Abstract
The rapidly expanding Zika virus (ZIKV) epidemic has affected thousands of individuals with severe cases causing Guillain-Barré syndrome, congenital malformations, and microcephaly. Currently, there is no available vaccine or therapy to prevent or treat ZIKV infection. We evaluated whether sofosbuvir, an FDA-approved nucleotide polymerase inhibitor for the distantly related hepatitis C virus, could have antiviral activity against ZIKV infection. Cell culture studies established that sofosbuvir efficiently inhibits replication and infection of several ZIKV strains in multiple human tumor cell lines and isolated human fetal-derived neuronal stem cells. Moreover, oral treatment with sofosbuvir protected against ZIKV-induced death in mice. These results suggest that sofosbuvir may be a candidate for further evaluation as a therapy against ZIKV infection in humans.
1. INTRODUCTION
Zika virus (ZIKV) is a flavivirus of the Flaviviridae family that can be transmitted by the bite of female Aedes mosquitoes, through a sexual route, from a pregnant mother to her unborn fetus, by blood transfusion, or other bodily fluids (Anderson et al., 2016; Barzon et al., 2016; Miner et al., 2016; L. R. Petersen et al., 2016; Swaminathan et al, 2016). ZIKV was isolated initially from a rhesus monkey in the Zika forest near Entebbe, Uganda in 1947 (Dick, 1952; Dick et al., 1952), and is related closely to other viruses that cause significant global morbidity including yellow fever (YFV), Dengue (DENV), Japanese encephalitis (JEV), and West Nile (WNV) viruses (Lazear and Diamond, 2016). Although once an obscure virus that caused only sporadic outbreaks, ZIKV is now a major and emerging global health problem due to its epidemic spread in South, Central, and North America and ability to cause severe disease in utero and in adults.
Most symptomatic ZIKV infections cause a mild febrile illness associated with rash, arthralgia, and conjunctivitis (Lessler et al., 2016). The recent epidemics, however, have been linked to Guillian-Barré syndrome (GBS) in adults (Brasil et al., 2016; Cao-Lormeau et al., 2016; Oehler et al., 2014) and microcephaly in infants born to ZIKV-infected mothers (Araujo et al., 2016; Brasil et al., 2016; Brasil et al., 2016; Paploski et al., 2016; Rasmussen et al., 2016). ZIKV infection also can result in meningoencephalitis, shock syndrome (Zonneveld et al., 2016), spontaneous abortion, and intrauterine growth restriction (Carteaux et al., 2016; Miner et al., 2016). Despite the potentially devastating consequences of ZIKV to humans, currently there is no countermeasure to control infection and mitigate disease (E. E. Petersen et al., 2016). In light of the increasing global transmission of ZIKV, including locally-transmitted cases in the United States, the identification of potential antiviral drug targets and compounds with inhibitory activity has become urgent.
Similar to other flaviviruses, ZIKV is a ~10.8 kb positive-strand RNA virus containing 3′ and 5′ UTRs and a 5′ type 1 cap structure (Ye et al., 2016). After ZIKV enters into the host cell, the RNA genome serves as a template for translation of the viral polyprotein, which is later cleaved by host and viral proteases into ten constituent viral proteins. Three structural proteins (the capsid [C], pre-membrane [prM], and the envelope [E]) contribute to the formation of new viral particles, whereas the seven remaining nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) contribute to virus replication and immune evasion. ZIKV NS5 is divided into two domains. The N-terminus contains the methyltransferase and guanylyltransferase activities that contribute to formation of the 5′ cap structure (Bollati et al., 2009; Egloff et al., 2007; Geiss et al., 2009; Issur et al., 2009) and the C-terminus contains the RNA-dependent RNA polymerase (RdRp) that facilitates synthesis of new viral genomes (Choi et al., 2004; Grun and Brinton, 1986; Tan et al., 1996). The RdRp domain is an attractive target for antiviral drug design because human cells lack RdRp activity, resulting in fewer deleterious side effects from RdRp inhibitors (Deng et al., 2016; Zmurko et al., 2016; Zou et al., 2011). Targeting of the RdRp domain and its enzymatic activity with small molecule compounds has been a successful antiviral strategy for other related RNA viruses including hepatitis C virus (HCV), which also is a member of the Flaviviridae family. Sofosbuvir is an RdRp inhibitor that is approved by the Food and Drug Administration (FDA) for the treatment of HCV infection (Keating and Vaidya, 2014). Sofosbuvir is an orally available nucleotide analog inhibitor prodrug; in hepatocytes it is metabolized to 2′-F-2′-C-methyluridine monophosphate and converted to the active triphosphate form that inhibits HCV replication by acting as a chain terminator during synthesis of new viral genomes (Murakami et al., 2010; Sofia et al., 2010). A recent report suggested that sofosbuvir may be active against ZIKV in human neuroepithelial stem cells by demonstrating that ZIKV NS1 antigen staining was reduced by treatment with 20 μM and 100 μM sofosbuvir (Onorati et al., 2016).
Given the high degree of structural conservation within the RdRp domain of the Flaviviridae family members (Lim et al., 2015) and known activity of sofosbuvir against HCV, we evaluated its effects on ZIKV infection. Cell culture experiments showed that sofosbuvir inhibited replication of multiple ZIKV strains corresponding to different geographical lineages in human hepatoma and placental cell lines and in human fetal-derived neuronal stem cells. In vivo studies in mice suggested that treatment with sofosbuvir could protect against lethal ZIKV challenge. These results indicate that sofosbuvir may have promise as an antiviral for treatment of ZIKV infection in humans.
2. MATERIALS AND METHODS
2.1 Ethics statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Institutional Animal Care and Use Committee at the Washington University School of Medicine (Assurance Number: A3381-01).
2.2 Viruses
ZIKV strains PRVABC59 (Puerto Rico, 2015) was a kind gift of A. Brault (Centers from Disease Control and Prevention, Fort Collins, CO). ZIKV Dakar 41519 strain (Senegal, 1984) and Brazilian (Paraiba 2015) were provided from the World Reference Center for Emerging Viruses and Arboviruses (R. Tesh, University of Texas Medical Branch). ZIKV stocks were propagated in Vero cells after inoculating at a MOI of 0.1 and incubating for 72 h. Viral titers were quantified by plaque assay as previously described (Lazear et al., 2016) and stocks were stored at −80°C in single-use aliquots.
2.3 Cells
Vero (African Green Monkey kidney epithelial) cells and Huh-7 (human hepatocellular carcinoma) cells were maintained in Dulbecco’s modified Eagle medium. Jar (human placental choriocarcinoma) cells were maintained in RPMI 1640. All medium was supplemented with 10% fetal bovine serum (Atlas) and L-glutamine, and cells were incubated at 37°C in humidified incubators supplemented with 5% CO2. Fetal NSCs were obtained commercially from Clontech (Human Neural Cortex (Y40050) and Hindbrain (Y40060) Stem Cell Line Kits). The NSCs were maintained in Neurobasal®-A without phenol red (Thermo Fisher) with the addition of B27 supplement (1:100, Thermo Fisher, #12587010), N2 supplement (1:200, Invitrogen, #17502-048), 20 ng/ml FGF (R&D Systems 4114-TC-01M), 20 ng/ml EGF (R&D Systems 236-EG-01M), GlutaMax (Thermo Fisher, #35050061), and sodium pyruvate. The cells were plated into dishes pre-coated with laminin (10 μg/ml, Sigma # L2020). Cells were grown to near confluency (80 to 90%) prior to passage. For passaging, cells were rinsed gently with 1X PBS (without calcium and magnesium) and then accutase (Sigma, A6964) was added for 5 min at 37°C to allow detachment.
2.4 In vitro viral infection and drug treatment experiments
Jar and Huh-7 cells were plated in 12-well plates and allowed to attach overnight. Sofosbuvir (Sellekchem) was dissolved in dimethyl sulfoxide (DMSO) and diluted in DMEM media. At 50% cell confluence, sofosbuvir was diluted serially and added to cell medium at final concentrations ranging from 1 to 500 μM with a final concentration of 1% DMSO. Cells were infected at the same time of drug or vehicle administration with ZIKV PRVABC59, Dakar 41519, or Paraiba strains at a MOI of 0.1, and plates were incubated at 37°C for 72 h. All conditions were plated in triplicate, and DMSO vehicle only and uninfected cells were used as controls. Cell supernatants were stored at −80°C for subsequent analysis by plaque and qRT-PCR assays. Plaque assays were performed with Vero cells as described previously with minor modifications (Aliota et al., 2016). Briefly, samples were diluted in 10-fold dilutions and added to Vero cell monolayers in 24-well plates. Virus was allowed to infect for 1 h and then 1 ml of a 1:1 solution of 2X DMEM with 10% FBS and 1% agarose was added to cells. Plates were inverted and incubated for four days. Subsequently, 100 μl of 0.5% (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (MTT) in PBS was added, and plates were incubated for a further 24 h at 37°C before plaques were counted. qRT-PCR analysis was performed as previously described (Lazear et al., 2016). Sofosbuvir cytotoxicity was quantified as described previously using CellTiterGlo reagent (Promega) (Stahla-Beek et al., 2012) for all cell lines.
NSCs (105) were seeded into 24-well plates 12 h prior to infection. ZIKV Paraiba was added at a MOI 5 for 2 h followed by addition of sofosbuvir. In all experiments, mock infected cells were incubated in parallel. Forty-eight hours after ZIKV infection, cells were fixed with 2% PFA diluted in PBS for 10 min at room temperature and permeabilized with HBSS buffer (10 mM HEPES, 0.1% (w/v) saponin (Sigma), and 0.025% NaN3 for 10 min at room temperature). NSCs were transferred to a V-bottom plate and incubated for 1 h at 4°C with 2 μg/mL of ZV-64 mAb (Zhao et al., 2016). After washing, cells were incubated with an Alexa Fluor 647-conjugated goat anti-mouse IgG (Invitrogen) for 30 min, washed twice with HBSS buffer, processed on a FACS Array (BD Biosciences), and analyzed using FlowJo software (Tree Star).
2.5 Mouse experiments
C57BL/6J mice (5 week-old, Jackson Laboratories) were inoculated with ZIKV by subcutaneous (footpad) route with 105 FFU of mouse-adapted ZIKV Dakar 41519) (Govero et al., 2016) in a volume of 50 μl. Treatment with sofosbuvir was performed as follows: one day after ZIKV infection, mice were administered 33 mg/kg/day (0.67 mg of sofosbuvir per day for each 20 g mouse) of sofosbuvir dissolved in Kool-Aid or Kool-Aid vehicle control for seven consecutive days (+1, +2, +3, +4, +5, +6, and +7). Daily weights were measured and survival was monitored for a total 21 days.
2.6 Statistical analysis
All data were analyzed with GraphPad Prism software. Kaplan-Meier survival curves were analyzed by the log rank test, and weight loss and viral infections were compared using an ANOVA with a multiple comparisons test. A P value of < 0.05 indicated statistically significant differences.
3. RESULTS
3.1 Inhibition of ZIKV infection in cells by sofosbuvir
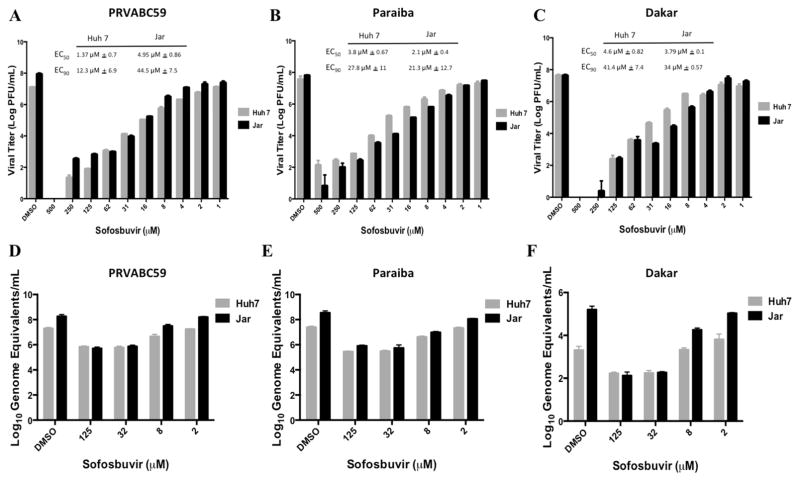
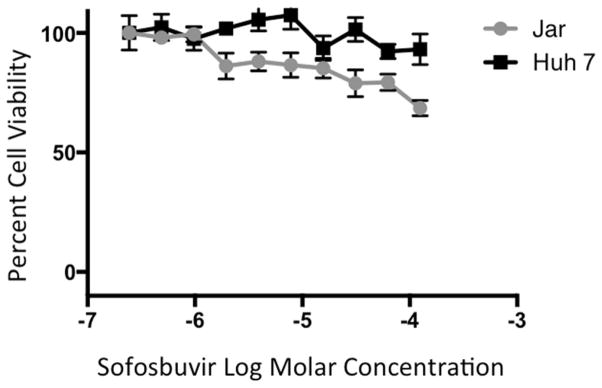
We tested whether sofosbuvir could inhibit the replication of ZIKV in cell culture. Increasing concentrations of sofosbuvir ranging from 1 to 500 μM were added to human Huh-7 hepatoma or Jar human placental choriocarcinoma cells. Cell cultures were inoculated at a multiplicity of infection (MOI) of 0.1 with different ZIKV strains (Dakar 41519 (Senegal, 1984), PRVABC59 (Puerto Rico 2015), or Paraiba (Brazil, 2015)) corresponding to African and American isolates. Three days later, cell culture supernatants were tested for viral yield by plaque and qRT-PCR assays. Plaque assay results revealed that high concentrations of sofosbuvir reduced infectious virus yield in both Huh-7 and Jar cells with EC50 values of 1 μM - 5 μM and EC90 values of 12 μM - 44 μM for all three ZIKV strains (Fig 1A–C). Analysis of viral RNA by qRT-PCR showed a similar inhibition by sofosbuvir, especially at higher concentrations of drug (Fig 1D–F). Importantly, the CC50 of sofosbuvir in these cells was > 200 μM (Fig 2), resulting in a selectivity index of ≥40.
Figure 1. Sofosbuvir reduces Zika virus titer in Huh-7 and Jar cells.
Huh-7 and Jar cells were treated with concentrations of Sofosbuvir from 500 μM to 1 μM and concurrently infected with ZIKV PRVABC59, Dakar 41519, or Paraiba strains at a MOI of 0.1. Plates were incubated at 37°C for 72 h and viral titers at each concentration were calculated by plaque assay (A–C) and qRT-PCR (D–F). Results are the average of three independent biological replicates with standard deviation shown.
Figure 2. Sofosbuvir cytotoxicity in Huh-7 and Jar cells.
Huh-7 and Jar cells were seeded in 96 well plates and allowed to attach overnight. Sofosbuvir was added from 200 μM to 0.4 μM and plates were incubated for 72 h. CellTiter-Glo reagent (Promega) was added in equal volume to media and wells were read for luminescence after 10 minutes at 37°C. Results are the average of three independent biological replicates with standard deviation shown.
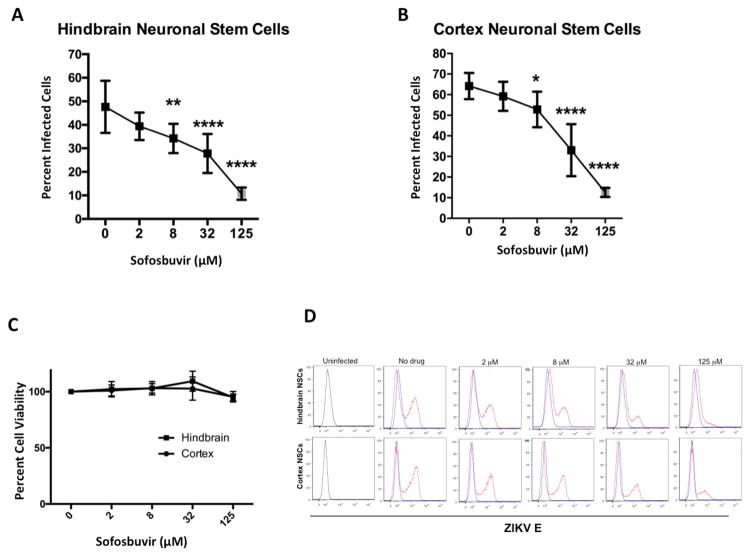
Sofosbuvir next was tested for antiviral effects in more physiologically relevant human fetal-derived hindbrain and cerebral cortex neuronal stem cells (NSCs). NSC were exposed to ZIKV Paraiba at an MOI of 5, treated with 2 μM – 125 μM sofosbuvir at time 0, incubated for 48 hours, and then analyzed for ZIKV E protein antigen by flow cytometry. Sofosbuvir significantly inhibited ZIKV infection of hindbrain (Fig 3A) or cerebral cortex (Fig 3B)-derived NSCs cells at concentrations of 8 μM or greater, with an EC50 of ~32 μM. Importantly, sofosbuvir was essentially non-toxic to both cell types (Fig 3C. The antiviral activity in the non-hepatocyte Jar placental cells and human NSCs confirmed that the sofosbuvir prodrug likely would be activated in non-hepatic tissues, which is critical as ZIKV targets other cell types in vivo including neuroprogenitor cells.
Figure 3. Sofosbuvir reduces ZIKV infection in human fetal-derived neuronal stem cells.
Human NSCs derived from fetal hindbrain or cerebral cortex were infected with ZIKV Paraiba at an MOI of 5 and treated with the indicated concentrations of sofosbuvir. A–B. Forty-eight hours later cells were harvested, fixed, permeabilized, stained with an anti-E protein antibody, and processed by flow cytometry. The data is pooled from three independent experiments, each performed in triplicate. The error bars indicate standard deviations. *, P < 0.05; **, P < 0.01; ****, P < 0.0001 (one-way ANOVA with multiple comparisons correction compared to 0 μM). C. Cytotoxicity analysis of sofosbuvir in human NSCs. NSCs were incubated with the indicated concentrations sofosbuvir for 48 hours, then cell viability was determined using CellTiter-Glo. The data is pooled from two independent experiments performed in duplicate. D. Flow cytometry histograms from data in panels A and B showing inhibitory effect of sofosbuvir against ZIKV infection in human NSCs. One representative experiment of three is shown.
3.2 In vivo protection with sofosbuvir
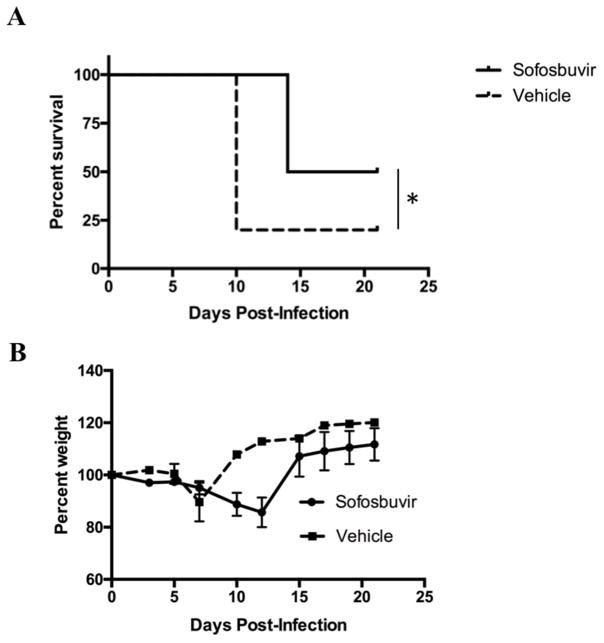
In recent studies, we generated models of ZIKV pathogenesis in mice deficient in type I IFN signaling (Lazear et al., 2016; Miner et al., 2016; Zhao et al., 2016). To evaluate whether sofosbuvir protects against ZIKV infection in vivo, we treated 5 week-old WT C57BL/6 mice beginning at day −1 with a single dose of anti-Ifnar1 antibody and then infected animals at day 0 with a mouse-adapted African ZIKV strain (Dakar 41519) that is more pathogenic in mice than isolates from Asia or the Americas (Lazear et al., 2016; Miner et al., 2016; Zhao et al., 2016). Sofosbuvir is orally bioavailable in humans, so we tested if sustained oral treatment by administration of drug in drinking water would provide protection against ZIKV in mice. Sofosbuvir (estimated at 33 mg/kg/day per oral route) or vehicle control were administered to mice via addition to their drinking water (supplemented with Kool-Aid® to promote oral intake) beginning at day +1 after ZIKV infection for seven consecutive days (from day +1 to +7). Therapeutic administration via drinking of sofosbuvir protected some of the animals against disease with greater overall survival rates observed compared to vehicle-treated controls (Fig 4A, P < 0.05). Mice that succumbed to ZIKV infection showed marked weight loss, whereas surviving infection maintained weight during the course of the experiment (Fig 4B).
Figure 4. Therapeutic effect of sofosbuvir in mice.
Five week-old WT C57BL/6 mice were treated at day −1 with 2 mg of anti-Ifnar1 blocking mAb. On day 0, animals were inoculated via a subcutaneous route in the footpad with 105 FFU of mouse-adapted ZIKV Dakar. On day 1, oral therapy was initiated with ~33 mg/kg/day sofosbuvir dissolved in Kool-Aid® or Kool-Aid® vehicle control. Survival (A) and aggregate body weights (B) were recorded. Results are pooled from two independent experiments with a total of n = 10 in each group. *, P < 0.05 (log-rank test).
4. DISCUSSION
ZIKV continues to spread in the Americas and other parts of the world. The severe clinical manifestations associated with ZIKV infection have prompted an urgent call for the development and implementation of effective anti-ZIKV therapeutics and vaccines. Although the need for an anti-ZIKV drug therapy is great, this challenge is occurring in the background of an absence of commercially available treatments for any member of the Flavivirus genus. Our study reports promising antiviral results against ZIKV infection with an existing, available FDA-approved HCV drug sofosbuvir.
A recent study suggested that sofosbuvir has activity against ZIKV (Onorati et al., 2016), but this study only looked at ZIKV NS1 staining in the presence of 20 μM and 100 μM sofosbuvir and did not assess the effect of sofosbuvir against viral replication. Therefore, demonstration of the efficacy of sofosbuvir against viral production and establishment of EC50 and therapeutic index values is critical for further evaluation of sofosbuvir as a potential anti-ZIKV agent. We found that ZIKV infectivity was significantly inhibited by sofosbuvir in vitro. Sofosbuvir treatment of different cell types of distinct lineage inhibited infection with a selectivity index of ≥40, indicating a substantial difference between toxic and therapeutic concentrations. Although studies with a more extensive panel of ZIKV strains is necessary, the similar responses to sofosbuvir by African and American ZIKV strains, which vary by 3 to 5% at the amino acid level, suggest that a range of circulating strains will be sensitive to treatment, increasing the likelihood that sofosbuvir will be useful over a broad geographic range.
Sofosbuvir is a nucleotide analog inhibitor that became commercially available in 2013 and is currently formulated in combination with ledipasvir (as Harvoni®) as a treatment for chronic HCV infection. In Huh-7.5 cells, sofosbuvir shows an EC50 of ~500–850 nM against genotype 1a and 3a HCV viruses, which is close to the EC50 of sofosbuvir against ZIKV in Huh-7 cells (1.37 uM – 4.6 uM) (Ramirez et al., 2014; Ramirez et al., 2016). Sofosbuvir has a high rate of viral clearance, relatively few side effects, and requires a shorter course of treatment compared to prior HCV therapies (Berden et al., 2014; Cholongitas and Papatheodoridis, 2014). Though sofosbuvir resistance development has been reported, and is mainly associated with S282T mutations in the HCV NS5B RdRp (Lam et al., 2012), it has been slower to develop compared to inhibitors of other HCV proteins such as the NS3/4A protease (Rong et al., 2010; Sarrazin and Zeuzem, 2010). This may be due to the high degree amino acid conservation in the RdRp domain and the lack of fitness observed in viruses with drug-induced RdRp mutations (Dutartre et al., 2006; Koonin and Dolja, 1993; Migliaccio et al., 2003). Studies examining the potential for sofosbuvir resistance to occur with ZIKV are currently underway. Moreover, as other candidate drug therapies against ZIKV are validated (Xu et al., 2016), combination studies with sofosbuvir could be initiated, which would be expected to limit resistance emergence.
Using a recently established ZIKV pathogenesis model in WT C57BL/6 mice treated with an anti-Ifnar1 blocking antibody (Lazear et al., 2016), we showed that sofosbuvir protected mice against ZIKV disease and lethality. Sofosbuvir is orally bioavailable and is administered via ingestion, so to provide a sustained dosing of drug we chose to administer sofosbuvir in drinking water. Therapeutic oral administration of sofosbuvir with a physiologically relevant dose (~33 mg/kg/day × 7 days) prevented weight loss and death in 50% of treated mice. However, higher concentrations of sofosbuvir via oral administration were not effective and resulted in toxicity to mice (J. Govero and M. Diamond, unpublished observations). There may be differences in the metabolism of sofosbuvir in mice compared to humans that result in less efficacy and/or greater toxicity in mice; whereas the pharmacokinetics/pharmacodynamics of sofosbuvir are well described in primates and humans (Osinusi et al., 2013; Regan et al., 2016; Rodriguez-Torres et al., 2013), studies in mice have not been published. Nonetheless, unpublished pharmacokinetic studies in mice by Gilead indicated that sofosbuvir (Sovaldi) had low stability in rodent serum, potentially due to high esterase activity (European Medicines Agency assessment report EMA/CHMP/688774/2013). While sofosbuvir efficacy in mice is modest, this report is the first in vivo demonstration that a small molecule therapeutic can protect animals against ZIKV infection. Many ZIKV pathogenesis studies currently are being conducted in mice, and rodents are considered first-line animal models for antiviral drug testing. Because sofosbuvir is less stable in rodent serum, its anti-ZIKV activity in vivo may be underestimated in mice. Demonstration of efficacy in small animal models is generally a prerequisite for therapeutic testing in non-human primates and human volunteers, and our findings provide justification for further in vivo testing. We suggest that the efficacy of sofosbuvir against ZIKV should be assessed further in non-human primates or human patients rather than in mice.
Although animal studies with sofosbuvir have failed to reveal evidence of fetal harm (Cada et al., 2014), there are no controlled data in human pregnancy. Sofosbuvir is recommended for use in combination with ribavirin (which can cause birth defects) and/or pegylated interferon (which displays abortifacient effects) to treat HCV infection and has therefore been classified as category B and not recommended for use in pregnant women. Therefore, the immediate use of sofosbuvir as a monotherapy for preventing microcephaly and other congenital malformations seems unlikely until the effects of sofosbuvir administration during pregnancy are well-understood. So what populations might benefit from treatment? Although clinical trials are needed, sofosbuvir might be used in men and non-pregnant women to prevent chronic persistent infection or damage in tissues (e.g., male reproductive tract (Govero et al., 2016; Mansuy et al., 2016; Turmel et al., 2016)) that could promote sexual transmission or diminish viremia to disrupt the infection cycle with Aedes aegypti mosquitoes. High-risk individuals entering into an endemic area for brief periods also might benefit from short courses of sofosbuvir prophylaxis. However, a key factor in sofosbuvir being used to prophylactically or therapeutically treat ZIKV infection is price. Currently, a 12-week regimen to treat chronic HCV infection costs approximately $84,000. This high cost may put sofosbuvir out of reach for many individuals in low-income areas unless pricing is reduced. Therefore, sofosbuvir treatment may be a valuable tool in combatting ZIKV spread.
5. CONCLUSIONS
We evaluated whether sofosbuvir, an FDA-approved nucleotide polymerase inhibitor for the distantly related hepatitis C virus, could have antiviral potential for ZIKV infection. Cell culture studies established that sofosbuvir efficiently inhibits replication and infection of several ZIKV strains in multiple human tumor cell lines and isolated human fetal-derived neuronal stem cells without significant drug toxicity. Moreover, oral treatment with sofosbuvir protected against ZIKV-induced death in mice. Sofosbuvir may be a candidate for further evaluation as a therapy against ZIKV infection in non-human primates and ultimately humans.
Highlights.
Sofosbuvir reduces replication of multiple ZIKV isolates in human liver and placental cells.
Sofosbuvir protects human neuronal stem cells from ZIKV infection.
Oral administration of sofosbuvir via drinking reduces ZIKV death in mice.
Sofosbuvir should be evaluated as an anti-ZIKV treatment in non-rodent species.
Acknowledgments
This work was in part supported by grants from the Colorado State University College Research Council, NIH R01 AI073755 to M.S.D., and NIH R01 AI114675 to B.J.G. We thank Dr. Jeremy Rich for support of Dr. Zhe Zhu.
Footnotes
AUTHOR CONTRIBUTIONS
K.M.B, J.G, Z.Z., M.S.D., and B.J.G. designed experiments. K.M.B, J.G., V.S., M.V, Z.Z., and B.J.G. performed experiments. K.M.B., J.G., M.V., B.J.G., M.S.D., and J.G. analyzed data. K.M.B., M.S.D, and B.J.G. wrote the first draft of the manuscript and all authors edited it subsequently.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016;10:e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KB, Thomas SJ, Endy TP. The Emergence of Zika Virus: A Narrative Review. Ann Intern Med. 2016;165:175–183. doi: 10.7326/M16-0617. [DOI] [PubMed] [Google Scholar]
- Araujo LM, Ferreira MLB, Nascimento OJ. Guillain-Barré syndrome associated with the Zika virus outbreak in Brazil. Arq Neuropsiquiatr. 2016;74:253–255. doi: 10.1590/0004-282X20160035. [DOI] [PubMed] [Google Scholar]
- Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, Palù G. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill. 2016;21:30316. doi: 10.2807/1560-7917.ES.2016.21.32.30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berden FAC, Kievit W, Baak LC, Bakker CM, Beuers U, Boucher CAB, Brouwer JT, Burger DM, van Erpecum KJL, van Hoek B, Hoepelman AIM, Honkoop P, Kerbert-Dreteler MJ, de Knegt RJ, Koek GH, van Nieuwkerk CMJ, van Soest H, Tan ACITL, Vrolijk JM, Drenth JPH. Dutch guidance for the treatment of chronic hepatitis C virus infection in a new therapeutic era. Neth J Med. 2014;72:388–400. [PubMed] [Google Scholar]
- Bollati M, Milani M, Mastrangelo E, Ricagno S, Tedeschi G, Nonnis S, Decroly E, Selisko B, de Lamballerie X, Coutard B, Canard B, Bolognesi M. Recognition of RNA cap in the Wesselsbron virus NS5 methyltransferase domain: implications for RNA-capping mechanisms in Flavivirus. J Mol Biol. 2009;385:140–152. doi: 10.1016/j.jmb.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Brasil P, Pereira JP, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, Carvalho de Sequeira P, Machado Siqueira A, Abreu de Carvalho LM, Cotrim da Cunha D, Calvet GA, Neves ES, Moreira ME, Rodrigues Baião AE, Nassar de Carvalho PR, Janzen C, Valderramos SG, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med NEJMo. 2016:a1602412. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil P, Sequeira PC, Freitas AD, Zogbi HE, Calvet GA, de Souza RV, Siqueira AM, de Mendonca MCL, Nogueira RMR, de Filippis AMB, Solomon T. Guillain-Barré syndrome associated with Zika virus infection. Lancet. 2016;387:1482. doi: 10.1016/S0140-6736(16)30058-7. [DOI] [PubMed] [Google Scholar]
- Cada DJ, Cong J, Baker DE. Sofosbuvir. Hosp Pharm. 2014;49:466–478. doi: 10.1310/hpj4905-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra JC, Desprès P, Fournier E, Mallet HP, Musso D, Fontanet A, Neil J, Ghawché F. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteaux G, Maquart M, Bedet A, Contou D, Brugières P, Fourati S, Cleret de Langavant L, de Broucker T, Brun-Buisson C, Leparc-Goffart I, Mekontso Dessap A. Zika Virus Associated with Meningoencephalitis. N Engl J Med. 2016;374:1595–1596. doi: 10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]
- Choi KH, Groarke JM, Young DC, Kuhn RJ, Smith JL, Pevear DC, Rossmann MG. The structure of the RNA-dependent RNA polymerase from bovine viral diarrhea virus establishes the role of GTP in de novo initiation. PNAS. 2004;101:4425–4430. doi: 10.1073/pnas.0400660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholongitas E, Papatheodoridis GV. Sofosbuvir: a novel oral agent for chronic hepatitis C. Ann Gastroenterol. 2014;27:331–337. [PMC free article] [PubMed] [Google Scholar]
- Deng Y-Q, Zhang N-N, Li C-F, Tian M, Hao J-N, Xie X-P, Shi P-Y, Qin C-F. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect Dis. 2016;3:ofw175. doi: 10.1093/ofid/ofw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GWA. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- Dick GWA, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Dutartre H, Bussetta C, Boretto J, Canard B. General catalytic deficiency of hepatitis C virus RNA polymerase with an S282T mutation and mutually exclusive resistance towards 2′-modified nucleotide analogues. Antimicrob Agents Chemother. 2006;50:4161–4169. doi: 10.1128/AAC.00433-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff M-P, Decroly E, Malet H, Selisko B, Benarroch D, Ferron F, Canard B. Structural and functional analysis of methylation and 5′-RNA sequence requirements of short capped RNAs by the methyltransferase domain of dengue virus NS5. J Mol Biol. 2007;372:723–736. doi: 10.1016/j.jmb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Geiss BJ, Thompson AA, Andrews AJ, Sons RL, Gari HH, Keenan SM, Peersen OB. Analysis of flavivirus NS5 methyltransferase cap binding. J Mol Biol. 2009;385:1643–1654. doi: 10.1016/j.jmb.2008.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH, Diamond MS. Zika virus infection damages the testes in mice. Nature. 2016 doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun JB, Brinton MA. Characterization of West Nile virus RNA-dependent RNA polymerase and cellular terminal adenylyl and uridylyl transferases in cell-free extracts. J Virol. 1986;60:1113–1124. doi: 10.1128/jvi.60.3.1113-1124.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issur M, Geiss BJ, Bougie I, Picard-Jean F, Despins S, Mayette J, Hobdey SE, Bisaillon M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA. 2009;15:2340–2350. doi: 10.1261/rna.1609709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating GM, Vaidya A. Sofosbuvir: first global approval. Drugs. 2014;74:273–282. doi: 10.1007/s40265-014-0179-7. [DOI] [PubMed] [Google Scholar]
- Koonin EV, Dolja VV. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- Lam AM, Espiritu C, Bansal S, Micolochick Steuer HM, Niu C, Zennou V, Keilman M, Zhu Y, Lan S, Otto MJ, Furman PA. Genotype and subtype profiling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus. Antimicrob Agents Chemother. 2012;56:3359–3368. doi: 10.1128/AAC.00054-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Diamond MS. Zika Virus: New Clinical Syndromes and Its Emergence in the Western Hemisphere. J Virol. 2016;90:4864–4875. doi: 10.1128/JVI.00252-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host and Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessler J, Chaisson LH, Kucirka LM, Bi Q, Grantz K, Salje H, Carcelen AC, Ott CT, Sheffield JS, Ferguson NM, Cummings DAT, Metcalf CJE, Rodriguez-Barraquer I. Assessing the global threat from Zika virus. Science. 2016;353:aaf8160–aaf8160. doi: 10.1126/science.aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SP, Noble CG, Shi P-Y. The dengue virus NS5 protein as a target for drug discovery. Antiviral Res. 2015;119:57–67. doi: 10.1016/j.antiviral.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16:405. doi: 10.1016/S1473-3099(16)00138-9. [DOI] [PubMed] [Google Scholar]
- Migliaccio G, Tomassini JE, Carroll SS, Tomei L, Altamura S, Bhat B, Bartholomew L, Bosserman MR, Ceccacci A, Colwell LF, Cortese R, De Francesco R, Eldrup AB, Getty KL, Hou XS, LaFemina RL, Ludmerer SW, MacCoss M, McMasters DR, Stahlhut MW, Olsen DB, Hazuda DJ, Flores OA. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J Biol Chem. 2003;278:49164–49170. doi: 10.1074/jbc.M305041200. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao Bin, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Sene A, Richner JM, Smith AM, Santeford A, Ban N, Weger-Lucarelli J, Manzella F, Rückert C, Govero J, Noguchi KK, Ebel GD, Diamond MS, Apte RS. Zika Virus Infection in Mice Causes Panuveitis with Shedding of Virus in Tears. Cell Rep. 2016;16:3208–3218. doi: 10.1016/j.celrep.2016.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami E, Tolstykh T, Bao H, Niu C, Steuer HMM, Bao D, Chang W, Espiritu C, Bansal S, Lam AM, Otto MJ, Sofia MJ, Furman PA. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J Biol Chem. 2010;285:34337–34347. doi: 10.1074/jbc.M110.161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, Baudouin L, Mallet H, Musso D, Ghawche F. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill. 2014;19 doi: 10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- Onorati M, Li Z, Liu F, Sousa AMM, Nakagawa N, Li M, Dell’Anno MT, Gulden FO, Pochareddy S, Tebbenkamp ATN, Han W, Pletikos M, Gao T, Zhu Y, Bichsel C, Varela L, Szigeti-Buck K, Lisgo S, Zhang Y, Testen A, Gao XB, Mlakar J, Popovic M, Flamand M, Strittmatter SM, Kaczmarek LK, Anton ES, Horvath TL, Lindenbach BD, Sestan N. Zika Virus Disrupts Phospho-TBK1 Localization and Mitosis in Human Neuroepithelial Stem Cells and Radial Glia. Cell Rep. 2016;16:2576–2592. doi: 10.1016/j.celrep.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinusi A, Meissner EG, Lee YJ, Bon D, Heytens L, Nelson A, Sneller M, Kohli A, Barrett L, Proschan M, Herrmann E, Shivakumar B, Gu W, Kwan R, Teferi G, Talwani R, Silk R, Kotb C, Wroblewski S, Fishbein D, Dewar R, Highbarger H, Zhang X, Kleiner D, Wood BJ, Chavez J, Symonds WT, Subramanian M, McHutchison J, Polis MA, Fauci AS, Masur H, Kottilil S. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013;310:804–811. doi: 10.1001/jama.2013.109309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paploski IAD, Prates APPB, Cardoso CW, Kikuti M, Silva MMO, Waller LA, Reis MG, Kitron U, Ribeiro GS. Time Lags between Exanthematous Illness Attributed to Zika Virus, Guillain-Barré Syndrome, and Microcephaly, Salvador, Brazil. Emerging Infect Dis. 2016;22:1438–1444. doi: 10.3201/eid2208.160496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen EE, Staples JE, Meaney-Delman D, Fischer M, Ellington SR, Callaghan WM, Jamieson DJ. Interim Guidelines for Pregnant Women During a Zika Virus Outbreak--United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:30–33. doi: 10.15585/mmwr.mm6502e1. [DOI] [PubMed] [Google Scholar]
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Li YP, Jensen SB, Pedersen J, Gottwein JM, Bukh J. Highly efficient infectious cell culture of three hepatitis C virus genotype 2b strains and sensitivity to lead protease, nonstructural protein 5A, and polymerase inhibitors. Hepatology. 2014;59:395–407. doi: 10.1002/hep.26660. [DOI] [PubMed] [Google Scholar]
- Ramirez S, Mikkelsen LS, Gottwein JM, Bukh J. Robust HCV Genotype 3a Infectious Cell Culture System Permits Identification of Escape Variants With Resistance to Sofosbuvir. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects--Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- Regan CP, Morissette P, Regan HK, Travis JJ, Gerenser P, Wen J, Fitzgerald K, Gruver S, DeGeorge JJ, Sannajust FJ. Assessment of the clinical cardiac drug-drug interaction associated with the combination of hepatitis C virus nucleotide inhibitors and amiodarone in guinea pigs and rhesus monkeys. Hepatology. 2016 doi: 10.1002/hep.28752. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Torres M, Lawitz E, Kowdley KV, Nelson DR, Dejesus E, McHutchison JG, Cornpropst MT, Mader M, Albanis E, Jiang D, Hebner CM, Symonds WT, Berrey MM, Lalezari J. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naïve patients with HCV genotype 1: a randomized, 28-day, dose-ranging trial. J Hepatol. 2013;58:663–668. doi: 10.1016/j.jhep.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Rong L, Dahari H, Ribeiro RM, Perelson AS. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci Transl Med. 2010;2:30ra32–30ra32. doi: 10.1126/scitranslmed.3000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrazin C, Zeuzem S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology. 2010;138:447–462. doi: 10.1053/j.gastro.2009.11.055. [DOI] [PubMed] [Google Scholar]
- Sofia MJ, Bao D, Chang W, Du J, Nagarathnam D, Rachakonda S, Reddy PG, Ross BS, Wang P, Zhang H-R, Bansal S, Espiritu C, Keilman M, Lam AM, Steuer HMM, Niu C, Otto MJ, Furman PA. Discovery of a β-d-2′-Deoxy-2′-α-fluoro-2′-β-C-methyluridine Nucleotide Prodrug (PSI-7977) for the Treatment of Hepatitis C Virus. J Med Chem. 2010;53:7202–7218. doi: 10.1021/jm100863x. [DOI] [PubMed] [Google Scholar]
- Stahla-Beek HJ, April DG, Saeedi BJ, Hannah AM, Keenan SM, Geiss BJ. Identification of a novel antiviral inhibitor of the flavivirus guanylyltransferase enzyme. J Virol. 2012;86:8730–8739. doi: 10.1128/JVI.00384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S, Schlaberg R, Lewis J, Hanson KE, Couturier MR. N Engl J Med. 2016;375:1907–1909. doi: 10.1056/NEJMc1610613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan BH, Fu J, Sugrue RJ, Yap EH, Chan YC, Tan YH. Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology. 1996;216:317–325. doi: 10.1006/viro.1996.0067. [DOI] [PubMed] [Google Scholar]
- Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, Leparc-Goffart I. Late sexual transmission of Zika virus related to persistence in the semen. Lancet. 2016;387:2501. doi: 10.1016/S0140-6736(16)30775-9. [DOI] [PubMed] [Google Scholar]
- Xu M, Lee EM, Wen Z, Cheng Y, Huang W-K, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, Jacob F, Nguyen HN, Itkin M, Hanna C, Shinn P, Allen C, Michael SG, Simeonov A, Huang W, Christian KM, Goate A, Brennand KJ, Huang R, Xia M, Ming G-L, Zheng W, Song H, Tang H. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016 doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Liu Z-Y, Han J-F, Jiang T, Li X-F, Qin C-F. Genomic characterization and phylogenetic analysis of Zika virus circulating in the Americas. Infect Genet Evol. 2016;43:43–49. doi: 10.1016/j.meegid.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, Fremont DH. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016:1–26. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJF, Neyts J. The Viral Polymerase Inhibitor 7-Deaza-2′-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Negl Trop Dis. 2016;10:e0004695. doi: 10.1371/journal.pntd.0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld R, Roosblad J, Staveren JWV, Wilschut JC, Vreden SGS, Codrington J. Three atypical lethal cases associated with acute Zika virus infection in Suriname. IDCases. 2016;5:49–53. doi: 10.1016/j.idcr.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G, Chen Y-L, Dong H, Lim CC, Yap LJ, Yau YH, Shochat SG, Lescar J, Shi P-Y. Functional analysis of two cavities in flavivirus NS5 polymerase. J Biol Chem. 2011;286:14362–14372. doi: 10.1074/jbc.M110.214189. [DOI] [PMC free article] [PubMed] [Google Scholar]