Abstract
Background:
This study was focused on the probability of carcinogen risk of low-level ingestion and dermal exposure with polycyclic aromatic hydrocarbons (PAHs) from drinking water in Tehran, capital of Iran.
Methods:
Concentrations of 16 PAHs were measured in the tap, bottled and heated tap water in four different seasons. Using a questionnaire-based survey, exposure with PAHs from drinking water was evaluated via direct ingestion, swimming, washing and showering. Finally, a comprehensive risk assessment was performed in four age groups. Rank correlation was used to represent variability in risk analysis and obtained coefficients were used for sensitivity analysis. In addition, Monte Carlo simulation was implemented to determine risk probability distributions and to calculate cumulative probability of the total risks in different age groups.
Results:
The lifetime average daily dose and the dermal absorbed dose were 0.69E-06 and 1.33E-05 mg/kg/day, respectively. The total estimated excess lifetime cancer risk (ELCR) of ingestion and dermal exposure were 1.57E-05 and 17.24E-05.
Conclusion:
Sum of estimated ingestion and dermal ELCR was 18.81E-05, which was higher than the acceptable value recommended by WHO. It means a total of 1504 lifetime cancer cases in residents of Tehran. Monte Carlo simulation indicated that risk probability above the acceptable level was 96.2% in dermal exposure. Moreover, sensitivity analysis indicated that tap water consumption (Pspearman >0.92) and washing activities (Pspearman>0.95) had the greatest correlation on the cancer risk.
Keywords: Drinking water, Risk assessment, Uncertainties, Iran
Introduction
Chemical can enter the body by various routes. The drinking water can be act as an important pathway for the entering of organic pollutants. Among all chemical, persistent organic pollutant such as PAHs, polychlorinated dibenzo dioxins/furans, polychlorinated biphenyles, organochlorine and organophosphorus pesticides poses the greatest risks to human health (1). Polycyclic aromatic hydrocarbons are one of the most widespread persistent organic pollutants in the water environment (2). Water pollution to PAHs is related to dry and wet atmospheric deposition (3), runoffs (4), municipal and industrial wastewaters (5) and petroleum spills (6). Laboratory experiments and epidemiological data had indicated that both short- and long-term exposure to PAHs could cause harmful health effects. PAHs can increase the risk of cancers (7) and capable to cause oxidative stress during its metabolism (8). Besides, they can DNA adduct formation (9).
Human carcinogen risk assessment (CRA) is a useful approach to quantify potential harmful effects of toxic chemicals on human health. CRA is implemented in four stages including hazard identification, dose-response assessment, exposure assessment and risk characterization (10). Hazard identification is based to determine whether exposure to a stressor can cause adverse effects in human. Dose-response assessment is the second step in risk assessment to evaluate the adverse effects associated with a biological, chemical or physical stressor. This stage consists of the analysis of the relationship between the amount of a stressor absorbed by organisms and the changes developed in reaction to the agent. Exposure assessment is critical section that deals with long-term, low-level exposure in environment. It is crucial for the identification and evaluation of health risks. The aim of exposure assessment is to identify and quantify exposures to chemical stressors that may cause health effects. This stage identifies the exposure that occurs in human populations. Risk characterization is final stage in risk assessment. It summarizes estimation of the probability of an adverse effect in human population. Finally, outcomes can express as excess lifetime cancer risk (11, 12).
Many chemical pollutants, such as THMs and PAHs can enter through three different routes to human body and cause health adverse effect. Ingestion is the most dominant route which followed by inhalation or dermal contact (13, 14). Some studies have investigated the health risk of exposure to PAHs from drinking water. They have estimated ELCR between 1.00E-05 to 4.50E-05 (15–17).
The most important action in CRA is determining the parameters that have effects on the exposure pathway. Since both skin contact and direct ingestion is resulted to entry of the PAHs into the body, in this research is evaluated the carcinogenic risk from direct ingestion of water (including tap water, bottled water, and heated tap water), showering and swimming in Tehran, the capital of Iran, using probabilistic techniques. In addition, Monte Carlo simulation and analysis were used to determine risk probability distributions and selection of parameters that had the most important effects on the risk.
This study was the first effort to provide information on the carcinogenic risk of PAHs in Iran and might be useful in developing strategies for carcinogenic risk management.
Materials and Methods
According to the Tehran Water and Wastewater Company, the distribution system of drinking water was divided into six districts. Four water samples were collected from each district in each season. The samples were directly taken from taps (99 samples) located in different parts of the distribution system. Bottled water samples were purchased from local retailer stores. Bottled water samples were comprised 22 products from different companies, including mineral bottled water (MBW) and bottled drinking water (BDW) that were packed in polyethylene terephthalate (44 samples). Heated water was prepared by boiling tap water for 3–10 minutes in laboratory (20 samples). Samples were collected in 1000 ml amber glass bottles with Teflon lined tops to prevent any kind of reaction after the sampling. Each sample was stored in an icebox at 4 °C while being transported to the laboratory. Water sampling carried out during the period July 2010–December 2011. Finally, sixteen priority PAHs, including naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Fl), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flu), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chy), benzo[a]pyrene (BaP), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), dibenzo[a,h]anthracene (DahA), indeno[1,2,3-cd]pyrene (IcdP) and benzo[g,h,i]perylene (BghiP) were analyzed in Central Laboratory, School of Public Health, Tehran University of Medical Sciences for each sample. Water samples were extracted using a solid phase extraction (SPE) system according to the established procedure by EPA (18, 19). Further information on PAHs extraction in the tap water is reported by Karyab et al. (20).
The PAHs extracted were analyzed by using a 3800 Varian gas chromatography coupled to a Varian Saturn 2200 mass spectrometer, equipped with a 30m×0.25mm i.d. WCOT CP-Sil 8 CB column. Calibration curves were plotted at seven concentration levels from 2 to 2000 ng/l with standard solutions containing all studied PAHs. Detection limit (DL) for individual PAHs, with a signal to noise ratio of 3, ranged from 0.8 to 2 ng/l. Concentrations that were below the DLs were assigned as not determined; in such cases, half of the DL value for that substance was considered for the calculations. To check the analytical recovery rate, ten ultra-pure water samples were spiked with 500 and 1000 ng/l of individual PAHs and extracted. The mean recovery rate for single PAHs was Nap (36.28%), Acy (68.1%), Ace (82.6%), Fl (59.1%), Phe (112.2%), Ant (97.0%), Flu (74.5%), Pyr (82.0%), BaA (79.7%), Chy (64.5%), BaP (69.7%), BbF (108.1%), BkF (117.8%), DahA (132.5%), IcdP (80.2%), and BghiP (47.83 %). With each series of samples, blank samples were analyzed to check contamination, check column performance, resolution, and the detection limits. Blank samples were included solvents, standard mixture of PAHs and ultra-pure extracted water. No detectable amount of PAHs was found in solvents and extracted ultra-pure water samples.
To represent variability, Monte Carlo simulation and sensitivity analysis were implemented by standard Model Risk software. Sensitivity analysis was performed with rank correlation to identify and selection of parameters that had the most important effects on the risk. The obtained cancer risks were compared in different exposure pathway using the spearman coefficient. Rank correlation assesses a possible association between variables. The correlation coefficient ranges from −1 to +1. Coefficient of 1.0 presents perfect correlation, 0 to 1.0 indicates variables tend to increase or decrease together, −1.0 to 0 indicates that one variable increases as the other decreases and −1.0 indicate that there are perfect negative or inverse correlation. Monte Carlo simulation, which is often used to address uncertainty in assessments of risks, was implemented to determine risk probability distributions. In addition, it was used to calculate cumulative probability of the total risks in different age groups and different exposure pathway. In addition, cancer risk was compared in different exposure pathway and age groups using the H independent test with SPSS package 17 software (Chicago, IL, USA).
To estimate the lifetime average daily dose (LADD), exposure assessments were conducted based on: a) the exposure factors that were prepared from the questionnaire; and b) the concentrations of PAHs that were measured in tap, bottled, and heated tap water. Toxic equivalency factors (TEF) were applied to convert the concentrations of multi-component PAHs into BaPeq concentration. TEF is an estimate of the relative toxicity of a PAH compound compared to Bap (21).
A questionnaire survey was conducted to investigate the exposure factors, including water intake rates as well as the frequency and the duration of dermal exposure via hand washing, showering, and swimming. After interviewing and training, standard drinking-scaled cups were delivered to the participants. Then data were collected on the behavior of the 3368 participants in four seasons, which were selected randomly during the Tehran city. To obtain age-dependent adjustment factors (ADAF), the study was conducted on four standard age groups, including less than 2, 2-<6, 6-<6, and older than16 (22). Finally, lifetime average daily dose (LADD) and dermal adsorbed dose (DAD) was calculated. The equation used to calculate LADD was adapted from USEPA (23), which is expressed in Eq. 1.
| (Eq. 1) |
where, LADD is calculated as mg/kg.day, Ci is the concentration of BaPeq in water (mg/L), IR is the water intake rate (L/day), EF is the exposure frequency (350 day/year), ED is the exposure duration (years), BW is the body weight (kg), and AT is the averaging time (70 yr×365day/yr). The DAD during showering, hand washing, and swimming was calculated using Eqs. 2 and 3, which adapted from USEPA (2004):
Hence
| (Eq. 2) |
| (Eq. 3) |
Where, DAevent is the absorbed dose per event (mg/cm2.event), tevent is the event duration (hr/event), t* is the time to reach steady-state (hr), FA is the fraction of absorbed water (dimensionless), kp is the dermal permeability coefficient of the target compound in water (cm/hr), Cw is the concentration of BaPeq in water (mg/cm3), τevent is lag time per event (hr/event), DAD is the daily exposure dose of BaPeq through dermal absorption pathway (mg/kg.day), SA is the skin surface area available for contact (in washing: 875 cm2 for less than 2 year, 925 cm2 for those aged 2-<6, 975 cm2 for 6-<16, and 1360 cm2 for older than16; in showering and swimming: 5300 cm2 for less than 2, 7600 cm2 for those aged 2-<6, 15900 cm2 for those aged 6-<16, and 18150 cm2 for older than16), and EV is the event frequency (events/day).
Risk associated with ingestion and dermal exposure was calculated using the Eqs. 4 and 5, respectively, which was adapted from USEPA (23, 24). In addition, age-dependent adjustment factors were used to adjust the estimated excess risks in the four age groups.
| (Eq. 4) |
Where, oral CSF is the cancer slope factor of BaP for direct ingestion, which defines the relationship between dose of the BaP and the corresponding response. According to the Integrated Risk Information System (IRIS), the CSF for BaP fits the lognormal distribution, and the ingestion slope factor is in the range of 4.5 to 11.7 per (mg/kg.day), with a geometric mean of 7.3 per (mg/kg.day). Schneider et al. (2002) verified CSF value of 11.5 per (mg/kg.day) for mixture of PAHs.
| (Eq. 5) |
Where, ABSGI is the gastrointestinal absorption factor. It is assumed that in the direct ingestion 100% of BaP is absorbed, whereas in the dermal contact, the ABSGI factor is 0.89. Monte Carlo simulation was implemented to analyze the uncertainties and determination of its impact on the risk estimated. In addition, sensitivity analysis was used to identify the most important of exposure pathway by calculating rank correlation. To ensure the consistency of the results, simulation was run with 5000 iterations, which has been demonstrated that have high reliability (25).
Results
The concentration of single PAHs in the distribution system ranged from not quantifiable to 438.96 ng/l. Except Fl, Ant, Flu, and Phy, all PAHs were identified in the water samples. The maximum single PAHs concentration (ng/l) was found for Chy (438.96), IcdP (277.51), BkF (203.75), DahA (114.61), BghiP (67.74), and Nap (63.10). Chy occurred most frequently in water samples (60.6 %). It was followed by Nap (46.5%) and DahA (31.3 %). 44.5–100% of individual PAHs had vaporized from the spiked samples in heated tap water. The total PAHs concentration in heated tap water was 6.30 ng/l. The mean concentration of Total PAHs in MBW and BDW was 20.54 and 32.20 ng/l, respectively. Nap, Fl, Phe, BaA, BbF, BkF and IcdP were identified in the bottled water.
Based on TEFs, the BaPeq concentrations in tap water, bottled water, and heated tap water are presented in Table 1. In the selected scenario, the mean of BaPeq concentration was used for calculating LADD and DAD, which were 15.90, 4.84, and 0.45 ng/L in tap water, bottled water, and heated tap water, respectively.
Table 1:
Concentration of Benzo (a) Pyrene equivalent in different water sources (ng/L)
| BaPeq (ng/l) | Tap water | Bottled water | Heated tap water | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| District 1 | District 2 | District 3 | District 4 | District 5 | District 6 | MBW | DBW | Total | ||
| Max. | 107.20 | 68.11 | 25.71 | 65.69 | 23.72 | 20.10 | 30.8 | 7.78 | 14.2 | 2.33 |
| Min. | 1.96 | 3.29 | 3.27 | 1.13 | 3.24 | 3.10 | 3.41 | 3.41 | 3.41 | 0.11 |
| Mean | 29.44 | 21.55 | 11.67 | 19.43 | 11.56 | 12.00 | 5.08 | 4.35 | 4.84 | 0.45 |
| Median | 4.30 | 7.41 | 8.85 | 5.45 | 9.64 | 12.41 | 3.42 | 3.55 | 3.50 | 0.18 |
Exposure parameters in different age groups are presented in Table 2. The annual average direct tap water consumption was 1.75 liter per capita per day. The share of tap water, bottled water, and heated tap water was 58%, 16%, and 26%, respectively. The frequency of dermal exposure with drinking water via hand washing, showering, and swimming was 3.59±1.10, 0.4±0.11, and 0.0021±0.3 events per day, respectively. In addition, the duration of dermal exposure with drinking water via hand washing, showering, and swimming was 1.69±0.71, 24.92±9.65, and 63.05±18.38 minutes per event, respectively. As presented in Table 3, Sum of LADD and DAD was 6.97×10−7 and 1.33×10−5, respectively. The DAD/LADD ratio was 19/1, which indicates higher intake rate of PAHs through dermal exposure. For both ingestion and dermal exposure, the maximum intake rate was observed for the older than 16 years. Additionally, the minimum PAHs intake was observed for those aged less than 2.
Table 2:
Exposure parameters of ingestion and dermal exposure to PAHs in the different exposure pathway (mean ± SD)
| Exposure pathway | Unit | Age groups (yr) | ||||
|---|---|---|---|---|---|---|
| < 2 | 2- < 6 | 6- <16 | > 16 | |||
| Direct water ingestion | Tap | L/capita/day | 0.45±0.12 | 0.51±0.14 | 1.12±0.27 | 1.23±0.27 |
| Bottled | 0.22±0.06 | 0.29±0.06 | 0.27±0.10 | 0.30±0.15 | ||
| Heated | 0.28±0.12 | 0.27±0.06 | 0.38±0.13 | 0.54±0.14 | ||
| Dermal exposure | Washing | Event/day | 2.97±1.03 | 2.84±0.49 | 3.04±0.73 | 3.89±1.13 |
| Showering | 3.27±0.47 | 2.67±0.44 | 2.85±0.63 | 2.72±1.03 | ||
| Swimming | ND | ND | 0.03±0.12 | 0.10±0.38 | ||
| Dermal exposure | Washing | Min/event | 1.24±0.81 | 1.77±0.77 | 1.71±0.48 | 1.73±0.73 |
| Showering | 17.36±8.72 | 24.20±8.53 | 26.64±7.68 | 27.02±9.49 | ||
| Swimming | ND* | ND | 90 | 58.15±15.29 | ||
Not determined
Table 3:
Lifetime average daily dose and dermal adsorb dose if ingestion and dermal exposure to PAHs from drinking water (mg/kg/d)
| Exposure pathway | components | < 2 | 2- < 6 | 6- < 16 | > 16 | sum |
|---|---|---|---|---|---|---|
| Ingestion | Tap water | 8 -10×2.93 | 8 -10×6.64 | 8 -10×3.46 | 7 -10×4.63 | 7 -10×6.37 |
| Bottled water | 9 -10×4.38 | 8 -10×1.15 | 9 -10×5.76 | 8 -10×3.46 | 8 -10×5.62 | |
| Heated water | 10 -10×2.35 | 10 -10×4.44 | 10 -10×3.35 | 9 -10×2.57 | 9 -10×3.58 | |
| sum | 8 -10×3.39 | 8 -10×7.84 | 8 -10×8.42 | 7 -10×5.01 | 7 -10×6.97 | |
| Dermal | washing | 7 -10×1.13 | 7 -10×2.71 | 7 -10×1.16 | 7 -10×1.56 | 6 -10×2.10 |
| showering | 7 -10×3.98 | 6 -10×1.10 | 6 -10×1.39 | 6 -10×8.39 | 5 -10×1.11 | |
| swimming | ND | ND | 9 -10×6.05 | 7 -10×1.04 | 7 -10×1.09 | |
| sum | 7 -10×5.11 | 6 -10×1.37 | 6 -10×1.55 | 6 -10×9.90 | 5 -10×1.33 |
The results for multi-pathway risk assessment are given in Table 4. The excessive cancer risks due to ingestion of tap water, bottled water, and heated tap water were estimated to be 1.36E-05, 0.15E-05, and 0.06E-05, respectively. In addition, the excessive cancer risk for washing, showering, and swimming were 2.71E-5, 14.45E-5, and 0.14E-5. Sum of lifetime cancer risk of dermal absorption and oral ingestion was 17.24E-05 and 1.57E-5, respectively.
Table 4:
The estimated ingestion and dermal ELCR for exposure to PAHs in the water sources
| Exposure pathway | Components | < 2 | 2- < 6 | 6- < 16 | > 16 | sum |
|---|---|---|---|---|---|---|
| Ingestion | Tap water | 5 -10×0.34 | 5 -10×0.23 | 5 -10×0.35 | 5 -10×0.47 | 5 -10×1.36 |
| Bottled water | 5 -10×0.05 | 5 -10×0.04 | 5 -10×0.02 | 5 -10×0.04 | 5 -10×0.15 | |
| Heated water | 5 -10×0.003 | 5 -10×0.002 | 5 -10×0.001 | 5 -10×0.003 | 5 -10×0.06 | |
| Sum | 5 -10×0.40 | 5 -10×0.28 | 5 -10×0.29 | 5 -10×0.59 | 5 -10×1.57 | |
| Dermal | Washing | 6 -10×1.46 | 6 -10×3.50 | 6 -10×2.08 | 6 -10×2.01 | 5 -10×2.71 |
| Showering | 6 -10×5.15 | 5 -10×1.43 | 5 -10×1.81 | 5 -10×10.7 | 5 -10×14.45 | |
| Swimming | ND | ND | 6 -10×0.08 | 6 -10×1.34 | 5 -10×0.14 | |
| Sum | 5 -10×0.66 | 5 -10×1.78 | 5 -10×2.03 | 5 -10×12.85 | 5 -10×17.24 | |
| Total ELCR | 5 -10×1.06 | 5 -10×2.06 | 5 -10×2.32 | 5 -10×13.44 | 5 -10×18.81 |
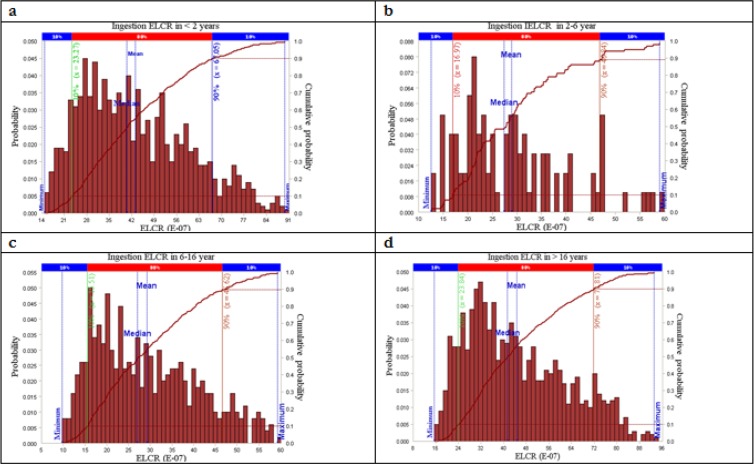
Simulation of the direct ingestion risk of PAHs from drinking water is shown in Fig. 1. As shown in Fig. 1-a, the cancer risk is ranged from 0.18E-5 to 2.7E-5 in the age group of less than 2 (P=0.01–0.07). In this category, the risk value of 0.61E-5 had the highest probability. The simulated cancer risk of direct ingestion for the age group of 2-<6 is shown in Fig. 1-b. The figure is indicated that the cancer risk is varied between 0.2E-5 and 2E-5, which the value of 0.51E-5 having the maximum probability (P=0.008–0.08). In the age group of 6-<16, the simulated risk is ranged from 0.04E-5 to 2.02E-5 and that the value of 0.35E-5 having the highest probability. Finally, in the age group of older than16, the simulated cancer risk was between 0.12E-5 and 3.3E-5, with the cancer risk of 0.69E-5 having the highest probability (Fig. 1-d).
Fig. 1:
Distriution of excess lifetime cancer risk attributable to direct ingestion of PAHs in drinking water
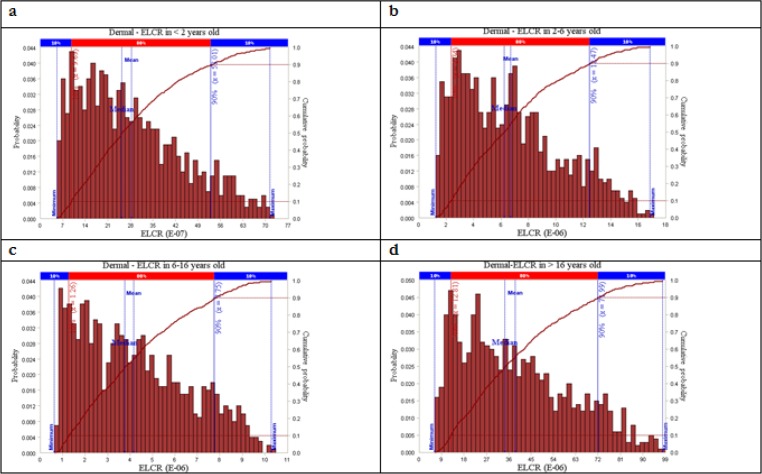
Risk probability distribution of dermal ELCR is illustrated in Fig. 2. As presented in Fig. 2-a, in the less than 2, the dermal cancer risk was ranged from 0.007E-5 to 0.72E-5. In this category, the risk value of 0.1E-5 had the highest probability. The dermal cancer risk simulated for the age group of 2-<6 is shown in Fig. 2-b. Accordingly, the cancer risk is varied from 0.1E-5 to 1.6E-5 and that the risk value of 0.2E-5 having the maximum probability. For the age group of 6-<16, the simulated cancer risk ranged from 0.8E-5 to 1E-5 (Fig. 2-c) and that the risk value of 1.3E-5 having highest probability. As shown in Fig. 2-d, in the age group of older than16, the simulated cancer risk is ranged from 0.1E-5 to 1.05E-5, which the value of 0.13E-5 having the maximum probability.
Fig. 2:
Distriution of excess lifetime cancer risk attributable to dermal exposure with PAHs in drinking water
Discussion
Point estimation showed that the cancer risk of exposure through direct ingestion was 1.57E-05. It was 1.5 times higher than the acceptable risk level of 1.00E–05 defined by the WHO. Among water sources, it was found that tap water had the highest risk (1.36E-5); its cancer risk was 1.36 times higher than the acceptable risk level. These could be explained by the fact that the water in-take rate and PAHs concentrations in the tap water samples were higher than bottled and heated tap water. Strong relationships were detected between exposure pathway and ELCR (df =5, chi-square=18.36, P= 0.003). In different exposure pathway, maximum differences was detected between showering and heated tap water consumption (P=0.027).
Obtained results indicated that dermal-ELCR of PAHs was 17.24E-5, which was 17.24 times higher than the acceptable risk level. Furthermore, dermal-ELCR was almost 11 times higher than that of the ingestion-ELCR. These could be explained by the fact that dermal exposure and its cancer slope factor are higher than those of ingestion intake are. In a dose-response study (26), the dermal toxicity of PAHs was 3.4 times higher than that of the direct ingestion. The above finding is consistent with another study (13), that the dermal cancer risk was 5.42 times higher than the ingestion risk. Besides, obtained results by Qu et al., (27) were similar the present study, which means of dermal-ELCR in adults was estimated 17 times more than direct-ELCR of PAHs in water (3.85E-10 versus 5.39E-9). Different results had been obtained from exposure to PAHs in traffics. Chen and Liao (25) had reported inhalation-ELCR to 2.7-fold of skin contact-ELCR. Similar estimations are provided by Gungormus et al. (28).
In all of the age groups, estimated ingestion-ELCR is indicated that the age group of older than16 had the highest risk (0.59E-05), followed by the age group of less than 2 (0.4E-05). These could be explained by the higher water intake rate in older than16 and higher ADAF in the less than 2. In addition, the ingestion cancer risk from different drinking water sources was in the following order: tap water > bottled water > heated tap water.
The obtained results indicated that the maximum dermal-ELCR was estimated in the older than 16, which was followed by in the age groups of 6-<16, 2-<6, and less than 2.
In all pathway of exposure, showering in the older than16 had the highest cancer risk, which was followed by the risk of showering in the age group of 6-<16, the risk of showering in the age group of 2-<6, and the risk of tap water ingestion in the age group of older than 16. Although washing and showering could threat human heath, our results indicated that the primary concern is showering (14.45E-5) that followed by washing (2.71E-5) and swimming (0.14E-5). These could be explained by body area and event frequency exposure in older than 16. The obtained risk shows that each year approximately 21.49 cancers could get from the daily intake of PAHs in polluted drinking water in populations of Tehran.
To improve the credibility of the estimated cancer risks, Monte Carlo simulation was applied to identify the uncertainties in the risk assessment. It was implemented to determine risk probability distributions and to calculate cumulative probability of the total risks in different age groups and exposure pathway. As presented in Figs. 1 and 2, the dermal absorption in the residents older than 16 had the highest probability cancer risk, with the mean and median of 3.86E-05 and 3.45E-05, respectively. In addition, the lowest cancer risk belonged to the category of dermal exposure for the residents less than 2.
The cumulative risk showed that in all age groups the probability of ingestion risk value above acceptable risk level was zero (Fig. 1). As shown in Fig. 2, the probability of dermal risk values being above 1.00E-05 was 96.2%, 0.39%, and 20.78% for the age groups of >16, 6-<16, and 2-<6, respectively. Additionally, similar to the ingestion risk, for the age group of <2, the probability of cumulative risk being above 1.00E–05 was zero.
Sensitivity analysis was conducted to evaluate and selection of parameters that had the most important effects on the risk. Rank correlation was used for the sensitivity analysis of the exposure pathways. As presented in Table 5, analysis indicated that tap water had the greatest impact on the ingestion-ELCR (Pspearman =0.92–0.98). In dermal-ELCR, washing activities had more correlation than swimming and showering (Pspearman =0.95–0.98).
Table 5:
Spearman coefficient of rank correlation for ingestion and dermal excess lifetime cancer risk
| Age groups (yr) | Ingestion ELCR | Dermal ELCR | ||||
|---|---|---|---|---|---|---|
| Tap water | Bottler water | Heated water | Washing | Showering | Swimming | |
| <2 | 0.92 | −0.05 | 0.05 | 0.95 | 0.03 | 0.01 |
| 2-<6 | 0.96 | −0.2 | 0.1 | 0.95 | 0.03 | 0.01 |
| 6-<16 | 0.98 | 0.08 | 0.05 | 0.98 | −0.03 | 0.01 |
| >16 | 0.96 | −0.05 | 0.05 | 0.98 | −0.05 | 0.03 |
Conclusion
The carcinogenic risk of PAHs in the drinking water was higher than the acceptable level. The sum of ingestion and dermal-ELCR was 18.81E-05, which was approximately 18.8 times higher than the acceptable level proposed by the WHO. The highest cumulative risk probability of above 1.00E–05 was observed in dermal exposure in older than16 (96.2%). The obtained results suggested that the dermal exposure of PAHs in water sources poses threats to human health more than direct water ingestion. Therefore, intervention, remediation or further serious action should be paid for reducing the adverse effects of dermal exposure in activities such as swimming and bathing in polluted water.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
This study was a PhD dissertation. We thank the Tehran University of Medical Sciences, for financially supporting this research under grant No: 90-02-27-14151. The authors declare that there is no conflict of interests.
References
- 1.Ritter L, Solomon K, Sibley P, Hall K, Keen P, Mattu G, Linton B. (2002). Sources, pathways and relative risks of contaminants in surface water and groundwater: a perspective prepared for the Walkerton inquiry. J Toxicol Environ Health A, 65(1):1–142. [DOI] [PubMed] [Google Scholar]
- 2.Patrolecco L, Ademollo N, Capri S, Pagnotta R, Polesello S. (2010). Occurrence of priority hazardous PAHs in water, suspended particulate matter, sediment and common eels (Anguilla anguilla) in the urban stretch of the River Tiber (Italy). Chemosphere, 81(11): 1386–1392. [DOI] [PubMed] [Google Scholar]
- 3.Olivella MA. (2006). Polycyclic aromatic hydrocarbons in rainwater and surface waters of Lake Maggiore, a subalpine lake in Northern Italy. Chemosphere, 63(1): 116–131. [DOI] [PubMed] [Google Scholar]
- 4.Ngabe B, Bidleman TF, Scott GI. (2000). Polycyclic aromatic hydrocarbons in storm runoff from urban and coastal South Carolina. Sci Total Environ, 255(1–3): 1–9. [DOI] [PubMed] [Google Scholar]
- 5.Martins CC, Bícego MC, Mahiques M.M, Figueira RC, Tessler M.G, Montone RC. (2011). Polycyclic aromatic hydrocarbons (PAHs) in a large South American industrial coastal area (Santos Estuary, Southeastern Brazil): Sources and depositional history. Mar Pollut Bull, 63(5–12): 452–458. [DOI] [PubMed] [Google Scholar]
- 6.Vidal M, Domínguez J, Luís A. (2011). Spatial and temporal patterns of polycyclic aromatic hydrocarbons (PAHs) in eggs of a coastal bird from northwestern Iberia after a major oil spill. Sci Total Environ, 409(13): 2668–2673. [DOI] [PubMed] [Google Scholar]
- 7.Anderson LM, Diwan BA, Fear NT, Roman E. (2000). Critical windows of exposure for children's health: cancer in human epidemiological studies and neoplasm in experimental animal models. Environ Health Perspect, 108(Suppl . 3): 573–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh VK, Patel DK, Jyoti, Ram S, Mathur N, Siddiqui MKJ. (2008). Blood levels of polycyclic aromatic hydrocarbons in children and their association with oxidative stress indices: An Indian perspective. Clin Biochem, 41(3): 152–161. [DOI] [PubMed] [Google Scholar]
- 9.Gurbani D, Bharti SK, Kumar A, Pandey AK, Ana FREE, Verma A, Husain Khan A, Patel DK, Mudiam MKR, Jain S.K, Roy R, Dhawan A. (2013). Polycyclic aromatic hydrocarbons and their quinones modulate the metabolic profile and induce DNA damage in human alveolar and bronchiolar cells. Int J Hyg Environ Health, 216(5): 553–565. [DOI] [PubMed] [Google Scholar]
- 10.WHO (2011). Guidelines for Drinking-water Quality. fourth edition. Available from: http://apps.who.int/iris/bitstream/10665/44584/1/9789241548151_eng.pdf.
- 11.WHO (2001). Human exposure assessment, an introduction. Available from: www.imm.ki.se/publ/PDF/HEAboken.pdf.
- 12.EPA (2005). Guidelines for Carcinogen Risk Assessment, Risk Assessment Forum U.S. Environmental Protection Agency. Available from: www.epa.gov/risk_assessment/guidance.htm.
- 13.Wu B, Zhang Y, Zhang X, Chen S. (2011). Health risk assessment of polycyclic aromatic hydrocarbons in the source water and drinking water of China: Quantitative analysis based on published monitoring data. Sci Total Environ, 410–411: 112–118. [DOI] [PubMed] [Google Scholar]
- 14.Pardakhti AR, Bidhendi GR, Torabian A, Karbassi A, Yunesian M. (2011). Comparative cancer risk assessment of THMs in drinking water from well water sources and surface water sources. Environ Monit Assess, 179(1–4):499–507. [DOI] [PubMed] [Google Scholar]
- 15.Pongpiachan, Tipmanee D, Deelaman W, Muprasit J, Feldens P, Schwarzer K. (2013). Risk assessment of the presence of polycyclic aromatic hydrocarbons (PAHs) in coastal areas of Thailand affected by the 2004 tsunami. Mar Pollut Bull, 76 (1–2): 370–378. [DOI] [PubMed] [Google Scholar]
- 16.Aziz F, Syed JH, Malik RF, Katsoyiannis A, Mahmood A, Li J, Zhang G, Jones KC. (2014). Occurrence of polycyclic aromatic hydrocarbons in the Soan River, Pakistan: Insights into distribution, composition, sources and ecological risk assessment. Ecotoxicol Environ Saf, 109: 77–84. [DOI] [PubMed] [Google Scholar]
- 17.Gute BD, Grunwald GD, Basak SC. (1999). Prediction of the dermal penetration of polycyclic aromatic hydrocarbons (PAHs): a hierarchical QSAE approach. SAR QSAR. SAR QSAR Environ Res, 10(1): 1–15. [DOI] [PubMed] [Google Scholar]
- 18.USEPA (2010). Drinking Water Analytical Methods, Analytical Methods Approved for Drinking Water Compliance Monitoring. https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P100J7BF.txt.
- 19.Li N, Lee HK. (2001). Solid-phase extraction of polycyclic aromatic hydrocarbons in surface water: Negative effect of humic acid. J Chromatogr A, 921(2): 255–263. [DOI] [PubMed] [Google Scholar]
- 20.Karyab H, Yunesian M, Nasseri S, Mahvi A.H, Ahmadkhaniha R., Rastkari N, Nabizadeh R. (2013). Polycyclic Aromatic Hydrocarbons in drinking water of Tehran. J J Environ Health Sci Eng, 11 (1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nisbet ICT, LaGoy PK. (1992). Toxic Equivalency Factors (TEFs) for Polycyclic Aromatic Hydrocarbons (PAHs). Regul Toxicol Pharmacol, 16 (3): 290–300. [DOI] [PubMed] [Google Scholar]
- 22.USEPA (2012). Handbook for Implementing the Supplemental Cancer Guidance. Available from: https://www.epa.gov/risk/risk-assessment-guidelines.
- 23.USEPA (1989). Risk Assessment Guidance for Superfund Volume I Human Health Evaluation Manual (Part A). Available from: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part.
- 24.USEPA (2004). Risk Assessment Guidance for Superfund, Human Health Evaluation Manual, (Part E, Supplemental Guidance for Dermal Risk Assessment). Available from: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-e.
- 25.Chen SC, Liao CM. (2006). Health risk assessment on human exposed to environmental polycyclic aromatic hydrocarbons pollution sources. Sci Total Environ, 366(1): 112–123. [DOI] [PubMed] [Google Scholar]
- 26.Knafla A., Phillipps KA, Brecher RW, Petrovic S, Richardson M. (2006). Development of a dermal cancer slope factor for benzo[a]pyrene. Regul Toxicol Pharmacol, 45(2): 159–168. [DOI] [PubMed] [Google Scholar]
- 27.Qu C, Li B, Wu H, Wang S, Giesy JP. (2015). Multi-pathway assessment of human health risk posed by polycyclic aromatic hydrocarbons. Environ Geochem Health, 37(3): 587–601. [DOI] [PubMed] [Google Scholar]
- 28.Gungormus E, Tuncel S, Hakan Tecer L, Sofuoglu SC. (2014). Inhalation and dermal exposure to atmospheric polycyclic aromatic hydrocarbons and associated carcinogenic risks in a relatively small city. Ecotoxicol Environ Saf, 108: 106–113. [DOI] [PubMed] [Google Scholar]