Abstract
Urbanization causes both changes in community composition and evolutionary responses, but most studies focus on these responses in isolation. We performed an integrated analysis assessing the relative contribution of intra- and interspecific trait turnover to the observed change in zooplankton community body size in 83 cladoceran communities along urbanization gradients quantified at seven spatial scales (50–3200 m radii). We also performed a quantitative genetic analysis on 12 Daphnia magna populations along the same urbanization gradient. Body size in zooplankton communities generally declined with increasing urbanization, but the opposite was observed for communities dominated by large species. The contribution of intraspecific trait variation to community body size turnover with urbanization strongly varied with the spatial scale considered, and was highest for communities dominated by large cladoceran species and at intermediate spatial scales. Genotypic size at maturity was smaller for urban than for rural D. magna populations and for animals cultured at 24°C compared with 20°C. While local genetic adaptation likely contributed to the persistence of D. magna in the urban heat islands, buffering for the phenotypic shift to larger body sizes with increasing urbanization, community body size turnover was mainly driven by non-genetic intraspecific trait change.
This article is part of the themed issue ‘Human influences on evolution, and the ecological and societal consequences’.
Keywords: Anthropocene, body size, Daphnia magna, eco-evolutionary partitioning metrics, intraspecific trait variation, urbanization
1. Introduction
In the past decade, a growing number of empirical studies have provided evidence that micro-evolutionary change can occur at the same temporal scale as ecological change and, therefore, can influence ecological dynamics [1]. The importance of eco-evolutionary feedbacks in biological responses to changing environments caused by, for example, interactions with antagonists (e.g. [2]), or by human disturbance, such as agriculture [3] and climate change (e.g. [4]) led to the development of eco-evolutionary partitioning metrics. These metrics quantify the relative contribution of evolutionary and ecological processes to trait change and have revealed that effect sizes of evolutionary change can be equal to or even higher than those of ecological change [2,5,6]. Most eco-evolutionary studies, however, focus on population level dynamics (e.g. [7,8]) and only a few studies quantify the influence of evolutionary change on community structure [6,9,10] beyond specific predator–prey or host–parasite interactions (e.g. [2]). In addition, most community-level studies focus on intraspecific phenotypic variation [11,12] rather than also quantifying the genotypic component to trait change.
Urbanization has been shown to elicit both ecological and evolutionary responses in different organism groups, impacting biodiversity patterns and ecosystem functioning [13,14]. At the community level, a significant decrease in mammal, reptile, amphibian, invertebrate and plant species richness has been observed in highly urbanized areas [14]. At the level of individual populations, a broad range of adaptive behavioural, morphological and physiological responses of urban populations have been reported (reviewed in [15]). For example, the plant species Crepis sancta shows a reduced settling success of dispersing seed types and a significant increase in non-dispersing seed types in urban areas associated with increased habitat fragmentation [16,17]. Tüzün et al. [18] recently reported altered behavioural responses to a pesticide in replicated urban populations of the damselfly Coenagrion puella, suggesting local adaptation to higher pesticide concentrations in urban environments. There is a strong need to integrate evolutionary and ecological responses to urbanization [19]. This integration should not only include an assessment of the importance of intraspecific trait variation (ITV; i.e. within species trait variation among environments that is a result of either phenotypic plasticity, ontogenetic shifts or genetic adaptation) relative to species trait turnover (SPT; i.e. trait change caused by the change in relative abundances of species; it thus reflects trait change caused by interspecific trait variation) along the urbanization gradient, but should also clarify how much of ITV is the result of genetic responses compared with environmental modulation through phenotypic plasticity or ontogenetic change. In addition, there is a need to account for scale effects of urbanization.
Because human-dominated landscapes generate intense and novel selection pressures on very short spatial and temporal scales, urban areas provide a powerful system to monitor evolutionary trait change and the reciprocal interplay with ecological dynamics across taxa [19–22]. Cities differ from rural areas in hydrology, atmospheric chemistry, microclimate, nutrient concentrations, land use and vegetation cover [23–25]. Urban areas are characterized by higher eutrophication, soil and water contamination by organic pollutants and heavy metals, noise and light pollution, and usually have a low overall habitat quality and connectivity [26,27]. One of the striking environmental changes linked to urbanization is the significant increase in ambient surface temperature compared with rural surroundings (‘urban heat island effect’, UHI; [28,29]). These have also been studied in detail in the northern provinces of Belgium (Flanders) during the past decade [30]. The average difference in summer mean surface temperature between cities and rural areas in Flanders ranged between +1.4°C and +4.5°C (with extreme observations up to 8°C differences in summer day temperature) and was positively correlated with the level of impervious surfaces.
As a result of the increased average and extreme temperatures, we expect that urban dwellers will show a reduced body size in many organism groups to better optimize physiological oxygen transport and respiration processes (‘temperature–size rule’; [31]). Body size is a key trait in freshwater zooplankton, as it is a strong determinant of SPT along environmental gradients (e.g. fish predation pressure; [32,33]) as well as of performance in competition and top-down control of algae [34,35]. The presence of large zooplankton species, such as the water flea Daphnia magna, can prevent shifts from the clear-water to the turbid stable state in ponds and shallow lakes [36]. Oxygen limitation can, however, be severe in aquatic systems (e.g. [37]). It is currently not known to what extent urban zooplankton populations show a reduced body size compared with rural populations, to what extent patterns in body size variation run parallel for intra- and interspecific trait turnover, and to what degree changes in body size along urbanization gradients also have a genetic component.
We here performed a partitioning analysis to better understand the underlying processes leading to variation in body size in zooplankton communities along strong urbanization gradients using the variation partitioning method developed by Lepš et al. [38] and adapted by Kichenin et al. [39] and Lajoie & Vellend [12]. This method enables us to assess the relative importance of ITV versus SPT to community body size turnover along the urbanization gradient. Moreover, we adapted this method to integrate both genetic and non-genetic ITV, which constitutes a novel approach towards disentangling genotypic trait variation (GTV) from other ITV components and SPT. To do so, we combined zooplankton community field data and common garden experimental data on one focal species, D. magna. Our overall aim was to disentangle the underlying mechanisms shaping community trait–environment tracking along urbanization gradients. Environmental filtering is expected to lead to community body size turnover along the urbanization gradient, and we here want to assess to what extent this is caused by intra- versus interspecific trait variation. Our specific hypotheses are (i) that ITV contributes significantly to the observed community trait turnover, (ii) that the patterns in intra- and interspecific body size variation covary along the urbanization gradient, and (iii) that evolutionary processes (i.e. genetic change) contribute significantly to intraspecific and overall community trait turnover in this system. Given the importance of body size for top-down control of algae, community body size turnover with increasing urbanization resulting in an increased dominance of small zooplankton species or an evolutionary reduction in body size in dominant species could negatively affect community grazing efficiency and thereby water quality of urban ponds.
2. Material and methods
A detailed description of Material and Methods can be found in electronic supplementary material, A.
(a). Data acquisition
(i). Habitat selection
A total of 84 permant shallow ponds were sampled in Flanders (Belgium; latitude 51°00′ 00″ N, longitude 4° 30′00″ E, period May–July 2013; sampling locations are given in electronic supplementary material, figure SA1) involving gradients in urbanization along the cities of Antwerpen, Brussel, Gent and Leuven. Urbanization, assessed as percentage built-up area in 200 × 200 m and 3 × 3 km plots (%BA, i.e. surface area taken by buildings, houses and industrial infrastructure, excluding roads and parking lots) is considered high from 10% BA upwards. Ponds represented approximately equal number of estimated low (rural), intermediate and high urbanized ponds at both spatial scales. %BA was later quantified more precisely at seven separate radii around each pond (50, 100, 200, 400, 800, 1600 and 3200 m), allowing possible scale-dependent effects (e.g. a local ‘park cooling’ effect versus the more regional ‘urban heat island effect’) to be disentangled. After sampling, loggers were installed in two urban and two rural ponds, to monitor water temperature on a daily basis throughout the year (2013–2014).
(ii). Zooplankton community composition and intraspecific trait variation for body size
From each pond, a 20–40 l zooplankton sample (filtered over 64 µm) was taken using a tube sampler. At least 300 individuals were identified in each sample [40,41] of which body size was measured on 15 individuals of each species present in the sample. Densities were expressed as individuals per litre. For the focal species D. magna, an additional set of 15 adults was measured to calculate the average adult body size. A list of species present across all ponds and their mean body size are given in electronic supplementary material, table SA1; the number of species in each pond is given in electronic supplementary material, table SA2.
(iii). Genetic variation and phenotypic plasticity in body size in Daphnia magna
We exposed 12 D. magna populations inhabiting ponds along the urbanization gradient (%BA at a radius of 3200 m) to 20°C and 24°C in a common garden experiment to mimic the temperature gradient along the urbanization gradient (with 20°C reflecting the average July maximum day temperature in rural ponds observed over a two-week period; see electronic supplementary material, table SA3, figure SA2). A total of 432 lines (12 populations × 6 lineages × 2 temperatures × 3 replicates) were grown in the laboratory under standardized laboratory conditions (water baths at 20 ± 0.6°C, 14 L : 10 D photoperiod, dechlorinated tap water, fed 1 × 105 cells ml−1 of the green algae Acutodesmus obliquus (strain number: CCAP 276/3A, formerly known as Scenedesmus obliquus) daily, and 80% medium refreshment every other day) to obviate interference from the source habitat conditions through (grand) maternal effects. Twelve neonates (less than 24 h old, second to fourth clutch) were transferred to 500 ml jars placed in temperature controlled water baths (20 ± 0.6°C or 24 ± 0.4°C), with the same photoperiod, feeding and medium refreshment regimes as described above. Size at maturity was scored at the stage of first adult instar (four individuals per cohort). We checked genotypic uniqueness of all lineages by screening variation at 27 microsatellite markers [42]. Lineages identical at all loci were considered to belong to the same clone and their data were pooled. Pond location, urbanization level and the number of clones used in our experiment are given in electronic supplementary material, table SA4.
(b). Statistical analysis
(i). Data
Exploratory analyses indicated different patterns of the cladoceran community body size change depending on whether the communities were dominated by small- or large-bodied taxa (see §3). We divided the data into two subsets. The large-species dominated community subset (n = 34, large species having an average body size > 1 mm, see e.g. [32]; species included: D. magna, D. obtusa, D. pulex and the chydorid Eurycercus lamellatus), involving communities having more than 5% of large species in terms of abundances (a 5% cut-off value is inspired by the fact that their body size is large, so that in terms of biomass their contribution is more substantial), and the small-species dominated community subset (n = 50; all other ponds). All analyses on communities presented below were done on all three datasets (i.e. the full dataset and the two subsets). For the analyses involving genetic variation in D. magna, only the large-species subset was used as community dataset. Data analyses were conducted with the R software v. 3.2.3 for Windows [43]. Outliers and influential data points were detected using the Cook's distance, the outlier test function (‘car’ package, [43]), and visual screening by plotting the model residuals versus leverage and plotting all the data points. The justification for outlier removal as well as the results of analyses without outlier removal are described in the electronic supplementary material, B.
(ii). Quantifying the relative importance of intraspecific trait variation and interspecific trait turnover on the change in cladoceran community body size
We calculated the local community body size for a community j first as the abundance-weighted average of the local body sizes of all species present in the local community (i.e. 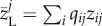 with qij the relative abundance and zij the local average body size value of species i of community j). Second, we calculated it as the abundance-weighted average using the metacommunity-wide average body size of all species (i.e. the average body size of a species across all communities where it is present;
with qij the relative abundance and zij the local average body size value of species i of community j). Second, we calculated it as the abundance-weighted average using the metacommunity-wide average body size of all species (i.e. the average body size of a species across all communities where it is present; 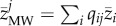 with qij the relative abundance of a species i of community j and
with qij the relative abundance of a species i of community j and 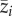 the metacommunity-wide average body size value of species i across the communities where it is present).
the metacommunity-wide average body size value of species i across the communities where it is present).
We partitioned the variation explained by ITV and SPT to the total trait variation along the urbanization gradients using the method described by Lajoie & Vellend [12] (figure 1). We built three regression models. In the SPT + ITV model, the abundance-weighted average community body sizes using the local species body size distributions for each community was regressed against percentage built-up area, reflecting the effect of both ITV of each species and species turnover along the gradient. In the SPT model, the abundance-weighted average community body sizes using the metacommunity-wide species body sizes were regressed against percentage built-up area, accounting for the changes in the relative abundances of species and species replacements along the gradient. Determining the effect of ITV on community trait turnover along the gradient was then done by subtracting the SPT model from the SPT + ITV model, i.e. 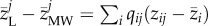 . We evaluated all models at all seven spatial scales and for the three different sets of communities (i.e. the full dataset and the two subsets). Based on the Akaike information criterion (AIC), we log-transformed percentage built-up area for all models. To better meet the assumption of normality for the regression analyses, the abundance-weighted average community body sizes were also log-transformed. One extreme outlier was detected in the ITV model and removed from the full dataset (n = 83; see electronic supplementary material, figure SA3). For the subset of communities dominated by large species two outliers were removed (n = 32, see electronic supplementary material, figure SA3), and for the subset of communities dominated by small species one outlier was removed (n = 49; see electronic supplementary material, figure SA3). Bootstrap analyses were performed to assess variation in our estimates of the contribution of ITV and SPT (figure 2; solid line). The variance in community weighted means of body size attributable to SPT and to ITV was assessed as the ratio of the regression sum of squares of the SPT model (SSRSPT) or the ITV model (SSRITV) over the total sum of squares of the model including both SPT and ITV (SSTSPT+ITV). This quantifies the contribution of SPT and ITV, respectively, to the total explained variance in community average body size along the urbanization gradient. The proportional variation explained by ITV was determined using the formula: SSRITV/(SSRITV + SSRSPT) [12].
. We evaluated all models at all seven spatial scales and for the three different sets of communities (i.e. the full dataset and the two subsets). Based on the Akaike information criterion (AIC), we log-transformed percentage built-up area for all models. To better meet the assumption of normality for the regression analyses, the abundance-weighted average community body sizes were also log-transformed. One extreme outlier was detected in the ITV model and removed from the full dataset (n = 83; see electronic supplementary material, figure SA3). For the subset of communities dominated by large species two outliers were removed (n = 32, see electronic supplementary material, figure SA3), and for the subset of communities dominated by small species one outlier was removed (n = 49; see electronic supplementary material, figure SA3). Bootstrap analyses were performed to assess variation in our estimates of the contribution of ITV and SPT (figure 2; solid line). The variance in community weighted means of body size attributable to SPT and to ITV was assessed as the ratio of the regression sum of squares of the SPT model (SSRSPT) or the ITV model (SSRITV) over the total sum of squares of the model including both SPT and ITV (SSTSPT+ITV). This quantifies the contribution of SPT and ITV, respectively, to the total explained variance in community average body size along the urbanization gradient. The proportional variation explained by ITV was determined using the formula: SSRITV/(SSRITV + SSRSPT) [12].
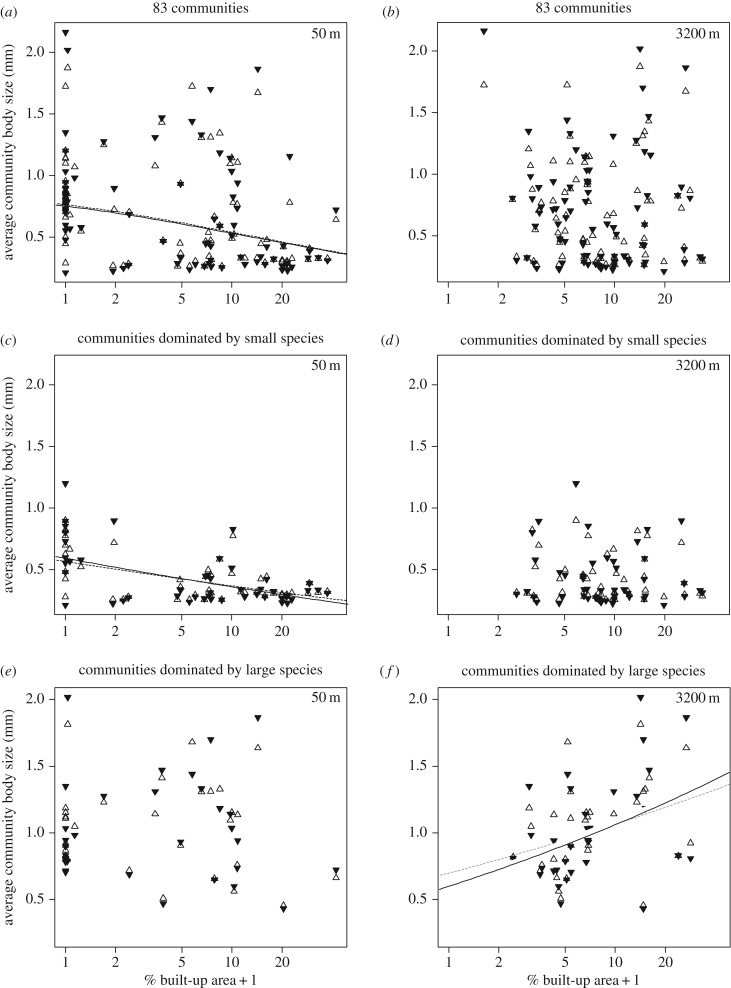
Figure 1.
Relationships between body size variation and percentage built-up area (%BA + 1, plotted on a log-scale) among the 83 communities (a,b), the subset of communities dominated by small species (n = 49; c,d) and the subset of communities dominated by large species (n = 32; e,f), where average community body size is calculated using the local trait values of the species (filled triangles) and using the metacommunity-wide species trait values (unfilled triangles). Results are given for urbanization assessed at the local (50 m; a,c,e) and regional (3200 m; b,d,f) spatial scale. Significant relationships (p < 0.05) between body size variation and percentage built-up area for the community body sizes calculated using the local and metacommunity-wide species trait values are shown by black solid and dashed lines, respectively. p < 0.1 are shown in grey lines. p-values are given in electronic supplementary material, table SA5.
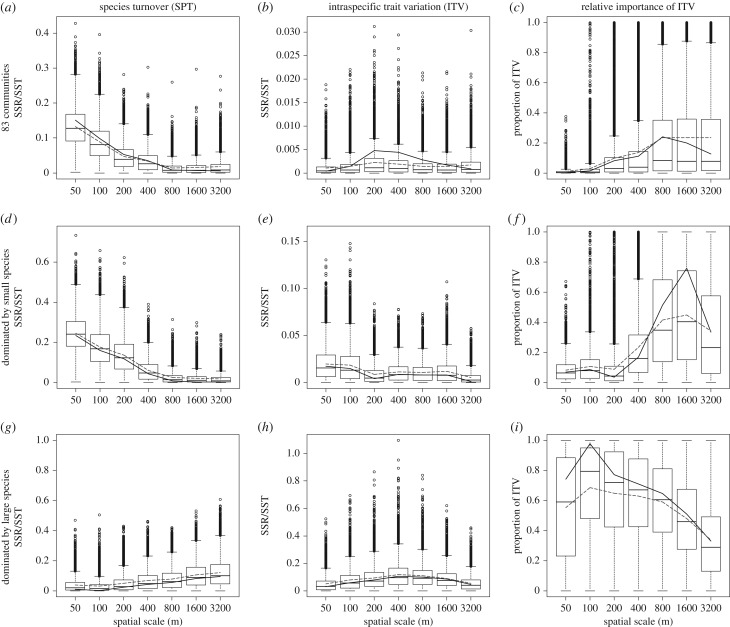
Figure 2.
Bootstrap results of partitioning intraspecific and interspecific sources of body size variation along the urbanization gradient as quantified at different spatial scales for the total set of 83 communities (a–c), the subset of communities dominated by small species (n = 49; d–f) and the subset of communities dominated by large species (n = 32; g–i). The black solid line represents the variability of the total model explained by species turnover (SPT; a,d,g) or intraspecific trait variation (ITV; b,e,h) of the observed data sample and is calculated by dividing the regression sum of squares of the SPT model (SSRSPT) or the ITV model (SSRITV) by the total sum of squares (SST) of the SPT + ITV model. The relative explanatory power of ITV to community body size turnover along the urbanization gradient is calculated as SSRITV/(SSRITV + SSRSPT) and is for the observed data sample given by the black solid line (c,f,i). The dashed lines represent the average of 10 000 bootstrap samples. Box plots show the distributions of the bootstrap estimates.
(iii). Genetic variation and phenotypic plasticity in body size in Daphnia magna
We tested for the effect of urbanization and temperature exposure on genotypic values (this term is clarified in electronic supplementary material, Box SA1) of D. magna size at maturity with clone (nested in population) and population included as random effects in our linear mixed-effect model (using the ‘lme4’ and ‘car’ packages in R to compute approximate F-test statistics and p-values for fixed effects; [43,44]). Built-up area was log-transformed. We applied the restricted maximum-likelihood estimation method (REML), and corrected the degrees of freedom for fixed effects using the Kenward–Roger approximation. Tests of normality were performed and assumptions were met (electronic supplementary material, A, Material and Methods). Significance of random effects was tested by model comparison (Wald's χ2 and p-values computed using the ‘car’ package; [43,45]).
(iv). The relative contribution of genotypic trait variation, non-genetic intraspecific trait variation and interspecific trait turnover to the change in cladoceran community body size
To disentangle the relative contribution of genotypic trait variation (GTV) and non-genetic trait variation (i.e. phenotypic plasticity or ontogenetic shifts; ITVPLASTICITY/OTHER) to the change in average community body size along the urbanization gradients, we modified the method of Lajoie & Vellend [12] and included the genotypic trait values for 12 D. magna populations at 20°C and 24°C. Of the 10 communities containing D. magna in the community dataset, seven communities were shared between both datasets. For the other three D. magna communities, genotypic trait values were estimated using the regression function from the common garden experiment (electronic supplementary material, A). We determined the contributions of genotypic and non-genetic trait variation along the urbanization gradient, by rewriting the phenotypic trait value zi for a species i as the sum of its genotypic trait value (GTV; 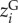 ), its plasticity response to temperature (ITVPLAST-T;
), its plasticity response to temperature (ITVPLAST-T; 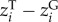 ) and its plasticity response to other environmental conditions present in the field or ontogenetic changes (ITVOTHER;
) and its plasticity response to other environmental conditions present in the field or ontogenetic changes (ITVOTHER; 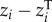 ), i.e.
), i.e.
| 2.1 |
with 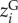 the genotypic trait value calculated as the average of the trait values in the 20°C and 24°C treatments, and
the genotypic trait value calculated as the average of the trait values in the 20°C and 24°C treatments, and 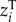 the estimated effect of plasticity in response to temperature (T), based on an expected relationship between percentage of built-up area and temperature (detailed in electronic supplementary material, A). Substituting equation (2.1) into the ITV model of Lajoie & Vellend [12] results for each community j consisting of sj species in
the estimated effect of plasticity in response to temperature (T), based on an expected relationship between percentage of built-up area and temperature (detailed in electronic supplementary material, A). Substituting equation (2.1) into the ITV model of Lajoie & Vellend [12] results for each community j consisting of sj species in
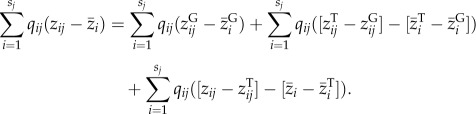 |
2.2 |
To calculate the abundance-weighted average community body size for these 10 communities, we used the genotypic trait values of D. magna, combined with the metacommunity-wide body sizes for all other species present in the local communities. As a result, the effects of ITV and GTV only reflect variation in the focal species D. magna. To determine the contribution of GTV, temperature-related phenotypic plasticity and phenotypic variation due to other environmental conditions or demographic population structure along the urbanization gradient, a regression analysis was performed on each of the three terms in the right-hand side of equation (2.2). To determine the proportional contribution of each trait component (GTV, ITVPLAST-T, ITVOTHER or SPT) a similar formula was used as in Lajoie & Vellend [12]. Two communities identified as outliers were excluded from this analyis; the results including these communities are presented in electronic supplementary material, B.
3. Results
(a). Intraspecific trait variation versus interspecific trait turnover
For the set of 83 communities (one outlier community removed, figure SA3 shows plots with outliers included), we found a significant negative relation between the abundance-weighted average community body size and percentage built-up area, but only when we quantified built-up area at small spatial scales (50 m, β1 = −0.077, p < 0.001, figure 1a; 100 m, β1 = −0.059, p = 0.006; other spatial scales p > 0.05; electronic supplementary material, table SA5 and figure SA4). We observed a decrease in the contribution of variation explained by SPT to the total variation in community body size along the urbanization gradient with increasing spatial scale at which urbanization was determined (figure 2a, electronic supplementary material, table SA5). We observed a slight increase in the contribution of variation explained by ITV at larger spatial scales, with highest contributions at the 200–800 m scales (figure 2b, electronic supplementary material, table SA5). The relative importance of ITV versus SPT increased with spatial scale, but the impact of ITV was not significant (figure 2c, electronic supplementary material, table SA5).
For the set of communities dominated by small species, a similar significant negative relation between abundance-weighted average community body size and percentage built-up area was found at smaller spatial scales (50 m, β1 = −0.068, p < 0.001, figure 1c; 100 m, β1 = −0.057, p < 0.001; 200 m, β1 = −0.051, p = 0.007; other spatial scales p > 0.05; electronic supplementary material, table SA5 and figure SA4). SPT accounted for 23.34% to 11.76% of the total variation in average community body size along the urbanization gradient at small spatial scales, but declined rapidly to 0.21% at the largest spatial scale (figure 2d; electronic supplementary material, table SA5). There is a tendency for ITV to explain a larger portion of the observed average community body size turnover along the urbanization gradient at smaller spatial scales (but marginally non-significant, even at the 50 m scale; 1.73%, p = 0.052; figure 2e; electronic supplementary material, table SA5). The relative importance of ITV to the combined contribution to variation explained by ITV and SPT accounted for 6.91% at this scale and increased with increasing spatial scale.
For the set of communities dominated by large species, we observed a very different pattern, with an increase in average community body size with percentage built-up area, which was only significant when built-up area is assessed at the larger spatial scales (400 m, β1 = 0.057, p = 0.046; 800 m, β1 = 0.066, p = 0.034; 1600 m, β1 = 0.085, p = 0.020; 3200 m, β1 = 0.110, p = 0.024, figure 1f; scales below 400 m p > 0.05; electronic supplementary material, table SA5 and figure SA4). For this subset of communities, ITV explained a significant portion (8.48–11.63%) of the turnover in observed average community body size along the urbanization gradient at larger spatial scales (400–1600 m; figure 2h; electronic supplementary material, table SA5). At all spatial scales except 3200 m, ITV explained more variation along the urbanization gradient than SPT.
(b). Evolution and phenotypic plasticity of body size in Daphnia magna
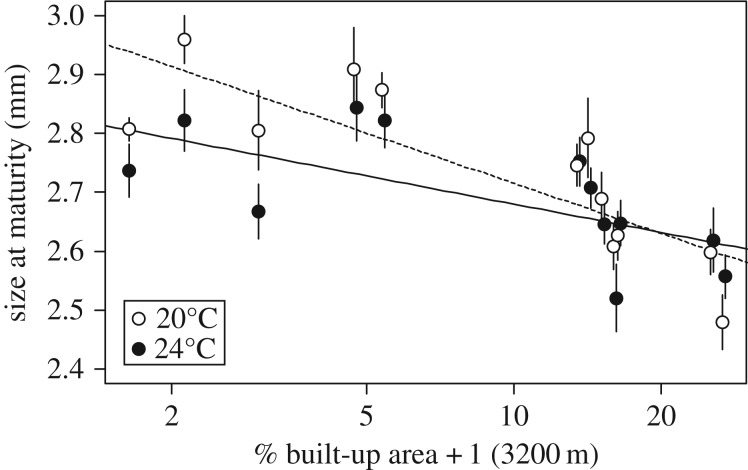
Genotypic trait values for size at maturity of D. magna populations decreased with increasing levels of urbanization (β1 = −0.123 ± 0.027 s.e., F1/8 = 14.730, p = 0.004; figure 3). A significant temperature effect was found, with individuals cultured at 24°C having an average smaller size at maturity (F1/273 = 6.432, p = 0.012) than individuals cultured at 20°C. In addition, there was a significant interaction effect between temperature and built-up area (β1= 0.054 ± 0.019 s.e., F1/273 = 8.221, p = 0.004; figure 3). Some of the more urban populations showed a similar to even larger size at maturity at 24°C compared with 20°C, while rural populations showed a clear reduction in size at maturity at 24°C (figure 3). Moreover, the difference in size at maturity between 20°C and 24°C was larger in rural than in urban populations. Model comparison showed a significant effect of clone (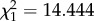 , p < 0.001) and population (
, p < 0.001) and population (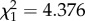 , p = 0.036), which were included as random factors in the model. Clonal temperature reaction norms for each population along the urbanization gradient are shown in electronic supplementary material, figure SA5).
, p = 0.036), which were included as random factors in the model. Clonal temperature reaction norms for each population along the urbanization gradient are shown in electronic supplementary material, figure SA5).
Figure 3.
Regression of average population genotypic values of size at maturity (mm) ±1 s.e. of 12 D. magna populations along percentage built-up area (%BA +1, assessed at the 3200 m scale, plotted on a log-scale). Empty symbols and the dotted line denote size at maturity at 20°C, while filled symbols and the solid line refer to size at maturity at 24°C as determined in a common garden experiment using two to six clones per population.
(c). The relative contribution of genotypic trait variation, non-genetic intraspecific trait variation and interspecific trait turnover
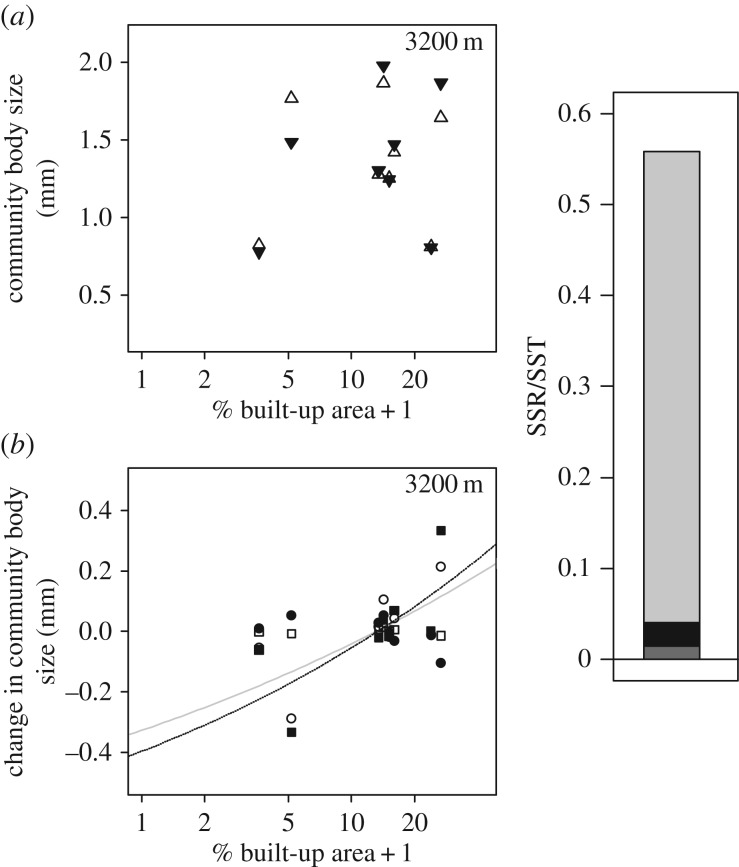
For communities in which D. magna was present, a tendency towards an increased average community body size was detected with increasing levels of urbanization (figure 4a, 3200 m scale), similar to the pattern found for the total set of communities dominated by large species. Owing to the low number of communities, however, the full model was not significant (SPT + ITV, p = 0.423, electronic supplementary material, table SA6; for results without outlier removal, see electronic supplementary material, table SB5 and figure SB8). ITV of D. magna explained 32.43% of the total observed trait turnover along the urbanization gradient (marginally non-significant, p = 0.056, electronic supplementary material, table SA6). ITV was clearly the larger contributor, explaining 95.78% of the total variation explained by both ITV and SPT together. Including genotypic trait values at 20°C and 24°C in the analysis enabled us to disentangle three types of ITV along the urbanization gradient: GTV, non-genetic phenotypic trait variation due to increased temperatures (ITVPLAST-T) and non-genetic phenotypic trait variation due to increased temperatures (ITVOTHER; figure 4b). Only the latter component showed a significant contribution to the observed increase in average community body size (electronic supplementary material, table SA6, p = 0.047). ITVOTHER explained 92.89% of the total explained variation by all trait components, while GTV and SPT contributed 4.55% and 2.56%, respectively. Regression analysis on the difference in D. magna body size measurements on all individuals (i.e. including juveniles) versus adults only plotted against degree of urbanization, suggests that the large impact of ITVOTHER might be due to a change in relative abundance of juveniles along the urbanization gradient (β1 = −0.048, p = 0.032; electronic supplementary material, figure SA6).
Figure 4.
(a) Relationship between average community body size variation and percentage built-up area (%BA + 1, 3200 m, plotted on a log-scale) of eight D. magna communities (two outliers were removed, PL25-red and TP-Blap1-riv similar to the general analysis of all large-species dominated communities). Community average body size is calculated using the local trait values of D. magna (filled triangles; note: for all other species we used community-wide average body size values) or the community-wide average D. magna body size (unfilled triangles). (b) Relationships between intraspecific trait variation (ITV, unfilled circles, grey solid line, p < 0.1), genetic trait variation (GTV, filled circles), plasticity response to temperature (ITVPLAST-T, unfilled squares), and plasticity response to other cues (ITVOTHER, filled squares, black dotted line, p < 0.05) with percentage built-up area (%BA + 1, 3200 m, plotted on a log-scale). p-Values are given in electronic supplementary material, table SA6. (c) Visualization of the relative importance of SPT (dark grey), GTV (black), ITVPLAST-T (not visible due to its small value) and ITVOTHER (light grey) to the total amount of variation in community body size along the urbanization gradient explained by all components of ITV and SPT.
4. Discussion
We observed an overall decrease in average community body size of cladoceran communities in response to urbanization. The overall pattern as well as the relative contribution of intra- and interspecific trait variation to this pattern depended, however, strongly on the spatial scale at which urbanization was quantified and whether or not the communities were dominated by large species. For one of these large species, D. magna, a common garden experiment revealed a significant genetic decline in size at maturity with increasing levels of urbanization around the ponds. In addition, the animals matured at a smaller size when reared at 24°C compared with 20°C. Disentangling the effects of all intraspecific trait components (i.e. GTV, plasticity in response to temperature, plasticity related to other environmental variables and ontogenetic shifts) revealed that the community trait change along the urbanization gradient was mainly driven by intraspecific trait shifts not related to genotype or temperature.
In our dataset of 83 ponds, the average cladoceran community body size declined with increasing urbanization, which is in line with our expectations given that urban areas represent heat islands [28,30] and that higher temperatures favour smaller body sizes [46,47]. Our pilot temperature logging study of ponds along the urbanization gradient offers the first additional evidence of aquatic ecoystems being impacted by the urban heat island effect as the average summer (July–August) maximum water temperature of 2014 differed by 4.03°C between two of the most urban and two of the most rural ponds along the gradient (electronic supplementary material, figure SA2). The decrease in community body size was only significant for built-up area assessed in the immediate surroundings of the pond (50–200 m radius). This most likely reflects built-up area in the immediate vicinity of the pond being the best predictor of extreme temperatures, while parks and large gardens in the cities experience somewhat cooler temperatures [48–50]. We, however, found that cladoceran communities characterized by a relatively high relative abundance (more than 5%) of large-bodied species displayed a different pattern. Average community body size of these communities increased with increasing built-up area at larger spatial scales (400 m and more). This pattern is opposite to the pattern found for the total set of communities and for the communities dominated by small-bodied species. We can only speculate on why we find an opposite pattern for communities dominated by large-bodied species. One possible explanation might be that urban ponds on the one hand tend to be rich in nutrients [26] while, on the other hand, by being relatively isolated they can be characterized by a reduced presence of zooplanktivorous fish or increased relative importance of small, gape-limited invertebrate predators. These factors would favour large-bodied zooplankton [51,52]. The fact that this pattern is only observed when urbanization is quantified at larger spatial scales (400 m radius or more) reflects regional effects, and is in line with the predator hypothesis. Dispersal barriers can prevent specific predators being present; this would result in urbanization effects acting at a larger scale independently of the presence of built-up area in the immediate vicinity of the study habitats. Ponds in city parks or other urban green infrastructure are indeed still relatively isolated from each other, compared with rural ponds.
We used the variation partitioning method described by Lajoie & Vellend [12] to assess the relative importance of intra- versus interspecific trait variation to the observed community body size turnover along the urbanization gradient. For the total set of communities as well as for the communities dominated by small-bodied species, we found significant contributions of variation explained by SPT and ITV when urbanization was assessed at small spatial scales, with SPT being the more important contributor to explained variation. For the communities dominated by small-bodied species, the relative contribution of ITV to the total body size turnover along the urbanization gradient increased at larger spatial scales (400 m and more), but at these scales the regression models were non-significant. For communities dominated by large-bodied species, the contributions of SPT and ITV to body size turnover along the urbanization gradient were of the same order of magnitude at larger spatial scales (ITV accounted for 51% of the total amount of explained variation at 1600 m and 33% at 3200 m). The contribution of ITV, however, became even larger at intermediate (71% at 400 m and 65% at 800 m; ITV models significant) and smaller spatial scales (even up to 98% at 100 m).
For the large-bodied water flea D. magna, we quantified genetic variation in size at maturity in a common garden experiment. As predicted, we found that body size declined with increasing levels of urbanization. This result is in line with other studies that provided evidence for reductions in body size with increasing urbanization in carabid species [53] and a few other terrestrial organisms, such as house sparrows and tropical frogs (Passer domesticus [54]; Dendropsophus ebraccatus [55]). The opposite pattern of increased body size with higher levels of urbanization has, however, been reported for the orb weaving spider Nephila plumipes [56]. Whereas these former studies focused on patterns of phenotypic trait variation, our study is the first to show a genetic based reduction in body size along rural–urban gradients in aquatic ecosystems. We also observed a significant phenotypic plasticity response to temperature, with D. magna individuals maturing at a smaller size when reared at 24°C compared with 20°C. This observation is in line with expectations based on the urban heat island effect and oxygen constraints [31]. Especially in aquatic organisms, a reduction in body size with increasing temperatures can be necessary, as oxygen is limited in supply in aquatic systems and its solubility in water decreases while organismal metabolic rates increase with increasing temperature [37]. The temperature loggers in the two urban and two rural ponds yielded an average temperature difference during the summer months (July–August) of approximately 4.03°C (see electronic supplementary material, figure SA2). Assuming an urban heat island effect of approximately 4°C, the combined effect of genetic based change and phenotypic plasticity would result in a total reduction in size at maturity of almost 10% (from 2.87 mm to 2.60 mm) in urban compared with rural populations. Finally, we also observed a genotype by environment interaction between population (different populations along the urbanization gradient) and phenotypic plasticity in response to rearing temperature. This likely reflects a pattern of local adaptation through the evolution of phenotypic plasticity. More specifically, while the urban populations show a clear pattern of reduced mean size at maturity compared with rural populations, their phenotypic plasticity in size at maturity in response to increased temperature is much less strong. Our study adds to the evidence of pronounced genetic differentiation and local adaptation of natural Daphnia populations over short time periods and at microgeographic scales in relation to natural and human-induced environmental changes [57,58]. We also provide evidence of specific urbanization-driven evolutionary change, as has been reported for a few other species [18,59].
Modifying the variation partitioning method used in Lajoie & Vellend [12] enabled us to integrate all three levels of trait variation (individual plasticity, genotype-dependent trait values and species-specific trait values) that shape eco-evolutionary dynamics along environmental gradients [11]. Assessing the contribution of GTV, phenotypic plasticity in response to a given environmental condition (here temperature; ITVPLAST-T) and a rest component of ITV (reflecting phenotypic plasticity to unmeasured environmental conditions or ontogenetic variation linked to individual growth and development; ITVOTHER) to the observed community body size turnover, led to three important conclusions. First, most variation in average community body size along the studied urbanization gradient was due to ITV rather then SPT. Second, among the components of ITV, the largest contributor was the component linked to phenotypic plasticity induced by other environmental variables than temperature and those reflecting ontogenetic growth. The latter explanation is more likely as our measurements of body size in the field samples involved randomly sampled individuals and thus were not limited to adults. There is a large difference in body size between neonate (approx. 0.6–0.7 mm) and adult (up to 5 mm) D. magna [60], so that differences in longevity, age distribution and the relative abundance of adults and juveniles can have an important impact on average community body size. Third, we found an opposite pattern of increasing body size with urbanization in field collected D. magna compared with the decreasing genotypic values of body size with increasing levels of urbanization in the same species. This provides evidence for phenotypic and genotypic countergradient variation for body size [61] along an urbanization gradient in this species, and would lead to an idiosyncratic pattern of the observed ITV along the urbanization gradient [62]. We note that the phenotypic trait shift observed in the field likely reflects an ontogenetic shift and is not due to phenotypic plasticity in response to temperature, as this latter response positively covaried with the genetic differences. We speculate that the countergradient genetic shift to smaller size at maturity in urbanized areas is important for survival of the D. magna populations in urban ponds. This genetic response may allow survival at higher temperatures in an environment that simultaneously leads to larger individuals by enabling longer lifespans (e.g. due to reduced predation) in this species that is characterized by indeterminate growth. While genetic changes only contributed less than 5% to the total change in community body size along the urbanization gradient, evolution still accounted for double the percentage of variation explained by SPT. Our analysis also reveals a case of hidden evolution, i.e. evolution obscured by ecological change as measured in the field. This reflects the complex interplay between environmental, ecological, phenotypic and genotypic processes, often leading to cryptic eco-evo dynamics [63].
Zooplankton are key grazers of phytoplankton in ponds and lakes [64]. Small-bodied zooplankton are less efficient grazers than large-bodied species, especially large-bodied Daphnia species [32,34,51]. Recent experimental work with Belgian zooplankton species confirmed that assemblies of large cladoceran species can have an up to sevenfold higher impact on phytoplankton biomass than assemblies of small cladoceran species of the same biomass [35]. The here observed reduction in average body size with increasing urbanization at the level of the community, as well as in terms of genotypic values at the population level and phenotypic plasticity at the level of individual genotypes, may therefore lead to reduced top-down control of phytoplankton, increasing the probability of a shift to the turbid, phytoplankton dominated state [36,65]. Moreover, in combination with the urban heat island effect and an increased number of extreme temperature events, the reduced capacity to control blooms of toxic cyanobacteria such as Microcystis [66] could generate health risks.
(a). Knowledge gaps and future directions
Overall, our results show that ITV can be an important driver of total community body size turnover along strong environmental gradients, in this case urbanization. Depending on the spatial scale at which urbanization was quantified, 50% or more of community turnover in cladoceran body size could be explained by ITV. We also showed that the large-bodied cladoceran D. magna genetically adapts to the degree of urbanization and remains smaller at maturity in ponds in urbanized areas. In real landscapes, this effect on body size is expected to be even more pronounced given that the animals also show phenotypic plasticity for smaller size at maturity with increasing temperatures. Yet, our analysis of the size distribution of D. magna individuals in samples taken from the field shows an increase in body size with increasing urbanization, which might reflect changes in age distribution along the urbanization gradient. In our integrated analysis, it was this phenotypic increase in body size of D. magna with increasing urbanization that was the major determinant of the urbanization-driven change in average community body size. Genotypic trait change contributed relatively little to the overall change in average community body size along the rural–urban gradient. Yet the observed genetic trait shift towards smaller sizes at maturity with increasing urbanization is likely important to the success of D. magna in urban ponds, as the countergradient evolution enables this species to deal with the higher temperatures in settings that support a phenotypic shift to larger individuals. Our integrated analysis revealed striking patterns of body size turnover along urbanization gradients in response to the urban heat island effect, mediated by evolutionary and non-evolutionary ITV as well as by changes in the relative abundances of species. Moreover, we detected a number of surprises and complexities such as the differences in responses between communities dominated by small versus large species, the cryptic eco-evolutionary dynamics, and the fact that ITV likely associated with ontogenetic shifts in one species has a stronger impact on community trait turnover than shifts in species composition. These complexities need to be considered in future studies and can strongly impact predictions of trait shifts in the face of environmental change.
Our extended partitioning approach allows combining field data with detailed quantitative genetic analyses obtained from common garden experiments. It can be applied to any trait and provides a highly valuable tool to study eco-evolutionary dynamics along environmental gradients in time and space. In this study, we integrated genotypic and phenotypic data of one species, but ideally, GTV of all important member species of the community should be included to better grasp the interplay between evolutionary and non-evolutionary drivers of ITV and their relative importance to SPT in explaining community responses to environmental gradients.
Our analyses are limited to one sampling campaign. We acknowledge that including multiple data points through time would likely provide more power to detect the underlying drivers of the observed trait change along the urbanization gradient and correct for seasonal variation. This could help in generating more specific predictions on the causes and consequences of community trait shifts associated with urbanization. Our results indicate that the eco-evolutionary dynamics may have important consequences for ecosystem functions and ecosystem services provided by urban ponds. Quantifying these ecological and socio-economic consequences of ITV in urban ponds was beyond the scope of the present study, but should be tackled in future work.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Marina Alberti and the editors for providing us with the opportunity to present our work in this special volume. We also thank two anonymous reviewers, Kiyoko Gotonda and Andrew P. Hendry for their thoughtful and constructive comments. We thank Fabio T. Hanashiro and Matthias Vanhamel for their contribution to the fieldwork. We thank Edwin van den Berg for his expert identifications and measuring cladoceran zooplankton in the samples. We thank all partners in the SPEEDY consortium for their help in the overall design and running of the SPEEDY programme on quantifying population and community responses to urbanization.
Data accessibility
Data available from the Dryad Digital Repository, data package title: ‘Eco-evolutionary dynamics in urbanized landscapes: evolution, species sorting and the change in zooplankton body size along urbanization gradients’ ([67], http://dx.doi.org/10.5061/dryad.237hs).
Authors' contributions
L.D.M., C.S., K.I.B., J.M.T.E. and L.G. designed the study. K.I.B., J.M.T.E. and A.T.G. collected the field data. K.I.B. carried out the common garden experiments. K.I.B. and L.G. analysed the data with input of C.S., L.D.M. and A.T.G. C.S. and L.D.M. contributed to trouble shooting. K.I.B. and L.G. wrote the first and revised version of the manuscript, with strong input from L.D.M. and C.S. All authors provided comments on subsequent versions of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
K.I.B. and C.S. are supported by the Fund for Scientific Research-Flanders (FWO Vlaanderen), L.G. and J.M.T.E. are supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT Vlaanderen, grant nos 131454 and 121625), A.T.G. received a doctoral scholarship from Brazil through ‘Science Without Borders—CNPq’ (grant no. 245629/2012-2). We gratefully acknowledge financial support from BELSPO sponsored IAP project SPEEDY and KU Leuven Research Council excellence financing PF/2010/07.
References
- 1.Hendry AP. 2016. Eco-evolutionary dynamics. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Becks L, Ellner SP, Jones LE, Hairston NG. 2012. The functional genomics of an eco-evolutionary feedback loop: linking gene expression, trait evolution, and community dynamics. Ecol. Lett. 15, 492–501. ( 10.1111/j.1461-0248.2012.01763.x) [DOI] [PubMed] [Google Scholar]
- 3.Loeuille N, Barot S, Georgelin E, Kylafis G, Lavigne C. 2013. Chapter 6. Eco-evolutionary dynamics of agricultural networks: implications for sustainable management. Ecol. Netw. Agric. World 49, 339–435. ( 10.1016/B978-0-12-420002-9.00006-8) [DOI] [Google Scholar]
- 4.Van Doorslaer W, et al. 2009. Local adaptation to higher temperatures reduces immigration success of genotypes from a warmer region in the water flea Daphnia. Glob. Change Biol. 15, 3046–3055. ( 10.1111/j.1365-2486.2009.01980.x) [DOI] [Google Scholar]
- 5.Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127. ( 10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 6.Pantel JH, Duvivier C, De Meester L. 2015. Rapid local adaptation mediates zooplankton community assembly in experimental mesocosms. Ecol. Lett. 18, 992–1000. ( 10.1111/ele.12480) [DOI] [PubMed] [Google Scholar]
- 7.Phillips BL, Shine R. 2004. Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proc. Natl Acad. Sci. USA 101, 17 150–17 155. ( 10.1073/pnas.0406440101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U. 2004. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428, 932–935. ( 10.1038/nature02430) [DOI] [PubMed] [Google Scholar]
- 9.Bassar RD, et al. 2010. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc. Natl Acad. Sci. USA 107, 3616–3621. ( 10.1073/pnas.0908023107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews B, Aebischer T, Sullam KE, Lundsgaard-Hansen B, Seehausen O. 2016. Experimental evidence of an eco-evolutionary feedback during adaptive divergence. Curr. Biol. 26, 483–489. ( 10.1016/j.cub.2015.11.070) [DOI] [PubMed] [Google Scholar]
- 11.Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J. 2012. The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252. ( 10.1016/j.tree.2011.11.014) [DOI] [PubMed] [Google Scholar]
- 12.Lajoie G, Vellend M. 2015. Understanding context dependence in the contribution of intraspecific variation to community trait–environment matching. Ecology 96, 2912–2922. ( 10.1890/15-0156.1) [DOI] [PubMed] [Google Scholar]
- 13.Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. 2006 From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191. ( 10.1016/j.tree.2005.11.019) [DOI] [PubMed] [Google Scholar]
- 14.McKinney ML. 2008. Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst. 11, 161–176. ( 10.1007/s11252-007-0045-4) [DOI] [Google Scholar]
- 15.Donihue CM, Lambert MR. 2015. Adaptive evolution in urban ecosystems. Ambio 44, 194–203. ( 10.1007/s13280-014-0547-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheptou P-O, Carrue O, Rouifed S, Cantarel A. 2008. Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta. Proc. Natl Acad. Sci. USA 105, 3796–3799. ( 10.1073/pnas.0708446105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dubois J, Cheptou P-O. 2017. Effects of fragmentation on plant adaptation to urban environments. Phil. Trans. R. Soc. B 372, 20160038 ( 10.1098/rstb.2016.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tüzün N, Debecker S, Op de Beeck L, Stoks R. 2015. Urbanisation shapes behavioural responses to a pesticide. Aquat. Toxicol. 163, 81–88. ( 10.1016/j.aquatox.2015.04.002) [DOI] [PubMed] [Google Scholar]
- 19.Alberti M. 2015. Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol. Evol. 30, 114–126. ( 10.1016/j.tree.2014.11.007) [DOI] [PubMed] [Google Scholar]
- 20.Cheptou P-O, Hargreaves AL, Bonte D, Jacquemyn H. 2017. Adaptation to fragmentation: evolutionary dynamics driven by human influences. Phil. Trans. R. Soc. B 372, 20160037 ( 10.1098/rstb.2016.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendry AP, Gotanda KM, Svensson EI. 2017. Human influences on evolution, and the ecological and societal consequences. Phil. Trans. R. Soc. B 372, 20160028 ( 10.1098/rstb.2016.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lancaster LT, Morrison G, Fitt RN. 2016. Life history trade-offs, the intensity of competition, and coexistence in novel and evolving communities under climate change. Phil. Trans. R. Soc. B 372, 20160046 ( 10.1098/rstb.2016.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberti M, Marzluff JM, Shulenberger E, Bradley G, Ryan C, Zumbrunnen C. 2003. Integrating humans into ecology: opportunities and challenges for studying urban ecosystems. BioScience 53, 1169–1179. ( 10.1641/0006-3568(2003)053%5B1169:ihieoa%5D2.0.co;2) [DOI] [Google Scholar]
- 24.Kowarik I. 2011. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut 159, 1974–1983. ( 10.1016/j.envpol.2011.02.022) [DOI] [PubMed] [Google Scholar]
- 25.Gilbert O. 2012. The ecology of urban habitats. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 26.Kaye JP, Groffman PM, Grimm NB, Baker LA, Pouyat RV. 2006. A distinct urban biogeochemistry? Trends Ecol. Evol. 21, 192–199. ( 10.1016/j.tree.2005.12.006) [DOI] [PubMed] [Google Scholar]
- 27.Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM. 2008. Global change and the ecology of cities. Science 319, 756–760. ( 10.1126/science.1150195) [DOI] [PubMed] [Google Scholar]
- 28.Oke TR. 1973. City size and the urban heat island. Atmos. Environ. 7, 769–779. ( 10.1016/0004-6981(73)90140-6) [DOI] [Google Scholar]
- 29.Arnfield AJ. 2003. Two decades of urban climate research: a review of turbulence, exchanges of energy and water, and the urban heat island. Int. J. Climatol. 23, 1–26. ( 10.1002/joc.859) [DOI] [Google Scholar]
- 30.De Ridder K, Maiheu B, Wouters H, van Lipzig N. 2015. Indicatoren van het stedelijk hitte-eiland in Vlaanderen, studie uitgevoerd in opdracht van de Vlaamse Milieumaatschappij MIRA/2015/05, VITO. See http://www.milieurapport.be/Upload/main/0_Klimaatrapport/2015-05_MIRA_UHI_eindrapport_TW2.pdf. [Google Scholar]
- 31.Atkinson D. 1994. Temperature and organism size: a biological law for ectotherms? Adv. Ecol. Res. 25, 1–58. ( 10.1016/S0065-2504(08)60212-3) [DOI] [Google Scholar]
- 32.Brooks JL, Dodson SI. 1965. Predation, body size, and composition of plankton. Science 150, 28–35. ( 10.1126/science.150.3692.28) [DOI] [PubMed] [Google Scholar]
- 33.Kerfoot W, Sih A. 1987. Predation: direct and indirect impacts on aquatic communities. Hanover, New Hampshire: University Press of New England. [Google Scholar]
- 34.Tessier AJ, Leibold MA, Tsao J. 2000. A fundamental trade-off in resource exploitation by Daphnia and consequences for plankton communities. Ecology 81, 826–841. ( 10.1890/0012-9658(2000)081%5B0826:AFTOIR%5D2.0.CO;2) [DOI] [Google Scholar]
- 35.Gianuca AT, Pantel JH, De Meester L. 2016. Disentangling the effect of body size and phylogenetic distances on zooplankton top-down control of algae. Proc. R. Soc. B 283, 20160487 ( 10.1098/rspb.2016.0487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheffer M, Hosper S, Meijer M, Moss B, Jeppesen E. 1993. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 8, 275–279. ( 10.1016/0169-5347(93)90254-M) [DOI] [PubMed] [Google Scholar]
- 37.Horne CR, Hirst A, Atkinson D. 2015. Temperature-size responses match latitudinal-size clines in arthropods, revealing critical differences between aquatic and terrestrial species. Ecol. Lett. 18 327–335. ( 10.1111/ele.12413) [DOI] [PubMed] [Google Scholar]
- 38.Lepš J, de Bello F, Šmilauer P, Doležal J. 2011. Community trait response to environment: disentangling species turnover vs intraspecific trait variability effects. Ecography, 34, 856–863. ( 10.1111/j.1600-0587.2010.06904.x) [DOI] [Google Scholar]
- 39.Kichenin E, Wardle DA, Peltzer DA, Morse CW, Freschet GT. 2013. Contrasting effects of plant inter- and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 27, 1254–1261. ( 10.1111/1365-2435.12116) [DOI] [Google Scholar]
- 40.Amoros C. 1984. Introduction practique á la systèmatique des organismes des eaux continentales françaises. Crustacès cladocères. Bull. Mens. Soc. Linn. Lyon 53, 72–107, 120–144. [Google Scholar]
- 41.Flössner D. 2000. Die Haplopoda und Cladocera (ohne Bosminidae) Mitteleuropas. Leiden, The Netherlands: Backhuys. [Google Scholar]
- 42.Jansen B, Geldof S, De Meester L, Orsini L. 2011. Isolation and characterization of microsatellite markers in the waterflea Daphnia magna. Mol. Ecol. Resour. 11, 418–421. ( 10.1111/j.1755-0998.2010.02970.x) [DOI] [PubMed] [Google Scholar]
- 43.R Core Development Team. 2015. R: a language and environment for statistical computing. Vienna, Austria: R Core Development Team. [Google Scholar]
- 44.Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823 ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 45.Fox J, Weisberg S. 2010. An R companion to applied regression. Beverley Hills, CA: Sage. [Google Scholar]
- 46.Angilletta MJ, Steury TD, Sears MW. 2004. Temperature, growth rate, and body size in ectotherms: fitting pieces of a life-history puzzle. Integr. Compar. Biol. 44, 498–509. ( 10.1093/icb/44.6.498) [DOI] [PubMed] [Google Scholar]
- 47.Kozłowski J, Czarnołeski M, Danko M. 2004. Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr. Compar. Biol. 44, 480–493. ( 10.1093/icb/44.6.480) [DOI] [PubMed] [Google Scholar]
- 48.Yu C, Hien WN. 2006. Thermal benefits of city parks. Energy Build. 38, 105–120. ( 10.1016/j.enbuild.2005.04.003) [DOI] [Google Scholar]
- 49.Ren Z, He X, Zheng H, Zhang D, Yu X, Shen G, Guo R. 2013. Estimation of the relationship between urban park characteristics and park cool island intensity by remote sensing data and field measurement. Forests 4, 868–886. ( 10.3390/f4040868) [DOI] [Google Scholar]
- 50.Alavipanah S, Wegmann M, Qureshi S, Weng Q, Koellner T. 2015. The role of vegetation in mitigating urban land surface temperatures: a case study of Munich, Germany during the warm season. Sustainability 7, 4689–4706. ( 10.3390/su7044689) [DOI] [Google Scholar]
- 51.Dodson SI. 1974. Zooplankton competition and predation: an experimental test of the size-efficiency hypothesis. Ecology 55, 605–613. ( 10.2307/1935150) [DOI] [Google Scholar]
- 52.Rabus M, Waterkeyn A, Van Pottelbergh N, Brendonck L, Laforsch C. 2011. Interclonal variation, effectiveness and long-term implications of Triops-induced morphological defences in Daphnia magna Strauss. J. Plankt. Res. 34, 152–160. ( 10.1093/plankt/fbr092) [DOI] [Google Scholar]
- 53.Weller B, Ganzhorn JU. 2004. Carabid beetle community composition, body size, and fluctuating asymmetry along an urban-rural gradient. Basic Appl. Ecol. 5, 193–201. ( 10.1078/1439-1791-00220) [DOI] [Google Scholar]
- 54.Liker A, Papp Z, Bókony V, Lendvai A. 2008. Lean birds in the city: body size and condition of house sparrows along the urbanization gradient. J. Anim. Ecol. 77, 789–795. ( 10.1111/j.1365-2656.2008.01402.x) [DOI] [PubMed] [Google Scholar]
- 55.Matías-Ferrer N, Escalante P. 2015. Size, body condition, and limb asymmetry in two hylid frogs at different habitat disturbance levels in Veracruz, Mexico. Herpetol. J. 25, 169–176. [Google Scholar]
- 56.Lowe EC, Wilder SM, Hochuli DF. 2014. Urbanisation at multiple scales is associated with larger size and higher fecundity of an orb-weaving spider. PLoS ONE 9, e105480 ( 10.1371/journal.pone.0105480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geerts A. et al. . 2015. Rapid evolution of thermal tolerance in the water flea Daphnia. Nat. Clim. Change 5, 665–668. ( 10.1038/nclimate2628) [DOI] [Google Scholar]
- 58.Stoks R, Govaert L, Pauwels K, Jansen B, De Meester L. 2016. Resurrecting complexity: the interplay of plasticity and rapid evolution in the multiple trait response to strong changes in predation pressure in the water flea Daphnia magna. Ecol. Lett. 19, 180–190. ( 10.1111/ele.12551) [DOI] [PubMed] [Google Scholar]
- 59.Partecke J, Gwinner E. 2007. Increased sedentariness in European blackbirds following urbanization: a consequence of local adaptation? Ecology 88, 882–890. ( 10.1890/06-1105) [DOI] [PubMed] [Google Scholar]
- 60.Ebert D. 1991. The effect of size at birth, maturation threshold and genetic differences on the life-history of Daphnia magna. Oecologia 86, 243–250. ( 10.1007/BF00317537) [DOI] [PubMed] [Google Scholar]
- 61.Conover DO, Schultz ET. 1995. Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol. Evol. 10, 248–252. ( 10.1016/S0169-5347(00)89081-3) [DOI] [PubMed] [Google Scholar]
- 62.Albert CH, Thuiller W, Yoccoz NG, Soudant A, Boucher F, Saccone P, Lavorel S. 2010. Intraspecific functional variability: extent, structure and sources of variation. J. Ecol. 98, 604–613. ( 10.1111/j.1365-2745.2010.01651.x) [DOI] [Google Scholar]
- 63.Kinnison MT, Hairston NG, Hendry AP. 2015. Cryptic eco-evolutionary dynamics. Ann. NY Acad. Sci., 1360, 120–144. ( 10.1111/nyas.12974) [DOI] [PubMed] [Google Scholar]
- 64.Jeppesen E, Jensen JP, Søndergaard M, Lauridsen T. 1999. Trophic dynamics in turbid and clearwater lakes with special emphasis on the role of zooplankton for water clarity. In Shallow lakes' 98, pp. 217–231. Berlin, Germany: Springer. [Google Scholar]
- 65.Scheffer M, Carpenter S, Foley JA, Folke C, Walker B. 2001. Catastrophic shifts in ecosystems. Nature 413, 591–596. ( 10.1038/35098000) [DOI] [PubMed] [Google Scholar]
- 66.Kosten S, et al. 2012. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Change Biol. 18, 118–126. ( 10.1111/j.1365-2486.2011.02488.x) [DOI] [Google Scholar]
- 67.Brans KI, Govaert L, Engelen JMT, Gianuca AT, Souffreau C, De Meester L. 2016. Data from: Eco-evolutionary dynamics in urbanized landscapes: evolution, species sorting and the change in zooplankton body size along urbanization gradients. Dryad Digital Repository. ( 10.5061/dryad.237hs) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository, data package title: ‘Eco-evolutionary dynamics in urbanized landscapes: evolution, species sorting and the change in zooplankton body size along urbanization gradients’ ([67], http://dx.doi.org/10.5061/dryad.237hs).