Vemurafenib (Zelboraf™, Genentech, CA) is a highly effective oral chemotherapy agent for patients with metastatic melanoma who carry the BRAF V600E mutation. Side effects of this protein kinase inhibitor (PKI) include arthralgia, rash, and fatigue which are reported in up to one-third of treated patients. Mild abnormalities in liver biochemistries were reported with vemurafenib use in 30% of subjects, 11% developed severe laboratory abnormalities and acute liver failure has been reported (Table 1). Herein, a case of severe vemurafenib induced granulomatous hepatitis leading to chronic cholestatis is reported along with a review of the hepatoxicity of other PKI’s.
Table 1.
Hepatotoxicity of approved protein kinase inhibitors.
| Drug | Approved indication(s) | % AST/ALT elevation [% grade 3–4] |
Latency to liver injury onset | Fatal hepatic failure reported |
|---|---|---|---|---|
| Axitinib | RCC | 22 [<1] | N/A | No |
| Bosutinib | CML | 20 [6–10] | Median 30–33 days | No |
| Cabozantinib | Medullary thyroid cancer | 86 [3–6] | N/A | No |
| Ceritinib | Non-small cell lung cancer | 27 [<1] | N/A | No |
| Crizotinib | Non-small cell lung cancer | 76 [17] | <2 months | Yes |
| Dabrafenib | Metastatic melanoma | 25 [0.5]a | N/A | No |
| Dasatinib | CML, ALL | 0–5 [0] | N/A | No |
| Erlotinib | Non-small cell lung cancer, pancreatic cancer | 35–45 [10–14]* | <2–4 weeks | Yesˆ |
| Gefitinib | Non-small cell lung cancer | 11–40 [2–5] | N/A | No |
| Ibrutinib | B cell malignancies | None reported | N/A | No |
| Idelalisib | CLL | 35–50 [14] | <12 weeks | Yes (Black box warning) |
| Imatinib | CML, ALL, hypereosinophlic syndrome, myelodysplasia, GIST | 6–12 [3–6] | 12–77 days | Yes |
| Lapatinib | HER-2-positive breast cancer | 37–53 [2–6] | Days to months | Yes (Black Box warning) |
| Lenvatinib | Thyroid cancer, metastatic RCC | [5] | N/A | Yes |
| Nilotinib | CML | 72 [4] | N/A | No |
| Nintedanib | Non-small cell lung cancer | 14 | N/A | No |
| Palbociclib | ER-pos HER2-neg breast cancer | None reported | N/A | No |
| Pazopanib | RCC, soft tissue sarcoma | 46–53 [7–12] | <18 weeks | Yes (Black box warning) |
| Ponatinib | CML, AML | 56 [8] | N/A | Yes (Black box warning) |
| Regorafenib | Colorectal cancer | 58–65 [5–6] | N/A | Yes (Black box warning) |
| Ruxolitinib | myelofibrosis | 25 [<1] | N/A | No |
| Sorafenib | RCC, hepatocellular carcinoma, thyroid cancer | 21–59 [2–4] | N/A | No |
| Sunitinib | GIST, RCC, pancreatic neuroendocrine tumor | 56–78 [3–5] | N/A | Yes (Black box warning) |
| Tofacitinib | Rheumatoid arthritis | None reported | N/A | No |
| Trametinib | Metastatic melanoma | 60 [2] | N/A | No |
| Vandetanib | Medullary thyroid cancer | 51 [2] | N/A | No |
| Vemurafenib | Melanoma with BRAF mutations | 30 [11] | 3 to 6 weeks | No |
RCC, renal cell cancer; CML, chronic myeloid leukemia; ALP, alkaline phosphatase; ALL, acute lymphoblastic leukemia; GIST, gastrointestinal stromal tumor; ER-pos, estrogen receptor positive; HER2-pos, human epidermal growth factor receptor 2 positive N/A= Not available
alkaline phosphatase elevations;
when used in combination with gemcitabine for pancreatic cancer (4% AST/ALT elevation with monotherapy);
when used in Childs B cirrhosis
Case Report
A 69 year old Caucasian man with metastatic melanoma began vemurafenib 240 mg twice daily that was titrated to 720 mg twice daily after two weeks. Six weeks after starting therapy, the patient was hospitalized with vomiting and abdominal pain with a serum alanine aminotransferase (ALT) 45 U/L, aspartate aminotransferase (AST) 36 U/L, alkaline phosphatase (ALP) 209 U/L and total bilirubin 1.3 mg/dl. Despite drug discontinuation, his liver enzymes peaked 7 days later with an ALT 170 U/L, AST 132 U/L, ALP 663 U/L and bilirubin of 6.0 mg/dl. Serologies against hepatitis A, B, C, E, CMV, EBV, anti-smooth muscle, ANA and AMA as well as quantitative HCV RNA were negative. An abdominal CT scan revealed splenomegaly without biliary dilation. There was no eosinophilia and the INR remained normal throughout.
Concomitant medications started greater than 6 months prior included atenolol, naproxen, and tadalafil. He denied alcohol use and reported no dietary or herbal supplement use. A liver biopsy 7 days after DILI onset revealed cholestatic injury with granulomas and eosinophils (Figure 1). The patient improved clinically but the ALP remained elevated at 302 IU/L with a normal bilirubin eight months after presentation. The causality score in the Drug Induced Liver Injury Network protocol was highly likely for vemurafenib hepatotoxicity. The patient subsequently received dabrafenib 100 mg twice daily with good disease response but it was later discontinued after 12 months due to inflammatory arthritis and uveitis.
Figure 1.
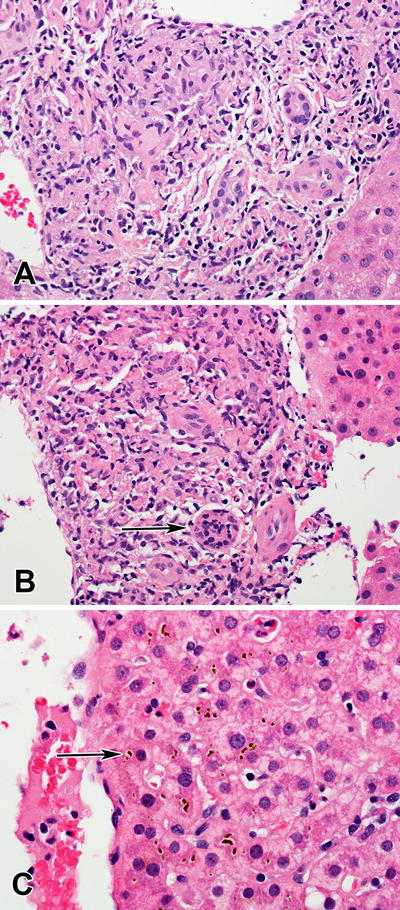
Granulomatous hepatitis associated with Vemurafenib use. A. Poorly formed granulomatous inflammation in a portal area. (H&E, 400×) B. Lymphocytic infiltration of the bile ducts (arrow). (H&E, 400×) C. Canalicular cholestasis (arrow) in zone 3. (H&E, 600×)
Discussion
Protein kinases are a family of regulatory enzymes that catalyze phosphorylation of specific intracellular protein residues leading to changes in protein function including cell proliferation. For this reason, the PKIs have become a major focus of cancer drug development. PKIs can target tyrosine and/or serine/threonine residues. Although tyrosine kinase inhibitor (TKI) use has expanded dramatically in the last five years, most of these agents have been associated with hepatotoxicity during clinical trials and several have been withdrawn from the market for severe hepatotoxicity (Table 1). Although vemurafenib is a serine-threonine kinase inhibitor, it appears to have many of the same hepatotoxic effects as TKIs (1,2).
The mechanism of injury with vemurafenib hepatotoxicity is unknown. Acholestatic or mixed pattern of liver injury was more common at presentation compared to a hepatocellular injury pattern (65% vs 35%) in 63 patients with vemurafenib hepatotoxicity (2). Most patients presented within 2 months of drug initiation and the estimated reporting rate of vemurafenib hepatotoxicity was 5.13 (95% CI, 3.8–6.4) per 1000-patient years’ exposure.
Confidently identifying drug induced liver injury (DILI) in oncology patients receiving chemotherapy can be challenging. Malignant involvement of the liver, ischemic hepatopathy, cholestasis of sepsis, HBV reactivation, herpes hepatitis and non-chemotherapy associated DILI must all be considered. Therefore, many clinicians obtain a liver biopsy to help exclude competing causes of liver injury and confirm a diagnosis of DILI. Vemurafenib-associated DILI can be treated by dose reduction or discontinuation. In the aforementioned case series, 17 patients (27 %) were continued on vemurafenib at a decreased dose and 25 patients were rechallenged after discontinuation, with 20 being able to complete therapy (2).
There is a potential for synergistic hepatotoxicity when PKI’s are used with other potentially hepatotoxic chemotherapeutic agents. In one study of 10 patients with metastatic melanoma who received both vemurafenib and ipilibumab, a CTLA4 inhibitor, 7 developed treatment-limiting DILI within five weeks with prominent auto-immune hepatitis like features (3). Although the hepatotoxic effects of PKI’s are well documented, use of these agents will likely continue to increase due to their dramatic clinical benefit observed in individual patients with otherwise fatal malignancies. Therefore, consulting physicians should become familiar with the hepatotoxicity profile of this rapidly expanding class of agents via use of electroniclectronic databases such as LiverTox (http://livertox.nih.gov/).
Acknowledgments
Funding source: Dr. Fontana is a site investigator in the Drug Induced Liver Injury Network (DILIN) which is structured as an U01 cooperative agreement supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) (U01DK065184 (University of Michigan [Ann Arbor]). Additional support was provided by the Intramural Division of the National Cancer Institute (NCI), NIH.
Abbreviations
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- DILI
drug induced liver injury
- DILIN
Drug induced liver injury network
- PKI
Protein kinase inhibitor
- TKI
Tyrosine-kinase inhibitor
References
- 1.Shah RR. Hepatotoxicity of Tyrosine Kinase Inhibitors: Clinical and Regulatory Perspectives. Drug Safety. 2013;36:491–503. doi: 10.1007/s40264-013-0048-4. [DOI] [PubMed] [Google Scholar]
- 2.Munson MLG, Sagi LS, Morley R, Aldairy W, Shih M, Tucker E. Drug-induced Liver Injury associated with Vemurafenib Treatment. (Abstract) Hepatology. 2015;62(Suppl 1) #1920. [Google Scholar]
- 3.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with Combination of Vemurafenib and Ipilimumab. N Engl J Med. 2013;368:1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]


