Abstract
Background
Although body mass index (BMI) has been used in risk stratification for lung resection, many models only take obesity into account. Recent studies have demonstrated that underweight patients also experience increased postoperative complications. We explored the relationship of extremes of BMI to outcomes after lung resection for non-small cell cancer.
Methods
Patients in the Society of Thoracic Surgeons General Thoracic Surgery Database (2009 to 2014) undergoing elective lung resection for cancer were evaluated. Multivariable logistic regression was used to adjust for potential confounders including functional status and spirometry.
Results
We evaluated 41,446 patients (median 68 years of age; 53% female) grouped by BMI: underweight (<18.5 kg/m2; 3.0%), normal (18.5 to 24.9 kg/m2; 33.5%), overweight (25 to 29.9 kg/m2; 35.4%), obese I (30 to 34.9 kg/m2; 18.1%), obese II (35 to 39.9 kg/m2; 6.4%), and obese III (≥40 kg/m2; 3.6%). Pulmonary complication rates were higher in underweight and obese III patients compared to normal BMI patients (p < 0.001). On multivariable analysis, compared to patients with normal BMI, being underweight was associated with an increased risk of pulmonary complications (adjusted odds ratio [OR]: 1.41, 95% confidence interval [CI]: 1.16 to 1.70) and any postoperative event (adjusted OR: 1.44, 95% CI: 1.26 to 1.64). Obese III patients had an increased risk of any major postoperative complication (adjusted OR: 1.18, 95% CI: 1.02 to 1.36). Overweight and obese class I to II patients had a lower risk of pulmonary complications and any postoperative event.
Conclusions
BMI is associated with postoperative complications after lung resection for cancer. Being underweight or severely overweight is associated with an increased risk of complications, whereas being overweight or moderately obese appears to have a protective effect.
The obesity incidence in the United States has been increasing since the late 20th century, with 30% to 40% of adults currently classified as overweight and 35% classified as obese [1–3]. Increasing body mass is associated with comorbid conditions that adversely affect life expectancy and overall medical expenditures [4, 5]. Recent investigation has demonstrated that this relationship is also apparent among patients undergoing surgical resection of lung cancer, specifically that operating time is increased among patients with higher body mass index (BMI) [6–8], and some have also reported that the incidence of pulmonary complications is higher in these patients [9, 10]. In contrast, a few reports found that overall surgical outcomes were similar for overweight or obese patients compared to normal weight patients [11–15] and in some studies overweight or obese patients actually had better outcomes than non-obese patients [8, 13, 14]. The overall association of overweight or obese status and outcomes of lung cancer resection remains difficult to define.
Our understanding of the relationship of underweight status to outcomes after lung resection for cancer is similarly limited. Being underweight is associated with poor nutritional status, sarcopenia and related weakness of respiratory muscles, and frailty [16–18]. Such factors could adversely influence outcomes after major lung resection, as has been recently shown [8, 13].
The relationship of BMI to outcomes after lung resection is complex and incompletely understood. The present study was performed to better characterize the relationship of extremes of body mass index to surgical outcomes after lung cancer resection.
Materials and Methods
We queried the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database (Versions 2.081 and 2.2) for all patients undergoing elective major lung resection between January 1, 2009, and June 30, 2014. Data were collected for demographic, physiologic, operative and outcome variables. For patients undergoing more than one operation, only the first operation was included. Patients were excluded if they underwent carinal sleeve resection, extrapleural pneumonectomy, resection of apical (Pancoast) lung tumors including the chest wall, and wedge resections. Additional exclusion criteria included missing important data (age, BMI, gender or discharge mortality), patients less than 19 years of age, patients in American Society of Anesthesiologists class VI at the time of surgery, and operations for occult or stage 0 pathological stage disease.
Outcomes included operative mortality and complications. Operative mortality was defined as death during hospitalization or within 30 days of the index operation. Major complications were categorized as pulmonary (atelectasis requiring bronchoscopy, pneumonia, adult respiratory distress syndrome, initial ventilator support more than 48 hours, reintubation, tracheostomy, and other), cardiovascular (deep venous thrombosis, atrial arrhythmia requiring treatment, ventricular arrhythmia requiring treatment, myocardial infarction, pulmonary embolism, other major cardiovascular event), infectious (empyema, sepsis, other infection requiring intravenous antibiotics), other (central neurologic event, delirium, unexpected admission to the intensive care unit, new renal failure or increase in creatinine twofold greater than preoperative value), and surgical (air leak greater than 5 days’ duration, blood transfusion, chylothorax requiring drainage or medical therapy, pneumothorax, unexpected return to the operating room, bronchopleural fistula). We also analyzed 2 composite variables termed “any major postoperative complication” consisting of operative mortality, pulmonary, cardiovascular or other complications as defined previously, and “any postoperative event” that included any complication listed for an individual patient, major or otherwise.
BMI was analyzed as a categorical variable based on previously defined World Health Organization criteria: underweight (<18.5 kg/m2), normal (18.5 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2), obese I (30 to 34.9 kg/m2), obese II (35 to 39.9 kg/m2), and obese III (≥40 kg/m2) [19]. Univariable analyses compared patients in different BMI categories to each other using the Wilcoxon rank sum test (number of groups = 2) or Kruskal-Wallis test (number of groups >2) for continuous variables and the chi-square test for categorical variables. A logistic regression model using generalized estimating equations was created to determine the association of BMI and outcomes utilizing complete case analysis. Covariates included in the model came from a previously developed and validated STS risk model [20]. There were 4.7% missing values for pathologic staging, 5.3% missing values for FEV1 % predicted, 6.9% missing values for weight loss within 3 months, and less than 4% missing values for the other covariates. Patients with missing data were excluded from the multivariate analysis. We compared excluded cohort with included cohort, and found no evidence of clinically meaningful differences. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Elective lung cancer resection was performed in 41,446 patients meeting our inclusion criteria. The largest fraction of patients was overweight (35.4%), while 3.0% were underweight, 33.5% had a normal BMI, 18.1% were categorized as obese I, 6.4% were categorized as obese II, and 3.6% were categorized as obese III (Table 1). The majority of patients had a lobectomy performed for pathologic stage I disease. Compared to normal weight patients, underweight patients were significantly more likely to be women and have chronic obstructive pulmonary disease. Diabetes, hypertension, and coronary artery disease tended to be more common with increasing BMI category. Underweight patients were more likely to be recent smokers and to have a worse performance status.
Table 1.
Demographics and Clinical Characteristics
| Variable | Overall (N = 41,446) | Underweight (n = 1,248) | Normal (n = 13,880) | Overweight (n = 14,668) | Obese I (n = 7,507) | Obese II (n = 2,659) | Obese III (n = 1,484) | P Value |
|---|---|---|---|---|---|---|---|---|
| Age, y | 66.6 ± 10.4 | 64.5 ± 10.8 | 66.7 ± 10.8 | 67.4 ± 10.2 | 66.4 ± 9.9 | 65.1 ± 9.8 | 62.3 ± 10.9 | <0.0001 |
| Gender | <0.0001 | |||||||
| Male | 19,479 (47.0) | 358 (28.7) | 5,818 (41.9) | 7,807 (53.2) | 3,783 (50.4) | 1,197 (45.0) | 516 (34.8) | |
| Female | 21,967 (53.0) | 890 (71.3) | 8,062 (58.1) | 6,861 (46.8) | 3,724 (49.6) | 1,462 (55.0) | 968 (65.2) | . |
| Race | <0.0001 | |||||||
| Caucasian | 36,121 (87.7) | 1,053 (84.9) | 11,870 (86.1) | 12,932 (88.7) | 6,649 (89.2) | 2,334 (88.2) | 1,283 (87.1) | |
| Black | 3,588 (8.7) | 141 (11.4) | 1,183 (8.6) | 1,172 (8.0) | 662 (8.9) | 267 (10.1) | 163 (11.1) | . |
| Hispanic | 2 (0.0) | 0 (0.0) | 1 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | . |
| Asian | 903 (2.2) | 34 (2.7) | 549 (4.0) | 265 (1.8) | 43 (0.6) | 7 (0.3) | 5 (0.3) | . |
| Native Am | 56 (0.1) | 2 (0.2) | 17 (0.1) | 17 (0.1) | 12 (0.2) | 7 (0.3) | 1 (0.1) | . |
| Other | 283 (0.7) | 6 (0.5) | 97 (0.7) | 110 (0.8) | 43 (0.6) | 15 (0.6) | 12 (0.8) | . |
| Hawaiian/Pac | 41 (0.1) | 1 (0.1) | 13 (0.1) | 19 (0.1) | 4 (0.1) | 4 (0.2) | 0 (0.0) | . |
| Mixed | 187 (0.5) | 3 (0.2) | 62 (0.4) | 61 (0.4) | 42 (0.6) | 11 (0.4) | 8 (0.5) | |
| ASA risk class | <0.0001 | |||||||
| I | 164 (0.4) | 6 (0.5) | 63 (0.5) | 65 (0.4) | 20 (0.3) | 5 (0.2) | 5 (0.3) | |
| II | 6,382 (15.4) | 159 (12.8) | 2,430 (17.5) | 2,332 (15.9) | 1,042 (13.9) | 289 (10.9) | 130 (8.8) | . |
| III | 30,842 (74.4) | 946 (75.9) | 10,113 (72.9) | 10,907 (74.4) | 5,693 (75.8) | 2,054 (77.3) | 1,129 (76.1) | . |
| IV | 4,032 (9.7) | 135 (10.8) | 1,264 (9.1) | 1,358 (9.3) | 745 (9.9) | 310 (11.7) | 220 (14.8) | . |
| V | 14 (0.0) | 1 (0.1) | 5 (0.0) | 2 (0.0) | 6 (0.1) | 0 (0.0) | 0 (0.0) | . |
| Performance status | <0.0001 | |||||||
| 0 | 16,896 (40.8) | 441 (35.3) | 5,715 (41.2) | 6,234 (42.5) | 2,973 (39.6) | 1,013 (38.1) | 520 (35.1) | |
| 1 | 22,738 (54.9) | 712 (57.1) | 7,561 (54.5) | 7,856 (53.6) | 4,230 (56.4) | 1,521 (57.2) | 858 (58.0) | . |
| 2 | 1,475 (3.6) | 70 (5.6) | 502 (3.6) | 481 (3.3) | 238 (3.2) | 101 (3.8) | 83 (5.6) | . |
| 3 | 265 (0.6) | 22 (1.8) | 79 (0.6) | 75 (0.5) | 51 (0.7) | 20 (0.8) | 18 (1.2) | . |
| 4 | 31 (0.1) | 3 (0.2) | 6 (0.0) | 10 (0.1) | 10 (0.1) | 1 (0.0) | 1 (0.1) | . |
| 5 | 12 (0.0) | 0 (0.0) | 6 (0.0) | 3 (0.0) | 1 (0.0) | 2 (0.1) | 0 (0.0) | . |
| Coronary artery disease | 8,812 (21.4) | 164 (13.2) | 2,401 (17.5) | 3,439 (23.6) | 1,850 (24.8) | 671 (25.5) | 287 (19.4) | <0.0001 |
| Cerebrovascular disease | 3,270 (8.0) | 105 (8.5) | 1,120 (8.2) | 1,207 (8.3) | 576 (7.7) | 184 (7.0) | 78 (5.3) | 0.0005 |
| Congestive heart failure | 1,163 (2.8) | 23 (1.9) | 289 (2.1) | 412 (2.8) | 254 (3.4) | 117 (4.4) | 68 (4.6) | <0.0001 |
| Hypertension | 25,200 (61.1) | 550 (44.4) | 7,199 (52.2) | 9,175 (62.8) | 5,160 (69.0) | 1,994 (75.2) | 1,122 (75.7) | <0.0001 |
| Diabetes mellitus | 7,506 (18.3) | 81 (6.6) | 1,390 (10.1) | 2,570 (17.7) | 1,936 (26.0) | 931 (35.3) | 598 (40.4) | <0.0001 |
| Renal insufficiency history | 0.0040 | |||||||
| No renal insufficiency | 40,664 (98.3) | 1,221 (98.2) | 13,643 (98.5) | 14,378 (98.2) | 7,360 (98.2) | 2,599 (97.8) | 1,463 (98.6) | |
| Creatinine ≥ 2 | 490 (1.2) | 9 (0.7) | 131 (0.9) | 189 (1.3) | 106 (1.4) | 40 (1.5) | 15 (1.0) | . |
| Dialysis of any type | 230 (0.6) | 13 (1.0) | 82 (0.6) | 79 (0.5) | 32 (0.4) | 18 (0.7) | 6 (0.4) | . |
| COPD | 14,781 (36.0) | 639 (51.7) | 5,194 (37.8) | 4,963 (34.2) | 2,559 (34.4) | 936 (35.6) | 490 (33.2) | <0.0001 |
| DLCO % predicted | 73.2 ± 21.3 | 64.7 ± 21.6 | 71.5 ± 21.8 | 74.1 ± 21.1 | 74.5 ± 20.6 | 75.9 ± 20.2 | 75.4 ± 20.5 | <0.0001 |
| FEV1 % predicted | 81.5 ± 20.3 | 75.5 ± 21.1 | 81.8 ± 21.0 | 82.3 ± 20.4 | 81.4 ± 19.6 | 79.5 ± 18.6 | 78.8 ± 18.8 | <0.0001 |
| Approach | <0.0001 | |||||||
| Thoracotomy | 20,771 (50.1) | 657 (52.6) | 6,805 (49.0) | 7,240 (49.4) | 3,819 (50.9) | 1,389 (52.3) | 861 (58.1) | |
| Thoracoscopy | 20,659 (49.9) | 591 (47.4) | 7,071 (51.0) | 7,422 (50.6) | 3,686 (49.1) | 1,268 (47.7) | 621 (41.9) | . |
| Preoperative chemotherapy | 3,859 (9.4) | 108 (8.7) | 1,427 (10.4) | 1,397 (9.6) | 616 (8.3) | 203 (7.7) | 108 (7.3) | <0.0001 |
| Preoperative radiation therapy | 3,302 (8.0) | 95 (7.7) | 1,236 (9.0) | 1,167 (8.0) | 550 (7.4) | 161 (6.1) | 93 (6.3) | <0.0001 |
| Pathological stage | 0.0349 | |||||||
| Stage I A/B | 26,708 (67.6) | 775 (65.5) | 8,924 (67.4) | 9,429 (67.4) | 4,888 (68.1) | 1,717 (68.1) | 975 (70.0) | |
| Stage II A/B | 7,386 (18.7) | 247 (20.9) | 2,529 (19.1) | 2,594 (18.6) | 1,300 (18.1) | 481 (19.1) | 235 (16.9) | . |
| Stage III A/B | 5,071 (12.8) | 156 (13.2) | 1,655 (12.5) | 1,851 (13.2) | 932 (13.0) | 301 (11.9) | 176 (12.6) | . |
| Stage IV | 332 (0.8) | 6 (0.5) | 134 (1.0) | 107 (0.8) | 55 (0.8) | 24 (1.0) | 6 (0.4) | . |
| Smoking status | <0.0001 | |||||||
| Never smoker | 6,142 (14.8) | 113 (9.1) | 2,093 (15.1) | 2,084 (14.2) | 1,110 (14.8) | 443 (16.7) | 299 (20.2) | |
| Quit smoking >1 month | 25,250 (60.9) | 525 (42.1) | 7,582 (54.6) | 9,430 (64.3) | 5,005 (66.7) | 1,763 (66.3) | 945 (63.7) | . |
| Recent smoker | 10,046 (24.2) | 610 (48.9) | 4,203 (30.3) | 3,151 (21.5) | 1,390 (18.5) | 453 (17.0) | 239 (16.1) | . |
Values are mean ± SD or n (%).
ASA = American Society of Anesthesiologists; COPD = chronic obstructive pulmonary disease; DLCO = diffusing capacity for carbon monoxide; FEV1 = forced expiratory volume in 1 second; SD = standard deviation.
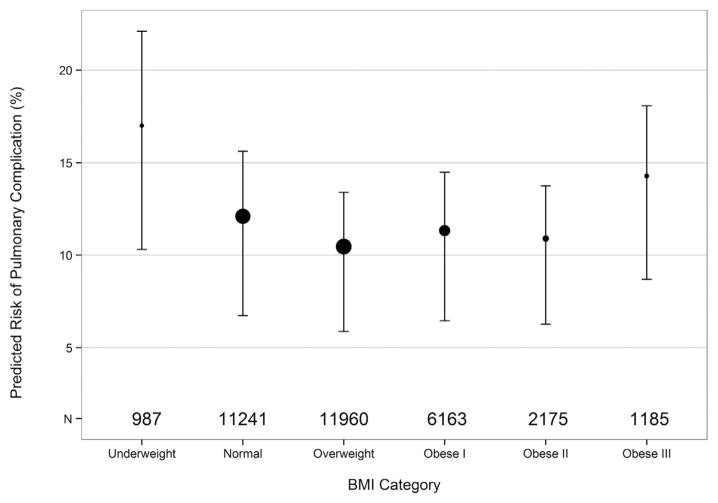
The incidence of operative mortality was low (1.7%), and did not differ significantly by BMI category (Table 2). Pulmonary complications were most frequent in underweight and obese III patients and were decreased in overweight and obese I and II patients relative to normal BMI patients (Fig 1). Surgical complications were most frequent in underweight patients and decreased with increasing BMI. Infectious complications were statistically higher in underweight patients and lowest in obese patients. Although there was no significant difference by BMI for the any major complication category, the incidence of any postoperative event differed significantly, with the highest percentage in the underweight group.
Table 2.
Univariate Analysis of Operative Outcomes
| Operative Outcome | All | Underweight | Normal | Overweight | Obese I | Obese II | Obese III | p Value |
|---|---|---|---|---|---|---|---|---|
| Primary procedure | 0.0019 | |||||||
| Segmentectomy | 2,030 (4.9) | 83 (6.7) | 648 (4.7) | 690 (4.7) | 400 (5.3) | 133 (5.0) | 76 (5.1) | |
| Lobectomy | 34,779 (83.9) | 1,018 (81.6) | 11,661 (84.0) | 12,308 (83.9) | 6,286 (83.8) | 2,269 (85.4) | 1,237 (83.5) | . |
| Sleeve lobectomy | 745 (1.8) | 16 (1.3) | 239 (1.7) | 272 (1.9) | 129 (1.7) | 53 (2.0) | 36 (2.4) | . |
| Bilobectomy | 1,632 (3.9) | 59 (4.7) | 568 (4.1) | 544 (3.7) | 305 (4.1) | 95 (3.6) | 61 (4.1) | . |
| Pneumonectomy | 2,244 (5.4) | 72 (5.8) | 760 (5.5) | 848 (5.8) | 385 (5.1) | 107 (4.0) | 72 (4.9) | . |
| Procedure time, min | 198.5 ± 136.1 | 200.3 ± 160.5 | 195.4 ± 139.7 | 198.1 ± 130.4 | 201.5 ± 132.3 | 204.8 ± 145.3 | 202.8 ± 135.2 | <0.0001 |
| Total intensive care unit days | 2.0 (4.6) | 2.8 (6.7) | 2.2 (5.4) | 1.9 (4.0) | 1.9 (4.0) | 1.9 (4.2) | 2.2 (3.9) | <0.0001 |
| Postoperative length of stay, days | 6.2 ± 7.1 | 8.0 ± 10.0 | 6.6 ± 7.9 | 5.9 ± 6.3 | 5.9 ± 7.1 | 5.8 ± 5.6 | 6.2 ± 5.1 | <0.0001 |
| Pulmonary complications | 4,774 (11.5) | 211 (16.9) | 1,684 (12.1) | 1,552 (10.6) | 828 (11.0) | 294 (11.1) | 205 (13.8) | <0.0001 |
| Cardiovascular complications | 6,087 (14.7) | 174 (13.9) | 1,998 (14.4) | 2,203 (15.0) | 1,108 (14.8) | 392 (14.7) | 212 (14.3) | 0.6934 |
| Infectious complications | 1,012 (2.4) | 48 (3.8) | 380 (2.7) | 318 (2.2) | 169 (2.3) | 57 (2.1) | 40 (2.7) | 0.0004 |
| Other complications | 2,876 (6.9) | 93 (7.5) | 1,012 (7.3) | 990 (6.8) | 494 (6.6) | 180 (6.8) | 107 (7.2) | 0.3297 |
| Surgical complications | 8,994 (21.7) | 446 (35.7) | 3,701 (26.7) | 2,916 (19.9) | 1,296 (17.3) | 398 (15.0) | 237 (16.0) | <0.0001 |
| Operative mortality | 698 (1.7) | 23 (1.8) | 240 (1.7) | 246 (1.7) | 121 (1.6) | 42 (1.6) | 26 (1.8) | 0.9759 |
| Any major complication | 10,161 (24.5) | 333 (26.7) | 3,437 (24.8) | 3,548 (24.2) | 1,813 (24.2) | 646 (24.3) | 384 (25.9) | 0.2551 |
| Any postoperative event | 16,028 (38.7) | 622 (49.8) | 5,763 (41.5) | 5,470 (37.3) | 2,700 (36.0) | 917 (34.5) | 556 (37.5) | <0.0001 |
Values are n (%) or mean ± SD.
SD = standard deviation.
Fig 1.
Risk of pulmonary complications stratified by body mass index (BMI) category. The size of each circle represents the number of patients (N) and the place of each circle indicates the mean in that group. Vertical bars indicate interquartile range (25th and 75th percentiles).
Following multivariable adjustment for extent of resection, American Society of Anesthesiologists class, performance status, renal dysfunction, induction chemotherapy, steroid use, age, urgent procedure, male gender, and FEV1 [20], compared to patients with normal BMI, overweight patients had a lower risk of operative mortality, and overweight and obese I to II patients had a lower risk of pulmonary complications and any postoperative event (Table 3). Obese III patients had an increased risk of any major postoperative complication compared to normal weight patients. Being underweight was independently associated with an increased risk of pulmonary complications and any postoperative event.
Table 3.
Multivariable Analysis of Outcomes Stratified by BMI
| Outcome | Underweight (<18.5 kg/m2) | Overweight (25–29.9 kg/m2) | Obese I (30–34.9 kg/m2) | Obese II (35–39.9 kg/m2) | Obese III (≥40 kg/m2) | Overall P value |
|---|---|---|---|---|---|---|
| Operative mortality | 1.12 (0.72–1.76) 0.6100 |
0.77 (0.64–0.93) 0.0065 |
0.85 (0.67–1.07) 0.1603 |
0.91 (0.64–1.29) 0.5984 |
1.35 (0.83–2.19) 0.2333 |
0.0581 |
| Any pulmonary complication |
1.41 (1.16–1.70) 0.0005 |
0.81 (0.74–0.88) <0.0001 |
0.87 (0.78–0.96) 0.0042 |
0.80 (0.68–0.93) 0.0038 |
1.12 (0.95–1.34) 0.1862 |
<0.0001 |
| Any major complication | 1.10 (0.94–1.28) 0.2539 |
0.90 (0.85–0.97) 0.0027 |
0.94 (0.87–1.01) 0.1110 |
0.96 (0.85–1.09) 0.5623 |
1.18 (1.02–1.36) 0.0283 |
0.0030 |
| Any postoperative event |
1.44 (1.26–1.64) <0.0001 |
0.79 (0.74–0.83) <0.0001 |
0.77 (0.72–0.82) <0.0001 |
0.70 (0.63–0.78) <0.0001 |
0.89 (0.79–1.01) 0.0716 |
<0.0001 |
Values are adjusted odds ratio (95% confidence interval) and P value. Outcomes that are statistically significant are in bold font.
BMI = body mass index.
Comment
The increasing rate and high prevalence of obesity in the United States is mirrored in the population of patients undergoing lung resection [21]. The conduct of thoracic surgery in obese patients takes longer and sometimes requires special perioperative considerations and instrumentation. Intuitively, prolonged surgery, decreased mobility, impaired diaphragm motion, and comorbidities associated with obesity should be associated with increased risk for lung resection. However, published reports demonstrate conflicting results regarding this issue [7–15].
Our analysis found that being overweight or mildly to moderately obese was not associated with an increase in operative risk. Patients in these categories had a lower incidence of complications such as operative mortality (overweight group only), pulmonary complications, and any postoperative event. These findings echo those of some other studies [8, 13, 14], suggesting the possibility that the obesity paradox is applicable to lung resection patients. However, not all categories of obesity were protective from complications. Patients in the obese III category, although evidencing a trend toward lower mortality and a decrease in any postoperative events, did experience a trend towards increased pulmonary complications and had a significantly increased risk of any major complication. Thus, overweight or mild to moderate obese status does confer an overall surgical advantage, whereas extreme obesity is associated with increased risk. The variability in outcomes may be related to the fact that high BMI fails to distinguish among body composition phenotypes that have an important impact on surgical risk and outcomes [22]. Reliance on increased BMI category alone to assess surgical risk of major lung resection is fraught with challenges.
There is growing recognition that underweight patients who require lung resection may pose a greater challenge for patient selection and perioperative management because of the increased incidence of adverse outcomes in this group [8, 13]. The current study identified underweight status as being associated with an increased risk of pulmonary complications and the occurrence of any postoperative event. Underweight patients did have an increased incidence of chronic obstructive pulmonary disease and of worse performance status compared to other BMI categories, but these factors were adjusted for in the multivariable analysis that demonstrated in increased risk for the underweight group. Whether being underweight was related to recent weight loss or was a chronic condition was not evaluable in this data set. The increased incidence of adverse events in the underweight group may be related to decreased strength of muscles of respiration, inactivity, fatigue, and overall weakness.
In contrast to varying phenotypes that are found among overweight and obese patients, being underweight is likely a reasonable surrogate for sarcopenia and is associated with a high incidence of frailty. Sarcopenia (low core muscle mass) is associated with decreased functional status and frailty, and the latter two conditions are associated with increased perioperative risk after major lung resection [23–26]. It is interesting to note that functional status and strength can be improved through a short-term exercise program [27, 28]. Exercise may be an appropriate intervention in clinical research aimed at decreasing perioperative complications in groups identified as being at increased risk for major lung resection.
Given the large data set used for this study, it is possible to identify statistically significant findings that may not be clinically important. We believe that our findings with regard to the relationship of extremes of BMI to outcomes after major lung resection are clinically important. The overall p value listed in Table 3 reflects the strength of the overall association of the BMI categories with the outcomes, and the odds ratios reflect the magnitude of those associations.
Potential shortcomings of our study include the imprecision of using BMI as a metric for outcomes. Body composition is often unrelated to BMI—obese patients may be sarcopenic or physically fit unrelated to BMI. Similarly, underweight patients may be chronically underweight or underweight as a result of recent weight loss. It is likely that chronically underweight patients with stable core muscle mass and functional status have substantially less risk than do patients who have experienced recent weight loss and a decrease in their functional status. The use of BMI as a categorical rather than a continuous variable constrained the analyses to pre-defined descriptive categories, the names of which have implications that may not be applicable to the current U.S. population. Not all patients in the STS General Thoracic Surgery Database had data on BMI, but the number was fortunately low (2.3%) and did not have an impact on the study outcomes. Similarly, not all patients with BMI data had sufficient information on clinical covariates to permit their use in the multivariable analyses. We believe that the patient data that were used for this purpose were representative of the overall cohort.
BMI is associated with postoperative complications after lung resection for cancer. Being underweight or severely overweight is associated with an increased risk of complications, whereas being overweight or moderately obese appears to have a protective effect. These findings may assist physicians in selecting patients for major lung resection and in providing appropriate resources for perioperative care. The use of exercise to mitigate effects of underweight status on surgical outcomes should be investigated in this population.
Acknowledgments
This work was funded by the Society of Thoracic Surgeons National Database. Brian C. Gulack, MD, is supported by the NIH funded Cardiothoracic Surgery Trials Network, 5U01HL088953-05.
Footnotes
Presented at the Fifty-second Annual Meeting of the Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016.
Winner of the Richard E. Clark Award for General Thoracic Surgery.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Colditz GA. Prevalence of overweight and obesity in the United States, 2007–2012. JAMA Intern Med. 2015;175:1412–3. doi: 10.1001/jamainternmed.2015.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Blume SW, Huang JC, Hammer M, Ganz ML. Prevalence and healthcare costs of obesity-related comorbidities: evidence from an electronic medical records system in the United States. J Med Econ. 2015;4:1–9. doi: 10.3111/13696998.2015.1067623. [DOI] [PubMed] [Google Scholar]
- 4.Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L NEDCOM the Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Ann Intern Med. 2003;138:24–32. doi: 10.7326/0003-4819-138-1-200301070-00008. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein EA, Brown DS, Wrage LA, Allaire BT, Hoerger TJ. Individual and aggregate years-of-life-lost associated with overweight and obesity. Obesity (Silver Spring) 2010;18:333–9. doi: 10.1038/oby.2009.253. [DOI] [PubMed] [Google Scholar]
- 6.St Julien JB, Aldrich MC, Sheng S, et al. Obesity increases operating room time for lobectomy in the Society of Thoracic Surgeons database. Ann Thorac Surg. 2012;94:1841–7. doi: 10.1016/j.athoracsur.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mungo B, Zogg CK, Hooker CM, et al. Does obesity affect the outcomes of pulmonary resections for lung cancer? A National Surgical Quality Improvement Program analysis Surgery. 2015;157:792–800. doi: 10.1016/j.surg.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Zogg CK, Mungo B, Lidor AO, et al. Influence of body mass index on outcomes after major resection for cancer. Surgery. 2015;158:472–85. doi: 10.1016/j.surg.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Launer H, Nguyen DV, Cooke DT. National perioperative outcomes of pulmonary lobectomy for cancer in the obese patient: a propensity score matched analysis. J Thorac Cardiovasc Surg. 2013;145:1312–8. doi: 10.1016/j.jtcvs.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Petrella F, Radice D, Borri A, et al. The impact of preoperative body mass index on respiratory complications after pneumonectomy for non-small-cell lung cancer. Results from a series of 154 consecutive standard pneumonectomies. Eur J Cardiothorac Surg. 2011;39:738–44. doi: 10.1016/j.ejcts.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Smith PW, Wang H, Gazoni LM, Shen KR, Daniel TM, Jones DR. Obesity does not increase complications after anatomic resection for non-small cell lung cancer. Ann Thorac Surg. 2007;84:1098–106. doi: 10.1016/j.athoracsur.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Dhakal B, Eastwood D, Sukumaran S, et al. Morbidities of lung cancer surgery in obese patients. J Thorac Cardiovasc Surg. 2013;146:379–84. doi: 10.1016/j.jtcvs.2013.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson MK, Im HK, Watson S, Johnson E, Wigfield CH, Vigneswaran WT. Association of body mass index and outcomes after major lung resection. Eur J Cardiothorac Surg. 2014;45:e94–9. doi: 10.1093/ejcts/ezu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas PA, Berbis J, Falcoz PE, et al. EPITHOR Group. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg. 2014;45:652–9. doi: 10.1093/ejcts/ezt452. [DOI] [PubMed] [Google Scholar]
- 15.Paul S, Andrews W, Osakwe NC, et al. Perioperative outcomes after lung resection in obese patients. Thorac Cardiovasc Surg. 2015;63:544–50. doi: 10.1055/s-0034-1383720. [DOI] [PubMed] [Google Scholar]
- 16.Reijnierse EM, Trappenburg MC, Leter MJ, et al. The association between parameters of malnutrition and diagnostic measures of sarcopenia in geriatric outpatients. PLoS One. 2015;10:e0135933. doi: 10.1371/journal.pone.0135933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65:377–81. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 18.Lan CC, Su CP, Chou LL, Yang MC, Lim CS, Wu YK. Association of body mass index with exercise cardiopulmonary responses in lung function-matched patients with chronic obstructive pulmonary disease. Heart Lung. 2012;41:374–81. doi: 10.1016/j.hrtlng.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. [Accessed November 27, 2015];BMI classification. 2006 Available at: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 20.Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90:875–83. doi: 10.1016/j.athoracsur.2010.03.115. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson MK, Vigneswaran WT. Changes in patient presentation and outcomes for major lung resection over three decades. Eur J Cardiothorac Surg. 2008;33:497–501. doi: 10.1016/j.ejcts.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care. 2015;18:535–51. doi: 10.1097/MCO.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 23.Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183:40–6. doi: 10.1016/j.jss.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 24.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res. 2013;183:104–10. doi: 10.1016/j.jss.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Miller AL, Min LC, Diehl KM, et al. Analytic morphomics corresponds to functional status in older patients. J Surg Res. 2014;192:19–26. doi: 10.1016/j.jss.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsiouris A, Horst HM, Paone G, Hodari A, Eichenhorn M, Rubinfeld I. Preoperative risk stratification for thoracic surgery using the American College of Surgeons National Surgical Quality Improvement Program data set: functional status predicts morbidity and mortality. J Surg Res. 2012;177:1–6. doi: 10.1016/j.jss.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 27.Marsh AP, Chmelo EA, Katula JA, Mihalko SL, Rejeski WJ. Should physical activity programs be tailored when older adults have compromised function? J Aging Phys Act. 2009;17:294–306. doi: 10.1123/japa.17.3.294. [DOI] [PubMed] [Google Scholar]
- 28.Drey M, Zech A, Freiberger E, et al. Effects of strength training versus power training on physical performance in prefrail community-dwelling older adults. Gerontology. 2012;58:197–204. doi: 10.1159/000332207. [DOI] [PubMed] [Google Scholar]