Abstract
Chronic alcohol use has widespread effects on brain morphometry. Alcohol dependent individuals are often diagnosed with comorbid substance use disorders. Alterations in brain morphometry may be different in individuals that are dependent on alcohol alone and individuals dependent on alcohol and other substances. We examined subcortical brain volumes in 37 individuals with alcohol dependence only (ADO), 37 individuals with polysubstance use disorder (PS), and 37 healthy control participants (HC). Participants underwent a structural MR scan and a model-based segmentation tool was used to measure the volume of 14 subcortical regions (bilateral thalamus, caudate, putamen, globus pallidus, hippocampus, amygdala, and nucleus accumbens). Compared to HC, ADO had smaller volume in the bilateral hippocampus, right nucleus accumbens, and right thalamus. PS only had volume reductions in the bilateral thalamus compared to HC. PS had a larger right caudate compared to ADO. Subcortical volume was negatively associated with drinking measures only in the ADO group. This study confirms the association between alcohol dependence and reductions in subcortical brain volume. It also suggests that polysubstance use interacts with alcohol use to produce limited subcortical volume reduction and at least one region of subcortical volume increase. These findings indicate that additional substance use may mask damage through inflammation or may function in a protective manner, shielding subcortical regions from alcohol-induced damage.
Keywords: Alcohol use disorder, Brain structure, MRI, Polysubstance use disorder, Subcortical
Introduction
It has been well established that alcohol use disorders (AUD) are associated with large changes in brain morphometry (Buhler and Mann, 2011). Postmortem studies have demonstrated that alcohol dependent individuals have reduced brain weight (Harper and Blumbergs, 1982), decreased cerebral white matter (Harper and Corbett, 1990), and reduced number of cortical neurons (Harper and Corbett, 1990), particularly the pyramidal neurons located in the prefrontal cortex (Kril et al., 1997). Additional neuronal loss has been reported in the brains of alcohol dependent individuals, including loss of anterior insular von Economo neurons, a specialized neuronal population implicated in neuropsychiatric conditions involving emotional and social deficits (Senatorov et al., 2015).
Magnetic resonance imaging (MRI) has provided a non-invasive tool to further explore volumetric and morphometric changes associated with alcohol use disorders. A recent meta-analysis identified consistent gray matter atrophy in the bilateral prefrontal cortex, bilateral insula, and bilateral posterior cingulate cortex in the brains of alcohol dependent individuals (Xiao et al., 2015). Subcortical regions are also negatively impacted by heavy alcohol use. Individuals with alcohol dependence have reduced volume in subcortical regions including the hippocampus (Agartz et al., 1999; Sullivan et al., 1995; Wrase et al., 2008), amygdala (Wrase et al., 2008), caudate and putamen (Sullivan et al., 2005), and nucleus accumbens (Makris et al., 2008; Wrase et al., 2008).
Individuals with alcohol dependence often present with comorbid substance use disorders (Moss et al., 2010), yet relatively few studies have investigated the association between comorbid alcohol and substance use and brain volume. Individuals with alcohol dependence and comorbid psychostimulant dependence had increased white matter volume and increased frontal gray matter volume compared to individuals with alcohol dependence alone (Mon et al., 2014). Both individuals with alcohol dependence alone and individuals with comorbid psychostimulant dependence had decreased parietal white matter, occipital white matter, and thalamic gray matter volume, compared to light drinking controls (Mon et al., 2014). Short-term abstinent alcohol dependent individuals had smaller subcortical volume in the nucleus accumbens, amygdala, caudate, thalamus, and hippocampus, compared to individuals with alcohol dependence and comorbid stimulant disorder (Fein and Fein, 2013). These differences were largely not present in a similar population undergoing long-term abstinence (Fein and Fein, 2013), indicating the potential for some structural recovery during abstinence. Polysubstance dependent individuals have decreased gray matter in the orbitofrontal cortex (Pennington et al., 2015; Tanabe et al., 2009), bilateral prefrontal lobe (Grodin et al., 2013; Liu et al., 1998), and occipital lobe (Zois et al., 2016), compared to control participants. However, both the Tanabe and Liu studies were broadly focused on polysubstance users and included participants without an AUD diagnosis.
Furthermore, both alcohol dependent individuals and substance dependent individuals have impairments in cognitive functioning. A recent meta-analysis found cognitive impairment in eleven domains in short-term abstinent individuals with AUD, including verbal fluency, speed of processing, working memory, attention, problem solving, inhibition, verbal learning, verbal memory, visual learning, visual memory, and visuospatial processing (Stavro et al., 2013). Men with polysubstance dependence show neuropsychological deficits in domains including attention, inhibitory control, visual memory, abstract reasoning, mental flexibility, conceptualization, and motor planning (Cunha et al., 2010; Medina et al., 2004). Women with polysubstance use disorder perform worse on verbal learning tasks than matched control participants (Medina et al., 2006). Cocaine use disorder has been associated with deficits in visual learning and memory, executive functioning, impulsivity, verbal learning and memory, visuospatial abilities, and working memory (Potvin et al., 2014). Cognitive deficits are also present in active, non-abstinent drug users. Active cocaine users performed worse than healthy controls on attention-switching working memory tasks, displaying a particular deficit in visuospatial tasks (Kubler et al., 2005), and active heavy drinkers showed impairments on a memory and visuospatial index (Green et al., 2010).
Alcohol dependence is often comorbid with other substance use disorders. However, there has been limited research on the effect of comorbid alcohol and drug dependence on subcortical volume and previous studies have only included individuals with alcohol dependence and comorbid stimulant use disorders. We compared subcortical brain volume in three groups: individuals with alcohol dependence only (ADO), individuals with polysubstance use disorder (PS; all diagnosed with alcohol dependence and at least two additional substance use disorders), and healthy control participants (HC). We further sought to determine if subcortical regional volumes were related to cognitive and drinking measurements. We focused on subcortical structures as they are implicated in domains disrupted by addiction, including reward (nucleus accumbens, amygdala), cognition (hippocampus), habit formation (caudate, putamen, globus pallidus), and compulsive behavior (thalamus). We examined the following hypotheses: (1) Both ADO and PS individuals have decreased subcortical volumes compared to HC; (2) PS individuals have increased subcortical volume compared to ADO individuals; and (3) In ADO and PS individuals, subcortical volumes negatively correlate with drinking measures and directly correlate with poor cognitive performance.
Materials and Methods
All recruitment and testing procedures were reviewed and approved by the National Institute on Alcohol Abuse and Alcoholism Institutional Review Board. After complete detoxification and withdrawal, experimental procedures (psychometric interviews and MRI) were explained and all participants provided written informed consent to participate.
Participants
Individuals with alcohol dependence (n = 74, 32 Female), ages 21–64, were admitted to an inpatient alcohol treatment program. Alcohol dependent participants were divided based on their comorbid substance use into individuals with alcohol dependence only (ADO; n = 37, 16 female) and individuals with polysubstance use disorder (PS; n = 37, 16 female). All PS reported alcohol as their problem substance and were diagnosed with at least 2 comorbid substance use disorders (see Table 1 and Table S1). Community-recruited healthy control participants (HC; n = 37, 16 Female), ages 21 – 58, with no history of significant medical illness or psychiatric disorders, were also included for comparison.
Table 1.
Demographic characteristics.
| Characteristics | HC (n = 37) | ADO (n = 37) | PS (n = 37) | Pairwise comparisons |
|---|---|---|---|---|
| Male/Female | 21/16 | 21/16 | 21/16 | HC = ADO = PS (NS) |
| Age (years) | 39.00 ± 8.15 | 40.16 ± 9.16 | 38.24 ± 7.64 | HC = ADO = PS (NS) |
| BMI | 25.51 ± 4.18 | 26.74 ± 4.44 | 26.16 ± 5.35 | HC = ADO = PS (NS) |
| Lifetime Total Alcohol (kg) | 11.64 ± 16.97 | 467.03 ± 470.64 | 619.15 ± 479.41 | HC < ADO (p < 0.001) |
| HC < PS (p < 0.001) | ||||
| ADO = PS (NS) | ||||
| Heavy Drinking (years) | - | 10.28 ± 7.46 | 11.98 ± 7.15 | ADO = PS (NS) |
| Age of Onset of Heavy Drinking | - | 25.32 ± 9.48 | 22.86 ± 7.35 | ADO = PS (NS) |
| Alcohol Abstinence (days) | - | 21 ± 5 | 22 ± 3 | ADO = PS (NS) |
| Smokers, n (%) | 4 (11) | 20 (54) | 24 (65) | HC < ADO (χ2 < 0.001) |
| HC < PS (χ2 < 0.001) | ||||
| ADO = PS (NS) | ||||
| Smoking (Years) | 10.25 ± 8.14 | 17.03 ± 10.47 | 22.79 ± 7.99 | HC = ADO = PS (NS) |
| Cigarette Packs/Day | 0.64 ± 0.46 | 0.64 ± 0.54 | 1.30 ± 0.49 | HC = ADO (NS) |
| HC < PS (p < 0.05) | ||||
| ADO < PS (p < 0.001) | ||||
| Psychiatric comorbidities, n (%) | 0 (0) | 28 (76) | 27 (73) | HC < ADO (χ2 < 0.001) |
| HC < PS (χ2 < 0.001) | ||||
| ADO = PS (NS) | ||||
| Alanine Transaminase (ALT) | 26.03 ± 12.16 | 53.25 ± 51.49 | 45.75 ± 36.16 | HC < ADO (p = 0.003) |
| HC < PS (p = 0.003) | ||||
| ADO = PS (NS) | ||||
| Aspartate Transaminase (AST) | 26.19 ± 7.01 | 57.53 ± 59.39 | 54.39 ± 68.26 | HC < ADO (p = 0.002) |
| HC < PS (p = 0.016) | ||||
| ADO = PS (NS) | ||||
| Albumin | 4.36 ± 0.45 | 4.40 ± 0.44 | 4.12 ± 0.43 | HC = ADO (NS) |
| HC > PS (p = 0.02) | ||||
| ADO > PS (p = 0.008) | ||||
| Education (years) | 16.51 ± 2.95 | 14.46 ± 2.57 | 12.85 ± 2.09 | HC > ADO (p = 0.002) |
| HC > PS (p < 0.001) | ||||
| ADO > PS (p = 0.003) | ||||
|
| ||||
| Psychiatric Comorbidities | HC (n = 37) | ADO (n = 37) | PS (n = 37) | |
|
| ||||
| Major Depressive Disorder, n (%) | 0 (0) | 25 (68) | 24 (65) | |
| Anxiety Disorder, n (%) | 0 (0) | 15 (41) | 20 (54) | |
| PTSD, n (%) | 0 (0) | 12 (32) | 14 (38) | |
| OCD, n (%) | 0 (0) | 9 (24) | 12 (32) | |
| Eating Disorder, n (%) | 0 (0) | 1 (3) | 2 (5) | |
|
| ||||
| Drug Use | HC (n = 37) | ADO (n = 37) | PS (n = 37) | |
|
| ||||
| Dependence/Abuse | Dependence/Abuse | Dependence/Abuse | ||
| Cocaine, n (%) | - | - | 24 (65) / 9 (24) | |
| Amphetamine, n (%) | - | - | 8 (22) / 3 (8) | |
| Cannabis, n (%) | - | - | 28 (76) / 0 (0) | |
| Hallucinogen, n (%) | - | - | 10 (27) / 12 (37) | |
| Sedative, n (%) | - | - | 8 (22) / 6 (16) | |
| Opioid, n (%) | - | - | 7 (19) / 6 (16) | |
| Tobacco, n (%) | 4 (11) / 0 (0) | 20 (54) / 0 (0) | 24 (65) / 0 (0) | |
Abbreviations: HC, Healthy control participants; ADO, individuals with alcohol dependence only; PS, individuals with polysubstance use disorder; PTSD, Posttraumatic Stress Disorder, OCD, Obsessive-Compulsive Disorder
Participants with an estimated IQ below 80, who had neurological abnormalities, had a history of psychotic symptoms, or were not eligible for an MRI scan were not included in the sample. All participants were assessed with the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), which determined that each inpatient met criteria for alcohol dependence and that the comparison participants did not meet criteria for current axis I disorders. A urine sample was collected to verify drug abstinence. All participants received a physical examination to ensure good general physical and neurological health. A social worker administered a semi-structured lifetime drinking history interview to each participant. Alcohol use history was divided into epochs of use patterns according to the respondent's history. We calculated three drinking history parameters from these epochs: 1) age at onset of heavy drinking, defined a priori as the age at which the subject reported first consuming 90 drinks in a 1-month period; 2) years of heavy drinking, defined as the cumulative total contiguous or noncontiguous months during which the participant drank 90 or more drinks per month; and 3) estimated lifetime alcohol consumption (in kg), which is a summation of all alcohol ingestion, including during periods where ingestion did not reach 90 drinks per month. Demographic information can be found in Table 1.
Cognitive Tests
Cognitive tests were conducted to measure estimated intelligence quotient (IQ), memory, inhibitory control, and visual attention and task switching. Cognitive tests were conducted on average 11.52 ± 21.48 days before the MR scan for HC and 1.68 ± 1.36 days before the MR scan for AD participants (ADO = 2.00 ± 1.47; PS = 1.40 ± 1.22). IQ was estimated from two sub-scales, vocabulary and block design, from the Wechsler Adult Intelligence Scale – Revised (WAIS-R; (Wechsler, 1981)). The Buschke Selective Reminding Test (SRT; (Buschke and Fuld, 1974)) was used to evaluate memory impairment. In this task, a list of 12 words was read to the participant who was asked to recall these words. The participant was then reminded of the words that they did not recall. This procedure was repeated 8 times. We calculated the total number of trials required to learn the word list as a measure of episodic memory function. The Stroop Color Word Test (SCWT; (Stroop, 1935)) was used to evaluate inhibitory control. Participants were asked to say the color of ink of a color word, where the word meanings and ink colors were mismatched. We used the color-word T-score to measure inhibitory control. Finally, the Trail Making Test (TMT; (Reitan, 1992)) was administered to measure visual attention and task switching. Visual attention was measured in Part A (Trails A), where participants were instructed to draw lines to connect consecutively numbered circles as fast as possible. Visual attention and task switching were measured in Part B (Trails B), where participants were instructed to draw lines to connect alternatively numbered and lettered circles. We used the number of seconds required to complete Trails A and Trails B (see Table 2).
Table 2.
Cognitive Measures.
| Characteristics | Group | Score | Contrast | Effect Size | p-value |
|---|---|---|---|---|---|
| WAIS IQ Estimate | HC | 110.27 ± 8.81 | HC = ADO | 0.35 | 0.21 |
| ADO | 106.02 ± 14.80 | HC > PS | 1.43 | <0.001 | |
| PS | 97.71 ± 8.81 | ADO > PS | 0.68 | 0.004 | |
| Selective Reminding Test (number total trials) | HC | 7.39 ± 1.29 | HC = ADO | 0.07 | 0.84 |
| ADO | 7.47 ± 1.01 | HC = PS | 0.37 | 0.21 | |
| PS | 7.84 ± 1.17 | ADO = PS | 0.34 | 0.27 | |
| Stroop Color Word | HC | 51.61 ± 7.32 | HC = ADO | 0.47 | 0.18 |
| ADO | 48.29 ± 6.93 | HC < PS | 0.68 | 0.03 | |
| PS | 46.56 ± 7.59 | ADO = PS | 0.24 | 0.44 | |
| Trails A (seconds) | HC | 30.61 ± 11.76 | HC = ADO | 0.43 | 0.10 |
| ADO | 36.14 ± 13.92 | HC = PS | 0.03 | 0.90 | |
| PS | 30.97 ± 10.18 | ADO = PS | 0.42 | 0.10 | |
| Trails B (seconds | HC | 60.65 ± 18.66 | HC < ADO | 0.57 | 0.03 |
| ADO | 77.43 ± 36.82 | HC < PS | 0.74 | 0.005 | |
| PS | 84.22 ± 41.26 | ADO = PS | 0.17 | 0.51 |
MRI acquisition
All subjects were scanned using a 1.5 T General Electric MRI scanner (General Electric, Milwaukee, WI) and a standard head coil. Whole-brain high-resolution coronal structural scans were collected using a T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) pulse sequence with matrix 256 × 256 × 124, repetition time (TR) = 100 ms, echo time (TE) = 12 ms, field of view (FOV) = 24 cm, and voxel size of (0.9375 × 0.9375 × 2.0) mm3.
Cortical and Subcortical Analysis
The segmentation of intracranial volume (ICV) into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) was performed using an intensity-adaptive algorithm. Based on the information gathered from the histogram of T1-weighted images, an unsupervised K-means clustering procedure separated various tissue regions (Momenan et al., 1997). GM, WM, and CSF were then ICV-normalized.
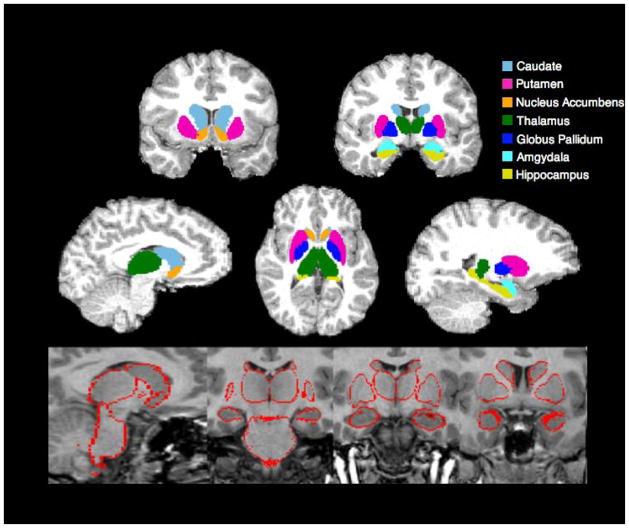
Volumetric analysis of subcortical structures was carried out using FSL’s Integrated Registration and Segmentation Tool (FIRST), which is a model-based segmentation and registration tool (Patenaude et al., 2011). The shape/appearance models used in FIRST were constructed from manually segmented images provided by the Center for Morphometric Analysis, Massachusetts General Hospital, Boston, MA. Images underwent a two-stage affine registration to MNI152 space at a 1x1x1 mm resolution. The first stage registered images to a template using a 12 degrees of freedom registration. The second stage applied a 12 degrees of freedom registration using an MRNI142 subcortical mask that excluded voxels outside the subcortical regions. Following registration, images were transformed back to native space using inverse transformation in order for segmentation to be performed with the original voxel intensities. Images were then segmented into 14 subcortical structures (bilateral thalamus, caudate, globus pallidus, putamen, hippocampus, amygdala, nucleus accumbens) and volumetric masks were created. Boundary voxels were converted into interior voxels and overlapping segmentations were corrected by comparing intensity distributions in overlapping segmentations. Subcortical segmentation was visually inspected for quality assurance, and participants with uncorrected outliers were excluded from further analysis (see Figure 1 for subcortical segmentation example). Volumes for the subcortical regions were extracted using fslstats and were ICV-normalized using the following equation: (tissue-volume/ICV) * 1000.
Figure 1.
Example Subcortical Segmentation
Top panel: Subcortical segmentation and registration generated by FIRST overlaid on a skull stripped brain.
Bottom panel: Subcortical segmentation output used for quality assurance.
Group volumetric analysis was conducted using JMP software (version 10.0.0; SAS Institute, Cary, North Carolina). Multivariate analysis of covariance (MANCOVA) examined group differences separately across cortical and subcortical volumes. Age and smoking status were significant predictors of variance for cortical volumes and were included as covariates. Age and education were significant predictors of variance for subcortical volume and were included as covariates. The direction of the effect of age and education can be found in the supplement. Sex and other Axis I disorders (see Table 1) were not significant predictors of subcortical or cortical volume variance and were not included as covariates. Although Axis I disorders were not a significant predictor of variance, we performed a secondary analysis excluding participants with a PTSD diagnosis (Table S2 and S3), because we have previously found an effect of comorbid PTSD on brain structure (Grodin et al., 2013). The MANCOVAs were followed up with post-hoc analyses to identify group differences, post-hoc Tukey HSD (honest significant difference) t-tests were conducted, and Bonferroni corrections were applied. Effect sizes were calculated using Cohen’s d (Cohen, 1988). We conducted associations between cortical and subcortical volumes and cognitive and drinking measures using Pearson’s correlations. To limit the number of comparisons, we only calculated correlations using regions that showed significant group differences.
Results
Demographic and Cognitive Results
There were no group differences on participant age or BMI. Unsurprisingly, both ADO and PS had greater lifetime alcohol consumption than HC. There were no differences between ADO and PS on lifetime alcohol consumption, age of heavy drinking onset, or years of heavy drinking. There were a greater number of smokers in the ADO and PS groups compared to HC, but no differences between ADO and PS. There were also a greater number of comorbid psychiatric diagnoses (excluding alcohol and substance dependence) in ADO and PS compared to HC, but there no group differences between ADO and PS. Both ADO and PS had elevated ALT and AST compared to HC, with no differences between ADO and PS. PS had decreased albumin compared to HC and ADO. There were significant differences between all groups on years of education, with HC having the highest years of education and PS having the lowest. PS also had a lower estimated IQ than HC and ADO. On cognitive measures, HC performed better than PS on the Stroop Color Word Test. ADO and PS had worse performance on the Trails B (task switching) compared to HC. There were no group differences in Buschke Selective Reminding Test performance or in Trails A (visual attention) performance.
Volumetric Results
Cortical and Subcortical Volumes
MANCOVA revealed a significant main effect of group (F(2,102) = 5.48, p < 0.001), age (F(2,102) = 13.46, p < 0.001), and smoking status (F(2,102) = 3.93, p = 0.01) on cortical volumes. There were significant group effects on GM volume and CSF volume (see Table 3).
Table 3.
Gray Matter, White Matter, Cerebrospinal Fluid, and Intracranial Volume Group measurements.
| Region | Group Effect: F-value (p-value) | Group | Volume | Contrast | Effect Size | p-value |
|---|---|---|---|---|---|---|
| GM | 13.15 (<0.001) | HC | 362.74 ± 49.10 | HC > ADO | 0.80 | <0.001 |
| ADO | 325.11 ± 45.00 | HC > PS | 0.75 | <0.001 | ||
| PS | 332.55 ± 28.40 | ADO = PS | 0.20 | 0.79 | ||
| WM | 0.16 (0.85) | HC | 314.44 ± 47.82 | HC = ADO | 0.28 | 0.38 |
| ADO | 303.17 ± 32.72 | HC = PS | 0.23 | 0.69 | ||
| PS | 325.12 ± 45.00 | ADO = PS | 0.56 | 0.87 | ||
| CSF | 5.42 (0.006) | HC | 153.52 ± 23.85 | HC < ADO | 0.66 | 0.006 |
| ADO | 171.87 ± 31.51 | HC = PS | 0.18 | 0.50 | ||
| PS | 157.82 ± 25.14 | ADO = PS | 0.49 | 0.09 | ||
| ICV (mL) | HC | 1,393.53 ± 146.53 | HC > ADO | 0.52 | <0.001 | |
| ADO | 1,323.69 ± 119.69 | HC > PS | 0.58 | <0.001 | ||
| PS | 1,307.85 ± 150.92 | ADO = PS | 0.12 | 0.57 |
Abbreviations: GM, gray matter; WM, white matter; CSF, cerebrospinal fluid; ICV, intracranial volume
MANCOVA revealed a significant main effect of group (F(2,104) = 2.74, p < 0.001), age (F(2,104) = 2.56, p < 0.004), and education (F(2,104) = 1.95, p = 0.04) p < 0.001) on subcortical volumes. There were significant group effects on the left thalamus, right thalamus, left caudate, right caudate, left putamen, right globus pallidus, left hippocampus, right hippocampus, left amygdala, left nucleus accumbens, and right nucleus accumbens (see Table 4).
Table 4.
Subcortical volumes for each group, effect sizes, and p-values for follow-up tests
| Subcortical Region | Group Effect: F-value (p-value) | Group | Volume | Contrast | Effect Size | p-value uncorrected | p-value corrected |
|---|---|---|---|---|---|---|---|
| L Thalamus | 8.16 (<0.001) | HC | 6.88 ± 0.67 | HC = ADO | 0.55 | 0.02 | |
| ADO | 6.56 ± 0.48 | HC > PS | 0.69 | 0.002 | 0.03 | ||
| PS | 6.49 ± 0.44 | ADO = PS | 0.15 | 0.42 | |||
| R Thalamus | 8.07 (<0.001) | HC | 6.68 ± 0.58 | HC > ADO | 0.67 | 0.002 | 0.03 |
| ADO | 6.27 ± 0.64 | HC > PS | 0.56 | 0.003 | 0.04 | ||
| PS | 6.39 ± 0.44 | ADO = PS | 0.22 | 0.93 | |||
| L Caudate | 3.65 (0.03) | HC | 2.80 ± 0.48 | HC = ADO | 0.60 | 0.03 | |
| ADO | 2.57 ± 0.25 | HC = PS | 0.56 | 0.36 | |||
| PS | 2.73 ± 0.36 | ADO = PS | 0.34 | 0.65 | |||
| R Caudate | 7.82 (<0.001) | HC | 2.77 ± 0.42 | HC = ADO | 0.54 | 0.07 | |
| ADO | 2.58 ± 0.27 | HC = PS | 0.40 | 0.31 | |||
| PS | 2.92 ± 0.32 | ADO < PS | 1.15 | 0.002 | 0.03 | ||
| L Putamen | 3.27 (0.04) | HC | 4.05 ± 0.53 | HC = ADO | 0.51 | 0.05 | |
| ADO | 3.82 ± 0.35 | HC = PS | 0.07 | 0.86 | |||
| PS | 4.02 ± 0.31 | ADO = PS | 0.60 | 0.27 | |||
| R Putamen | 3.04 (0.05) | HC | 4.01 ± 0.55 | HC = ADO | 0.54 | 0.04 | |
| ADO | 3.76 ± 0.35 | HC = PS | 0.15 | 0.62 | |||
| PS | 3.94 ± 0.34 | ADO = PS | 0.52 | 0.45 | |||
| L Globus Pallidus | 3.01 (0.05) | HC | 1.46 ± 0.16 | HC = ADO | 0.29 | 0.37 | |
| ADO | 1.42 ± 0.11 | HC = PS | 0.00 | 0.41 | |||
| PS | 1.46 ± 0.12 | ADO = PS | 0.35 | 0.99 | |||
| R Globus Pallidus | 3.23 (0.04) | HC | 1.48 ± 0.16 | HC = ADO | 0.42 | 0.08 | |
| ADO | 1.42 ± 0.12 | HC = PS | 0.14 | 0.05 | |||
| PS | 1.46 ± 0.12 | ADO = PS | 0.33 | 0.88 | |||
| L Hippocampus | 7.59 (<0.001) | HC | 3.13 ± 0.31 | HC > ADO | 0.79 | 0.001 | 0.01 |
| ADO | 2.88 ± 0.32 | HC = PS | 0.33 | 0.16 | |||
| PS | 3.04 ± 0.23 | AD = PS | 0.57 | 0.39 | |||
| R Hippocampus | 5.51 (0.005) | HC | 3.15 ± 0.30 | HC > ADO | 0.62 | 0.003 | 0.04 |
| ADO | 2.96 ± 0.31 | HC = PS | 0.24 | 0.16 | |||
| PS | 3.09 ± 0.20 | ADO = PS | 0.50 | 0.67 | |||
| L Amygdala | 3.71 (0.03) | HC | 1.25 ± 0.16 | HC = ADO | 0.13 | 0.80 | |
| ADO | 1.23 ± 0.15 | HC = PS | 0.33 | 0.03 | |||
| PS | 1.20 ± 0.14 | ADO = PS | 0.21 | 0.09 | |||
| R Amygdala | 2.69 (0.07) | HC | 1.19 ± 0.16 | HC = ADO | 0.47 | 0.19 | |
| ADO | 1.12 ± 0.14 | HC = PS | 0.34 | 0.08 | |||
| PS | 1.13 ± 0.19 | ADO = PS | 0.06 | 0.78 | |||
| L Nucleus Accumbens | 3.85 (0.02) | HC | 0.45 ± 0.09 | HC = ADO | 0.70 | 0.02 | |
| ADO | 0.39 ± 0.08 | HC = PS | 0.12 | 0.82 | |||
| PS | 0.44 ± 0.08 | ADO = PS | 0.62 | 0.17 | |||
| R Nucleus Accumbens | 7.34 (0.001) | HC | 0.41 ± 0.09 | HC > ADO | 0.82 | <0.001 | 0.01 |
| ADO | 0.34 ± 0.08 | HC = PS | 0.37 | 0.04 | |||
| PS | 0.38 ± 0.07 | ADO = PS | 0.53 | 0.68 |
Healthy Controls vs. Individuals with Alcohol Dependence Only
HC had greater GM volume compared to ADO (p < 0.001), while ADO had greater cerebrospinal fluid compared to HC (p < 0.001; see Table 3). There were four subcortical regions with significant group differences. HC had significantly greater subcortical volume in the bilateral hippocampus, right thalamus, and right nucleus accumbens compared to ADO (all p < 0.05, corrected; see Table 4).
Healthy Controls vs. Individuals with Polysubstance Use Disorder
HC had greater GM volume compared to PS (p < 0.001; Table 3). There were two subcortical regions that showed significant group differences: the left and right thalamus (both p < 0.05, corrected). HC had significantly greater volume in these regions compared to PS (see Table 4).
Individuals with Alcohol Dependence Only vs. Individuals with Polysubstance Use Disorder
ADO and PS did not differ on cortical measurements, although there was a trend towards increased CSF in ADO. There was one subcortical region that showed significant difference between ADO and PS. PS had significantly larger right caudate volume compared to ADO (p = 0.001, corrected; see Table 4).
Correlation Results
Cognitive Measures
Gray matter volume was positively correlated with IQ (r = 0.47, p = 0.01) and negatively correlated with TMT Trails A scores (r = −0.32, p = 0.04) in HC. In ADO, gray matter volume was positively correlated with Stroop color-word T-scores (r = 0.51, p = 0.03). In PS, gray matter volume was positively correlated with IQ (r = 0.32, p = 0.04).
In HC, Stroop color-word T-scores were positively correlated with left thalamus volume (r = 0.54, p = 0.02), right thalamus volume (r = 0.47, p = 0.04), right caudate volume (r = 0.48, p = 0.04), and right nucleus accumbens volume (r = 0.53, p =0.02). In ADO, IQ scores were correlated with right caudate volume (r = 0.48, p = 0.04) and TMT Trails B scores were negatively correlated with right hippocampal volume (r = −0.34, p = 0.04). In PS, Buschke SRT scores were positively correlated with right thalamus volume (r = 0.36, p = 0.04).
Drinking Measures
CSF volume was positively correlated with lifetime alcohol consumption (r = 0.42, p = 0.02) in HC. There were no other significant correlations between cortical volume and drinking measures.
In ADO, bilateral hippocampal volume was positively correlated with age of heavy drinking onset (left hippocampus: r = 0.38, p = 0.02; right hippocampus: r = 0.30, p = 0.04), and negatively correlated with lifetime alcohol consumption (left hippocampus: r = −0.41, p = 0.01; right hippocampus: r = −0.33, p = 0.04). Years of heavy drinking was negatively correlated with left hippocampal volume (r = −0.45, p = 0.005) and right nucleus accumbens volume (r = −0.31, p = 0.03), in ADO. There were no correlations between drinking measures and volumetric measures in PS.
Discussion
We conducted a cross-sectional analysis of subcortical volumes in three groups: individuals with alcohol dependence only, individuals with polysubstance use disorder, and a comparison group of healthy controls. To our knowledge, this is the first neuroimaging study to compare subcortical volumes in alcohol dependent individuals with and without multiple additional substance use disorder diagnoses. We report four major findings. First, compared to healthy controls, individuals with alcohol dependence only had reduced volume in the bilateral hippocampus, right thalamus, and right nucleus accumbens. Second, compared to healthy controls, individuals with polysubstance use disorder had reduced volume in the bilateral thalamus. Third, individuals with polysubstance use disorder had larger right caudate volume compared to individuals with alcohol dependence only. Finally, there were associations between cortical and subcortical volumes, neurocognitive measures, and drinking measures. Correlations between drinking measures and subcortical volumes were only present in individuals with alcohol dependence only.
ADO and PS had cortical volume reductions compared to HC. Both alcohol dependent groups had smaller GM compared to HC, but only ADO had larger CSF. GM volume reductions have been reported in both alcohol dependent individuals (Mon et al., 2014) and polysubstance users (Liu et al., 1998). Despite both ADO and PS having decreased GM, only the ADO individuals had increased CSF compared to HC. Increased CSF volume has been shown to predict gray matter deficits in alcohol dependent individuals (Le Berre et al., 2015). The decrease in GM volume and increase in CSF volume in ADO are suggestive of a hydoccephalus ex vacuo process, where brain atrophy results in a compensatory enlargement of CSF (Le Berre et al., 2015). PS did not have this compensatory CSF enlargement, perhaps due to their larger (though not statistically significant) WM volume. Furthermore, there was a significant group x smoking interaction; smoking had an effect on GM in HC, but there were not additional effects of smoking in ADO or PS (see Supplement for details).
ADO and PS also had subcortical volume reduction compared to HC. ADO had subcortical volume reductions in the bilateral hippocampus, right nucleus accumbens, and right thalamus, while PS only had decreased volume in the bilateral thalamus. In general, ADO had the smallest subcortical volumes and HC had the largest, with PS falling in the middle. Additionally, the effect sizes for the volume reductions were larger in the ADO group than in the PS group. The largest effect was in the right nucleus accumbens, where HC had significantly larger accumbal volumes compared to ADO. Several studies have reported reduced accumbal volume in alcohol dependent individuals (Fein and Fein, 2013; Makris et al., 2008; Wrase et al., 2008). Furthermore, a preclinical study reported reductions in accumbal volume in alcohol-naïve rats with a genetic predisposition to alcohol addiction (Gozzi et al., 2013), suggesting that accumbal volume reductions may be a pre-existing condition resulting in a vulnerability to alcohol addiction. ADO individuals also had decreased hippocampal volume, which is consistent with the literature on the hippocampal volume and alcohol use disorder (Agartz et al., 1999; Fein and Fein, 2013; Sullivan et al., 1995; Wrase et al., 2008), and corresponds to preclinical findings of hippocampal neurodegeneration due to alcohol exposure (Geil et al., 2014). There was also decreased volume in the right thalamus in both ADO and PS individuals, and decreased left thalamic volume in PS individuals. Several studies have reported reductions in thalamic volume in AD individuals (Fein and Fein, 2013; Kril et al., 1997; Mon et al., 2014), and thalamic volume has been shown to negatively correlate with alcohol consumption (Kril et al., 1997). Thalamic volume reductions have been reported in individuals with alcohol dependence and comorbid psychostimulant dependence (Mon et al., 2014), however another study investigating a similar population did not find a decrease in thalamus volume (Fein and Fein, 2013). This discrepancy may be due to differences in abstinence duration between studies. In the present study, PS individuals were abstinent for roughly three weeks. The population investigated by Mon and colleagues were abstinent for about a month, while the population investigated by Fein and Fein were abstinent for a longer period, about two to three months. Therefore, the decreased thalamic volume in PS individuals may only be present during short-term abstinence that is recovered after two-three months of abstinence. Reduced thalamic volume has also been reported in opioid dependent individuals, and alcohol consumption in those individuals was negatively associated with thalamic volume, indicating that increasing alcohol consumption in drug users may result in further decreases in thalamic gray matter (Reid et al., 2008). The thalamus was the only subcortical region with significant volume reduction in both alcohol dependent groups. The thalamus is implicated in compulsive behaviors, as compulsive self-stimulation has been seen in patients with stimulating electrodes implanted in their thalamus (Portenoy et al., 1986), and it serves as an important relay station from the striatum to the orbitofrontal cortex. Dysfunction in the striato-thalamo-orbitofrontal circuit has been hypothesized to result in compulsive behavior and increased drug-seeking motivation seen in addiction (Volkow and Fowler, 2000).
The right caudate was the only region that showed a significant difference between the alcohol dependent groups. The right caudate was larger in the PS compared to ADO, replicating a previous finding in individuals with ADO and AD with a comorbid stimulant disorder (Fein and Fein, 2013). There are two potential explanations for the larger caudate volume in PS individuals. First, polysubstance use may have a neuroprotective effect on the caudate, potentially mediated cannabis use. Cannabinoids inhibit the production of pro-inflammatory mediators and reduce glutamate excitotoxicity (O'Sullivan and Kendall, 2010), and alcohol-induced neurodegeneration has been attributed to increases in oxidative stress and to glumatergic exicitotoxicity (Crews, 2008; Lovinger, 1993). However, the addition of cannabis use as a covariate in the MANCOVA model did not effect the results (i.e. there was no main effect of cannabis use on subcortical volume and the same subcortical regions showed significant group differences). Alternatively, inflammation caused by stimulant use may have masked the effects of alcohol on this region (Clark et al., 2013). Previous studies have identified increases in caudate volume in stimulant dependent individuals, including cocaine dependence (Ersche et al., 2011) and methamphetamine dependence (Jernigan et al., 2005). A positron emission tomography (PET) study found increased activated microglia, increasing the production of pro-inflammatory cytokines, in the striatum of chronic methamphetamine users (Sekine et al., 2008). The cognitive results provide support for the stimulant inflammation hypothesis, as PS individuals were equal or worse in cognitive measures compared to ADO individuals. If cannabis were protecting the brain from alcohol-related damage, PS would likely show a better performance than ADO on these measures. Brain volume has a positive correlation (r = 0.33) with intelligence in healthy individuals (McDaniel, 2005), an effect which was replicated in the present study. Furthermore, regional volume has been shown to have positive associations with cognitive measures. Thalamic volume was positively associated with cognitive speed in young and middle-aged individuals (Van Der Werf et al., 2001) and caudate, putamen, and globus pallidus volumes were positively associated with task switching accuracy (Verstynen et al., 2012). However, brain volume is not always positively associated with cognition and longitudinal studies on multiple groups of alcohol and drug users will be required to understand the mechanism of increased brain volume in polysubstance use.
While the right caudate was the only region to show significant differences between alcohol dependent groups, there was also a reduction in subcortical volume in the bilateral hippocampus and right nucleus accumbens in ADO that was not present in the PS. Although there was no significant volumetric difference between ADO and PS in these regions, there is likely a similar mechanism functioning to increase the volume or mask volumetric damage of these regions in the PS individuals. Fein and colleagues examined a similar population of short-term abstinent individuals with alcohol dependence and individuals with comorbid substance use and reported a large number of group differences, with their stimulant using group showing larger volume in the nucleus accumbens, amygdala, caudate, thalamus, and hippocampus (Fein and Fein, 2013). The population included in this study differs from the Fein study; here we included a population abusing at least two substances in addition to alcohol, while their population was restricted to only alcohol dependent individuals with comorbid stimulant dependence. Their alcohol dependent only population was also slightly older and both alcohol dependent groups had undergone a longer period of abstinence than our participants. Additionally, the Fein study combined the volume of the right and left subcortical regions, preventing laterality comparisons. These methodological and demographic differences may have resulted in Fein and Fein finding more significant group differences. Our PS population abused a wider range of substances, some of which may have masked the effect of others. Further, an older population may be more susceptible to the negative effects of alcohol. Pfefferbaum and colleagues have reported an age related increase in GM volume loss in alcohol dependent individuals that was unrelated to length of illness or lifetime intake of ethanol (Pfefferbaum et al., 1992). Sample sizes for the alcohol dependent groups were roughly equal in both studies, indicating that statistical power differences are unlikely to be responsible for differences.
Subcortical volumes were correlated with alcohol drinking measures in ADO, but not in PS. Associations were found between the bilateral hippocampus, right nucleus accumbens, and age of heavy drinking onset, years of heavy drinking, and lifetime alcohol consumption; all supporting the hypothesis that alcohol consumption is negatively associated with regional subcortical volume. Despite the lack of differences between ADO and PS on drinking measures, there were no associations between drinking measures and subcortical volumes in PS. Fein and colleagues also reported negative associations between subcortical volumes and alcohol use measures in ADO individuals, but not in PS individuals (Fein and Fein, 2013). There are several potential explanations for why there are no associations between PS individuals and drinking measures. First, polysubstance use may alter the effect of alcohol on subcortical volumes, masking or protecting regions from alcohol-induced damage. Second, polysubstance use itself may alter subcortical volume, potentially in a different direction than alcohol’s effect. Third, polysubstance use may change the association between alcohol use measures and subcortical volumes. Fourth, subcortical volume increases or reductions may be a premorbid condition, particularly in PS individuals, that confer vulnerability to addiction.
This study has several limitations, which should be considered. First, this study was cross-sectional and cannot reveal if reductions in subcortical volume are a direct effect of alcohol (or drug) consumption, or if volume reductions are a premorbid condition. Furthermore, the drinking measure association analysis relied on self-report, which may be inaccurate, particularly in heavy alcohol users. We also did not collect information regarding quantity and frequency of substance use in the substance-using group, preventing us from investigating associations between substance use and brain volume. Additionally, we were unable to fully investigate the impact of smoking on brain volume, as there were very few healthy control smokers. Future studies will need to include healthy control smokers to thoroughly examine the potential interaction between smoking, alcohol and/or polysubstance dependence, and brain volume. We also did not examine the influence of genetic polymorphisms on brain morphometry in this sample; future research should include this component to further elucidate differences between polysubstance using and alcohol using populations.
In summary, our present findings provide evidence for subcortical volume reductions in both individuals with alcohol dependence only (ADO) and individuals with polysubstance use disorder (PS), compared to healthy controls. ADO had volume reductions in the bilateral hippocampus, right nucleus accumbens, right thalamus, and GM and had increased volume in the CSF; while PS only had volume reductions in the bilateral thalamus and GM. We also found a difference in caudate volume between ADO and PS, where ADO showed decreased right caudate volume compared to PS. There were significant associations between subcortical volume and drinking measures in ADO, which were not present in PS. This is the first study to investigate the effect of alcohol and multiple comorbid substance dependence on subcortical volume. This study provides evidence that polysubstance use should be accounted for when studying the alcohol dependent population, and that comorbid substance use may underlie or mask volumetric differences previously reported in alcohol dependent individuals.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institutes of Health.
Footnotes
Disclosure/Conflict of Interest:
The authors state that they have no conflicts of interest and have nothing to declare.
Authors Contribution:
EG and RM were responsible for the study concept and design. EG performed data analysis. EG and RM interpreted the findings. EG drafted the manuscript. RM provided critical revision of the manuscript for intellectual content. Both authors critically reviewed the content and approved final version for publication.
References
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiat. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcoholism, clinical and experimental research. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Buschke H, Fuld PA. Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology. 1974;24:1019–1025. doi: 10.1212/wnl.24.11.1019. [DOI] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotoxicity research. 2013;23:174–188. doi: 10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Crews FT. Alcohol-Related Neurodegeneration and Recovery Mechanisms From Animal Models. Alcohol Res Health. 2008;31:377–388. [PMC free article] [PubMed] [Google Scholar]
- Cunha PJ, Nicastri S, de Andrade AG, Bolla KI. The frontal assessment battery (FAB) reveals neurocognitive dysfunction in substance-dependent individuals in distinct executive domains: Abstract reasoning, motor programming, and cognitive flexibility. Addictive behaviors. 2010;35:875–881. doi: 10.1016/j.addbeh.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain : a journal of neurology. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Fein D. Subcortical volumes are reduced in short-term and long-term abstinent alcoholics but not those with a comorbid stimulant disorder. NeuroImage Clinical. 2013;3:47–53. doi: 10.1016/j.nicl.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geil CR, Hayes DM, McClain JA, Liput DJ, Marshall SA, Chen KY, Nixon K. Alcohol and adult hippocampal neurogenesis: promiscuous drug, wanton effects. Progress in neuro-psychopharmacology & biological psychiatry. 2014;54:103–113. doi: 10.1016/j.pnpbp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Agosta F, Massi M, Ciccocioppo R, Bifone A. Reduced limbic metabolism and fronto-cortical volume in rats vulnerable to alcohol addiction. NeuroImage. 2013;69:112–119. doi: 10.1016/j.neuroimage.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Garrick T, Sheedy D, Blake H, Shores EA, Harper C. The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcoholism, clinical and experimental research. 2010;34:443–450. doi: 10.1111/j.1530-0277.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Grodin EN, Lin H, Durkee CA, Hommer DW, Momenan R. Deficits in cortical, diencephalic and midbrain gray matter in alcoholism measured by VBM: Effects of co-morbid substance abuse. NeuroImage Clinical. 2013;2:469–476. doi: 10.1016/j.nicl.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Corbett D. Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients--a quantitative Golgi study. Journal of neurology, neurosurgery, and psychiatry. 1990;53:856–861. doi: 10.1136/jnnp.53.10.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Blumbergs PC. Brain weights in alcoholics. Journal of neurology, neurosurgery, and psychiatry. 1982;45:838–840. doi: 10.1136/jnnp.45.9.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. The American journal of psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. The European journal of neuroscience. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Pitel AL, Chanraud S, Beaunieux H, Eustache F, Martinot JL, Reynaud M, Martelli C, Rohlfing T, Pfefferbaum A, Sullivan EV. Sensitive biomarkers of alcoholism's effect on brain macrostructure: similarities and differences between France and the United States. Frontiers in human neuroscience. 2015;9:354. doi: 10.3389/fnhum.2015.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacol. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Excitotoxicity and Alcohol-Related Brain-Damage. Alcoholism-Clinical and Experimental Research. 1993;17:19–27. doi: 10.1111/j.1530-0277.1993.tb00720.x. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Marinkovic K, Breiter HC, Gasic GP, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel MA. Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- Medina KL, Shear PK, Schafer J. Memory functioning in polysubstance dependent women. Drug and alcohol dependence. 2006;84:248–255. doi: 10.1016/j.drugalcdep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Medina KL, Shear PK, Schafer J, Armstrong TG, Dyer P. Cognitive functioning and length of abstinence in polysubstance dependent men. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2004;19:245–258. doi: 10.1016/S0887-6177(03)00043-X. [DOI] [PubMed] [Google Scholar]
- Momenan R, Hommer D, Rawlings R, Ruttimann U, Kerich M, Rio D. Intensity-adaptive segmentation of single-echo T1-weighted magnetic resonance images. Human brain mapping. 1997;5:194–205. doi: 10.1002/(SICI)1097-0193(1997)5:3<194::AID-HBM4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Abe C, Gazdzinski S, Pennington D, Schmidt T, Meyerhoff DJ. Structural brain differences in alcohol-dependent individuals with and without comorbid substance dependence. Drug and alcohol dependence. 2014;144:170–177. doi: 10.1016/j.drugalcdep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Prospective follow-up of empirically derived Alcohol Dependence subtypes in wave 2 of the National Epidemiologic Survey on Alcohol And Related Conditions (NESARC): recovery status, alcohol use disorders and diagnostic criteria, alcohol consumption behavior, health status, and treatment seeking. Alcoholism, clinical and experimental research. 2010;34:1073–1083. doi: 10.1111/j.1530-0277.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 2010;215:611–616. doi: 10.1016/j.imbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt TP, Abe C, Mon A, Meyerhoff DJ. Alcohol Use Disorder with and without Stimulant Use: Brain Morphometry and Its Associations with Cigarette Smoking, Cognition, and Inhibitory Control. Plos One. 2015;10 doi: 10.1371/journal.pone.0122505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism, clinical and experimental research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Jarden JO, Sidtis JJ, Lipton RB, Foley KM, Rottenberg DA. Compulsive thalamic self-stimulation: a case with metabolic, electrophysiologic and behavioral correlates. Pain. 1986;27:277–290. doi: 10.1016/0304-3959(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. Journal of addiction medicine. 2014;8:368–376. doi: 10.1097/ADM.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Reid AG, Daglish MR, Kempton MJ, Williams TM, Watson B, Nutt DJ, Lingford-Hughes AR. Reduced thalamic grey matter volume in opioid dependence is influenced by degree of alcohol use: a voxel-based morphometry study. Journal of psychopharmacology. 2008;22:7–10. doi: 10.1177/0269881107080795. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov VV, Damadzic R, Mann CL, Schwandt ML, George DT, Hommer DW, Heilig M, Momenan R. Reduced anterior insula, enlarged amygdala in alcoholism and associated depleted von Economo neurons. Brain : a journal of neurology. 2015;138:69–79. doi: 10.1093/brain/awu305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addiction biology. 2013;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biological psychiatry. 2005;57:768–776. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior Hippocampal Volume Deficits in Nonamnesic, Aging Chronic-Alcoholics. Alcoholism-Clinical and Experimental Research. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PA, Vuurman E, Uylings HB, Jolles J. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysis. Brain research Cognitive brain research. 2001;11:377–385. doi: 10.1016/s0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Lynch B, Miller DL, Voss MW, Prakash RS, Chaddock L, Basak C, Szabo A, Olson EA, Wojcicki TR, Fanning J, Gothe NP, McAuley E, Kramer AF, Erickson KI. Caudate Nucleus Volume Mediates the Link between Cardiorespiratory Fitness and Cognitive Flexibility in Older Adults. Journal of aging research. 2012;2012:939285. doi: 10.1155/2012/939285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cerebral cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Psychological Corporation; New York: 1981. [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. The American journal of psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Xiao P, Dai Z, Zhong J, Zhu Y, Shi H, Pan P. Regional gray matter deficits in alcohol dependence: A meta-analysis of voxel-based morphometry studies. Drug and alcohol dependence. 2015;153:22–28. doi: 10.1016/j.drugalcdep.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Zois E, Kiefer F, Lemenager T, Vollstadt-Klein S, Mann K, Fauth-Buhler M. Frontal cortex gray matter volume alterations in pathological gambling occur independently from substance use disorder. Addiction biology. 2016 doi: 10.1111/adb.12368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.