Abstract
Background:
The objective of this study was to determine the residual concentrations of ethion and imidacloprid in cucumbers grown in greenhouse. The effect of some simple processing procedures on both ethion and imidacloprid residues were also studied.
Methods:
Ten active greenhouses that produce cucumber were randomly selected. Ethion and imidacloprid as the most widely used pesticides were measured in cucumber samples of studied greenhouses. Moreover, the effect of storing, washing, and peeling as simple processing procedures on both ethion and imidacloprid residues were investigated.
Results:
One hour after pesticide application; the maximum residue levels (MRLs) of ethion and imidacloprid were higher than that of Codex standard level. One day after pesticide application, the levels of pesticides were decreased about 35 and 31% for ethion and imidacloprid, respectively, which still were higher than the MRL. Washing procedure led to about 51 and 42.5% loss in ethion and imidacloprid residues, respectively. Peeling procedure also led to highest loss of 93.4 and 63.7% in ethion and imidacloprid residues, respectively. The recovery for both target analytes was in the range between 88 and 102%.
Conclusion:
The residue values in collected samples one hour after pesticides application were higher than standard value. The storing, washing, and peeling procedures lead to the decrease of pesticide residues in greenhouse cucumbers. Among them, the peeling procedure has the greatest impact on residual reduction. Therefore, these procedures can be used as simple and effective processing techniques for reducing and removing pesticides from greenhouse products before their consumption.
Keywords: Ethion, Imidacloprid, Greenhouse, Cucumber, Residuals
Introduction
Pesticides are commonly applied in agriculture to improve productivity and pest control. Food consumption is one of the most common routes of pesticide exposure in consumers (1, 2). In general, only 0.1% of pesticides effect on the target species (3, 4). Generally, pesticides are classified into four categories including organochlorine, organophosphate, carbamates and pirethroid pesticides. Ethion is an organophosphate (OP) and non-systemic pesticide, which affects through the inhibition of acetylcholinesterase (AChE) enzyme of the nervous systems in the target animals (5, 6). Ethion is widely used in agriculture to pest control such as mites, aphid, thrips, etc. (7). Pesticide residues on fruits and vegetables can constitute a potential risk to consumers, and considered as a human health concern. If the pesticides are used as recommended on the label of the product, its residues may not exceed from the maximum residue levels (MRLs), otherwise poisoning would be inevitable. Symptoms of ethion poisoning include nausea, or vomiting, anxiety, restlessness, lacrimation and excessive sweating. Severe poisoning can cause an inability to breathe. Death can also occur from direct mouth contact with ethion in some cases (8). Imidacloprid (also known as confidor) was commonly used for the control of sucking pests (9), which is a systemic pesticide and effects on the central nervous system by blocking nicotinic acetylcholine receptors (10–13). The imidacloprid has also adverse effects on human health. Liver and thyroid gland toxicity was reported by several chronic and sub-chronic studies (14). In the recent years, excessive use of pesticides and the increases of the number of spraying, have been resulted in the development of resistance to pesticides in pest populations (15). On the other hand, the extensive use of pesticides in agriculture increases their residues in agricultural products.
Due to these negative effects, Maximum Residue Limits (MRLs) were set by international organizations as the criteria to prevent the adverse health effects of pesticide residues. Accordingly, the MRLs were set to be 0.5 mg/kg and 1 mg/kg for ethion and imidacloprid, respectively (9, 16).
The Hamadan province is located in the west of Iran and has suitable environmental conditions for agriculture. In this province, total land area under greenhouse cultivation was about 97 hectares in 2014 (8). Greenhouse cucumber is considered as a major vegetable crop grown in greenhouse on a large scale. Due to the diversity of agricultural products and development of greenhouse land, the use of pesticides has obviously increased. According to the available data, 219 tons of insecticides, 116 tons fungicides, 19 tons of acaricides and 124 tons of herbicides have been used at Hamadan in 2014 (8). According to the literature and Hamadan province Agriculture Jihad Organization report about active greenhouses, ethion and imidacloprid were identified as the most widely used pesticides for pest control (8, 9).
Several studies have been conducted on the pesticides residues in various agricultural products. In a similar study, Spanish researchers measured a large number of pesticides in citrus, other fruits and vegetables (17). The pesticide residues were studied in field-sprayed and processed fruits and vegetables and reported that the commercial processing procedures led to a large reduction in the level of pesticide residues in the finished products (18). Furthermore, the food processing and some home preparation procedures such as washing, peeling and cooking can reduce or remove residues of insecticides and other pesticides remaining on food crops (19–22).
Therefore, the main objective of this study was to determine the residual level of ethion and imidacloprid in cucumbers grown in greenhouse. The effects of some simple processing methods on both pesticide residues were also studied.
Methods
Sampling
In this cross-sectional study, based on the land area under greenhouse cultivation, ten active greenhouses producing cucumber were randomly selected in Hamadan City. In selected greenhouses, ethion and imidacloprid were used to pest control. In the first step, to determine the pesticide adsorption rate in cucumbers grown of greenhouse after one hour of pesticide application, the samples were collected randomly from the lower, middle, and upper rows of bushes. About one kg of cucumbers were collected from each greenhouse and transported to the laboratory by a polyethylene plastic bag (23). In the next step, to study the effect of some simple procedures such as washing (with drinking water for 2 min), peeling (with a kitchen knife dipped in acetone for a short time), and storing at 4 °C (refrigerator common temperature, for 24–48 h) on the pesticide residues (16, 24), five kg of cucumbers were taken from each greenhouse before market supplying. Moreover, control samples (without any processing) were taken in each step to compare the results. All samples (including control samples) have the repeatability in terms of temperature, sampling time, technician, and spraying conditions except that control samples did not get any processing. To study the storage effects, samples were stored in polyethylene bag at 4 °C (in the refrigerator). Two samples were taken from each greenhouse and divided into six parts and the experiments were repeated three times for each sample. Accordingly, eighteen samples were taken from each greenhouse and totally 180 samples were analyzed during the study.
Chemicals
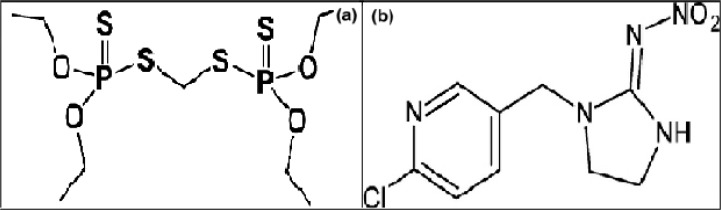
GC and HPLC analytical grade of ethion (CAS Number 563-12-2 and Empirical Formula C9H22O4P2S4) and imidacloprid (CAS Number 138261-41-3 and Empirical Formula C9H10ClN5O2) were purchased from Sigma-Aldrich (Germany). Fig. 1 shows the chemical structure of ethion (a) (25) and imidacloprid (b) (9). Acetone and acetonitrile were purchased from Merck (Darmstadt, Germany). Anhydrous magnesium sulfate (with chemical formula: MgSO4) and sodium acetate (with chemical formula: CH3COONa) were also purchased from Merck
Fig. 1:
Chemical structure of Ethion (a) and Imidacloprid (b)
Method of extraction and recovery studies
Sample preparation and pesticide residues extraction were carried out using the quick, east, cheap, effective, rugged and safe (QuEChERS) method (20, 26). In this method, 1000 g of cucumbers were crushed and homogenized in a high speed laboratory homogenizer. Then, 10 g of homogenized sample with 10 mL of acetonitrile were mixed in a 50 mL centrifuge tube. Finally, the obtained sample was centrifuged for 1 min. Then, 4 g of anhydrous magnesium sulfate and 1.5 g of sodium acetate were added to centrifuged sample. The final sample was centrifuged for 1 min. The upper layer containing 30 mg of GCB and 150 mg of anhydrous magnesium sulfate was mixed and then centrifuged at 4000 rpm for 5 min. Filtered extract was concentrated to 1 mL using a rotator device and then 10 μL of concentrated sample were injected to GC-MS. 20 μL of concentrated sample was also injected to HPLC device. The efficiency of extraction method was monitored by checking the recovery of both representative and blanks samples and the recovery of spiked samples (samples having known concentrations of both pesticides) at different levels, using internal standard in each unknown processed sample to assess the accuracy (in terms of recovery) and suitability of the whole analytical process. After homogenization, blank samples were spiked by addition of appropriate volumes of both pesticide standard mixtures in solution. Furthermore, to check instrument drift, a middle range standard was also injected regularly (including intra-day precision) for the minimum of five calibration standard solutions. Recovery tests were replicated three times for each fortification level.
Pesticide residue determination
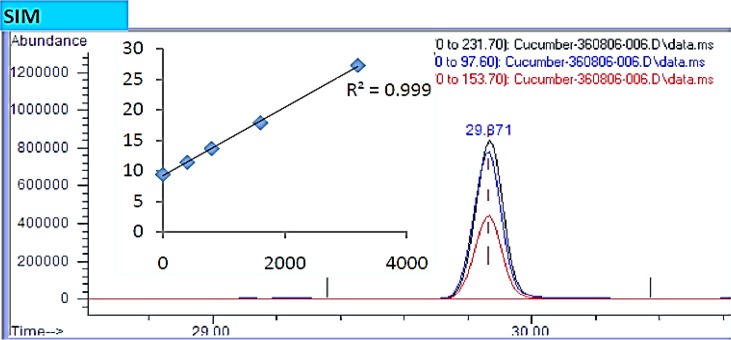
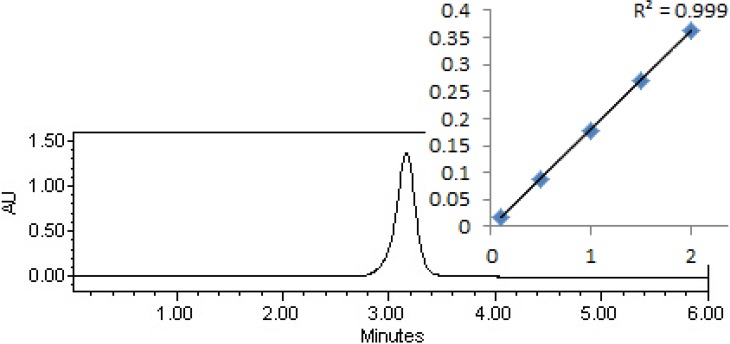
GC/MS device (model 7890 A, American) with a column dimensions of 0.25–30 mm I.D was used to measure the ethion residues in cucumber samples (26). The mobile phase was helium with a purity of 99%. The HPLC (model K2600, Germany-Knauer) equipped with an UV detector at a wavelength of 270 nm and Waters column C18 (25 mm×4.6 mm) was used to measure imidacloprid residue with the mobile phase of acetonitrile/water (60/40 v/v). Calibration curves and cucumber fortified samples were prepared by using working standard solutions through direct injection into the GC and HPLC column. Fig. 2 and 3 show the GC-MS chromatogram of standard solution and calibration curve of ethion and the HPLC chromatogram of standard solution and calibration curve of imidacloprid, respectively.
Fig. 2:
GC-MS chromatogram of standard solution and calibration curve of ethion
Fig. 3:
HPLC chromatogram of standard solution and calibration curve of imidacloprid
Quality assurance and quality control
In order to ensure the reliability of results, all the samples (including control samples) were analyzed in duplicates for quality assurance/quality control criteria (e.g., detection and determination limits, and reproducibility) before running sample analyses. The limit of detection (LOD, mg/kg) of each analytes was determined as the lowest concentration giving a response three times the standard deviation of the baseline noise, which defined, based on the analysis of three control samples. The limit of quantification (LOQ, mg/kg) was determined as the lowest concentration of a given compound giving a response quantified with relative standard deviation (RSD) lower than 20% which the latter was also used to evaluation of precision (9). RSD was measured by analyzing the samples with known concentration and comparing the results with the true values. Standard reference materials, i.e. GC and HPLC analytical grade of ethion and imidacloprid were purchased from Sigma-Aldrich (Germany) and were used for calibration and analytical control and the pesticides residues were identified relative to external standards.
Statistical analysis
The obtained data were analyzed using two-way ANOVA by SPSS software (IBM®, version 20, Chicago, IL, USA).
Results
Method performance and validation
The analytical efficient method of QuEChERS was used to determine pesticides residues in cucumbers. The mean recovery for all analytes and external standards were found to be consistent and varied from 88.4% to 102.2%, demonstrating the efficiency of this method, which allowed the identification, and quantification of pesticide present in the samples. The relative standard deviation (RSD) values were generally below 10% in all cases and are acceptable to further analysis. Calibration for quantification was carried out by use of external standard calibration curves, which were linear with correlation coefficients of 0.999 for both analytes. The LOD and LOQ for both analytes in the cucumbers samples ranged between 0.002 and 0.024 mg/kg, ensuring the values much lower than the MRLs values (9, 25).
Effect of storage time on pesticide residues
As shown in Table 1, one hour after the pesticide application, the maximum and minimum concentrations of ethion residues were 1.512 and 1.314 mg/kg (in greenhouses No. 1 and 4, respectively). One day after the pesticide application, the maximum and minimum concentration of ethion residues in samples supplied to the market were seen in greenhouses No. 1 and 4, with the corresponding values of 0.975 and 0.867 mg/kg, respectively. As a result, the percentage reduction of ethion residue was about 35%; however, it still was more than the codex standard level of 0.5 mg/kg. One hour after imidacloprid application, the maximum and minimum concentrations of residues were 1.802 and 1.568 mg/kg (in greenhouses No. 2 and 4, respectively). One day after the pesticide application and in the market, the maximum and minimum concentrations of imidacloprid residues were 1.207 and 1.113 mg/kg, respectively, which represented a reduction of 31%, however, these values were still more than the codex standards of 1 mg/kg.
Table 1:
Concentrations and percentages reduction of ethion and imidacloprid residues in cucumber samples of greenhouses No. 1 to 5, one hour after application until one day after market broadcast
| Pesticide | Pre-harvest time | Mean residuals concentration (mg kg−1) (standard deviation) Greenhouse No. | Mean reduction in residuals concentration (%) | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Ethion | 1h | 1.512 ± 0.028 | 1.488 ± 0.031 | 1.421 ± 0.027 | 1.314 ± 0.033 | 1.354 ±0.029 | - |
| 1day | 0.975 ± 0.014 | 0.952 ± 0.016 | 0.924 ± 0.013 | 0.867 ± 0.015 | 0.907 ±0.014 | 35 | |
| Imidacloprid | 1h | 1.723 ± 0.028 | 1.80 ± 0.030 | 1.672 ± 0.026 | 1.568 ± 0.034 | 1.630 ±0.029 | - |
| 1day | 1.172 ± 0.013 | 1.207 ± 0.014 | 1.154 ± 0.012 | 1.113 ± 0.015 | 1.118 ±0.013 | 31 | |
Effect of storage process, washing and peeling on the residual on the both pesticide residues
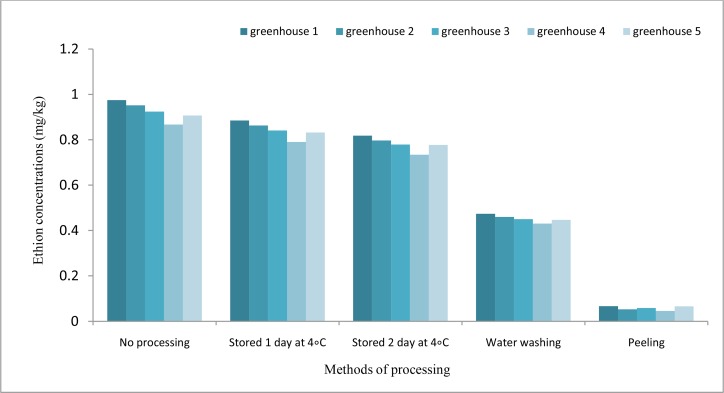
The effect of storage, washing, and peeling procedures on the ethion residue are shown in Fig. 4. The storage procedure at 4 °C for 1 day decreased by 9.2, 9.4, 9.02, 8.9, and 8.31% from the initial ethion residues in greenhouses No. 1, 2, 3, 4, and 5, respectively. On average, 9% reduction was observed in 1 day after storing at 4 °C, which this reduction was increased by 15.5% in 2 d after storing. Washing with tap water reduced 51.4-50.4% from the initial ethion residues in the same greenhouses. In such samples, the ethion residues were also reduced to 0.066 and 0.045 mg/kg by the peeling process, respectively. Therefore, approximately 94% of ethion residues were removed from the initial residues by peeling process.
Fig. 4:
Maximum concentration of ethion residues by storage at 4 °C, washing and peeling process in comparison with supplied sample to the market
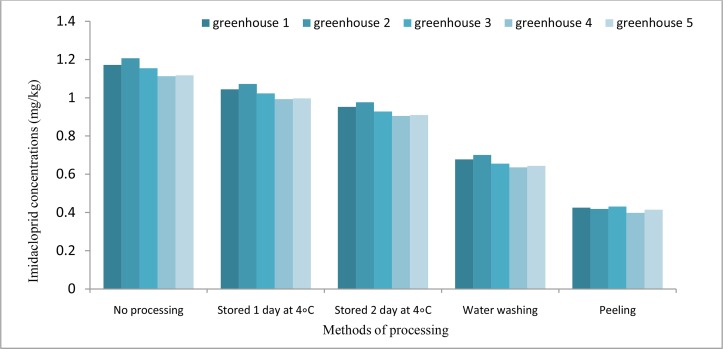
Fig. 5 shows the effect of storage, water washing, and peeling procedures on the imidacloprid residues. According to the results, one day after pesticide application, on average about 11.1% loss in imidacloprid residues was observed. Two days after pesticide application, the maximum concentration of imidacloprid residues was 0.976 mg/kg in greenhouse No. 2, and the minimum concentration was 0.905 mg/kg in greenhouse No. 4. Therefore, on average about 18.9% of the residuals was removed in such greenhouses. Washing procedure led to 41.9 and 42.8% reduction in imidacloprid residues for greenhouse No. 2 and 4, respectively. Moreover, the peeling process reduced the amount of imidacloprid residues to 0.418 and 0.398 mg/kg in the greenhouses No. 2 and 4, respectively. In other words, the peeling procedure reduced the amount of imidacloprid residues by 65.4 and 64.2% from the initial level in such samples.
Fig. 5:
Maximum concentration of imidacloprid residues by storage at 4 °C, washing and peeling process in comparison with supplied sample to the market
Discussion
The performance characteristics of the method (including linearity, repeatability, recovery, precision, and accuracy) were successful, with mean recovery values of 95%, relative standard deviation (RSD) values of below 10% in all cases, and mean LOD and LOQ between 0.002 and 0.024 mg/kg, ensuring that these values significantly lower than the MRLs (9). Considering all of these factors in the validation of method, the methodology is highly efficient, selective, precise, and robust for the determination of pesticides in the samples (9, 25).
The residues of both pesticides (ethion and imidacloprid) decreased over the time and during storing periods. Based on the results, the maximum concentration of ethion residues in cucumber samples was seen one hour after pesticide application, and its concentration decreased with the increasing the storage time. This result is accordance with another (16) study on the persistence of ethion residues on cucumber, in which the initial ethion residues decreased more than 80 and 90% after 7 to 10 d, respectively. In the other study, the maximum level of pesticide residues was found after one hour of application (27). Furthermore, the comparison of the mean levels of pesticide residues showed a significant difference between collected samples from different greenhouses (P<0.001 for ethion and P=0.045 for imidacloprid). This may be related to a difference in used dosage, application method, and frequency during the harvesting period. The levels of pesticide residues in agricultural products are influenced by environmental conditions (28). In addition, they concluded that the pesticide residues decreased with the increase of storage time. Our results are in accordance with these studies. Accordingly, the increasing of storage time can be used as a simple processing procedure for decreasing pesticide residues in agricultural products like cucumbers grown in greenhouse.
As shown in Fig. 4 and 5, about 51% and 42.5% of ethion and imidacloprid residues were reduced by the washing procedure, which the comparison of the mean levels of pesticide residues indicated a statistically significant difference between washed and unwashed samples (P=0.044). The washing procedure is an important method to pesticide residue dissipation in both household and commercial food processing (29). The effect of this procedure depends on the type of pesticides and the locations affected. This group of pesticides (phosphorus) is classified as either contact or systemic pesticides. In contact pesticides, their residues remain on plant surfaces after application. Since, ethion is one of the contact pesticides; it was unable to penetrate the plant surface and remains on the outer layers of fruit. Furthermore, regarding the polar properties in water, ethion pesticides can be easily dissolved in water, then washed and removed from the surface of products (30, 31).
Peeling is one of the other important techniques in the processing of most fruits and vegetables. The comparison of the mean levels of ethion residues showed a significant difference between the peeled and unpeeled samples (P<0.001) (Fig. 4). Furthermore, the peeling procedure was more effective (≈93%) in removing ethion residues than that of washing procedure (only ≈51%). The most used pesticides for food products can penetrate the cuticle and wax layers after sparing (32). Their penetration can be associated with different chemical structures of the compounds. As previously mentioned, ethion is one of the contact and non-systemic pesticide (18), which is unable to penetrate the plant and remains on their surfaces. Therefore, it forms a deposit on the surfaces of leaves and fruit. Therefore, it can be removed from the surface of products by washing or peeling method. While, a similar trend was not observed in the use of washing and peeling processes for the imidacloprid residues. The imidacloprid is a systemic pesticide and has a high ability to penetrate plant tissues, so its residues remain in fruit tissues even after peeling procedure. As a result, the peeling procedure was more effective for reducing ethion residues than imidacloprid residues from the products (P=0.039). These results are consistent with the finding of Chavarri et al. (18), which reported that systemic insecticides can penetrate into the plant tissues and lead to different processing methods be less effective in imidacloprid residuals reduction (18).
Storing food in low temperatures is also a common procedure in the processing of most fruits and vegetables. As shown in Fig. 4, storing the samples (at 4 °C for day) led to ≈9% reduction in ethion residues. The comparison of the mean levels of pesticide residues showed a significant difference between the stored and un-stored samples (P<0.045). While, results indicated that the storing at 4 °C (for 2 d) was less effective for decreasing pesticide residues than other processing procedures. The storing for 2 d was unable to decrease the residual values to less than the MRLs. Similar results were observed for imidacloprid in which storing at 4 °C for one day reduced only 11% of its residues. Furthermore, storing for one day in greenhouses temperature led to more reduction (about 33%) in the pesticide residues than storing in refrigerator (about 10%). These results are consistent with the findings (32), however, they reported higher removal efficacy for storage process than that of this study. This difference can be largely attributed to differences in storage temperature or chemical formula of pesticides. The rate of pesticide degradation decreased with the decreasing storage temperature. Environmental temperature was more effective than the storing at 4 °C on the reduction of pesticide residues, which also confirmed in this study. At least three days is required to dissipate the imidacloprid residues below the MRLs and half-life (T1/2) of this pesticide was equal to 3.40 d (9). Dissipation rate in pepper grown in greenhouse was different for different pesticides and this can be related to the different chemical structures of the compounds (21).
Conclusion
The studied procedures as simple and effective processing methods could be used for reducing and removing pesticides on greenhouse products as home or commercial processing techniques.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
The authors would like to thank Hamadan University of Medical Sciences for financial and instrumental supports, and to thank Dr. Heshmati, the laboratory manager of Vice Chancellor for Food and Drug for his assistance in HPLC and GC setup and sample analysis. The authors have declared no conflict of interest.
References
- 1. Shokrzadeh M, Saberyan M, Saeedi Saravi S. (2008). Assessment of lead (Pb) and cadmium (Cd) in 10 samples of Iranian and foreign consumed tea leaves and dissolved beverages. Toxicol Environ Chem, 90( 5): 879–83. [Google Scholar]
- 2. Carvalho FP. (2006). Agriculture, pesticides, food security and food safety. Environ Sci Policy, 9( 7–8): 685–92. [Google Scholar]
- 3. Katagi T. (2010). Bioconcentration, bioaccumulation, and metabolism of pesticides in aquatic organisms . Reviews of environmental contamination and toxicology: Springer; p. 1– 132. [DOI] [PubMed] [Google Scholar]
- 4. Carriger JF, Rand GM. (2008). Aquatic risk assessment of pesticides in surface waters in and adjacent to the Everglades and Biscayne National Parks: II. Probabilistic analyses. Ecotoxicology, 17( 7): 680–96. [DOI] [PubMed] [Google Scholar]
- 5. Karalliedde L, Senanayake N. (1989). Organophosphorus insecticide poisoning. Br J Anaesth, 63( 6): 736–50. [DOI] [PubMed] [Google Scholar]
- 6. U.S. Environmental Protection Agency Pesticides: Reregistration, Ethion IRED Facts. EPA Web Site; 2012; Available from ; https://archive.epa.gov/pesticides/reregistration/web/html/ethion_red.html.
- 7. Abdel-Gawad H, Mahdy F, Hashad A, Elgemeie GH. (2014). Fate of 14C-Ethion Insecticide in the Presence of Deltamethrin and Dimilin Pesticides in Cotton Seeds and Oils, Removal of Ethion Residues in Oils, and Bioavailability of Its Bound Residues to Experimental Animals. J Agric Food Chem, 62( 51): 12287–93. [DOI] [PubMed] [Google Scholar]
- 8. Management and Planning Organization of Hamedan province Yearly statistical Report about Hamedan province; 2014.
- 9. Hassanzadeh N, Esmaili Sari A, Bahramifar N. (2012). Dissipation of imidacloprid in greenhouse cucumbers at single and double dosages spraying. J Agr Sci Tech, 14( 3): 557–64. [Google Scholar]
- 10. Maienfisch P, Angst M, Brandl F, Fischer W, Hofer D, Kayser H, et al. (2001). Chemistry and biology of thiamethoxam :a second generation neonicotinoid. Pest Manag Sci, 57( 10): 906–13. [DOI] [PubMed] [Google Scholar]
- 11. Maienfisch P, Huerlimann H, Rindlisbacher A, Gsell L, Dettwiler H, Haettenschwiler J, et al. (2001). The discovery of thiamethoxam: a second-generation neonicotinoid. Pest Manag Sci, 57( 2): 165–76. [DOI] [PubMed] [Google Scholar]
- 12. Elbert A, Nauen R, Leicht W. Imidacloprid, a novel chloronicotinyl insecticide: biological activity and agricultural importance. Insecticides with novel modes of action: Springer; 1998. p. 50– 73. [Google Scholar]
- 13. Wiesner P, Kayser H. (2000). Characterization of nicotinic acetylcholine receptors from the insects Aphis craccivora, Myzus persicae, and Locusta migratoria by radioligand binding assays: relation to thiamethoxam action. J Biochem Mol Toxicol, 14( 4): 221–30. [DOI] [PubMed] [Google Scholar]
- 14. Kunkel BA, Held DW, Potter DA. (2001). Lethal and sublethal effects of bendiocarb, halofenozide, and imidacloprid on Harpalus pennsylvanicus (Coleoptera: Carabidae) following different modes of exposure in turfgrass. J Econ Entomol, 94( 1): 60–7. [DOI] [PubMed] [Google Scholar]
- 15. Hemingway J, Small G, Monro A, Sawyer B, Asap H. (1992). Insecticide resistance gene frequencies in Anopheles sacharovi populations of the Cukurova plain, Adana Province, Turkey. Med Vet Entomol, 6( 4): 342–8. [DOI] [PubMed] [Google Scholar]
- 16. Singh G, Singh B, Battu R, Jyot G, Joia BS. (2007). Persistence of ethion residues on cucumber, Cucumis sativus (Linn.) using gas chromatography with nitrogen phosphorus detector. Bull Environ Contam Toxicol, 79( 4): 437–9. [DOI] [PubMed] [Google Scholar]
- 17. Bogialli S, Di Corcia A. (2007). Matrix solid-phase dispersion as a valuable tool for extracting contaminants from foodstuffs. J Biochem Biophys Methods, 70( 2): 163–79. [DOI] [PubMed] [Google Scholar]
- 18. Chavarri MJ, Herrera A, Arino A. (2004). Pesticide residues in field-sprayed and processed fruits and vegetables. J Sci Food Agr, 84( 10): 1253–9. [Google Scholar]
- 19. Chavarri MJ, Herrera A, Ariño A. (2005). The decrease in pesticides in fruit and vegetables during commercial processing. Int J Food Sci Tech, 40( 2): 205–11. [Google Scholar]
- 20. Ramezani MK, Shahriari D. (2015). Dissipation behaviour, processing factors and risk assessment for metalaxyl in greenhouse-grown cucumber. Pest Manag Sci, 71( 4): 579–83. [DOI] [PubMed] [Google Scholar]
- 21. Fenoll J, Ruiz E, Hellín P, Lacasa A, Flores P. (2009). Dissipation rates of insecticides and fungicides in peppers grown in greenhouse and under cold storage conditions. Food Chem, 113( 2): 727–32. [Google Scholar]
- 22. Keikotlhaile BM, Spanoghe P, Steurbaut W. (2010). Effects of food processing on pesticide residues in fruits and vegetables: a meta-analysis approach. Food Chem Toxicol., 48( 1): 1– 6. [DOI] [PubMed] [Google Scholar]
- 23. Chavarri MJ, Herrera A, Ariño A. (2004). Pesticide residues in field-sprayed and processed fruits and vegetables. J Sci Food Agr, 84( 10): 1253–9. [Google Scholar]
- 24. Krol WJ, Arsenault TL, Pylypiw HM, Incorvia Mattina MJ. (2000). Reduction of pesticide residues on produce by rinsing. J Agric Food Chem, 48( 10): 4666–70. [DOI] [PubMed] [Google Scholar]
- 25. Xia H, Ma X. (2006). Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresour Technol, 97( 8): 1050–4. [DOI] [PubMed] [Google Scholar]
- 26. Nguyen TD, Yun MY, Lee GH. (2009). Multiresidue Method for the Determination of 118 Pesticides in Vegetable Juice by Gas Chromatography− Mass Spectrometry and Liquid Chromatography− Tandem Mass Spectrometry. J Agric Food Chem, 57( 21): 10095–101. [DOI] [PubMed] [Google Scholar]
- 27. Nasr HM, Abbassy MA, Marzouk MA, Mansy AS. Pollution Effects & Control. 2014.
- 28. Okihashi M, Kitagawa Y, Akutsu K, Obana H, Tanaka Y. (2005). Rapid method for the determination of 180 pesticide residues in foods by gas chromatography/mass spectrometry and flame photometric detection. J Pest Sci, 30( 4): 368–77. [Google Scholar]
- 29. Kaushik G, Satya S, Naik S. (2009). Food processing a tool to pesticide residue dissipation–A review. Food Res Int, 42( 1): 26–40. [Google Scholar]
- 30. Barceló D. (1997). Trace Determination of Pesticides and their Degradation Products in Water. Elsevier. [Google Scholar]
- 31. Ohkawa H, Miyagawa H, Lee PW. (2007). Pesticide chemistry: crop protection, public health, environmental safety. John Wiley & Sons. [Google Scholar]
- 32. Abou-Arab A. (1999). Behavior of pesticides in tomatoes during commercial and home preparation. Food Chem, 65( 4): 509–14. [Google Scholar]