiSepio et al., 1999). Human keratinocytes have an autonomous VD3 pathway and can convert VD3 to its hormonally active form, calcitriol (Lehmann, 2005).
Skin, despite its strong barrier properties provides several advantages as a route of delivery. Dermal drug delivery via topical application can localize a high concentration of active in the upper skin layers—which is the site of action for many drugs that treat dermatological disorders—and also minimize systemic exposure of the drug. Successful drug delivery depends on the physicochemical characteristics of the active and its carriers in the formulation. One of the approaches for formulating unstable and highly lipophilic compounds is by using particulate systems. Many studies have been focused on using nanoparticles for enhanced topical delivery of small and large molecules. A broad spectrum of particles (including liposomes, solid lipid nanoparticles (Jenning et al., 2000), polymeric micelles (Kilfoyle et al., 2012), microemulsions (Bhatia et al., 2013), and liquid crystalline nanoparticles (Madheswaran et al., 2013), (Angelova et al., 2011) have been studied for delivery of drugs and cosmetic actives to the skin. These delivery systems may be capable of providing enhanced solubility of the active, chemical and/or physical protection of the active, sustained and controlled release of the drug, and eventual enhancement of the biological absorption of the drug (Zhang et al., 2013).
A unique class of polymeric nanospheres, made of a family of biocompatible, amphiphilic tyrosine-derived ABA-triblock copolymers has been developed and characterized at The New Jersey Center for Biomaterials, Rutgers-The State University of New Jersey (Bourke and Kohn, 2003), (Sheihet et al., 2007). The chemical structure of these copolymers is composed of hydrophobic B-block i.e., oligomers of desaminotyrosyl-tyrosine ester (DTR) and diacid (Choueka et al.) and hydrophilic poly(ethylene glycol) (PEG) A-blocks. These PEG-b-oligo(DTR-XA)-b-PEG triblock copolymers undergo self-assembly in an aqueous environment to form polymeric micelles referred to as TyroSpheres. They have very low critical aggregation concentration of 2.6×10−7 g/ml and are capable of encapsulating hydrophobic compounds (Sheihet et al., 2005). Previous studies has demonstrated that TyroSpheres are not cytotoxic and drug encapsulated within these nanoparticles does not lose its activity (Nardin et al., 2004). Evaluation of this family of polymers has shown that the triblock polymer PEG5K-b-oligo(desaminotyrosyl-tyrosine octyl ester suberate)-b-PEG5K (DTO-SA/5K) is optimal for the encapsulation of lipophilic drugs (see graphical abstract). The drug-loaded TyroSpheres showed reproducible hydrodynamic diameter of approximately 70 nm and low polydispersity index (0.17), making this polymer the lead candidate evaluated for nanosphere applications (Sheihet et al., 2005). TyroSpheres have been previously evaluated for topical delivery of lipophilic drug/dye molecules (Nile Red (Batheja, 2011 #159), paclitaxel (Kilfoyle et al., 2012), cyclosporine A (Goyal et al., 2015), and adapalene (Ramezanli et al., 2016)) to treat dermatological diseases such as psoriasis and acne. Additionally, theses nanocarriers were able to localize in the hair follicles and release the drug in the pilosebaceous unit (Ramezanli et al., 2016).
VD3 is very hydrophobic (log P =9) and sensitive to many environmental factors (e.g. moisture, heat and light), which can induce isomerization or oxidation of its structure and adversely affecting its bioactivity (Ballard et al., 2007). Although the encapsulation of lipophilic compounds in nano-sized carriers has received significant attention in both the food and the pharmaceutical industry, few reports in literature are available for Vitamin D analogues (Guttoff et al., 2015), (Almouazen et al., 2013). Preparation of some of these carrier systems involves high temperature (Delaurent et al., 1998) (which would induce inactivation of VD) or toxic solvents e.g. tetrachloride or petroleum ether (Shi and Tan, 2002). In this study, we propose to use TyroSpheres for the topical delivery of VD3. VD3-TyroSpheres were fabricated and characterized for their size, binding and loading efficiencies, stability, drug release and permeation to human skin. To the best of our knowledge there is no published study on topical delivery of VD3 using nanocarriers. This work aims to address the need of a suitable carrier system for enhancing dermal delivery of VD3 and improving its stability in the formulation.
2. Materials and Methods
2.1 Materials
Suberic acid (SA), poly(ethylene glycol) monomethyl ether MW 5000 (PEG5K), Tween-80, Dimethylformamide (DMF), and Dulbecco’s Phosphate Buffered Saline (PBS), Cholecalciferol, and diethylene glycol monoethyl (Transcutol®) were purchased from Sigma Aldrich (St. Louis, MO). Dulbecco’s Modified Eagle Medium, high glucose (DMEM), trypsin (0.25% Trypsin-EDTA), Penicillin Streptomycin (10,000 U/mL), fetal bovine serum (FBS), and Dulbecco’s Phosphate Buffered Saline without calcium chloride and magnesium chloride (DPBS) were purchased from Life Technologies (Grand Island, NY). HPLC grade water, acetonitrile, and methanol were obtained from Fisher Scientific (Pittsburgh, PA). HaCaT cells were received as a generous gift from Dr. Qing Ren (Department of Radiation Oncology at Thomas Jefferson University). AlamarBlue® Reagent was obtained from AbD Serotec (Raleigh, NC). PEG5K-b-oligo(desaminotyrosyl-tyrosine octyl ester suberate)-b-PEG5K (Mn = 22.9 kDa, Mw = 31.9 kDa, molecular weight distribution (MWD) = 1.39 obtained from gel permeation chromatography) was synthesized according to previously published and established procedures at the New Jersey Center for Biomaterials, Rutgers-The State University of New Jersey. Their chemical structure and purity were confirmed by 1H NMR (Sheihet et al., 2005),(Sheihet et al., 2007). All reagents were used as received.
2.2. Preparation of VD3 loaded-TyroSphere formulations
ABA triblock copolymers composed of hydrophilic A blocks of poly(ethylene glycol) and hydrophobic B blocks of desaminotyrosyl-tyrosine octyl ester and suberic acid were used for preparation of TyroSpheres. Drug loaded-TyroSphere dispersion was prepared and purified by methods described previously (Batheja et al., 2011; Sheihet et al., 2007). The final VD3-TyroSphere formulation was obtained by redispersing the pellet formed from ultracentrifugation and filtering through 0.22 μm PVDF syringe filters (Merck Millipore). The formulations were protected from light.
2.3. VD3-TyroSpheres characterization
2.3.1. VD3 High Performance Liquid Chromatography (HPLC) assay method
An Agilent 1100 high-performance liquid chromatography (HPLC) system (Agilent Technologies, USA) equipped with a UV/Vis detector and a C18 column (Waters XBridge 3.5μm particle size, 4.6 × 50 mm) was used for chromatographic separations at 25 °C. A mixture of acetonitrile: water (isocratic A:B, 98:2) was applied as the mobile phase at a flow rate of 1 ml/min. The injection volume was 20 μL and the detection wavelength was set at 265 nm. Standard calibration curves were prepared at drug concentrations ranging from 0.1 to 100 μg/mL in both methanol and DMF. A full method validation was performed including variability, specificity, linearity, robustness, limit of detection, and limit of quantification.
2.3.2 VD3 Solubility
The solubility of VD3 in PBS media with or without surfactant Tween 80 (0.1–10% w/w) was determined and compared to the VD3-TyroSphere formulation. Super-saturated solutions of VD3 were prepared by adding excess amount of drug to each medium. Samples were vortexed and placed in a shaker water bath (100 rpm) at 37 °C for 24 h. The samples were then centrifuged and filtered through 0.45 μm PVDF filters (Whatman, Clifton, NJ), lyophilized, and re-dissolved in methanol. Drug concentrations in each solution were determined by HPLC technique. The solutions were prepared and stored in amber vials to protect the active against photodegradation.
2.3.3 Encapsulation and loading efficiency
VD3 concentration in the final purified nano-dispersion was measured by extracting VD3 from lyophilized aliquots of the VD3-TyroSphere formulation in methanol, followed by filtrating and running on HPLC (as described above). Binding efficiency and loading efficiency were calculated from the weights of VD3-TyroSphere samples and the drug concentrations using the equations below:
2.3.4 Size and size distribution
Particle size and polydispersity index (PDI) were determined by dynamic light scattering (Beckman Coulter Delsa™Nano) at 25°C. TyroSphere liquid dispersions were diluted 10 times for size measurement and were examined in triplicate by normalized intensity distribution for cumulants, size distribution, and polydispersity. An average of 50 measurements were recorded per replicate (Kilfoyle et al., 2012).
2.4 Cytotoxicity to Keratinocyte Cell Line
HaCaTs (a cell line of human keratinocytes) were cultured in a 37°C incubator with 97% RH and 10% CO2. DMEM with 10% FBS and 1% Penicillin/Streptomycin was applied as culture medium. When 90% confluency was reached, the cells were rinsed with DPBS, and trypsinized for passaging. The cells were seeded in 96-well plates at 3,000 cells/well seeding density. The cytotoxicity of VD3-TyroSpheres (at approximately 4% loading) and free VD3 (dissolved in DMSO and diluted in the medium) on HaCaT’s was tested at the concentration range of 1–50 μM. AlamarBlue® assay was performed to evaluate the 3-day cytotoxicity according to the method described by Kilfoyle et al. (Kilfoyle et al., 2012). The 50% inhibitory concentration of the formulation (IC50) was determined based on results of 3 independent experiments.
2.5 Drug release and diffusion through stratum corneum
Dermatomed human cadaver skin samples (500–700 μm) from the posterior torso of a male Caucasian donor were obtained from New York Firefighters Skin Bank (New York, NY). The frozen skin was thawed in room temperature and the epidermis was separated by immersing the skin pieces in deionized water and heating up to 60°C (Kligman and Christophers, 1963). Next, the epidermal layer was transferred to a Petri dish containing 0.025% trypsin solution and incubated at room temperature for 4 h. Then the viable layers of epidermis were removed by a cotton swab and the remaining SC membranes were rinsed with PBS (pH 7.4), dried in a desiccator overnight and stored at −20°C until use.
SC separated from human cadaver skin was mounted on vertical jacketed Franz diffusion cells with receiver volume of 5 ml and 0.64 cm2 donor-receptor area (PermeGear, Hellertown, PA). 20% DMF in PBS was added in the receptor compartment as the releasing medium, which was stirred with a magnetic bar at 300 rpm during the experiment at 37 °C. 200 μL of VD3-TyroSphere with 4 and 15 wt.% initial drug loading (containing 160 and 300 μg adapalene respectively) were applied on the SC in the donor compartment. At pre-determined time-points, 300 μL of releasing medium was collected from the receptor compartment and were replaced with the equivalent amount of the fresh release medium. The drug content in the samples was analyzed by HPLC.
2.6 Ex vivo skin distribution study on human cadaver skin
Dermatomed human skin samples (300–600 μm) from the posterior torso of a Caucasian male donor obtained from New York Firefighters Skin Bank (New York, NY) were used for skin distribution study. The permeation studies were carried out using vertical Franz diffusion cells with donor area of 0.64 cm2 (PermeGear, Inc., Hellertown PA) according to previously described methods (Kilfoyle et al., 2012). The skin samples were treated with either 200 μL VD3-TyroSphere liquid dispersion with 4 and 15 wt.% initial drug loading or VD3 solution in Transcutol® (1.5mg/ml, similar concentration to VD3-TyroSpheres 15%). The temperature of the receptor solution in the Franz cells was maintained at 37 °C. At predetermined time points (3, 6, and 12 h) the skin pieces were washed and removed from the Franz cells. Epidermal and dermal layers were manually separated using tweezers. VD3 was extracted in DMF from skin layers with the aid of BeadBug™ microtube homogenizer (Benchmark Scientific, Atkinson, NH). The drug content in epidermis, dermis, receptor media and donor compartment was determined by HPLC.
2.7 Stability of VD3 against photo degradation
VD3-TyroSphere dispersion and solution of VD3 in methanol (both about 200 μg/ml) were prepared for photochemical stability measurement. Samples were transferred into transparent glass vials and were placed in a safety cabinet exposed to 254 nm UV light. At various exposure time intervals 100 μL of sample was withdrawn from each treatment. The samples from VD3 in methanol were directly subjected for HPLC analysis. The VD3-TyroSphere samples were lyophilized, redispersed in methanol, filtrated, and analyzed by HPLC. This experiment was performed in triplicate.
2.8 Stability of the VD3-TyroSphere during storage
Formulations with different target drug loadings (4, 8, and 15% VD3/copolymer w/w) were prepared and stored at 4 °C for 6 months. Particle size and drug content were measured at a series of time-points up to 6 months. The formulations were also visually analyzed for existence of any precipitations. Short-term stability of VD3 in TyroSphere formulations and VD3 in Transcutol® (1mg/ml) at room temperature was also assessed. The formulations were kept in amber vials to protect the active from photo-induced degradation.
2.9 Statistic analysis
Statistical analysis was performed using Prism Version 6 (GraphPad software, La Jolla California). Student t-test and one-way analysis of variance ANOVA followed by Tukey’s post hoc was applied to determine the difference among the groups in every study. For skin distribution study, two-way ANOVA was used to compare drug delivery from different formulations at different time points. For all analysis, a P value of less than 0.05 derived from a two-tailed test was considered significant unless specified.
Results and Discussion
3.1 VD3-TyroSphere formulations characteristics
DTO-SA copolymers are composed of hydrophilic PEG segments and hydrophobic desaminotyrosyl alkyl ester segment. The polymers self-assemble to form polymeric micelles (TyroSpheres) in aqueous media. TyroSpheres have a core-shell structure with hydrophobic blocks making the core and hydrophilic blocks stabilizing the system in an aqueous environment. This structure allows TyroSpheres to “stabilize” significant amounts of hydrophobic drug in an aqueous dispersion. Previous studies reported that TyroSpheres were able to encapsulate a wide range of hydrophobic agents and significantly enhance their solubility (Batheja et al., 2011),(Kilfoyle et al., 2012). VD3 is a hydrophobic molecule and practically insoluble in aqueous media such as PBS. It has logP of approximately 9.09 and a molecular weight of 384.64. The molecular structure of VD3 has 7 rotatable bonds, 1 hydrogen acceptor, and 1 hydrogen donor. VD3 was loaded in TyroSpheres with initial drug to polymer input (w/w) ranging between approximately 1% and 30%. Table 1 provides the solubility results of VD3 in PBS with presence of Tween 80 or TyroSpheres. There has been substantial increase in the aqueous solubility by means of TyroSpheres and with 30% initial drug loading the drug concentration was approximately 3.5 mg/ml in the nano-dispersion.
Table 1.
Average solubility of vitamin D3 in PBS ± surfactant and TyroSpheres at room temperature.
| Sample content | Concentration of vitamin D3 (μg/mL) |
|---|---|
| PBS, pH 7.4 | < limit of quantitation |
| PBS + 0.01% (w/w) Tween 80 | 4.1 |
| PBS + 0.1% (w/w) Tween 80 | 55.0 |
| PBS + 1.0% (w/w) Tween 80 | 141.4 |
| 4% loaded in TyroSpheres | 564.7 |
| 30% loaded in TyroSpheres | 3483.7 |
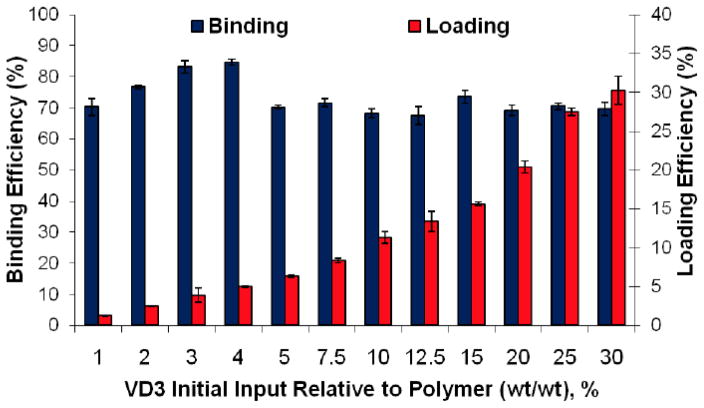
VD3 binding and loading efficiencies in TyroSpheres are presented in Figure 1. The average encapsulation efficiency was about 70%. The drug-to-polymer ratio highly influences the drug loading. As the drug initial input increased, higher loading was obtained in the TyroSpheres. TyroSpheres encapsulated VD3 to a maximum of 30.2 wt%. The high drug loading efficiency is due to high affinity of the VD3 structure to the hydrophobic section of the copolymer. These results are in good agreement with the reported outcomes in a computational modeling study (Costache et al., 2009). In this model, docking studies revealed that the high binding affinity of VD3 to the core of nanospheres is the result of hydrogen bonding and hydrophobic interactions between drug and the copolymer. VD3 is less rigid than other studied molecules including paclitaxel and curcumin, and its flexibility and extremely high hydrophobicity allowed for many hydrophobic interactions within the hydrophobic core of TyroSpheres.
Figure 1.
% Binding and loading efficiencies of vitamin D3 (VD3) in TyroSphere formulations The results are shown as mean ± SD, n ≥ 3.
Previous studies showed that drug loaded TyroSpheres have average diameter of about 60–70 nm, as measured by DLS technique; this is regardless of the encapsulated drug or drug concentration (Kilfoyle et al., 2012),(Goyal et al., 2015). However, the loading of VD3 had a small impact on the size of TyroSpheres. It is evident in Table 2 that larger nanoparticles were obtained by increasing VD3 initial loading. For samples above 25% VD3 input, a milky white dispersion was formed, which replaced the slightly hazy nano-suspensions seen below 25% input. The PDI in all cases was below 0.19. Filtration of TyroSphere liquid dispersion with PVDF syringe filters (0.22 μm Merck Millipore Ltd,) in the last step of their preparation did not have a significant effect on the resulting particle size.
Table 2.
Particle size and particle size polydispersity index (PDI) of Vitamin D3 (VD3) loaded in TyroSpheres measured by dynamic light scattering (DLS). The TyroSpheres were examined by normalized intensity distribution for average diameter, PDI, and size distribution. The data is based on the average of 3 replications.
| % VD3 initial loading | Average Diameter (nm) | PDI | D10 (nm) | D50 (nm) | D90 (nm) |
|---|---|---|---|---|---|
| 1.3 | 64.5 | 0.18 | 37.3 | 68.5 | 128.9 |
| 2.5 | 65.0 | 0.17 | 38 | 69.0 | 125.8 |
| 3.9 | 65.2 | 0.15 | 38.8 | 67.6 | 121.9 |
| 5.0 | 64.5 | 0.15 | 38.9 | 67.0 | 118.2 |
| 6.4 | 64.3 | 0.13 | 39 | 66.6 | 116.2 |
| 8.4 | 67.2 | 0.15 | 39.7 | 69.9 | 126.7 |
| 11.3 | 67.0 | 0.14 | 40.1 | 69.7 | 123.9 |
| 13.4 | 67.7 | 0.14 | 40.9 | 70.6 | 124.1 |
| 15.7 | 66.4 | 0.13 | 40.1 | 69.0 | 119.8 |
| 20.4 | 68.2 | 0.16 | 39.9 | 71.0 | 129.7 |
| 27.5 | 69.1 | 0.16 | 40.5 | 72.1 | 132.1 |
| 30.2 | 73.4 | 0.18 | 42.1 | 76.8 | 144.8 |
3.2 Cytotoxic effect of VD3 loaded and unloaded-TyroSpheres on HaCaTs
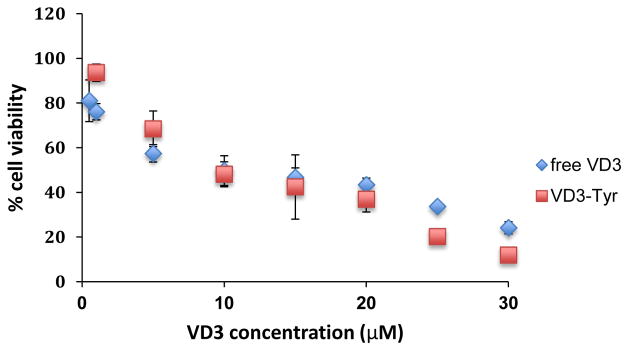
In order to test whether encapsulated VD3 preserves its activity in TyroSpheres, we evaluated cytotoxic effect of VD3 (in solution vs. entrapped in TyroSpheres) on HaCaTs. AlamarBlue® assay has been used to assess metabolic activity of HaCaTs following exposure to the tested chemicals. The conversion of the fluorescent Resazurin in AlamarBlue® to Resorufin, in response to cellular metabolic activity, is a marker for cell viability. TyroSpheres that were not loaded with drug did not show cytotoxicity on keratinocytes at concentration range of 1–30 μM/mL. Figure 2 depicts % cell viability of HaCaTs following 3-day treatment with either free VD3 or VD3-TyroSphres. The IC50 of free VD3 and VD3-TyroSpheres were calculated as 9.3±1.2 μM and 12.0±3.0 μM respectively. Thus, no significant change in metabolic activity of HaCaTs was observed between the two treatment groups, indicating that TyroSpheres do not induce short-term cytotoxicity on keratinocytes and the encapsulated VD3 does not lose its activity.
Figure 2.
Viability of HaCaT keratinocyte cell line exposed to free VD3 and VD3 encapsulated in TyroSpheres (VD3-Tyr). The % viability was measured by AlamarBlue® assay based on metabolic activity of cells.
3.3 Release of VD3 from TyroSpheres
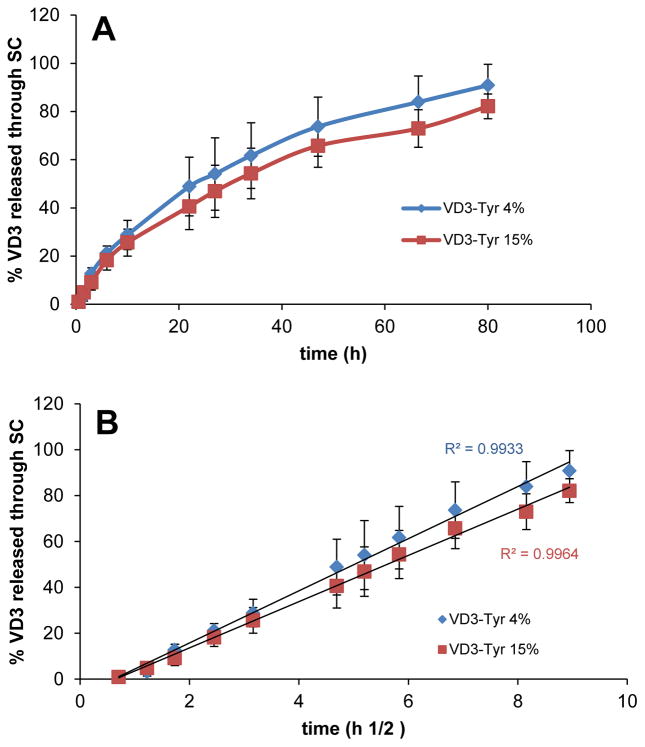
The ability of a carrier to release the drug is very important and is usually measured in vitro using a semi-permeable membrane in an aqueous environment. However, this method for drug release cannot predict the behavior of nanocarriers in contact with SC, which has lipophilic nature. Additionally, for VD3-TyroSpheres, when release studies were conducted with dialysis cassette in an aqueous environment (PBS+1% Tween 80 w/v), VD3 was not detected in the release compartment. This was not unexpected, as the water solubility of VD3 is very low and it is not stable in PBS. Therefore, in order to mimic skin application and better understand VD3 release behavior from TyroSpheres in a lipophilic environment, we studied drug release and diffusion through SC. Organic solvents are occasionally used as release media for highly hydrophobic and prone-to-hydrolysis compounds. In our experiment, 20% DMF in PBS was applied in the receptor chamber as the release medium. This is to ensure that the released VD3 can permeate through SC and be stabilized in the receptor chamber. Figure 4 depicts the cumulative % VD3 release from TyroSpheres which occurred in a sustained manner. To ensure that DMF diffusion from the receptor compartment to the donor compartment did not affect the stability of VD3 loaded TyroSpheres, we measured the size of the TyroSpheres in the donor compartment throughout the release experiment. The change in particle size of TyroSphere dispersion applied on SC was negligible, demonstrating that VD3 loaded TyroSphere dispersion was stable in the donor compartment. Therefore, the drug release did not occur due to dissociation and degradation of the nanoparticles. The VD3 detected in the receptor compartment is the portion of drug released from TyroSpheres and diffused across the SC. The influence of drug loading on release rate from TyroSpheres was also studied. VD3 release profiles from both 4 wt.% and 15wt.% VD3-TyroSpheres were very similar and there was no significant difference in the % drug release (p<0.05). Therefore, the drug loading did not affect the release kinetic. According to Figure 3.A, the cumulative drug release was not linear. We fit the release data to Higuchi square root model, where the cumulative drug release has a linear correlation with root of time (R2=0.99) irrespective of drug loading (see Figure 3.B). This indicates that the drug release from TyroSpheres is controlled by diffusion and concentration gradient, but not polymer erosion.
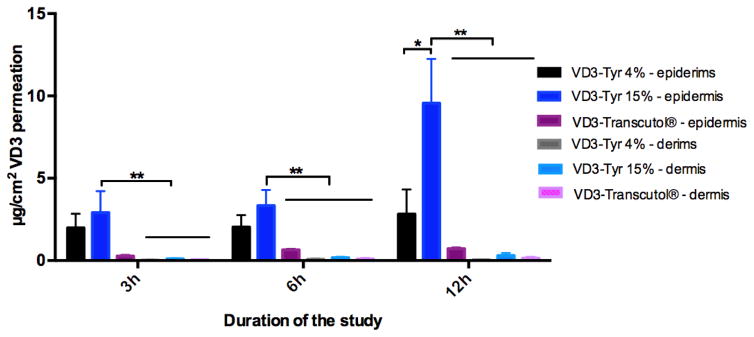
Figure 4.
Skin distribution of Vitamin D3 loaded in TyroSphere (VD3-Tyr) at 4 and 15 wt% initial loading and VD3 dissolved in Transcutol® (containing about 0.8, 1.5 and 1.5 mg/mL VD3 in the formulation). The bars show drug extracted from epidermis and dermis (μg per cm2 area of skin) following 3, 5 and 12 h of skin exposure to the formulation. The statistical data is expressed as mean ± SEM (n = 5), * P<0.05, ** P<0.01.
Figure 3.
Cumulative Vitamin D3 release from TyroSpheres and diffusion through stratum corneum (SC) A) as a function of time and B) as a function of the square root of time (Higuchi model). Study was done with 4 and 15 wt% initial loading VD3 in TyroSpheres (D3-Tyr). Data are shown as mean ± SD (n=5).
3.4 Skin distribution studies
Skin distribution study was conducted at 3, 6 and 12 h to assess TyroSphere ability to enhance delivery of VD3 to skin. Transcutol® has been reported to be an effective dermal penetration enhancer by enhancing drug solubility in the skin (Harrison et al., 1996). Thus, VD3 solution in Transcutol® (at similar drug concentration to VD3-TyroSphere 15wt%) was used to compare delivery efficiency of Transcutol® to that of TyroSpheres. Figure 4 provides the results of skin permeation study that were analyzed by HPLC. At all the time points, VD3 diffusion to epidermis, which is the target layer for VD3 delivery, was significantly higher than that to dermis for VD3-TyroSpheres 15 wt% formulation (p<0.01). Drug content in receptor compartment was below detection limit of our analytical technique, which was expected due to high lipophilicity of VD3. Following 6 h and 12 h exposure of VD3 formulations with human skin, VD3-TyroSpheres 15 wt% was found to be the most efficient formulation to deliver the drug to epidermis and dermis. According to Fick’s law of diffusion (J=-D×∂φ/∂x, where J is diffucion flux, D is diffusion coefficient, and dφ/dx is concentration gradient)(Zhang et al., 2013) it is expected that by increasing drug concentration in the formulation the diffusion flux increases, which explains higher VD3 delivery to skin layers from VD3-TyroSphere 15 wt% than 4wt% drug loading. By increasing the exposure time of skin with the formulation (from 6h to 12 h), significant increase in the VD3 diffusion to epidermis observed with VD3-TyroSphere 15 wt% (p<0.05), while VD3-Transcutol® with similar drug concentration did not show statistically significant increase in the delivery with longer application time. This observation is probably due to sustained release of VD3 from TyroSpheres that affects the rate of drug permeation to the skin and does not occur with VD3 solution in Transcutol®. The results from ex vivo skin distribution study are in agreement with previous published studies(Goyal et al., 2015; Kilfoyle et al., 2012; Sheihet et al., 2008) that confirmed TyroSpheres efficiency for topical drug delivery and show that these nanocarriers are more effective than some other dermal penetration enhancers, such as propylene glycol (Batheja et al., 2011) and Transcutol®. The exact mechanism behind dermal penetration enhancement effect of polymeric nanoparticles is still unknown. However according to the recent investigations in this area, this enhancement in skin penetration can be attributed to accumulation of nanoparticles in skin appendages, their penetration into the superficial layers of the SC, and/or higher concentration gradient due to higher apparent solubility of the loaded drugs in TyroSpheres (Chen-yu et al., 2012), (Desai et al., 2010).
3.5 Photodegradtion of VD3 in TyroSphere formulation
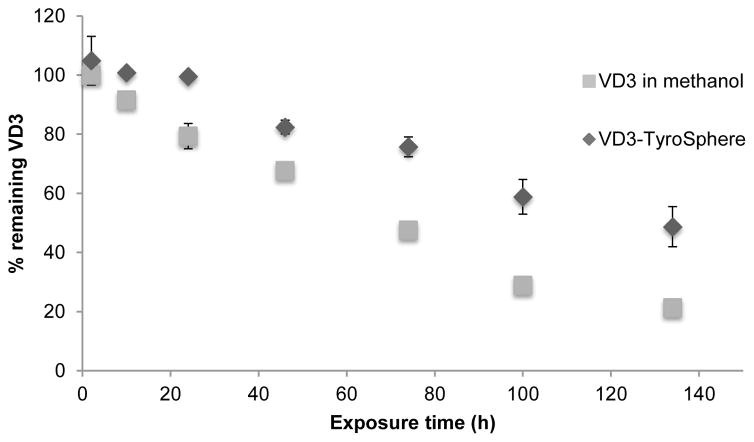
Photochemical stability of VD3 encapsulated in TyroSpheres was studied and compared with free VD3 dissolved in methanol. As it is shown in Figure 5, VD3 in methanol underwent photodegradation much faster than in TyroSphere formulation. In the first 24h exposure to UV light, no significant VD3 degradation was observed in TyroSphere formulation while nearly 20% of the drug was lost in methanol solution. After 134 h only 21% of VD3 was left in methanol formulation which is significantly lower than 49% VD3 left in TyroSphere formulation (p<0.01) Photodegradation of VD3 in both vehicles followed zero-order kinetics (R>0.97). The degradation rate constants were found to be −1.194 and −0.757 μg.ml−1.h−1 for VD3 in methanol and in TyroSpheres, respectively (Figure S1 in supplementary materials). The half-life of VD3 was calculated as 76 and 127 h in methanol solution and in TyroSpheres aqueous dispersion, respectively. Our results demonstrate that TyroSpheres improve stability of VD3 when exposed to UV light. This can be ascribed to the strong interaction of VD3 to the inner core of TyroSpheres(Costache et al., 2009). As a result, VD3 is protected from harsh environmental factors (like light and moisture) (Wichit et al., 2012). It is noteworthy that we chose methanol as a solvent that completely dissolves VD3 and other researchers have used it as a control solvent for evaluating photoprotective properties of the nanomaterial (Ourique et al., 2011),(Ourique et al., 2008). Light-induced degradation of VD3 can further be accelerated when VD3 is dispersed in aqueous media. Luo et al. (Luo et al., 2012) reported that only about 30% of VD3 remained when VD3 dispersion in water was exposed to UV light for 9.5h. Therefore, TyroSpheres not only enhance water solubility of VD3, but also substantially decrease degradation of this compound in the aqueous medium.
Figure 5.
Stability of VD3 encapsulated in TyroSpheres and dissolved in methanol (as control). The results are the average % of VD3 remained in the formulation.
3.6 Stability of VD3-TyroSphere formulations
Encapsulation of VD3 with TyroSpheres aims to protect VD3 from environmental factor (e.g. moisture and light) that affect its activity. Stability of VD3-TyroSphere formulations was studied by measuring particle size and drug content at different time points. During 6 months storage at 4°C, precipitation or change in turbidity of the colloidal dispersion was not visually observed. Additionally, there was no significant change in the average diameter size of nanoparticles (measured by DLS technique) and the particle size remained around 65 nm and 71 nm for both VD3-TyroSphere 4 and 15 wt% loading, respectively. The PDI was also less than 0.16 showing narrow size distribution and lack of agglomeration of TyroSpheres. TyroSpheres have micelle–like structure with hydrophobic core and hydrophilic shell. The highly hydrated outer shells of these polymeric micelles—due to the presence of PEG—can inhibit intermicellar aggregation of their hydrophobic inner cores (Opanasopit et al., 2007). As a result, TyroSpheres maintained a satisfactory aqueous stability irrespective of high contents of hydrophobic drug incorporated into their inner core
Following 6 months storage of VD3-TyroSpheres at 4°C, 93.2± 9.6 and 89.0±2.6 % of VD3 remained in 4 and 8 wt% VD3-TyroSphere formulations. Short-term stability assessment of VD3-TyroSpheres at 25°C confirmed that TyroSpheres can significantly increase drug stability in the formulation. As expected, the rate of VD3 degradation was less at 4°C compared to room temperature. After 2 months storing at room temperature, only 2% of VD3 was detected in Transcutol® (control vehicle for VD3 that was used in skin permeation study), while VD3 recovery in 4 and 15 wt% VD3-TyroSphere was 92 and 74% (Table S1). Our results suggest that at both storage conditions, the initial drug loading is related to the rate of VD3 degradation. This observation can be explained by the results of computational modeling study conducted by Costache et al. (Costache et al., 2009). They concluded that there are sites with different binding affinities to the drug in TyroSpheres. The sites with higher binding affinity are occupied first and by increasing the initial VD3 loading, these sites get saturated and the excess drug binds to sites with weaker affinities. During the storage, the portion of VD3 that is bonded weakly to the polymer, may release from TyroSpheres and leak to the surrounding aqueous medium, which results in its degradation. Therefore, VD3-TyroSpheres with lower drug loading (4 wt%) were found to be more stable than the high loading formulation (15 wt%).
Conclusions
There is limited work that has been performed on the topical delivery of VD3. In the present study we applied TyroSpheres as a novel carrier system to formulate VD3 for dermal delivery. We highlighted the importance of carrier selection to improve solubility, efficacy and stability of challenging-to-formulate drugs such asVD3. TyroSpheres were able to substantially enhance solubility of VD3 in aqueous medium without affecting activity of the drug. These biocompatible nanocarriers form a protective layer around the lipophilic drug that can protect it against environmental-induced degradation. Moreover, the skin delivery efficiency of TyroSpheres was found to be higher than some other dermal penetration enhancers, such as Transcutol®. This study provides evidence of TyroSpheres’ significant potential for targeted delivery of hydrophobic actives to skin layers.
Supplementary Material
Acknowledgments
We would like to acknowledge Dr. Larisa Sheihet for her valuable inputs and contributions for this project. Most of the studies reported in this manuscript were supported by Grant Number 5R01AR056079 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS - NIH). Additional funding was provided by the Center for Dermal Research CDR and the NJ Center for Biomaterials, Rutgers-The State University of New Jersey, Piscataway NJ.
Glossary
- SC
Stratum Corneum
- VD3
Vitamin D3
- HPLC
High Performance Liquid Chromatography
- DLS
Dynamic light scattering
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almouazen E, Bourgeois S, Jordheim LP, Fessi H, Briancon S. Nano-encapsulation of vitamin D3 active metabolites for application in chemotherapy: formulation study and in vitro evaluation. Pharmaceutical research. 2013;30:1137–1146. doi: 10.1007/s11095-012-0949-4. [DOI] [PubMed] [Google Scholar]
- Angelova A, Angelov B, Mutafchieva R, Lesieur S, Couvreur P. Self-assembled multicompartment liquid crystalline lipid carriers for protein, peptide, and nucleic acid drug delivery. Acc Chem Res. 2011;44:147–156. doi: 10.1021/ar100120v. [DOI] [PubMed] [Google Scholar]
- Ballard JM, Zhu L, Nelson ED, Seburg RA. Degradation of vitamin D3 in a stressed formulation: the identification of esters of vitamin D3 formed by a transesterification with triglycerides. J Pharm Biomed Anal. 2007;43:142–150. doi: 10.1016/j.jpba.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Barker JNWN, Ashton RE, Marks R, Harris RI, Berth-Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo-controlled, double-blind, dose-finding study with active comparator. British Journal of Dermatology. 1999;141:274–278. doi: 10.1046/j.1365-2133.1999.02975.x. [DOI] [PubMed] [Google Scholar]
- Batheja P, Sheihet L, Kohn J, Singer AJ, Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J Control Release. 2011;149:159–167. doi: 10.1016/j.jconrel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Bhatia G, Zhou Y, Banga AK. Adapalene microemulsion for transfollicular drug delivery. J Pharm Sci. 2013;102:2622–2631. doi: 10.1002/jps.23627. [DOI] [PubMed] [Google Scholar]
- Birlea SA, Costin GE, Norris DA. New insights on therapy with vitamin D analogs targeting the intracellular pathways that control repigmentation in human vitiligo. Med Res Rev. 2009;29:514–546. doi: 10.1002/med.20146. [DOI] [PubMed] [Google Scholar]
- Bourke SL, Kohn J. Polymers derived from the amino acid L-tyrosine: polycarbonates, polyarylates and copolymers with poly(ethylene glycol) Adv Drug Deliv Rev. 2003;55:447–466. doi: 10.1016/s0169-409x(03)00038-3. [DOI] [PubMed] [Google Scholar]
- Chen-yu G, Chun-fen Y, Qi-lu L, Qi T, Yan-wei X, Wei-na L, Guang-xi Z. Development of a quercetin-loaded nanostructured lipid carrier formulation for topical delivery. Int J Pharm. 2012;430:292–298. doi: 10.1016/j.ijpharm.2012.03.042. [DOI] [PubMed] [Google Scholar]
- Choueka J, Charvet JL, Koval KJ, Alexander H, James KS, Hooper KA, Kohn J. Canine bone response to tyrosine-derived polycarbonates and poly(L-lactic acid) J Biomed Mater Res. 1996;31:35–41. doi: 10.1002/(SICI)1097-4636(199605)31:1<35::AID-JBM5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Costache AD, Sheihet L, Zaveri K, Knight DD, Kohn J. Polymer-drug interactions in tyrosine-derived triblock copolymer nanospheres: a computational modeling approach. Mol Pharm. 2009;6:1620–1627. doi: 10.1021/mp900114w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaurent C, Siouffi AM, Pepe G. Cyclodextrin inclusion complexes with vitamin D-3: Investigations of the solid complex characterization. Chemia Analityczna. 1998;43:601–616. [Google Scholar]
- Desai P, Patlolla RR, Singh M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol Membr Biol. 2010;27:247–259. doi: 10.3109/09687688.2010.522203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSepio D, Chandraratna RA, Nagpal S. Novel approaches for the treatment of psoriasis. Drug Discov Today. 1999;4:222–231. doi: 10.1016/s1359-6446(99)01335-5. [DOI] [PubMed] [Google Scholar]
- Goyal R, Macri L, Kohn J. Formulation Strategy for the Delivery of Cyclosporine A: Comparison of Two Polymeric Nanospheres. Sci Rep. 2015;5:13065. doi: 10.1038/srep13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttoff M, Saberi AH, McClements DJ. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: factors affecting particle size and stability. Food Chem. 2015;171:117–122. doi: 10.1016/j.foodchem.2014.08.087. [DOI] [PubMed] [Google Scholar]
- Harrison JE, Watkinson AC, Green DM, Hadgraft J, Brain K. The relative effect of Azone and Transcutol on permeant diffusivity and solubility in human stratum corneum. Pharm Res. 1996;13:542–546. doi: 10.1023/a:1016037803128. [DOI] [PubMed] [Google Scholar]
- Hayhoe RPG, Henson SM, Akbar AN, Palmer DB. Variation of human natural killer cell phenotypes with age: Identification of a unique KLRG1-negative subset. Human Immunology. 2010;71:676–681. doi: 10.1016/j.humimm.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Jenning V, Gysler A, Schafer-Korting M, Gohla SH. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e V. 2000;49:211–218. doi: 10.1016/s0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- Kilfoyle BE, Sheihet L, Zhang Z, Laohoo M, Kohn J, Michniak-Kohn BB. Development of paclitaxel-TyroSpheres for topical skin treatment. J Control Release. 2012;163:18–24. doi: 10.1016/j.jconrel.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Archives of Dermatology. 1963;88:702–705. doi: 10.1001/archderm.1963.01590240026005. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Feldman D. Mechanisms of the Anti-Cancer and Anti-Inflammatory Actions of Vitamin D. Annual Review of Pharmacology and Toxicology. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- Lehmann B. The vitamin D3 pathway in human skin and its role for regulation of biological processes. Photochem Photobiol. 2005;81:1246–1251. doi: 10.1562/2005-02-02-IR-430. [DOI] [PubMed] [Google Scholar]
- Luo Y, Teng Z, Wang Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J Agric Food Chem. 2012;60:836–843. doi: 10.1021/jf204194z. [DOI] [PubMed] [Google Scholar]
- Madheswaran T, Baskaran R, Thapa RK, Rhyu JY, Choi HY, Kim JO, Yong CS, Yoo BK. Design and in vitro evaluation of finasteride-loaded liquid crystalline nanoparticles for topical delivery. AAPS PharmSciTech. 2013;14:45–52. doi: 10.1208/s12249-012-9888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- Nardin C, Bolikal D, Kohn J. Nontoxic block copolymer nanospheres: Design and characterization. Langmuir. 2004;20:11721–11725. doi: 10.1021/la0490285. [DOI] [PubMed] [Google Scholar]
- Nieves NJ, Ahrens JM, Plum LA, DeLuca HF, Clagett-Dame M. Identification of a Unique Subset of 2-Methylene-19-Nor Analogs of Vitamin D with Comedolytic Activity in the Rhino Mouse. Journal of Investigative Dermatology. 2010;130:2359–2367. doi: 10.1038/jid.2010.142. [DOI] [PubMed] [Google Scholar]
- Opanasopit P, Ngawhirunpat T, Rojanarata T, Choochottiros C, Chirachanchai S. N-phthaloylchitosan-g-mPEG design for all-trans retinoic acid-loaded polymeric micelles. Eur J Pharm Sci. 2007;30:424–431. doi: 10.1016/j.ejps.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Ourique AF, Melero A, de Bona da Silva C, Schaefer UF, Pohlmann AR, Guterres SS, Lehr CM, Kostka KH, Beck RC. Improved photostability and reduced skin permeation of tretinoin: development of a semisolid nanomedicine. Eur J Pharm Biopharm. 2011;79:95–101. doi: 10.1016/j.ejpb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Ourique AF, Pohlmann AR, Guterres SS, Beck RC. Tretinoin-loaded nanocapsules: Preparation, physicochemical characterization, and photostability study. Int J Pharm. 2008;352:1–4. doi: 10.1016/j.ijpharm.2007.12.035. [DOI] [PubMed] [Google Scholar]
- Picciano MF. Vitamin D Status and Health. Critical Reviews in Food Science and Nutrition. 2010;50:24–25. [Google Scholar]
- Ramezanli T, Zhang Z, Michniak-Kohn BB. Development and characterization of polymeric nanoparticle-based formulation of adapalene for topical acne therapy. Nanomedicine. 2016 doi: 10.1016/j.nano.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Sheihet L, Chandra P, Batheja P, Devore D, Kohn J, Michniak B. Tyrosine-derived nanospheres for enhanced topical skin penetration. Int J Pharm. 2008;350:312–319. doi: 10.1016/j.ijpharm.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Sheihet L, Dubin RA, Devore D, Kohn J. Hydrophobic drug delivery by self-assembling triblock copolymer-derived nanospheres. Biomacromolecules. 2005;6:2726–2731. doi: 10.1021/bm050212u. [DOI] [PubMed] [Google Scholar]
- Sheihet L, Piotrowska K, Dubin RA, Kohn J, Devore D. Effect of tyrosine-derived triblock copolymer compositions on nanosphere self-assembly and drug delivery. Biomacromolecules. 2007;8:998–1003. doi: 10.1021/bm060860t. [DOI] [PubMed] [Google Scholar]
- Shi XY, Tan TW. Preparation of chitosan/ethylcellulose complex microcapsule and its application in controlled release of vitamin D-2. Biomaterials. 2002;23:4469–4473. doi: 10.1016/s0142-9612(02)00165-5. [DOI] [PubMed] [Google Scholar]
- Wichit A, Tangsumranjit A, Pitaksuteepong T, Waranuch N. Polymeric micelles of PEG-PE as carriers of all-trans retinoic acid for stability improvement. AAPS PharmSciTech. 2012;13:336–343. doi: 10.1208/s12249-011-9749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdisciplinary Reviews-Nanomedicine and Nanobiotechnology. 2013;5:205–218. doi: 10.1002/wnan.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.