Abstract
Purpose
How micronutrients might influence risk of developing adenocarcinoma of the prostate has been the focus of a large body of research (especially regarding vitamins E, A, and D). Metabolomic profiling has the potential to discover molecular species relevant to prostate cancer etiology, early detection, and prevention, and may help elucidate the biologic mechanisms by which vitamins influence prostate cancer risk.
Methods
Prostate cancer risk data related to vitamins E, A, and D and metabolomics profiling from clinical, cohort, and nested case-control studies, along with randomized controlled trials, are examined and summarized, along with recent metabolomic data of the vitamin phenotypes.
Results
Higher vitamin E serologic status is associated with lower prostate cancer risk, and vitamin E genetic variant data support this. By contrast, controlled vitamin E supplementation trials have mixed results based on differing designs and dosages. Beta-carotene supplementation (in smokers) and higher circulating retinol and 25-hydroxy-vitamin D concentrations appear related to elevated prostate cancer risk. Our prospective metabolomics profiling of fasting serum collected 1-20 years prior to clinical diagnoses found lipid and energy/TCA cycle metabolites, including inositol-1-phosphate, lysolipids, alpha-ketoglutarate, and citrate, significantly associated with risk of aggressive disease.
Conclusions
Several active leads exist regarding the role of micronutrients and metabolites in prostate cancer carcinogenesis and risk. How vitamins D and A may adversely impact risk, and whether low-dose vitamin E supplementation remains a viable preventive approach, require further study.
Keywords: Prostate Cancer, Micronutrients, Vitamins, Metabolomics, vitamin E, vitamin D, vitamin A
Introduction
How micronutrients might influence risk of developing adenocarcinoma of the prostate has been the focus of a large body of research, including large randomized controlled supplementation trials, serologic analyses within prospective cohorts, laboratory experiments, and mechanistic studies. Although a wide range of vitamins and minerals have been pursued, more abundant and actionable data exist for vitamins E, A, and D. Additionally, metabolomic profiling is a relatively new analytic tool that measures a broad array of low molecular weight biochemicals in blood and other biospecimens [1,2] and that has the potential to discover molecular species relevant to both micronutrient status and cancer etiology, early detection, and prevention. These untargeted analyses are similar to other agnostic approaches, such as genome-wide association studies, that have successfully identified new biological pathways related to vitamin metabolism and potentially involved in carcinogenesis (e.g., prostate cancer). Recent available research related to prostate cancer risk and vitamins E, A, and D, as well as their metabolomic profiling, are presented in this article.
Vitamins and Prostate Cancer
Vitamin E
Vitamin E is a fat-soluble vitamin that occurs naturally in eight forms – four tocopherols and four tocotrienols - however, alpha-tocopherol is the only form considered in the establishment of dietary intake requirements. Vitamin E is commonly found in oils, whole grains, nuts, seeds, and some green leafy vegetables. Interestingly, because of the types of oils consumed in the United States, the intake of gamma-tocopherol is greater than that of alpha-tocopherol, while in Europe alpha-tocopherol intake is greater [3,4]. A primary biological role of vitamin E is in vivo anti-oxidation, but it also improves immunity, has anti-angiogenic properties, and inhibits protein kinase C activity, cell adhesion, and cell proliferation [5,6].
In the mid-1980s, epidemiological and laboratory evidence suggested that alpha-tocopherol and beta-carotene were associated with lower risks of lung and other cancers. In 1985, the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study controlled trial was launched to test these hypotheses. Finnish male smokers (n=29,133) were randomized to four intervention groups based on a 2×2 factorial design: 50 mg (50 IU) alpha-tocopheryl acetate (ATA) alone, 20 mg beta-carotene alone, both supplements, or placebo [7]. The trial reported a 32% reduction in prostate cancer incidence in the men receiving ATA (beta-carotene findings are discussed below)[8,9]. Subsequently, the Heart Outcomes Prevention Evaluation (HOPE) trial with 400 IU ATA daily [10] and the Physicians’ Health Study II (PHS-II) with 400 IU RRR-natural alpha-tocopherol supplementation every other day found no effect on prostate cancer incidence [relative risk (RR)= 0.98, 95% CI 0.76-1.26 and hazard ratio (HR)=0.97, 95% CI 0.85-1.09, respectively] [10,11]. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) tested 400 IU ATA daily, and although it initially reported no prostate cancer effect [12], further follow-up showed increased incidence (HR=1.17, 99% CI 1.004-1.36)[13]. Two other trials tested vitamin E in combination with other micronutrients – the SU.VI.MAX Trial using a 30 mg dose reported lower prostate cancer incidence among the men with normal baseline PSA [14], and the Heart Protection Study (HPS) found no incidence difference in response to 600 mg daily [15].
Several differences among these trials could account for the conflicting findings. For example, the vitamin E dosage in SELECT, HOPE, and HPS were at least 8-fold higher than in ATBC, and all ATBC participants were cigarette smokers, while only 4-25% were current smokers in the other trials [8,10,11,13-15]. It is of note that in PHS-II, prostate cancer incidence appeared elevated in the vitamin E arm among current smokers (HR=1.50, not significant). Another important factor was the pre-randomization prostate cancer screening in SELECT which probably eliminated most existing, clinically relevant adenocarcinomas (and hence <1% were stage T3 or higher and only 6-9% were Gleason 8-10) [13]. HPS (United Kingdom), SU.VI.MAX (France) and HOPE (multi-national) had no screening at entry, and while PHS-II also did not, it was conducted in the U.S. where routine PSA screening was common (and hence, fewer than expected advanced cases were diagnosed) [16]. By contrast, in the setting of little or no population PSA screening, the ATBC trial observed more advanced cases.
Most [17-27], but not all [28-30], epidemiologic evidence supports an inverse association between circulating vitamin E and supplementation and prostate cancer risk being limited to current or recent smokers [17-23], as well as those with more aggressive disease [17,23-27]. The most recent data supporting the ATBC trial findings for an ATA supplementation benefit comes from a pooled analysis of circulating nutrients and prostate cancer risk that included 11,000 cases and 18,000 controls from 15 cohort studies that showed alpha-tocopherol concentrations were inversely associated with overall prostate cancer risk (RR=0.86, 95% CI: 0.78, 0.94; P-trend=0.001 for highest vs. lowest quintile), and particularly with aggressive disease (RR=0.74, 95% CI: 0.59, 0.92; P-trend=0.001) [31].
An alternative approach we recently pioneered for testing micronutrient-cancer risk associations utilizes genetic proxies of vitamin biochemical phenotypes. Generally referred to as “Mendelian randomization” analyses, persons are classified regarding “exposures” based on the single nucleotide polymorphism (SNP) genetic variants related to that exposure phenotype and disease relative risks are calculated (for our purposes, lower or higher vitamin biochemical status in relation to cancer risk), which minimizes or eliminates potential confounding biases inherent in observational studies. With regard to vitamin E, we identified genetic variants related to higher serum alpha-tocopherol concentrations in a genome-wide association study (GWAS) [32], and demonstrated that a “high vitamin E” variant in a gene region involved in vitamin E transport (BUD13) was associated with lower prostate cancer risk (OR=0.75; 95% CI: 0.58, 0.98 per high vitamin E allele) [33]. Two other vitamin E genes (i.e., CYP4F2, and SCARB1) showed weaker associations with risk [33]. TTPA and SEC14L2, two other genes associated with circulating α-tocopherol, were not directly associated with prostate cancer risk in a case-only analysis of men with prostate cancer in California [34] or in the ATBC Study, although the effect of the ATA supplementation on prostate cancer risk differed by SEC14L2 genotype [35].
In summary, observational studies of circulating vitamin E and prostate cancer suggest that higher plasma/serum alpha-tocopherol may be associated with reduced risk for advanced disease or in particular subgroups such as smokers. This association is biologically plausible, but could also be due to confounding by other unmeasured risk factors. Some analyses of vitamin E genetic variants as proxies for biochemical status that avoid such biases are supportive of a protective association for vitamin E. Controlled supplementation trials have given mixed results, with low and high doses appearing protective and harmful, respectively, and populations routinely screened for prostate cancer may not appear to benefit from any inhibitory effects of supplementation on growth of sub-clinical tumors. Additional biomarker studies, including metabolomics profiling that evaluates serum status or response to vitamin E supplementation (described below), as well as Mendelian randomization studies, may provide further clues regarding biological mechanisms relevant to any effects on prostate carcinogenesis.
Vitamin A and Pro-Vitamin A Carotenoids
In addition to its central role in maintaining healthy vision, vitamin A compounds including retinol exhibit anti-neoplastic properties such as induction of apoptosis and cellular differentiation, and inhibition of cellular proliferation [36]. However, retinoids have also been shown to enhance tumor growth [37], possibly acting through the insulin-like growth factor I receptor [31] or sex steroids [38] to influence prostate cancer risk. Carotenoids are compounds found mainly in fruits and vegetables, and some can be enzymatically converted to vitamin A/retinol (i.e., beta-carotene, alpha-carotene, and beta-cryptoxanthin) [39]. Cancer preventive effects of carotenoids include their antioxidant/free-radical quenching activity and enhancement of immune surveillance, some of which derives from pro-vitamin A activity [40].
The ATBC trial described above found 23% higher prostate cancer incidence in men receiving 20 mg beta-carotene supplement daily [9], while two other trials reported no prostate cancer effect of 30 mg of beta-carotene (plus 25,000 IU retinyl palmitate) [41] or 50 mg beta-carotene every other day [42]. Previous studies have elucidated biological pathways that are influenced by beta-carotene supplementation and that may be relevant to cancer in smokers; e.g., increased cellular proliferation was implicated in one immunohistochemical analysis [43].
From the prospective ATBC cohort, we found an elevated prostate cancer risk with higher baseline circulating retinol concentrations (HR=1.19, 95% CI 1.03-1.36, for highest vs. lowest quintile) for 29,133 participants (including 2,041 incident cases) [38]. Risk was similarly elevated for aggressive disease, with the greatest risk increase was for men with consistently high retinol (HR=1.31, 95% CI 1.08-1.59) [38]. Prior to our publication, most observational studies between circulating retinol and pro-vitamin A carotenoids included relatively few cases and suggested either inverse or no associations with prostate cancer [38]. Only two of the older studies indicated elevated risks for higher vitamin A status: a small study of 12 cases [44], and the PHS with 578 cases (OR=1.56, 95% CI 1.07-2.27 for highest vs. lowest quintile)[25]. In PHS, alpha-carotene and beta-cryptoxanthin were not related to risk [25], but plasma beta-carotene appeared inversely associated with risk (OR=1.30, 95% CI 0.98-1.74 for lowest vs. highest quartile) [45].
The retinol findings in ATBC are supported by two other recent, larger analyses [31,46]. In the placebo arm of the Prostate Cancer Prevention Trial (PCPT), prostate cancer risk was significantly elevated with higher circulating retinol (OR = 1.30, 95% CI 1.00-1.68 for highest vs lowest quartile, n=974 cases) [46]. The risk association was stronger for high-grade disease (OR= 1.74, 95% CI 1.14-2.68). Risk was also elevated with higher circulating alpha-carotene (OR= 1.32, 95% CI 1.01-1.73 for highest vs lowest quartile). No associations were noted with retinol or carotenoid concentrations and prostate cancer in the finasteride arm [46]. A large pooled study of over 11,000 cases and 18,000 controls found that higher levels of retinol were associated with elevated risk of prostate cancer (OR=1.13, 95% CI 1.04-1.22 for highest vs lowest quintile) [31]. There were no associations with the pro-vitamin A carotenoids beta-carotene, alpha-carotene, or beta-cryptoxanthin [31], findings supported by a recent meta-analysis [47]. The findings observed in the larger studies suggest that most prior studies were underpowered to detect the 20-30% risk excess observed for higher vitamin A status. Smoking and prostate cancer screening do not appear to have influenced the findings. While GWAS studies have identified genetic variants related to higher serum carotenoid and retinol concentrations [48,49], these have yet to be tested in relation to prostate cancer risk.
In summary, beta-carotene supplementation (in smokers) and higher circulating retinol concentrations appear to be associated with elevated risk of prostate cancer. Examination of prostate cancer risk associations with genetic variants related to retinol status, and metabolomics profiling of beta-carotene supplementation and circulating retinol and beta-carotene (described below) could provide informative evidence to support or refute the findings.
Vitamin D
Vitamin D can be consumed via foods or supplements, or synthesized by the skin upon exposure to sunlight. Vitamin D is hydroxylated in the liver to 25-hydroxyvitamin D [25(OH)D, the circulating form of vitamin D that is considered the gold standard measure of vitamin D status and that is, therefore, measured in most epidemiologic studies], and then further hydroxylated in the kidney and other tissues to 1,25-dihydroxyvitamin D (the active form). The main biologic function of the active form of vitamin D is maintaining calcium homeostasis and regulating bone mineralization. However, vitamin D has also been shown experimentally to have anti-carcinogenic properties such as promoting apoptosis, differentiation, and immunomodulation, and inhibiting angiogenesis and proliferation [50].
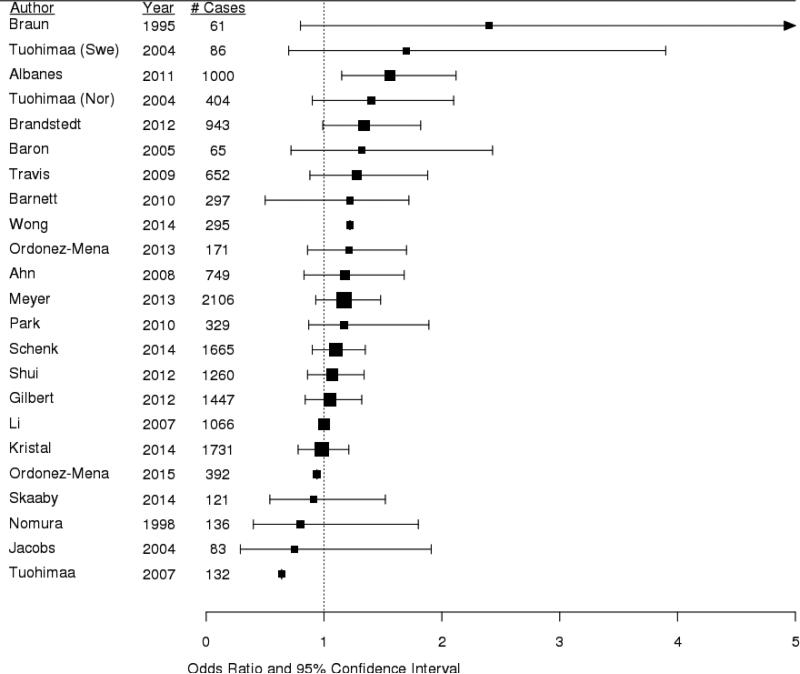
While past meta-analyses concluded that circulating vitamin D was not associated with prostate cancer risk [51-53], many individual studies indicated positive associations [54-61]. Our ATBC Study was one of the first to show a significantly elevated risk of prostate cancer with higher circulating 25(OH)D (OR =1.56, 95% CI 1.15-2.12 for highest vs. lowest quintile), with stronger risk among men with aggressive (i.e. ≥ stage III or ≥ 8 Gleason sum) tumors (OR=1.70, 95% CI 1.05-2.76) [62]. Furthermore, we found risk to be most elevated among men with simultaneously high concentrations of vitamin D binding protein (DBP), the major vitamin D transport molecule in circulation (OR= 1.81, 95% CI: 1.18–2.79 for highest vs. lowest quintile, p-trend = 0.001) [63]. The most recent meta-analysis that includes 12,000 cases concludes that higher 25(OH)D status is associated with a significant elevated risk of prostate cancer (OR=1.17, 95% CI 1.05-1.30) [64]. Two recent studies not included in the meta-analyses are the PCPT trial, which showed a non-significant positive association (OR=1.18, 95% CI 0.91–1.53 for highest vs. lowest quartile) [65], and the SELECT trial, which reported a U-shaped association, with the lowest risk for men with average vitamin D status (OR=0.74, 95% CI 0.59-0.92 for the third vs. first quintile) [66]. Figure 1 summarizes the current prospective data for 25(OH)D and prostate cancer risk.
Figure 1.
Forest plot of prospective epidemiologic studies of circulating 25(OH)D and risk of prostate cancer.
Square boxes represent the odds ratios, horizontal lines represent the 95% confidence intervals
Earlier studies of polymorphisms in candidate genes examined variation in the vitamin D receptor gene, VDR, as well as other vitamin D-pathway genes such as CYP27B1, GC, and CYP24A1, and did not find evidence of prostate cancer risk associations [67]. Using a similar GWAS approach to that used for vitamin E, we and others agnostically identified novel variants in GC, CYP24A1, CYP2R1, and DHCR7 related to circulating vitamin D [68,69]. Examining these genotypes in relation to prostate cancer risk within the NCI Breast and Prostate Cancer Cohort Consortium revealed that men carrying more variants related to low vitamin D status had lower risk of aggressive prostate cancer compared men genetically predisposed to have higher circulating 25(OH)D (OR=0.66, 95% CI 0.44-0.98 for polygenic score of four SNPs) [70], thereby further supporting a direct association between vitamin D status and prostate cancer.
There is surprisingly little clinical trial data regarding vitamin D supplementation and prostate cancer. Because of the interest in vitamin D and calcium on osteoporosis risk, several trials have only been conducted in post-menopausal women [71,72]. Another trial reported only total, colon, and respiratory cancer incidence [73]. The RECORD Trial was a 2×2 factorial design of 800 IU/day of vitamin D, 1000 mg calcium, both, or placebo, in which 85% of the participants were female. Although hazard ratios were not reported, 17 prostate cancers occurred in the vitamin D supplementation groups compared with 12 in the non-vitamin D groups [74], and a RR of 1.14 (0.68-2.95) was calculated by the U.S. Preventive Services Task Force based on the data [75]. The ongoing VITAL Trial testing daily supplementation with 2,000 IU vitamin D and/or 1 g of omega-3 fatty acid in a 2×2 factorial design should provide some prostate cancer incidence data in a few years [76].
As with the other nutrients discussed, many anti-cancer characteristics of vitamin D have been reported, but how it might promote carcinogenesis is speculative. Vitamin D can stimulate insulin synthesis and higher insulin status has been associated with higher prostate cancer risk [77]. The interaction we observed with vitamin D binding protein concentrations could indicate up-regulation of the megalin-cubilin cell membrane complex with a concomitant increase in SHBG-bound testosterone uptake [63]. As 25(OH)D concentrations are correlated with testosterone [78], future studies should evaluate 25(OH)D, insulin, and androgen hormones simultaneously.
In summary, some, but not all, observational studies indicate that circulating 25(OH)D concentration may be associated with an elevated risk of overall prostate cancer, although the association with more aggressive disease remains understudied. Analyses of genetic data support this possibility, and metabolomic characterization of circulating 25(OH)D may increase our understanding of the association (described below).
Metabolomic Profiling of Vitamin Status and Prostate Cancer Risk
Metabolomics is a powerful and promising tool for characterizing the broad array of low molecular weight biochemicals (i.e., <1 kDa) in humans and other organisms. It is currently employed both in targeted studies to quantify specific known metabolites of interest and in untargeted or “broad-spectrum” agnostic research of a wide range of phenotypes including diet and nutritional status, body mass index, tobacco use, and health outcomes such as type 2 diabetes, heart disease and cancer. Mass-spectrometry (MS) and nuclear magnetic resonance (NMR) platforms are the most commonly utilized [2]. The metabolites identified in such studies can have relevance to causal pathways, mediation, or underlying biological mechanisms, or may represent biochemicals not directly related to the phenotypes being examined. An increasing number of studies pursue the metabolomic profiling of cancer risk, progression and survival, and we have actively investigated profiling of response to controlled vitamin supplementation (e.g., in the ATBC trial), of vitamin biochemical status, and of prostate cancer risk. Findings from these recent studies are presented here.
Understanding metabolomic changes associated with vitamin supplementation or serologic status may provide further insights into the risk associations with prostate and other cancers. For example, an analysis in the ATBC Study comparing changes in the metabolome from pre- and post-supplementation blood samples in men receiving the trial beta-carotene supplement vs. those not receiving beta-carotene indicated altered xenobiotic metabolism [79]. This quantitative analysis of the pre- and post-supplementation serum identified 17 metabolites that were increased in response to beta-carotene, with xenobiotics being overrepresented (p=0.00004). Our findings pointed to the likely induction of cytochrome P450 enzymes, including possibly CYP1A2 and CYP2E1 [80,81], with implications regarding dietary supplement-prescription medication interactions. In an analysis in the same study sample and using the same analytic strategies, we examined changes in the metabolome in response to the trial ATA supplementation, identifying 24 altered metabolites (AM Mondul, et. al., submitted). Five vitamin E-related compounds were highly significantly impacted, including α-carboxyethyl-hydroxychroman (CEHC) sulfate, α-CEHC glucuronide, α-tocopherol, γ-tocopherol, and β-tocopherol. The structurally similar amino acids beta-alanine, ornithine, and N6-acetyllysine were also decreased by ATA supplementation. To our knowledge, no other studies have examined response to beta-carotene supplementation, and only two have examined response to vitamin E supplementation in humans, but these studies were very different from the above described work as they included only 10 subjects each and supplemented (including with almonds) for only 2 or 4 weeks [82,83].
Agnostic examination of the human metabolome in relation to vitamin serologic status represents a similar approach to elucidating correlates of nutritional status and nutrient-prostate cancer associations. For example, linear regression models in the ATBC Study identified 263 metabolites significantly associated with baseline serum retinol concentrations, including N-acetyltryptophan, 1-palmitoleoylglycerophosphocholine, 1-palmitoylglycerophosphoethanolamine, myo-inositol, urate, and the androgens 5alpha-androstan-3beta,17beta-diol sulfate and 4-androsten-3beta,17beta-diol monosulfate (D Albanes, et. al. unpublished). A similar study of fasting serum 25(OH)D found 117 metabolites significantly associated, the majority of which were lipids (SM Nelson, et. al., submitted). The strongest signals were for 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF), stearoyl-arachidonoyl-glycerophosphoethanolamine (GPPE), erucoyl-sphingomyelin, and two omega-3 essential fatty acids – eicosapentaenoate (EPA) and docosahexaenoate (DHA). Serum vitamin D was also associated with several amino acids, xenobiotics, and cofactor/vitamin metabolites. To our knowledge, there are no additional metabolomic studies of vitamins A or D serologic status, or of vitamin E supplementation. How the biochemicals identified in these analyses are related to metabolism of those vitamins or to prostate cancer risk requires investigation.
Several clinical studies have been conducted in prostate cancer patients to compare the metabolomics profiles of cases to non-cases, or across categories of clinical characteristics such as disease aggressiveness, recurrence, and survival. Recent reviews attempting to synthesize such post-diagnosis prostate cancer metabolomic data reveal diverse patient populations, study methods, laboratory platforms, and biological samples with mixed outcomes [84,85]. One focused on individual metabolite signals and found little replication across the clinical studies [85], while the other evaluated metabolite classes and metabolic pathways and noted patterns consistent with lipid and energy pathway dysregulation [84], similar to the pre-diagnostic prospective data described below. This apparent concordance at the pathway level across different study approaches is encouraging regarding our understanding of prostate cancer, and suggests it may be a more valuable approach for synthesizing data across studies than examining only individual metabolite signals.
To our knowledge, our research group has conducted and published the only two prospective studies of pre-diagnostic serum metabolome and subsequent risk of developing prostate cancer [86,87]. These studies evaluated pre-supplementation fasting serum collected from participants in the ATBC Study, described above [7], with the time from blood collection to prostate cancer diagnosis being 1-20 years. The first discovery analysis of 74 cases and 74 controls oversampled aggressive, stage III/IV or Gleason sum >=8 cases, and identified several biochemicals of interest with respect to risk, including 1-stearoylglycerol, a monoacylglycerolipid (or lysolipid) (OR=0.34, 95% CI=0.20 - 0.58, p=6.3×10−5) and alpha-ketoglutarate, an energy metabolite involved in the tricarboxylic acid (TCA) cycle (OR=0.53, 95% CI=0.35 – 0.81, p=0.003) [87]. Based on these novel findings, we conducted a larger study of 200 cases (of which 100 were aggressive cancers) and 200 controls and showed similar statistically significantly associations with aggressive disease for lipids (metabolite class p=0.041) and energy/TCA cycle metabolites (p=0.018). This included replication of the original study signal for alpha-ketoglutarate (OR=0.69, 95% CI=0.51-0.94, p=0.02), and identification of another TCA cycle member, citrate, among the top metabolites (OR=0.69, 95% CI=0.50 – 0.95, p=0.02)[86]. These strong findings for lipids and energy metabolites are consistent with a large body of basic and clinical research that indicates lipid and energy dysregulation in the development of many cancers, including prostate cancer [88-90]. Interestingly, we observed qualitative differences in the lipid sub-classes that were associated with prostate cancer risk diagnosed within 10 years of blood collection versus those diagnosed 10-20 years after blood collection [86]. Similar analyses in other prospective studies should help confirm and identify molecular species and pathways related to prostate cancer etiology, early detection, and prevention.
Moving forward in this emerging field will be challenging because the research is, by nature, heterogeneous in several respects. Metabolomics platforms or laboratory assays used for biospecimen analysis vary substantially and include NMR, high performance liquid chromatography (HPLC), MS in tandem with either liquid or gas chromatography (LC/MS or GC/MS), and enzyme-linked immunosorbent assay (ELISA) [91]. Metabolite limits of detection and sensitivity vary by analytical method, and sample preparation differences can introduce substantial variation in observed metabolite concentrations [91]. A wide variety of biological samples have been profiled for prostate cancer cases with diverse clinical and histopathological characteristics (e.g., stage and Gleason), including tissue from biopsies, radical prostatectomies, and distant metastases. Serum or plasma from both fasting and non-fasting participants, as well as urine can be assayed [84,85]. Metabolomic profiling of such different samples and populations might be expected to yield diverse and inconsistent findings that will require synthesis and a more comprehensive concept of disease development and progression. The timing of specimen collection with respect to the natural history of prostate cancer, as well as the comparison group chosen, should be considered carefully when interpreting and synthesizing results across studies.
Metabolomics is a relatively new approach to the study of prostate and other cancers. It has been widely used in clinical studies with the goal of identifying biomarkers for early detection and prognosis. It has, however, been far less utilized as a method for elucidating novel pathways in prostate cancer etiology or for evaluating vitamin biochemical status or response to supplementation. Despite substantial epidemiologic research of prostate cancer, there are few well-established modifiable risk factors, and further metabolomics studies may shed light on this malignancy. Additional studies of the relationships between exposures such as vitamins and the metabolome can provide novel insight into the biological mechanisms that underlie the influence of these exposures on prostate and other cancers. It is important to note that some of the analyses presented here may not be generalizable to other populations; the background of cigarette smoke exposure, the fasting status of the participants at blood collection, and the higher stage distribution of the prostate cancer cases due to the lack of routine PSA screening between 1985 and 2010 in Finland all contribute to the distinctiveness of the ATBC Study population. At the same time, it is important to consider how discordant findings across populations may yield clues that increase our understanding of cancer etiology and biological mechanisms as much as, if not more so, than consistent study results.
Conclusions
Substantial research into the role of lipid-soluble vitamins and metabolites in prostate cancer risk and carcinogenesis has provided several active leads that continue to be vigorously investigated. Although trial and observational study findings have not been entirely consistent, they have pointed to specific population, exposure, clinical, and methodological parameters that may provide further insights into the biological relationships and mechanisms of action. Specifically, how vitamins D and A may adversely impact the development of prostate cancer, and whether low-dose vitamin E supplementation remains a viable preventive approach, require further study. Newly discovered novel metabolomics patterns in men who are later diagnosed with prostate cancer should be replicated in diverse populations. It is anticipated that some of the underlying biology of the cumulative findings will represent breakthroughs that have translational relevance to prostate cancer prevention, early detection, and improved or more targeted therapeutic approaches.
Footnotes
Author's contributions
AM Mondul: Project development, manuscript writing/editing
SJ Weinstein: Project development, manuscript writing/editing
D Albanes: Project development, manuscript writing/editing
Conflict of Interest: The authors declare that they have no conflict of interest
Compliance with Ethical Standards:
All procedures performed by the authors in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards
This article does not contain any studies with animals performed by any of the authors.
Informed consent was obtained from all individual participants included in the referenced studies performed by the authors.
References
- 1.Nicholson JK, Lindon JC. Systems biology: Metabolomics. Nature. 2008;455(7216):1054–1056. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 2.O'Connell TM. Recent advances in metabolomics in oncology. Bioanalysis. 2012;4(4):431–451. doi: 10.4155/bio.11.326. [DOI] [PubMed] [Google Scholar]
- 3.Office of Dietary Supplements N Vitamin E fact sheet for health professionals. 2015 https://ods od nih gov/factsheets/VitaminE-HealthProfessional/
- 4.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol - an underestimated vitamin? Ann Nutr Metab. 2004;48(3):169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 5.Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002;76(4):703–716. doi: 10.1093/ajcn/76.4.703. [DOI] [PubMed] [Google Scholar]
- 6.Ricciarelli R, Zingg JM, Azzi A. Vitamin E: protective role of a Janus molecule. FASEB J. 2001;15(13):2314–2325. doi: 10.1096/fj.01-0258rev. [DOI] [PubMed] [Google Scholar]
- 7.The ATBC Cancer Prevention Study Group The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 8.Heinonen OP, Albanes D, Virtamo J, Taylor PR, Huttunen JK, Hartman AM, Haapakoski J, Malila N, Rautalahti M, Ripatti S, Maenpaa H, Teerenhovi L, Koss L, Virolainen M, Edwards BK. Prostate cancer and supplementation with alpha-tocopherol and beta-carotene: incidence and mortality in a controlled trial. J Natl Cancer Inst. 1998;90(6):440–446. doi: 10.1093/jnci/90.6.440. [DOI] [PubMed] [Google Scholar]
- 9.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 10.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293(11):1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 11.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301(1):52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, III, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr., Baker LH, Coltman CA., Jr. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein E, Thompson I, Tangen C, Crowley J, Lucia M, Goodman P, Minasian L, Ford L, Parnes H, Gaziano J, Karp D, Lieber M, Walther P, Klotz L, Parsons J, Chin J, Darke A, Lippman S, Goodman G, Meyskens F, Baker L. Vitamin E and the risk of prostate cancer. The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306(14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, Estaquio C, Hercberg S. Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial. Int J Cancer. 2005;116(2):182–186. doi: 10.1002/ijc.21058. [DOI] [PubMed] [Google Scholar]
- 15.Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 16.Gann PH. Randomized trials of antioxidant supplementation for cancer prevention: first bias, now chance--next, cause. JAMA. 2009;301(1):102–103. doi: 10.1001/jama.2008.863. doi:2008.863 [pii];10.1001/jama.2008.863 [doi] [DOI] [PubMed] [Google Scholar]
- 17.Cheng T, Barnett M, Kristal A, Ambrosone C, King I, Thornquist M, Goodman G, Neuhouser M. Genetic variation in myeloperoxidase modifies the association of serum alpha-tocopherol with aggressive prostate cancer among current smokers. J Nutr. 2011;141(9):1731–1737. doi: 10.3945/jn.111.141713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman GE, Schaffer S, Omenn GS, Chen C, King I. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from beta-carotene and retinol efficacy trial. Cancer Epidemiol Biomarkers Prev. 2003;12(6):518–526. [PubMed] [Google Scholar]
- 19.Eichholzer M, Stahelin HB, Gey KF, Ludin E, Bernasconi F. Prediction of male cancer mortality by plasma levels of interacting vitamins: 17-year follow-up of the prospective Basel study. Int J Cancer. 1996;66(2):145–150. doi: 10.1002/(SICI)1097-0215(19960410)66:2<145::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez C, Jacobs EJ, Mondul AM, Calle EE, McCullough ML, Thun MJ. Vitamin E supplements and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev. 2004;13(3):378–382. [PubMed] [Google Scholar]
- 21.Weinstein SJ, Wright ME, Pietinen P, King I, Tan C, Taylor PR, Virtamo J, Albanes D. Serum alpha-tocopherol and gamma-tocopherol in relation to prostate cancer risk in a prospective study. J Natl Cancer Inst. 2005;97(5):396–399. doi: 10.1093/jnci/dji045. [DOI] [PubMed] [Google Scholar]
- 22.Watters JL, Gail MH, Weinstein SJ, Virtamo J, Albanes D. Associations between alpha-tocopherol, beta-carotene, and retinol and prostate cancer survival. Cancer Res. 2009;69(9):3833–3841. doi: 10.1158/0008-5472.CAN-08-4640. doi:0008-5472.CAN-08-4640 [pii];10.1158/0008-5472.CAN-08-4640 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein SJ, Wright ME, Lawson KA, Snyder K, Mannisto S, Taylor PR, Virtamo J, Albanes D. Serum and dietary vitamin E in relation to prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1253–1259. doi: 10.1158/1055-9965.EPI-06-1084. [DOI] [PubMed] [Google Scholar]
- 24.Chan JM, Stampfer MJ, Ma J, Rimm EB, Willett WC, Giovannucci EL. Supplemental vitamin E intake and prostate cancer risk in a large cohort of men in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8(10):893–899. [PubMed] [Google Scholar]
- 25.Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, Stampfer MJ. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59(6):1225–1230. [PubMed] [Google Scholar]
- 26.Kirsh VA, Hayes RB, Mayne ST, Chatterjee N, Subar AF, Dixon LB, Albanes D, Andriole GL, Urban DA, Peters U. Supplemental and dietary vitamin E, beta-carotene, and vitamin C intakes and prostate cancer risk. J Natl Cancer Inst. 2006;98(4):245–254. doi: 10.1093/jnci/djj050. [DOI] [PubMed] [Google Scholar]
- 27.Peters U, Littman AJ, Kristal AR, Patterson RE, Potter JD, White E. Vitamin E and selenium supplementation and risk of prostate cancer in the Vitamins and lifestyle (VITAL) study cohort. Cancer Causes Control. 2008;19(1):75–87. doi: 10.1007/s10552-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 28.Wright ME, Weinstein SJ, Lawson KA, Albanes D, Subar AF, Dixon LB, Mouw T, Schatzkin A, Leitzmann MF. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1128–1135. doi: 10.1158/1055-9965.EPI-06-1071. [DOI] [PubMed] [Google Scholar]
- 29.Gill JK, Franke AA, Steven MJ, Cooney RV, Wilkens LR, Le ML, Goodman MT, Henderson BE, Kolonel LN. Association of selenium, tocopherols, carotenoids, retinol, and 15-isoprostane F(2t) in serum or urine with prostate cancer risk: the multiethnic cohort. Cancer Causes Control. 2009;20(7):1161–1171. doi: 10.1007/s10552-009-9304-4. doi:10.1007/s10552-009-9304-4 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Key TJ, Appleby PN, Allen NE, Travis RC, Roddam AW, Jenab M, Egevad L, Tjonneland A, Johnsen NF, Overvad K, Linseisen J, Rohrmann S, Boeing H, Pischon T, Psaltopoulou T, Trichopoulou A, Trichopoulos D, Palli D, Vineis P, Tumino R, Berrino F, Kiemeney L, Bueno-de-Mesquita HB, Quiros JR, Gonzalez CA, Martinez C, Larranaga N, Chirlaque MD, Ardanaz E, Stattin P, Hallmans G, Khaw KT, Bingham S, Slimani N, Ferrari P, Rinaldi S, Riboli E. Plasma carotenoids, retinol, and tocopherols and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition study. Am J Clin Nutr. 2007;86(3):672–681. doi: 10.1093/ajcn/86.3.672. [DOI] [PubMed] [Google Scholar]
- 31.Key TJ, Appleby PN, Travis RC, Albanes D, Alberg AJ, Barricarte A, Black A, Boeing H, Bueno-de-Mesquita HB, Chan JM, Chen C, Cook MB, Donovan JL, Galan P, Gilbert R, Giles GG, Giovannucci E, Goodman GE, Goodman PJ, Gunter MJ, Hamdy FC, Heliovaara M, Helzlsouer KJ, Henderson BE, Hercberg S, Hoffman-Bolton J, Hoover RN, Johansson M, Khaw KT, King IB, Knekt P, Kolonel LN, Le ML, Mannisto S, Martin RM, Meyer HE, Mondul AM, Moy KA, Neal DE, Neuhouser ML, Palli D, Platz EA, Pouchieu C, Rissanen H, Schenk JM, Severi G, Stampfer MJ, Tjonneland A, Touvier M, Trichopoulou A, Weinstein SJ, Ziegler RG, Zhou CK, Allen NE. Carotenoids, retinol, tocopherols, and prostate cancer risk: pooled analysis of 15 studies. Am J Clin Nutr. 2015;102(5):1142–1157. doi: 10.3945/ajcn.115.114306. doi:ajcn.115.114306 [pii];10.3945/ajcn.115.114306 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Major JM, Yu K, Wheeler W, Zhang H, Cornelis MC, Wright ME, Yeager M, Snyder K, Weinstein SJ, Mondul A, Eliassen H, Purdue M, Hazra A, McCarty CA, Hendrickson S, Virtamo J, Hunter D, Chanock S, Kraft P, Albanes D. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr296. doi:ddr296 [pii];10.1093/hmg/ddr296 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Major JM, Yu K, Weinstein SJ, Berndt SI, Hyland PL, Yeager M, Chanock S, Albanes D. Genetic variants reflecting higher vitamin e status in men are associated with reduced risk of prostate cancer. J Nutr. 2014;144(5):729–733. doi: 10.3945/jn.113.189928. doi:jn.113.189928 [pii];10.3945/jn.113.189928 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bauer SR, Richman EL, Sosa E, Weinberg V, Song X, Witte JS, Carroll PR, Chan JM. Antioxidant and vitamin E transport genes and risk of high-grade prostate cancer and prostate cancer recurrence. Prostate. 2013;73(16):1786–1795. doi: 10.1002/pros.22717. doi:10.1002/pros.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright ME, Peters U, Gunter MJ, Moore SC, Lawson KA, Yeager M, Weinstein SJ, Snyder K, Virtamo J, Albanes D. Association of variants in two vitamin e transport genes with circulating vitamin e concentrations and prostate cancer risk. Cancer Res. 2009;69(4):1429–1438. doi: 10.1158/0008-5472.CAN-08-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traber MG. Vitamin E. In: Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease. 9 edn. Lippincott Williams & Wilkins; Baltimore, MD: 1999. pp. 347–362. [Google Scholar]
- 37.Peehl DM, Feldman D. The role of vitamin D and retinoids in controlling prostate cancer progression. Endocr Relat Cancer. 2003;10(2):131–140. doi: 10.1677/erc.0.0100131. [DOI] [PubMed] [Google Scholar]
- 38.Mondul AM, Watters JL, Mannisto S, Weinstein SJ, Snyder K, Virtamo J, Albanes D. Serum retinol and risk of prostate cancer. Am J Epidemiol. 2011;173(7):813–821. doi: 10.1093/aje/kwq429. doi:kwq429 [pii];10.1093/aje/kwq429 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Office of Dietary Supplements N Vitamin A fact sheet for health professionals. 2015 https://ods od nih gov/factsheets/VitaminA-HealthProfessional/
- 40.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26(6):459–516. doi: 10.1016/j.mam.2005.10.001. doi:S0098-2997(05)00066-X [pii];10.1016/j.mam.2005.10.001 [doi] [DOI] [PubMed] [Google Scholar]
- 41.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr., Valanis B, Williams JH, Jr., Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 42.Cook NR, Le IM, Manson JE, Buring JE, Hennekens CH. Effects of beta-carotene supplementation on cancer incidence by baseline characteristics in the Physicians' Health Study (United States). Cancer Causes Control. 2000;11(7):617–626. doi: 10.1023/a:1008995430664. [DOI] [PubMed] [Google Scholar]
- 43.Wright ME, Groshong SD, Husgafvel-Pursiainen K, Genova E, Lucia MS, Wolff H, Virtamo J, Albanes D. Effects of beta-carotene supplementation on molecular markers of lung carcinogenesis in male smokers. Cancer Prev Res (Phila) 2010;3(6):745–752. doi: 10.1158/1940-6207.CAPR-09-0107. doi:1940-6207.CAPR-09-0107 [pii];10.1158/1940-6207.CAPR-09-0107 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coates RJ, Weiss NS, Daling JR, Morris JS, Labbe RF. Serum levels of selenium and retinol and the subsequent risk of cancer. Am J Epidemiol. 1988;128(3):515–523. doi: 10.1093/oxfordjournals.aje.a114999. [DOI] [PubMed] [Google Scholar]
- 45.Cook NR, Stampfer MJ, Ma J, Manson JE, Sacks FM, Buring JE, Hennekens CH. Beta-carotene supplementation for patients with low baseline levels and decreased risks of total and prostate carcinoma. Cancer. 1999;86(9):1783–1792. doi:10.1002/(SICI)1097-0142(19991101)86:9<1783::AID CNCR21>3.0.CO;2-N [pii] [PubMed] [Google Scholar]
- 46.Nash SH, Till C, Song X, Lucia MS, Parnes HL, Thompson IM, Jr., Lippman SM, Platz EA, Schenk J. Serum Retinol and Carotenoid Concentrations and Prostate Cancer Risk: Results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1507–1515. doi: 10.1158/1055-9965.EPI-15-0394. doi:1055-9965.EPI-15-0394 [pii];10.1158/1055-9965.EPI-15-0394 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Cui R, Xiao Y, Fang J, Xu Q. Effect of Carotene and Lycopene on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. PLoS One. 2015;10(9):e0137427. doi: 10.1371/journal.pone.0137427. doi:10.1371/journal.pone.0137427 [doi];PONE-D-15-16484 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi AM, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84(2):123–133. doi: 10.1016/j.ajhg.2008.12.019. doi:10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mondul AM, Yu K, Wheeler W, Zhang H, Weinstein SJ, Major JM, Cornelis MC, Mannisto S, Hazra A, Hsing AW, Jacobs KB, Eliassen H, Tanaka T, Reding DJ, Hendrickson S, Ferrucci L, Virtamo J, Hunter DJ, Chanock SJ, Kraft P, Albanes D. Genome-wide association study of circulating retinol levels. Hum Mol Genet. 2011;20(23):4724–4731. doi: 10.1093/hmg/ddr387. doi:ddr387 [pii];10.1093/hmg/ddr387 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.IARC . Vitamin D and Cancer. Lyon, France: 2009. [Google Scholar]
- 51.Yin L, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis of longitudinal studies: Serum vitamin D and prostate cancer risk. Cancer Epidemiol. 2009;33(6):435–445. doi: 10.1016/j.canep.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414–1424. doi: 10.1002/ijc.25439. doi:10.1002/ijc.25439 [doi] [DOI] [PubMed] [Google Scholar]
- 53.Gilbert R, Martin RM, Beynon R, Harris R, Savovic J, Zuccolo L, Bekkering GE, Fraser WD, Sterne JA, Metcalfe C. Associations of circulating and dietary vitamin D with prostate cancer risk: a systematic review and dose-response meta-analysis. Cancer Causes Control. 2011;22(3):319–340. doi: 10.1007/s10552-010-9706-3. doi:10.1007/s10552-010-9706-3 [doi] [DOI] [PubMed] [Google Scholar]
- 54.Park SY, Cooney RV, Wilkens LR, Murphy SP, Henderson BE, Kolonel LN. Plasma 25-hydroxyvitamin D and prostate cancer risk: the multiethnic cohort. Eur J Cancer. 2010;46(5):932–936. doi: 10.1016/j.ejca.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Travis RC, Crowe FL, Allen NE, Appleby PN, Roddam AW, Tjonneland A, Olsen A, Linseisen J, Kaaks R, Boeing H, Kroger J, Trichopoulou A, Dilis V, Trichopoulos D, Vineis P, Palli D, Tumino R, Sieri S, Bueno-de-Mesquita HB, van Duijnhoven FJ, Chirlaque MD, Barricarte A, Larranaga N, Gonzalez CA, Arguelles MV, Sanchez MJ, Stattin P, Hallmans G, Khaw KT, Bingham S, Rinaldi S, Slimani N, Jenab M, Riboli E, Key TJ. Serum vitamin D and risk of prostate cancer in a case-control analysis nested within the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Epidemiol. 2009;169(10):1223–1232. doi: 10.1093/aje/kwp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, Horst RL, Hollis BW, Huang WY, Shikany JM, Hayes RB. Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100(11):796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuohimaa P, Tenkanen L, Syvala H, Lumme S, Hakulinen T, Dillner J, Hakama M. Interaction of factors related to the metabolic syndrome and vitamin D on risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(2):302–307. doi: 10.1158/1055-9965.EPI-06-0777. [DOI] [PubMed] [Google Scholar]
- 58.Baron JA, Beach M, Wallace K, Grau MV, Sandler RS, Mandel JS, Heber D, Greenberg ER. Risk of prostate cancer in a randomized clinical trial of calcium supplementation. Cancer Epidemiol Biomarkers Prev. 2005;14(3):586–589. doi: 10.1158/1055-9965.EPI-04-0319. doi:14/3/586 [pii];10.1158/1055-9965.EPI-04-0319 [doi] [DOI] [PubMed] [Google Scholar]
- 59.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3):255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 60.Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, Stattin P, Harvei S, Hakulinen T, Luostarinen T, Dillner J, Lehtinen M, Hakama M. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004;108(1):104–108. doi: 10.1002/ijc.11375. [DOI] [PubMed] [Google Scholar]
- 61.Braun MM, Helzlsouer KJ, Hollis BW, Comstock GW. Prostate cancer and prediagnostic levels of serum vitamin D metabolites (Maryland, United States). Cancer Causes Control. 1995;6(3):235–239. doi: 10.1007/BF00051795. [DOI] [PubMed] [Google Scholar]
- 62.Albanes D, Mondul AM, Yu K, Parisi D, Horst RL, Virtamo J, Weinstein SJ. Serum 25-hydroxy vitamin D and prostate cancer risk in a large nested case-control study. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1850–1860. doi: 10.1158/1055-9965.EPI-11-0403. doi:10.1158/1055-9965.EPI-11-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinstein SJ, Mondul AM, Kopp W, Rager H, Virtamo J, Albanes D. Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of prostate cancer. Int J Cancer. 2013;132(12):2940–2947. doi: 10.1002/ijc.27969. doi:10.1002/ijc.27969 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z, Lin Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. Journal of Cancer Research and Clinical Oncology. 2014;140(9):1465–1477. doi: 10.1007/s00432-014-1706-3. doi:10.1007/s00432-014-1706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schenk JM, Till CA, Tangen CM, Goodman PJ, Song X, Torkko KC, Kristal AR, Peters U, Neuhouser ML. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1484–1493. doi: 10.1158/1055-9965.EPI-13-1340. doi:23/8/1484 [pii];10.1158/1055-9965.EPI-13-1340 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, Schenk JM, Thompson IM, Meyskens FL, Jr., Goodman GE, Minasian LM, Parnes HL, Klein EA. Plasma vitamin D and prostate cancer risk: results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1494–1504. doi: 10.1158/1055-9965.EPI-14-0115. doi:1055-9965.EPI-14-0115 [pii];10.1158/1055-9965.EPI-14-0115 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr. 2009;29:111–132. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 68.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–2745. doi: 10.1093/hmg/ddq155. doi:ddq155 [pii];10.1093/hmg/ddq155 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Jarvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hypponen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. doi:S0140-6736(10)60588-0 [pii];10.1016/S0140-6736(10)60588-0 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mondul AM, Shui IM, Yu K, Travis RC, Stevens VL, Campa D, Schumacher F, Ziegler RG, Bueno-de-Mesquita HB, Berndt SI, Crawford ED, Gapstur SM, Gaziano JM, Giovannucci E, Haiman C, Henderson BE, Hunter DJ, Johansson M, Key TJ, Le ML, Lindstrom S, McCullough M, Navarro C, Overvad K, Palli D, Purdue MP, Stampfer MJ, Weinstein SJ, Willett W, Yeager M, Chanock SJ, Trichopoulos D, Kolonel LN, Kraft P, Albanes D. Genetic variation in the vitamin D pathway in relation to risk of prostate cancer - Results from Breast and Prostate Cancer Cohort Consortium (BPC3). Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/1055-9965.EPI-13-0007-T. doi:1055-9965.EPI-13-0007-T [pii];10.1158/1055-9965.EPI-13-0007-T [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 72.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van HL, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 73.Trivedi D, Doll R, Khaw K. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, Grant AM, Campbell MK, Anderson FH, Cooper C, Francis RM, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA. Long-term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D(3) and/or calcium (RECORD trial). J Clin Endocrinol Metab. 2012;97(2):614–622. doi: 10.1210/jc.2011-1309. doi:jc.2011-1309 [pii];10.1210/jc.2011-1309 [doi] [DOI] [PubMed] [Google Scholar]
- 75.Fortmann SP, Burda BU, Senger CA, Lin J, Beil T, O'Connor E, Whitlock EP. Vitamin, Mineral, and Multivitamin Supplements for the Primary Prevention of Cardiovascular Disease and Cancer: A Systematic Evidence Review for the U.S. Preventive Services Task Force; Rockville, MD.: 2013. [PubMed] [Google Scholar]
- 76.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, Zaharris E, Macfadyen JG, Danielson E, Lin J, Zhang SM, Buring JE. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. doi: 10.1016/j.cct.2011.09.009. doi:S1551-7144(11)00245-X [pii];10.1016/j.cct.2011.09.009 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Albanes D, Weinstein SJ, Wright ME, Mannisto S, Limburg PJ, Snyder K, Virtamo J. Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst. 2009;101(18):1272–1279. doi: 10.1093/jnci/djp260. doi:djp260 [pii];10.1093/jnci/djp260 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 2010;73(2):243–248. doi: 10.1111/j.1365-2265.2009.03777.x. doi:CEN3777 [pii];10.1111/j.1365-2265.2009.03777.x [doi] [DOI] [PubMed] [Google Scholar]
- 79.Mondul AM, Sampson JN, Moore SC, Weinstein SJ, Evans AM, Karoly ED, Virtamo J, Albanes D. Metabolomic profile of response to supplementation with beta-carotene in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 2013;98(2):488–493. doi: 10.3945/ajcn.113.062778. doi:ajcn.113.062778 [pii];10.3945/ajcn.113.062778 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu C, Russell RM, Wang XD. Exposing ferrets to cigarette smoke and a pharmacological dose of beta-carotene supplementation enhance in vitro retinoic acid catabolism in lungs via induction of cytochrome P450 enzymes. J Nutr. 2003;133(1):173–179. doi: 10.1093/jn/133.1.173. [DOI] [PubMed] [Google Scholar]
- 81.Paolini M, Antelli A, Pozzetti L, Spetlova D, Perocco P, Valgimigli L, Pedulli GF, Cantelli-Forti G. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in beta-carotene supplemented rats. Carcinogenesis. 2001;22(9):1483–1495. doi: 10.1093/carcin/22.9.1483. [DOI] [PubMed] [Google Scholar]
- 82.Johnson CH, Slanar O, Krausz KW, Kang DW, Patterson AD, Kim JH, Luecke H, Gonzalez FJ, Idle JR. Novel metabolites and roles for alpha-tocopherol in humans and mice discovered by mass spectrometry-based metabolomics. Am J Clin Nutr. 2012;96(4):818–830. doi: 10.3945/ajcn.112.042929. doi:10.3945/ajcn.112.042929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong M, Lodge JK. A metabolomic investigation of the effects of vitamin E supplementation in humans. Nutrition & Metabolism. 2012;9(1):1–9. doi: 10.1186/1743-7075-9-110. doi:10.1186/1743-7075-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lloyd SM, Arnold J, Sreekumar A. Metabolomic profiling of hormone-dependent cancers: a bird's eye view. Trends Endocrinol Metab. 2015;26(9):477–485. doi: 10.1016/j.tem.2015.07.001. doi:S1043-2760(15)00134-4 [pii];10.1016/j.tem.2015.07.001 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trock BJ. Application of metabolomics to prostate cancer. Urol Oncol. 2011;29(5):572–581. doi: 10.1016/j.urolonc.2011.08.002. doi:S1078-1439(11)00244-4 [pii];10.1016/j.urolonc.2011.08.002 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mondul AM, Moore SC, Weinstein SJ, Karoly ED, Sampson JN, Albanes D. Metabolomic analysis of prostate cancer risk in a prospective cohort: The alpha-tocolpherol, beta-carotene cancer prevention (ATBC) study. Int J Cancer. 2015;137(9):2124–2132. doi: 10.1002/ijc.29576. doi:10.1002/ijc.29576 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mondul AM, Moore SC, Weinstein SJ, Mannisto S, Sampson JN, Albanes D. 1-stearoylglycerol is associated with risk of prostate cancer: results from serum metabolomic profiling. Metabolomics. 2014;10(5):1036–1041. doi: 10.1007/s11306-014-0643-0. doi:10.1007/s11306-014-0643-0 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dell' Antone P. Energy metabolism in cancer cells: how to explain the Warburg and Crabtree effects? Medical Hypotheses. 2012;79(3):388–392. doi: 10.1016/j.mehy.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 89.Nomura DK, Long JZ, Niessen S, Hoover HS, Ng SW, Cravatt BF. Monoacylglycerol lipase regulates a fatty acid network that promotes cancer pathogenesis. Cell. 2010;140(1):49–61. doi: 10.1016/j.cell.2009.11.027. doi:S0092-8674(09)01439-1 [pii];10.1016/j.cell.2009.11.027 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279(15):2610–2623. doi: 10.1111/j.1742-4658.2012.08644.x. doi:10.1111/j.1742-4658.2012.08644.x [doi] [DOI] [PubMed] [Google Scholar]
- 91.Lucarelli G, Rutigliano M, Galleggiante V, Giglio A, Palazzo S, Ferro M, Simone C, Bettocchi C, Battaglia M, Ditonno P. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer. Expert Rev Mol Diagn. 2015;15(9):1211–1224. doi: 10.1586/14737159.2015.1069711. doi:10.1586/14737159.2015.1069711 [doi] [DOI] [PubMed] [Google Scholar]