Abstract
Background
The U.S. infant mortality rate has been steadily decreasing in recent years as has the preterm birth rate; preterm birth is a major factor associated with death during the first year of life. The degree to which changes in gestational age-specific mortality and changes in the distribution of births by gestational age have contributed to the decrease in the infant mortality rate requires clarification.
Objectives
To better understand the major contributors to the 2007–2013 infant mortality decline for the total population, and for infants born to non-Hispanic black, non-Hispanic white, and Hispanic women.
Study Design
We identified births and infant deaths from 2007 and 2013 Centers for Disease Control and Prevention National Vital Statistics System’s period linked birth and infant death files. We included all deaths and births for which there was a reported gestational age at birth on the birth certificate of 22 weeks or greater. The decrease in the infant mortality rate was disaggregated such that all of the change could be attributed to improvements in gestational age-specific infant mortality rates and changes in the distribution of gestational age, by week of gestation, using the Kitagawa method. Sensitivity analyses were performed to account for records where obstetric estimate of gestational age was missing and for deaths and births less than 22 weeks gestation. Maternal race and ethnicity information was obtained from the birth certificate.
Results
The infant mortality rates after exclusions were 5.72 and 4.92 per 1000 live births for 2007 and 2013 respectively with an absolute difference of −0.80 (14% decrease). Infant mortality rates declined by 11% for non-Hispanic whites, by 19% for non-Hispanic blacks, and by 14% for Hispanics during the period. Compared to 2007, the proportion of births in each gestational age category was lower in 2013 with the exception of 39 weeks where there was an increase in the proportion of births from 30.1 percent in 2007 to 37.5 percent in 2013. Gestational age-specific mortality decreased for each gestational age category between 2007 and 2013 except 33 weeks and >42 weeks. About 31 percent of the decrease in the US infant mortality rate from 2007–2013 was due to changes in the gestational age distribution, and 69 percent was due to improvements in gestational age-specific survival. Improvements in the gestational age distribution from 2007–2013 benefitted infants of non-Hispanic white women (48%) the most, followed by infants of non-Hispanic black (31%) and Hispanic (17%) women.
Conclusions
Infant mortality improved between 2007 and 2013 as a result of both improvements in the distribution of gestational age at birth and improvements in survival after birth. The differential contribution of improvements in the gestational age distribution at birth by race and ethnicity suggests that preconception and antenatal health and health care aimed at preventing or delaying preterm birth may not be reaching all populations.
Keywords: Infant mortality, Kitagawa analysis, preterm birth
Condensation
The decrease in US infant mortality rate between 2007 and 2013 was attributed to changes in gestational age distribution and decreased gestational age-specific mortality.
Introduction
The U.S. infant mortality rate has been steadily decreasing in recent years from 6.75 per 1000 live births in 2007 to 5.96 per 1000 live births in 2013.1 During this same period, preterm birth rates also decreased. Based on gestational age recorded as “obstetric estimate” or “clinical estimate” on US birth certificates, the preterm birth rate fell from 10.44 percent in 2007 to 9.62 percent in 2013.2 Preterm birth is a major contributor to infant mortality. Two-thirds of all infant deaths occur among those infants born preterm and, based on conservative assumptions with respect to International Classification of Disease coding on death certificates and causal pathways between gestational age at birth and death in the first year of life, preterm-related mortality constitutes more than one-third of infant deaths.1,3 Hence an infant mortality rate for a given birth cohort can be seen as a function of the distribution of births by gestational age and the gestational age-specific mortality rate; changes in either or both of these parameters will result in change in the infant mortality rate.
It is not clear if the recent decrease in the infant mortality rate is driven by changes in the percentages of infants born preterm (distribution of births by gestational age), particularly at the earliest preterm gestations, changes in the risk of death at each gestational age (gestational age-specific mortality), or both. Moreover, in light of the persistent and well-documented disparities in preterm birth and infant mortality,1–2 it is not clear if changes in the two parameters of interest have been equivalent or disparate for non-Hispanic black, non-Hispanic white and Hispanic women and their infants. Preterm birth rates and infant mortality rates have decreased since 2007 for all women and their infants regardless of race and ethnicity. The aim of this study was to decompose the change in the US infant mortality rate into that proportion attributable to the change in the distribution of gestational age and the proportion attributable to gestational age-specific mortality for the total, non-Hispanic black, non-Hispanic white and Hispanic populations.
Materials and Methods
We used data from the Centers for Disease Control and Prevention National Vital Statistics System’s period linked birth and infant death files for 2007 and 2013.4 In this data set, information from death certificates for each person less than 365 days old in a given year is linked to the birth certificate. Hence, information on the birth certificate, including maternal race and ethnicity and gestational age at birth, can be used to augment the death data and these data comprise the numerator file. The denominator file consists of all live births in a given year. In 2007 and 2013, 98.4 and 99.0 percent of infant deaths respectively could be linked to a corresponding birth certificate. The number of infant deaths in the linked file are weighted to equal the sum of the linked plus unlinked infant deaths by age at death and state and these weights are applied during analysis to account for the small fractions of unlinked infant deaths, thus resulting in counts representing the entire population. The years 2007 and 2013 were chosen because 2007 is the first year that California, a state which has approximately 12 percent of births in the United States, reported gestational age at birth based on any criteria other than last menstrual period and 2007 was beginning of the decline in the US infant mortality rate. The most recent year linked birth infant death data is 2013. A detailed description of the linkage can be found elsewhere.4 This public use data set is derived from de-identified birth certificates and death certificates and hence fall outside the definition of human subjects. Therefore, this analysis was not subject to institutional review.
We included all deaths and births for which there was a reported gestational age at birth on the birth certificate of 22 weeks or greater. Although gestational age based on the last menstrual period (LMP) recorded on the birth certificate has been the traditional source of gestational age in national statistics, a large body of research demonstrates the superiority of the obstetric estimate over the LMP-based estimate and there appears to be little difference between the contemporary terms “obstetric estimate” and “clinical” estimates.2, 5 In 2014, the National Center for Health Statistics (NCHS) began using the obstetric estimate as the preferred measure of gestational age for national reporting. The obstetric estimate is defined by the NCHS as “the best estimate of the infant’s gestation in completed weeks based on the birth attendant’s final estimate of gestation”2 Hence, the first choice for gestational age in this analysis was based on the obstetric or clinical estimate (referred to hereafter as obstetric estimate). If the obstetric estimate of gestational age was missing and an LMP-based estimate was available, the LMP-based estimate was used as the estimate for gestational age. In 2007, 465 infant deaths and 13,452 births (1.5% and 0.3% of deaths and births respectively) and in 2013, 59 infant deaths and 3822 births (0.3% and 0.1% of deaths and births respectively) had LMP-based gestational age estimates due to missing obstetric estimates and available LMP-based estimates. For race- and ethnicity-specific analyses, maternal race and ethnicity was obtained from birth certificates and recorded as non-Hispanic black, non-Hispanic white and Hispanic. Race and ethnicity from the birth certificate is considered more reliable than from the death certificate because they are reported by the mother whereas the race and ethnicity of a decedent are reported by funeral directors and there may be variability in the sources of that information1.
Infant mortality was viewed as the product of the number of births at each gestational age (GA) and the gestational age-specific mortality. Hence the total infant mortality rate (IMR) can be expressed as:
It then follows that the infant mortality rate can be decomposed by the method of Kitagawa6:
where N1 and N2 are IMRs in 2013 and 2007 respectively; R1 and R2 are gestational age-specific mortality rates in 2013 and 2007 respectively; F1 and F2 are proportions of births at each gestational week for 2013 and 2007 respectively. The first half of the equation after the summation sign represents the proportion of the infant mortality rate attributable to the GA distribution and the second half the proportion attributable to the GA-specific mortality.
The numbers of deaths and births at each week of gestation 22 weeks and greater were tabulated from the numerator and denominator files respectively and the gestational age-specific mortality rates were calculated as the proportion of deaths to births in a given year. The proportion of births at each gestational age was calculated as the fraction of the total births for the year. The Kitagawa decomposition was tabulated and summed for the total, non-Hispanic black, non-Hispanic white and Hispanic populations. Sensitivity analyses that included births and deaths for which the obstetric estimate was available were done to account for births and deaths where LMP-based gestational age was substituted for missing obstetric gestational age. Also, because deaths and births with gestational age less than 22 weeks are included in US infant mortality rates, sensitivity analyses were done to account for the exclusion of these events.
Results
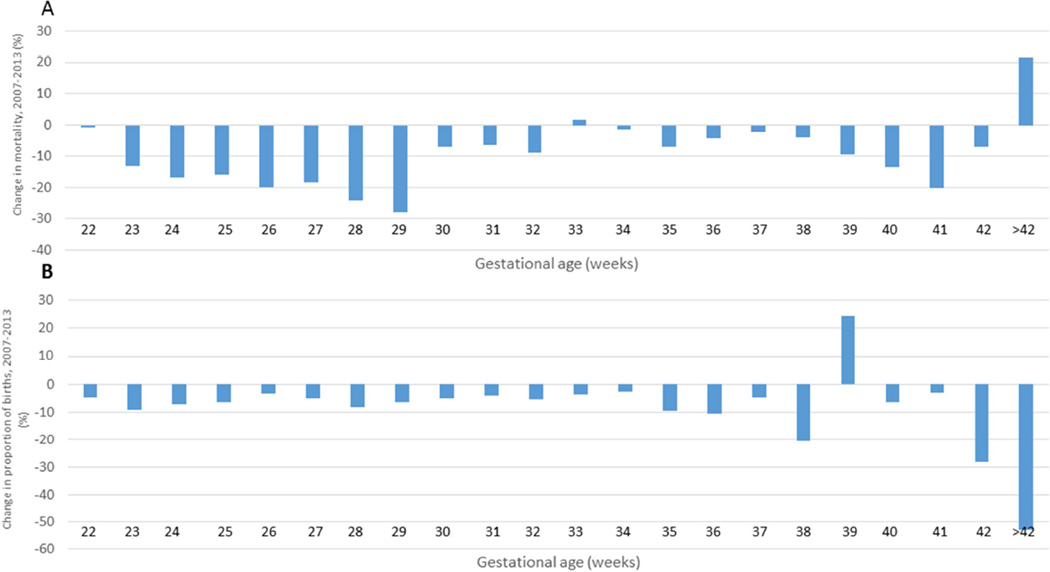
After excluding births (0.3% in 2007 and 0.2% in 2013) and deaths (15.5% in 2007 and 17.7% in 2013) less than 22 weeks and for whom gestational age information was missing, there were 24,633 infant deaths and 4,304,549 live births in 2007 and 19,301 infant deaths and 3,924,071 live births for 2013. The primary reason for excluding deaths was gestational age at birth less than 22 weeks (14.8% in 2007 and 16.9% in 2013). The infant mortality rates after exclusions were 5.72 and 4.92 per 1000 live births for 2007 and 2013 respectively with an absolute difference of −0.80 (14% decrease). The preterm birth rates (births 22–36 weeks) were 10.3 and 9.5 per 100 live births in 2007 and 2013 respectively. Compared to 2007, the proportion of births for each gestational age category was lower in 2013 except for 39 weeks where there was an increase in the proportion of births from 30.1 percent in 2007 to 37.5 percent in 2013. The gestational age-specific mortality decreased for each successive increase in gestational age between 2007 and 2013 except for gestational age 33 weeks where the increase was small (<2%) and gestational ages 42 weeks and greater where there were relatively few births and deaths (Table 1, Figure 1).
Table 1.
Infant deaths, births, gestational age-specific proportion of births and deaths, United States, 2007 and 2013.
| Gestational age (weeks) |
Infant deaths | Births | Proportion of total births |
GA-specific mortality (per 1000 births) |
||||
|---|---|---|---|---|---|---|---|---|
| 2007 | 2013 | 2007 | 2013 | 2007 | 2013 | 2007 | 2013 | |
| 22 | 2172 | 1872 | 2457 | 2132 | 0.00057 | 0.00054 | 883.87 | 878.01 |
| 23 | 2259 | 1619 | 3263 | 2695 | 0.00076 | 0.00069 | 692.39 | 600.75 |
| 24 | 2028 | 1426 | 4648 | 3933 | 0.00108 | 0.00100 | 436.23 | 362.54 |
| 25 | 1287 | 922 | 4904 | 4176 | 0.00114 | 0.00106 | 262.39 | 220.83 |
| 26 | 957 | 676 | 5436 | 4782 | 0.00126 | 0.00122 | 176.07 | 141.44 |
| 27 | 706 | 499 | 6276 | 5433 | 0.00146 | 0.00138 | 112.43 | 91.86 |
| 28 | 634 | 402 | 7673 | 6410 | 0.00178 | 0.00163 | 82.64 | 62.68 |
| 29 | 554 | 340 | 8484 | 7239 | 0.00197 | 0.00184 | 65.31 | 46.98 |
| 30 | 488 | 392 | 11510 | 9942 | 0.00267 | 0.00253 | 42.36 | 39.39 |
| 31 | 481 | 393 | 14360 | 12559 | 0.00334 | 0.00320 | 33.49 | 31.31 |
| 32 | 558 | 438 | 21922 | 18879 | 0.00509 | 0.00481 | 25.47 | 23.22 |
| 33 | 577 | 515 | 30734 | 26976 | 0.00714 | 0.00687 | 18.79 | 19.11 |
| 34 | 747 | 654 | 54765 | 48536 | 0.01272 | 0.01237 | 13.65 | 13.47 |
| 35 | 883 | 677 | 89862 | 74203 | 0.02088 | 0.01891 | 9.83 | 9.13 |
| 36 | 1233 | 960 | 179198 | 145778 | 0.04163 | 0.03715 | 6.88 | 6.58 |
| 37 | 1661 | 1410 | 382347 | 331962 | 0.08882 | 0.08460 | 4.35 | 4.25 |
| 38 | 2404 | 1674 | 887009 | 642452 | 0.20606 | 0.16372 | 2.71 | 2.61 |
| 39 | 2650 | 2725 | 1296392 | 1471159 | 0.30117 | 0.37491 | 2.04 | 1.85 |
| 40 | 1727 | 1273 | 986853 | 840503 | 0.22926 | 0.21419 | 1.75 | 1.51 |
| 41 | 534 | 378 | 280202 | 247856 | 0.06509 | 0.06316 | 1.90 | 1.52 |
| 42 | 71 | 43 | 22849 | 15004 | 0.00531 | 0.00382 | 3.11 | 2.89 |
| >42 | 23 | 12 | 3405 | 1462 | 0.00079 | 0.00037 | 6.75 | 8.21 |
| Total | 24,633 | 19301 | 4,304,549 | 3924071 | 1.00000 | 1.00000 | 5.72 | 4.92 |
GA, gestational age
Figure 1.
Percent change in gestational age-specific mortality, 2007–2013 (A) and percent decrease in proportion of births, 2007–2013 (B) for all births and deaths. Positive percentages indicate decreases and negative percentages are increases.
The results of the Kitagawa analysis by single weeks of gestational age are shown in Table 2. For each gestational age, the decomposition analysis demonstrates the contribution of changes in gestational age distribution and gestational specific mortality to the change in infant mortality. For example at age 22 weeks, 88% of the total decrease was due to changes in GA distribution and 12% was due to changes in GA specific mortality. At 33 and 42 and greater weeks gestation, the contribution to the overall decrease was completely driven by the change in the gestational age distribution for those weeks because gestational-age specific mortality increased for these groups. At 39 weeks, the overall decrease in infant mortality was completely driven by improvement in gestational age-specific mortality. We found that 31 percent of the overall infant mortality decrease was due to changes in the gestational age distribution and 69 percent was due to improvements in gestational age-specific survival.
Table 2.
Kitagawa decomposition of change in infant mortality, United States, 2007–2013.
| Gestational age (weeks) |
Contribution of GA distribution |
Contribution of GA-specific mortality |
Total | % of total change due to GA distribution |
% of total change due to GA-specific mortality |
|---|---|---|---|---|---|
| 22 | −0.024 | −0.003 | −0.027 | −88.1 | −11.9 |
| 23 | −0.046 | −0.066 | −0.112 | −41.0 | −59.0 |
| 24 | −0.031 | −0.077 | −0.108 | −28.7 | −71.3 |
| 25 | −0.018 | −0.046 | −0.064 | −28.4 | −71.6 |
| 26 | −0.007 | −0.043 | −0.050 | −14.0 | −86.0 |
| 27 | −0.008 | −0.029 | −0.037 | −20.4 | −79.6 |
| 28 | −0.011 | −0.034 | −0.045 | −24.1 | −75.9 |
| 29 | −0.007 | −0.035 | −0.042 | −16.8 | −83.2 |
| 30 | −0.006 | −0.008 | −0.013 | −42.7 | −57.3 |
| 31 | −0.004 | −0.007 | −0.012 | −38.1 | −61.9 |
| 32 | −0.007 | −0.011 | −0.018 | −38.1 | −61.9 |
| 33 | −0.005 | 0.002 | −0.003 | −179.5 | 79.5 |
| 34 | −0.005 | −0.002 | −0.007 | −68.5 | −31.5 |
| 35 | −0.019 | −0.014 | −0.033 | −57.2 | −42.8 |
| 36 | −0.030 | −0.012 | −0.042 | −72.1 | −27.9 |
| 37 | −0.018 | −0.008 | −0.027 | −68.4 | −31.6 |
| 38 | −0.113 | −0.019 | −0.132 | −85.3 | −14.7 |
| 39 | 0.144 | −0.065 | 0.079 | 181.9 | −81.9 |
| 40 | −0.025 | −0.052 | −0.077 | −32.0 | −68.0 |
| 41 | −0.003 | −0.024 | −0.028 | −11.9 | −88.1 |
| 42 | −0.004 | −0.001 | −0.005 | −81.4 | −18.6 |
| >42 | −0.003 | 0.001 | −0.002 | −137.0 | 37.0 |
| TOTALS | −0.250 | −0.554 | −0.804 | −31.1 | −68.9 |
GA, gestational age
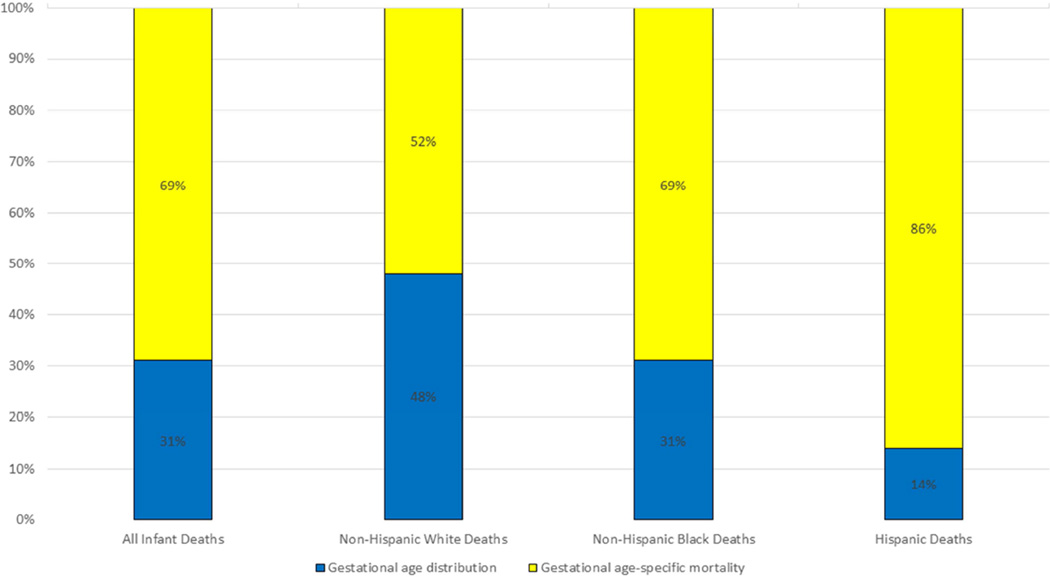
The infant mortality rate for non-Hispanic white infants born at 22 weeks and older fell from 4.91 to 4.37 (11%), for non-Hispanic black infants from 10.67 to 8.62 (19%) and for Hispanic infants from 4.76 to 4.08 (14%) per 1000 births between 2007 and 2013. The patterns at each gestational age were similar to the pattern in the total population in terms of the direction of change when the analysis was stratified by the three race-ethnicity groups although the magnitude of change at each gestational age differed (data not shown). Overall non-Hispanic white infants had a greater benefit from the change in the gestational age distribution than did non-Hispanic black and Hispanic infants. Forty-eight percent of the total decrease in infant mortality was attributed to improvements in the gestational age distribution for non-Hispanic white women while 31 percent and 14 percent of the decrease was attributed to such improvements for non-Hispanic black and Hispanic infants, respectively (Figure 2).
Figure 2.
Contributions to decline in infant mortality rates from 2007 to 2013 for births 22 weeks and greater
The overall contribution to changes in infant mortality rates by standard collapsed gestational age groupings is shown in Table 3. Not surprisingly, improvements for infants born at <32 weeks make the greatest contribution to the overall decrease in infant mortality for the entire population and for each race and ethnicity group. Thirty percent of the decrease in the infant mortality rate for the entire population was attributable to improvements for infants born late preterm (34–36 weeks; 10.1%) and early term (37–38 weeks; 19.7%). When broken down by race and ethnicity, almost 39% of the total decrease in infant mortality rate for non-Hispanic whites was attributed to improvements for infants born late preterm and early term while the contribution to declines in infant mortality rates for these gestational ages for non-Hispanic blacks and Hispanics were 21% and 24%.
Table 3.
Contribution by gestational age to the overall infant mortality decline 2007–2013, for the total population and for non-Hispanic white, Non-Hispanic black and Hispanic women
| Gestational age (weeks) |
Total population (%) |
Non-Hispanic white (%) |
Non-Hispanic black (%) |
Hispanic (%) |
|---|---|---|---|---|
| <32 | 63.4 | 60.8 | 73.4 | 61.9 |
| 32–33 | 2.6 | −0.6 | 3.5 | 4.3 |
| 34–36 | 10.1 | 8.9 | 9.6 | 9.4 |
| 37–38 | 19.7 | 29.7 | 11.0 | 14.5 |
| 39–41 | 3.2 | 0.2 | 2.1 | 8.6 |
| 42+ | 1.0 | 1.1 | 0.4 | 1.2 |
| Absolute decrease in infant mortality rate (per 1000 births) |
0.80 | 0.54 | 2.05 | 0.68 |
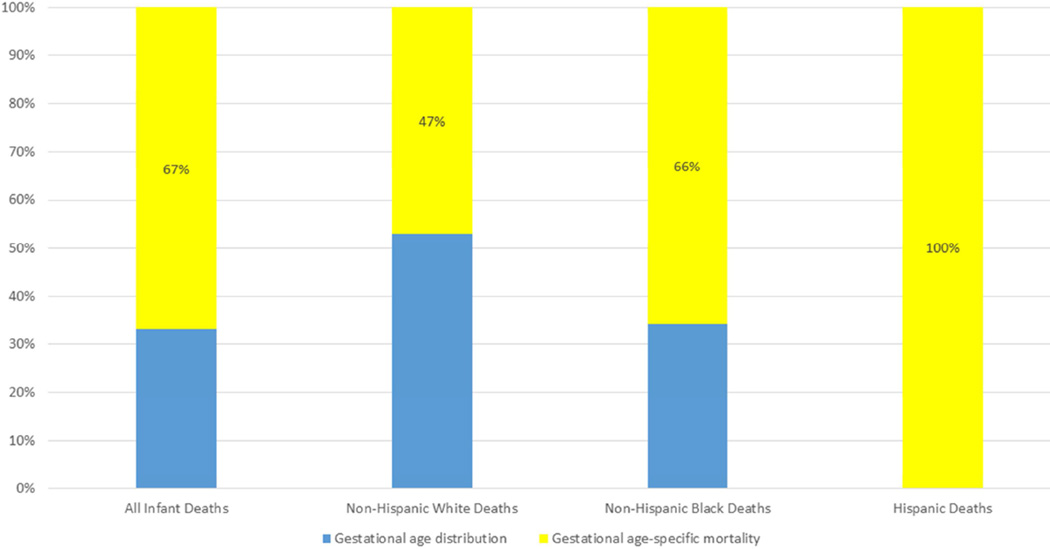
In sensitivity analyses, we examined the percent contribution by race and ethnicity when 1) births and deaths with missing obstetric gestational age (replaced with LMP-based gestational age in main analysis) were excluded, and 2) births and deaths that occurred at less than 22 weeks were included. In both cases, there was minimal change in the contributions of gestational-age distributions and gestational age-specific mortality for non-Hispanic black and non-Hispanic white infants. For Hispanic infants, the results for scenario 1 were similar to the main study. However in scenario 2, at <22 weeks gestation there were modest increases in the proportion of births (13.4%) and mortality rate (8.6%) from 2007 to 2013; therefore only decreases in gestational age-specific mortality contributed to the change in the infant mortality rates for Hispanic infants (Figure 3).
Figure 3.
Contributions to decline in infant mortality rates from 2007 to 2013 for all live births.
Comment
Infant mortality improved between 2007 and 2013 as a result of both improvements in the distribution of preterm births and improvements in survival after birth. Decreases in mortality at each week of preterm gestation, with the exception of a small increase at 33 weeks, contributed to the overall decrease and this occurred for non-Hispanic black, non-Hispanic white and Hispanic infants. While the changes in distribution of births and survival were most important at the earliest preterm gestations, substantial improvements also occurred for late preterm and early term infants. Accompanying the decrease in preterm births was an increase in the proportion of births at 39 weeks, when mortality rates are quite low. This shift has occurred concurrent with the accrual of evidence that infants born at early term gestations experience higher morbidity and mortality.7–8 Hence, this shift can be viewed through a positive lens. Still, because not all infant mortality can be attributed to gestational age at birth, as more births are shifted to 39 weeks, increased attention needs to be focused on causes of death common in this gestational age group, such as unintentional injuries, congenital anomalies, and sudden unexplained deaths.
Even modest improvement in survival at the earliest gestation, where gestational age-specific mortality is exceedingly high, contributes to overall decreases in infant mortality rates. Although not followed throughout infancy, a recent report from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network documented recent increases in survival to discharge for infants born extremely preterm, particularly for those born at 23 and 24 weeks. Moreover, survival to discharge without serious morbidities, a likely predictor of survival through infancy, increased for those born 25 through 28 weeks.9 Surely this trend represents improvements in care after birth, such as more appropriate use of intubation resulting in reductions in lung injury and better infection control practices resulting in reductions in late onset sepsis.9
Obstetrical practices also likely have impacts in achieving reductions in infant mortality, particularly in regard to improving the overall gestational age distribution. Just as small improvements in survival at high risk gestational ages makes an impact on overall infant mortality, declines in the proportions of births at these same high risk gestational ages may have substantial impacts on infant mortality. Shortly after the clinical trial demonstrating a salutary effect of 17 alpha-hydroxyprogesterone caproate (17-OHP) on the risk of preterm birth among women with a prior preterm birth, it was estimated that universal appropriate implementation of this intervention would have an important, but small effect on the overall preterm birth rate.10 However, viewing preterm birth as a dichotomous outcome does not account for small but important shifts in the distribution. Shifting a birth that may have occurred at 24 weeks to 28 weeks shifts the risk of mortality for that infant from 300–400 per 1000 births to less than 100 per 1000 births. While such phenomena are difficult to study rigorously, it is not inconceivable that appropriate use of 17-OHP, vaginal progestogens and cerclage are not just preventing preterm birth11 but also prolonging pregnancies to a gestation that better favors survival. Recent efforts that re-define response to such interventions, such as prolongation of gestation as opposed to singularly monitoring preterm birth, may help better understand this impact.12 Trials of interventions to prevent preterm birth should consider more subtle but important effects prior to discontinuing them when they fail to show an effect on the dichotomous outcome of preterm birth, as suggested by Mol and Byrne.13 Additionally, the consistent use of antenatal corticosteroids and increasing the availability of well-organized regional levels of care and referrals for women and infants will lead both to important prolongation of gestation and best care for neonates.14–15 Importantly, contemporary evidence supporting the appropriate avoidance of late preterm and early term deliveries16–18 and using such evidence to inform quality improvement efforts19–20 have resulted in a shift nationally to increase the proportion of births at 39 weeks gestation.
The strength of this analysis is that two full years of national data were used and thus it represents the recent infant mortality experience in the United States. The analysis was based on vital records and hence has limitations imposed by missing and misclassified information. A substantial fraction of deaths for each year occurred among infants born at less than 22 weeks gestation. Because the definition of live birth has no lower limit for birthweight or gestational age, these births can be legitimately included. However, because these infants did not have 100 percent mortality, even when the recorded gestational age was less than 20 weeks, we believe there was enough misclassification of gestational age to exclude them; our sensitivity analysis showed that this exclusion had only small effects and would not change our interpretation of the data. Similarly, results including substituted LMP-based estimates of gestational age for missing obstetric estimates did not differ from analyses excluding those birth and deaths. Finally, the period linked file is not strictly a cohort. Deaths that occur in a given calendar year are linked back to their birth certificates even if the birth occurred in the prior year while births are those that occur in the index year. Hence, not every death is included in the denominator as some of the infants in the denominator will die in the subsequent year. However, the denominator is huge (about 4 million births yearly) compared to the numerator. Moreover, identical infant mortality rates for 2013 were reported regardless of whether they are calculated using the period linked birth and infant death file or the unlinked multiple cause mortality files1, 21
Reasons for declining preterm birth rates have been posited to include changes in risk factors for preterm birth (e.g. maternal age, multiple births), interventions for prevention (progestogens, cerclage) and promulgation of evidence-based guidelines.11 Care practices aimed at reducing morbidity and mortality of extremely preterm neonates have demonstrated success.9, 22 Our analysis was an attempt to quantify the relative contributions of prolonging gestation and improving survival when preterm birth occurs. The successes are noteworthy but the work must continue. The finding that non-Hispanic black infants had the greatest improvement in infant mortality is encouraging given the longstanding high infant mortality rate for this population. However, in spite of the finding that the infant mortality and preterm birth rates for non-Hispanic black and Hispanics fell, improvements in gestation age-specific mortality was more salient for these groups. The benefit of the improved gestational age distribution was not as great for non-Hispanic black infants as for non-Hispanic white infants, and Hispanic infants had even less benefit from changes in gestational age distribution. While early term non-Hispanic white infants contributed 30 percent of the decrease in infant mortality rates, the same could not be said for non-Hispanic black infants (11%) and Hispanic infants (15%) did not receive the same benefit. To the degree that care practices influence pregnancy prolongation, this may represent issues of differential access and distribution of care, particularly for the Hispanic population. Moreover, in spite of improvements, the overall disparity in preterm birth and infant mortality between non- Hispanic black and non-Hispanic white women and their infants persists. Therefore, greater efforts must be made to develop the evidence for what is and is not working and to ensure that interventions are available to all.
Acknowledgments
This research was not funded
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
Presented as a poster at the Society for Maternal-Fetal Medicine’s 36th Annual Pregnancy Meeting, Atlanta, GA, February 4, 2016
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Mathews TJ, MacDorman MF, Thoma ME. National vital statistics reports. 9. Vol. 64. Hyattsville, MD: National Center for Health Statistics; 2015. Infant mortality statistics from the 2013 period linked birth/infant death data set. [PubMed] [Google Scholar]
- 2.Martin JA, Osterman MJK, Kirmeyer SE, Gregory ECW. National vital statistics reports. 5. Vol. 64. Hyattsville, MD: National Center for Health Statistics; 2015. Measuring gestational age in vital statistics data: Transitioning to the obstetric estimate. [PubMed] [Google Scholar]
- 3.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth top infant mortality in the United States. Pediatrics. 2006;118:1566–1573. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 4.National Vital Statistics System. [Accessed July 29, 2015];Linked Birth and Infant Death Data. ( http://www.cdc.gov/nchs/linked.htm)
- 5.Callaghan WM, Dietz PM. Differences in birthweight for gestational age distributions according to measures used to assign gestational age. Am J Epidemiol. 2010;171:826–836. doi: 10.1093/aje/kwp468. [DOI] [PubMed] [Google Scholar]
- 6.Lisonkova S, Hutcheon JA, Joseph KS. Sudden infant death syndrome: a re-examination of temporal trends. BMC Pregnancy Childbirth. 2012;12:59. doi: 10.1186/1471-2393-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nonmedically indicated early-term deliveries. Committee Opinion N0. 561. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2013;121:911–915. doi: 10.1097/01.AOG.0000428649.57622.a7. [DOI] [PubMed] [Google Scholar]
- 8.Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term pregnancy. A period of heterogeneous risk of infant mortality. Obstet Gynecol. 2011;117:1279–1287. doi: 10.1097/AOG.0b013e3182179e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA. 2013;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrini JR, Callaghan WM, Klebanoff M, Green NS, Lackritz EM, Howse JL, Schwarz RH, Damus K. Estimated effect of 17 alpha-hydroxyprogesterone caproate on preterm birth in the United States. Obstet Gynecol. 2005;105:267–272. doi: 10.1097/01.AOG.0000150560.24297.4f. [DOI] [PubMed] [Google Scholar]
- 11.Schoen CN, Tabbah S, Iams JD, Caughey AB, Berghella V. Why the United States preterm birth rate is declining. Am J Obstet Gynecol. 2015;213:175–180. doi: 10.1016/j.ajog.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Manuck TA, Rice MM, Bailit JL, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016 doi: 10.1016/j.ajog.2016.01.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mol B, Byrne R. Are we stopping preterm birth trials too early? Am J Obstet Gynecol. 2016;213:134–135. doi: 10.1016/j.ajog.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low-birth-weight and very preterm infants: a meta-analysis. JAMA. 2010;304:992–1000. doi: 10.1001/jama.2010.1226. [DOI] [PubMed] [Google Scholar]
- 15.American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine. Levels of maternal care. Am J Obstet Gynecol. 2015;212:259–271. [Google Scholar]
- 16.Bannerman CG, Fuchs KM, Young OM, Hoffman MK. Non-spontaneous late preterm birth: etiology and outcomes. Am J Obstet Gynecol. 2011;205:456.e1–456.e6. doi: 10.1016/j.ajog.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Salemi JL, Pathak EB, Salihu HM. Infant outcomes after elective early-term delivery compared with expectant management. Obstet Gynecol. 2016:657–666. doi: 10.1097/AOG.0000000000001331. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, et al. Effect of late preterm birth and maternal medical conditions on newborn morbidity risk. Pediatrics. 2008;121:e223–e232. doi: 10.1542/peds.2006-3629. [DOI] [PubMed] [Google Scholar]
- 19.Ohio Perinatal Quality Collaborative. 39-Weeks Charter Project. [Accessed March 31,2016]; https://www.opqc.net/projects/39%20weeks%202008. [Google Scholar]
- 20.Perinatal Quality Collaborative of North Carolina. [Accessed March 31, 2016];Eliminating Elective Deliveries under 39 Weeks Gestation. http://www.pqcnc.org/initiatives/39weeks. [Google Scholar]
- 21.Xu JQ, Murphy SL, Kochanek KD, Bastian BA. National vital statistics reports. 2. Vol. 64. Hyattsville, MD: National Center for Health Statistics; 2016. Deaths: Final data for 2013. [PubMed] [Google Scholar]
- 22.Backes CH, Rivera BK, Haque U, et al. A proactive approach to neonates born at 23 weeks of gestation. Obstet Gynecol. 2015;126:939–946. doi: 10.1097/AOG.0000000000001098. [DOI] [PubMed] [Google Scholar]