Abstract
For millennia, humans have imposed strong selection on domesticated crops, resulting in drastically altered crop phenotypes compared with wild ancestors. Crop yields have increased, but a long-held hypothesis is that domestication has also unintentionally decreased plant defences against herbivores. To test this hypothesis, we conducted a phylogenetically controlled meta-analysis comparing insect herbivore resistance and putative plant defence traits between crops and their wild relatives. Our database included 2098 comparisons made across 73 crops in 89 studies. We found that domestication consistently reduced plant resistance to herbivores, although the magnitude of the effects varied across plant organs and depended on how resistance was measured. However, domestication had no consistent effects on the specific plant defence traits underlying resistance, including secondary metabolites and physical feeding barriers. The values of these traits sometimes increased and sometimes decreased during domestication. Consistent negative effects of domestication were observed only when defence traits were measured in reproductive organs or in the plant organ that was harvested. These results highlight the complexity of evolution under domestication and the need for an improved theoretical understanding of the mechanisms through which agronomic selection can influence the species interactions that impact both the yield and sustainability of our food systems.
This article is part of the themed issue ‘Human influences on evolution, and the ecological and societal consequences’.
Keywords: agronomic selection, crop yield, herbivore resistance, plant defence theory, resource allocation, secondary metabolites
1. Introduction
Crop domestication and the concurrent global expansion of agriculture have dramatically altered ecosystems and have diverse evolutionary consequences [1]. One of these consequences is that interactions between plants and their insect herbivores can be significantly altered in agricultural systems. Crops often suffer intense pressure from herbivores [2], and a commonly invoked hypothesis explaining high damage levels is that crop evolution under domestication has altered plant traits in a way that has decreased resistance to herbivores [3–5]. However, it can be difficult to disentangle the relative importance of domestication per se versus agricultural practices in shaping plant–herbivore interactions [1]. One approach to isolating the effects of domestication is to compare crops and wild relatives, and such comparisons have often shown that crops are less resistant to herbivores than their wild counterparts (reviewed in [6,7]). However, most of these studies have focused on a few model crops, and comparisons within studies are generally made among a small number of crop varieties and wild species that represent a single domestication event. More recent work that focused on non-model crops (e.g. [8,9]) or examined resistance across multiple independent domestication events [10] has revealed inconsistent effects—domestication can variably increase, decrease or have no effect on herbivore resistance or plant defence traits. Thus, it is still unclear whether domestication does have a generalizable effect on plant–herbivore interactions.
The primary goal of this study was to assess how domestication affects: (i) plant resistance against insect herbivores (hereafter, herbivore resistance) and (ii) specific chemical and physical plant traits that may function as anti-herbivore defences (hereafter plant traits). We accomplished this using a phylogenetically explicit meta-analysis of 90 published studies that compared herbivore resistance or plant traits across 73 crops and their wild relatives. Furthermore, we examined how a number of eco-evolutionary factors could explain variation in the magnitude of domestication effects across studies. These factors included: (i) the measure of herbivore resistance or type of plant trait examined, (ii) which plant organ was measured, (iii) whether the organ measured was the primary organ harvested by humans, (iv) the extent of domestication of the crop, (v) the primary use of the crop, and (vi) the life history of the crop. Our results complement and extend recent narrative reviews on domestication and plant–herbivore interactions [6,7,11] by taking a phylogenetically controlled meta-analysis approach to comparisons between crops and their wild relatives, providing a robust test of the effects of domestication across crops with diverse evolutionary histories.
(a). Domestication effects on plant–herbivore interactions: a review of evolutionary mechanisms
Crop domestication is hypothesized to alter the outcome of plant–herbivore interactions through a number of evolutionary mechanisms. First, the genetic architecture of plants can be fundamentally changed during domestication by hybridization, genetic bottlenecks, alteration of reproductive strategies and increases in ploidy level, and these changes have complex consequences for the expression of plant traits [11–13]. Second, humans may directly select against traits that function in defence, including toxic, distasteful or anti-nutritive secondary metabolites or physical structures that make handling difficult [5,14]. Third, humans may select for traits that improve plant quality for insects, such as increased nutritional content [5,15,16]. Fourth, crops may evolve in response to altered selective regimes created by agricultural practices, such as the exclusion of herbivores with pesticides or the application of fertilizers [7,17]. Finally, humans may indirectly select against plant defences as we select for other traits of agronomic interest, such as higher yields or stress tolerance [3,4]. Indirect selection can occur if the traits under direct selection and defence are genetically correlated, i.e. through pleiotropy or genetic linkage, or phenotypically correlated because of resource-allocation trade-offs [3].
The hypothesis of resource-allocation trade-offs among growth, reproduction and defence is fundamental in plant defence theory [18] and predicts that selection for increased yield will result in fewer resources available for plants to invest in defence [3]. These potential trade-offs may also create a major challenge for crop breeders focused on improving resistance while maintaining high yields [19]. Its theoretical foundation and practical relevance have made the resource-allocation hypothesis an important driver of much of the research on domestication and plant–herbivore interactions (e.g. [3,10,20,21]). This hypothesis can also form the basis for many more nuanced predictions regarding how different defence traits will evolve in different plant organs and different evolutionary contexts.
(b). Eco-evolutionary factors predicted to drive variation in the domestication effect
(i). Type of response
Although plant defence is often conveniently discussed as if it were a single trait, defence typically involves suites of traits that can affect various aspects of herbivore behaviour and physiology [22]. Thus, depending on how herbivore resistance or plant defence traits are assessed, the observed effects of domestication may vary considerably. Our analysis included studies that assessed herbivore resistance using plant herbivory (e.g. herbivore abundance or damage to plant tissues) or using various measures of herbivore preference or performance. Because plant herbivory represents the most integrative estimate of how multiple plant traits affect multiple components of herbivore resistance, we predicted that any observed negative effects of domestication would be the strongest when resistance was measured based on levels of plant herbivory. Studies that assessed putative plant defence traits measured various chemical traits (e.g. alkaloids and phenolics) or physical traits (e.g. trichomes and leaf toughness) that are known or thought to provide a fitness benefit to plants in the presence of herbivores. Based on the hypothesis of resource-allocation trade-offs [3], more costly defences should be more likely to be lost during domestication. Physical defences are hypothesized to be more costly than chemical defences, because they are not recyclable and they directly compete with biomass ([23], but see [24]). Therefore, we predicted that domestication has reduced physical defences more strongly than chemical defences.
(ii). Plant organ measured
Plants are highly modular and can allocate chemical and physical defences differently among organs depending on both the costs and benefits of defending each organ [25]. In general, defence of reproductive organs may be more costly than leaf defence, because reproductive organs are typically photosynthetic sinks that are very costly to produce, requiring a substantial carbon and nutrient investment from the rest of the plant to complete their development [18]. High competition for resources in these rapidly developing organs could mean that any investment in defence has a direct cost in terms of reproductive fitness or, in the case of crops, yield [18]. Therefore, we predicted that domestication has reduced herbivore resistance and plant defence traits more strongly in reproductive organs than in vegetative organs.
(iii). Harvested and non-harvested organs
As humans select for increased palatability and ease of handing in harvested plant organs, they may be directly selecting against traits involved in defence. However, these selective pressures could have minimal effects on non-harvested organs. For example, sweet almonds have almost certainly experienced direct selection for reduced cyanogenic compounds in the kernels, but these compounds are still present in high concentrations in leaves and roots [26]. Harvested organs may also be more strongly affected by resource-allocation trade-offs. Although the hypothesis of resource-allocation trade-offs predicts that defence allocation will be reduced in all plant organs [3], there could be stronger localized trade-offs in the harvested organ, especially when yield increases are accomplished through gigantism of the harvested organ. Therefore, we predicted that domestication has reduced herbivore resistance and plant defence traits more strongly in harvested than in non-harvested organs.
(iv). Domestication extent
Domestication proceeds along a continuum from the formation of landraces to modern breeding [4]. Landraces have typically been developed in the native range of the crop with minimal agrochemical inputs and alongside herbivores that have a shared evolutionary history with the wild crop ancestor [4,6]. In this environment, herbivore resistance is likely to be maintained because herbivores can be important determinants of crop yield [2]. By contrast, modern agronomic breeding generally takes place in a near-optimum environment under insecticide-treated and fertilized conditions, where differences in the natural defences of plants would be less apparent [3,4]. Therefore, we predicted that domestication has reduced herbivore resistance and plant defence traits more strongly in modern varieties than in landrace varieties.
(v). Crop use
Selection for increased yield has been imposed on virtually all crops [4], including food and non-food (e.g. textiles), and, if resource-allocation trade-offs do occur, then they should occur in all types of crops [3]. However, food crops may have additionally experienced direct selection for increased palatability, and therefore we predicted that domestication will have reduced herbivore resistance and plant defence traits more strongly in food than non-food crops. Moreover, staple crops such as grains and legumes have formed the basis of human diets for thousands of years [4,27], and for much of this history they have been grown with minimal pesticide inputs where pest damage is likely to be a key determinant of yield. Furthermore, pest pressure on these crops may be relatively high because they are generally grown year after year, largely in mono-cultural stands. By contrast, specialty crops such as fruits and vegetables (defined in the culinary sense) are less essential to human survival and may have been subject to more direct selection for palatability and sweetness. For these reasons, we predicted that domestication will have reduced herbivore resistance and plant defence traits more strongly in specialty crops, such as culinary fruits and vegetables, than in staple crops, such as grains and legumes.
(vi). Plant life history
Although most research on domestication has focused on short-lived annuals from just a few families, domesticated crops include plants from greater than 150 families that are highly variable in their life-history traits [27,28]. For several reasons, we predicted that any negative effects of domestication on resistance and plant defence traits will be strongest for herbaceous annuals, intermediate for herbaceous perennials, and weakest for woody perennials. First, defence theory predicts that natural selection will act to minimize defence investment in short-lived plants that need to allocate heavily towards growth and reproduction in order to maximize fitness [18]. These selective pressures may be strongly magnified during human-mediated selection for fast-growing crops with maximum yield potential in a single growing season. Second, rapid generation times in annuals mean that annual crops may have diverged more from their wild ancestors than perennials, and defences may have been lost due to the combined effects of selection and genetic drift. Third, conscious and unconscious selection to maintain natural plant defences may be higher in long-lived crops because pest pressures may be higher—populations of pests can increase over time and common cultural pest control practices such as crop rotation are unavailable [29].
2. Material and methods
(a). Database assembly
To obtain a broad sampling of studies comparing herbivore resistance and putative plant defence traits in domesticated crops and wild relatives, we conducted the literature searches using Web of Science, Agricola and Google Scholar with various combinations of the search terms domesticat*, cultiva*, wild, ‘herbivor* resistance’, ‘insect resistance’, antibiosis, antixenosis, ‘secondary metabolite’, ‘secondary compound’ and ‘plant defen?e’. In addition, we searched the literature cited sections of several previous reviews and key studies on domestication and plant defence [3,6,7,10,11,27,28]. Articles were included in the database if they compared herbivore resistance or putative plant defence traits in a crop and a wild ancestor or relative. Complete criteria for study inclusion and data selection, the assembled database, and a complete list of references for all included studies are provided in the electronic supplementary material. To avoid bias and take full advantage of the available data, we often recorded multiple data points from a single study, including multiple types of relevant response variables and multiple comparisons made between different crop and wild relative accessions. These multiple comparisons also allowed us to place the differences between crops and wild relatives in the context of natural variation among and within wild species or among different crop varieties (see the electronic supplementary material for additional analyses exploring this variation). We accounted for the non-independence of data in the statistical analysis (see below).
For each crop/wild comparison, we recorded the means, variances and sample sizes of the relevant response variable measuring either herbivore resistance or plant traits. Herbivore resistance data were further categorized according to the type of response variable measured: herbivore performance, herbivore preference or plant herbivory. Herbivore performance was defined as any physiological herbivore response to plants, such as pupal mass, survival or development time. Herbivore preference was defined as any behavioural herbivore response to plants, such as oviposition preference or larval feeding choice. Plant herbivory included any measurement of herbivore damage, herbivore abundance or herbivore diversity on plants that could be due to the combined effects of herbivore preference and performance. Plant trait data were categorized as either chemical traits or physical traits. For chemical traits, we focused exclusively on constitutively produced non-volatile secondary metabolites due to limited data on volatile secondary metabolites and induced secondary metabolites.
We also documented additional variables that could mediate the impact of domestication. These included the plant organ in which resistance or defence traits were measured, whether the organ was a harvested or non-harvested part, the extent of domestication (landrace or modern cultivar), the primary crop use (including grains, legumes, oilseed, culinary fruits, vegetables or non-food) and the plant life history (herbaceous annual, herbaceous perennial and woody perennial).
(b). Effect size calculation
For each crop/wild comparison in the database, we calculated the domestication effect size using the Hedges' d metric. The metric is calculated as 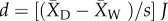 , where
, where 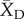 represents the mean of the domesticated crop,
represents the mean of the domesticated crop, 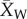 represents the mean of the wild relative, s represents the pooled standard deviation, and J is a correction factor for small sample size [30]. Both Hedges' d and its estimated sampling variance were calculated using the escalc function in the R package metafor [31]. When necessary we reversed the sign of the effect size so that a negative value of d always indicates a negative effect of domestication on plant defence traits or herbivore resistance.
represents the mean of the wild relative, s represents the pooled standard deviation, and J is a correction factor for small sample size [30]. Both Hedges' d and its estimated sampling variance were calculated using the escalc function in the R package metafor [31]. When necessary we reversed the sign of the effect size so that a negative value of d always indicates a negative effect of domestication on plant defence traits or herbivore resistance.
(c). Model construction
We fitted multilevel mixed-effects models using the rma.mv function in the R package metafor [31] that weighs each effect size by the inverse of its sampling variance plus the amount of residual heterogeneity not explained by moderators [31]. To account for the non-independence of data derived from the same species or same study, we included three sets of random effects terms in all models described below: (i) domesticated plant accession nested within domesticated species, (ii) wild plant accession nested within wild plant species nested within domesticated species and (iii) study identity. The complete R syntax for all analyses is available as part of the electronic supplementary material.
To account for the phylogenetic non-independence of data, we constructed a phylogeny of all crop species in the dataset that could be used to inform the correlation structure of the meta-analysis models (figure 1). The phylogenetic tree was constructed using the online phylogenetic query tool ‘Phylomatic’ [32], which uses a set of stored phylogenies to estimate relationships among a user-supplied list of taxa. If stored phylogenies were not available for a family, the genera nodes were connected directly to a polytomous family node [32]. To test for phylogenetic signal in the domestication effect size, we first estimated the mean effect size for each crop species by running separate random-effects meta-analysis models for each species. Random effects terms included: (i) domesticated accession, (ii) wild accession nested within wild species, and (iii) study identity. In cases where only a single comparison was available for a crop, we used the single effect size value as the estimate for that species. Once effect size estimates and associated variances were compiled for each crop species, we tested for phylogenetic signal in both the effect sizes and their associated variances using two metrics: Blomberg's K and Pagel's λ [33]. These analyses were conducted separately for the herbivore resistance data and the plant trait data using the phylosig function of the phytools package in R [33]. We found strong evidence of phylogenetic signal in the effect size estimates for the plant trait data (see results), and incorporated phylogenetic information in all subsequent models for consistency of interpretation across analyses. We used the vcv function in the R package ape [34] to compute the expected correlation structure of the effect sizes assuming a Brownian model of evolution across the phylogeny, and this structure was incorporated into the models via the rma.mv function in the R package metafor [31].
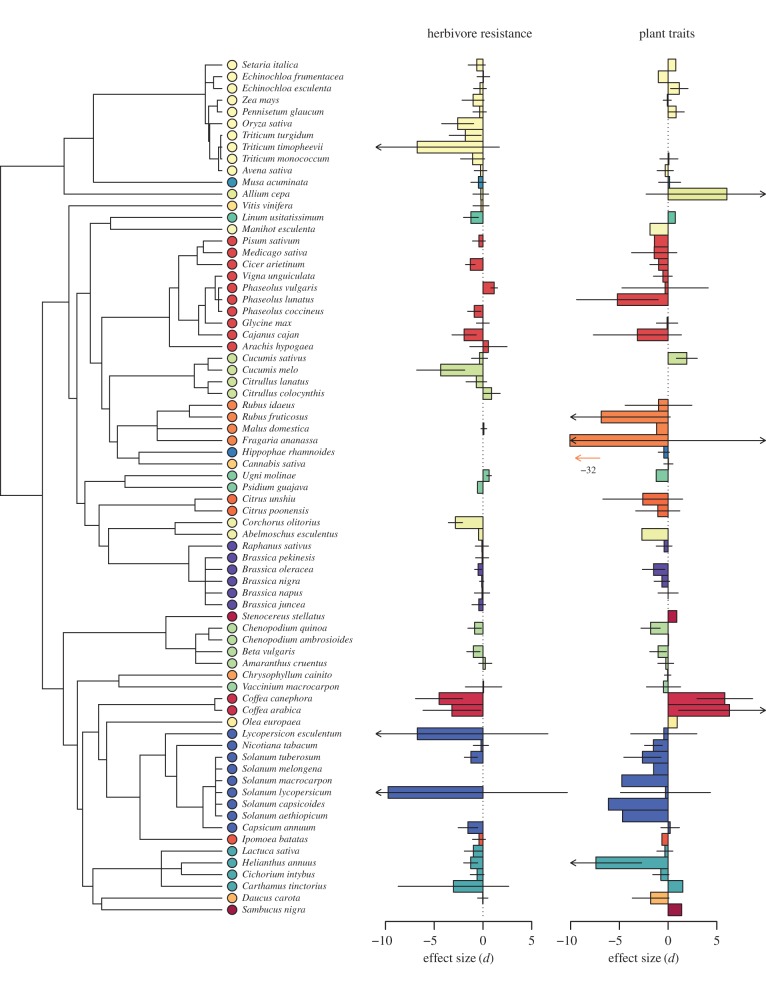
Figure 1.
Phylogeny of crop species included in the analysis with adjacent barplots showing the model-estimated mean effect sizes (Hedges' d) and 95% CIs for each crop. Negative values of d indicate a negative effect of domestication on herbivore resistance or putative plant defence traits. Arrows drawn at the ends of error bars indicate 95% CIs for Hedges' d that are outside the scale of the plotting region. For Fragaria annassa, the model-estimated mean effect size (−31.85) is also outside the plotting region. Colours on branch tips and adjacent bars correspond to plant families.
(d). Hypothesis testing and meta-regression
To address our primary research question of whether domestication impacts herbivore resistance and plant defence traits, we fitted random-effects models separately to the herbivore resistance data and the plant trait data using restricted maximum likelihood (REML). We considered model-estimated mean effect sizes with 95% confidence intervals (CIs) that did not cross zero as evidence for a significant effect of domestication.
We tested how various moderators influenced the magnitude of the domestication effect using meta-regression models, conducted separately for each moderator and for the herbivore resistance and plant trait data. Smaller subsets of data were used for some analyses depending on the availability of data (see the electronic supplementary material). When significant effects were detected for categorical moderators with more than two groups (e.g. different plant organs), the meta-analysis was followed by post-hoc comparisons among groups, carried out using the multcomp package in R [35]. To assess whether there was a significant effect of domestication for each group, we re-fitted models with no intercepts and the model coefficients and their associated CIs were used to determine whether the effect size was different from zero for each group.
(e). Heterogeneity statistics and bias analysis
For each mixed-effects model, we assessed residual heterogeneity using the QE statistic [30,31]. We found significant QE values for all models (p < 0.001, electronic supplementary material, table S1), suggesting the existence of additional important moderators that we did not include in our analyses. To assess the potential for publication bias to influence our conclusions, we used funnel plots and meta-regression models with study year as a moderator [30], both of which indicated a low probability that publication bias affected the observed patterns (electronic supplementary material, figures S1 and S2).
3. Results
(a). Domestication effects on herbivore resistance and plant defence traits
A phylogenetically controlled analysis revealed that domestication has a significant negative effect on herbivore resistance (figure 2a; electronic supplementary material, table S2a), but no overall effect on putative plant defence traits (figure 2b; electronic supplementary material, table S2b). These results are based on a large number of wild/domestic comparisons made across a diverse group of crops: herbivore resistance data included 1466 comparisons taken from 67 studies on 53 crop species and plant trait data included 632 comparisons taken from 43 studies on 60 crop species (electronic supplementary material, table S2). For the herbivore resistance data, there was no significant phylogenetic signal in either the effect sizes (Blomberg's K = 0.04, p = 0.84; Pagel's λ = 0.11, p = 0.41; figure 1) or the effect size variances (K = 0.05, p = 0.84; λ = 0.07, p = 0.61). For the plant trait data, a phylogenetic signal in the effect sizes was detected using both K and λ (K = 0.43, p = 0.04; λ = 0.99, p < 0.001; figure 1), and a signal was detected for the effect size variance using λ (K = 0.47, p = 0.20; λ = 1.005, p = 0.0002).
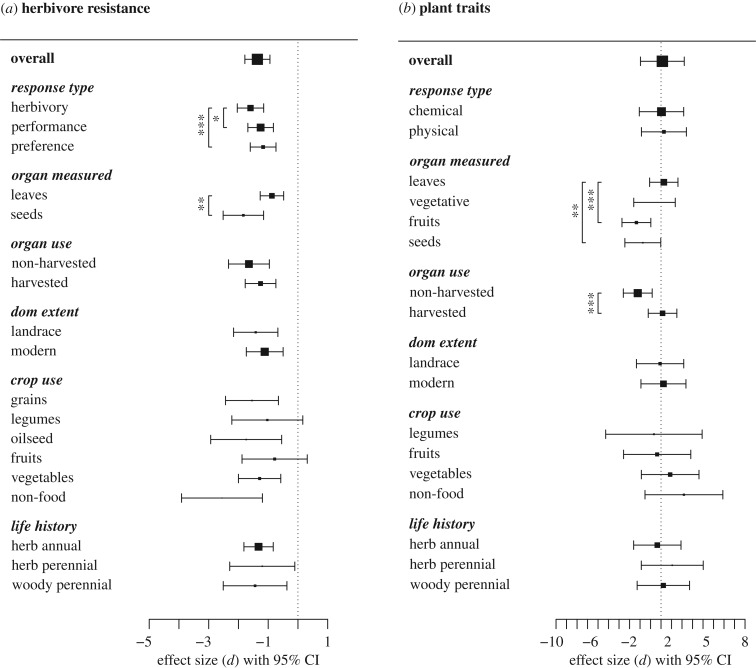
Figure 2.
Effect size (Hedges' d) estimates and 95% CIs showing the effects of domestication on herbivore resistance (a) and putative plant defence traits (b). Negative values of d indicate a negative effect of domestication on herbivore resistance or defence. Point sizes are proportional to the number of unique combinations of crop species and studies used to inform each estimate (see electronic supplementary material, table S2a, for complete sample size information).
(b). Factors driving variation in the domestication effect
(i). Type of response
The effect of domestication on herbivore resistance was dependent on the measure of resistance examined (QM = 13.60, p = 0.001; electronic supplementary material, table S1). Domestication had a negative effect on all three measures of herbivore resistance, but the effects were stronger for measures of plant herbivory than for measures of herbivore performance or preference (figure 2a; electronic supplementary material, table S2a). The effect of domestication on plant traits did not depend on the type of trait examined (QM = 0.51, p = 0.48; electronic supplementary material, table S1) and there was no significant effect for either chemical or physical traits (figure 2b; electronic supplementary material, table S2b).
(ii). Plant organ measured
The effect of domestication on herbivore resistance was dependent on which plant organ was measured (QM = 6.75, p = 0.01; electronic supplementary material, table S1). There were negative effects for both seeds and leaves, but the effect was nearly twice as strong for seeds (figure 2a; electronic supplementary material, table S2a). The effect of domestication on plant traits was also dependent on which plant organ was measured (QM = 142.56, p < 0.001; electronic supplementary material, table S1). Domestication significantly reduced putative defences in fruits and seeds and had no effect on leaves and other vegetative parts (figure 2b; electronic supplementary material, table S2b). Both fruits and seeds were significantly different from leaves (figure 2b).
(iii). Harvested and non-harvested organs
The effects of domestication on herbivore resistance were not different for harvested and non-harvested organs (QM = 1.27, p = 0.26; electronic supplementary material, table S1). By contrast, the effects of domestication on plant traits were strongly dependent on organ use (QM = 122.01, p < 0.001; electronic supplementary material, table S1). Domestication reduced putative defences in harvested organs, but had no effect on non-harvested organs (figure 2b; electronic supplementary material, table S2b).
(iv). Domestication extent
The effects of domestication on herbivore resistance and plant traits did not depend on the domestication extent (herbivore data: QM = 1.31, p = 0.25; plant data: QM = 0.52, p = 0.47; electronic supplementary material, table S1). For both landrace and modern crops, domestication reduced herbivore resistance, but had no effect on plant traits (figure 2; electronic supplementary material, table S2).
(v). Crop use
The effects of domestication on herbivore resistance and plant traits did not depend on the crop use (herbivore data: QM = 5.28, p = 0.38; plant data: QM = 2.02, p = 0.57; electronic supplementary material, table S1). Domestication reduced herbivore resistance in grains, oilseed crops, vegetables and non-food crops, but had no effect on culinary fruits or legumes (figure 2a). There was no effect of domestication on putative defence traits for any crop use category (figure 2b).
(vi). Life history
The effects of domestication on herbivore resistance and plant defence traits did not depend on the plant life history (herbivore data: QM = 0.10, p = 0.95; plant data: QM = 1.09, p = 0.58; electronic supplementary material, table S1). For all three life-history categories, domestication reduced herbivore resistance but had no effect on plant traits (figure 2; electronic supplementary material, table S2).
4. Discussion
(a). The general impact of crop domestication on plant–herbivore interactions
Domestication clearly has an overall negative effect on plant resistance to herbivores that is generalizable across crops with diverse evolutionary histories (figures 1 and 2). This finding is consistent with conclusions drawn in previous reviews [6,7] and has major implications for agricultural systems. Agricultural pests represent a critical challenge for food production, destroying an estimated 32% of potential crop yield despite all control efforts [2]. Our results show that evolutionary changes incurred via domestication can at least partly explain this intense pest pressure. Furthermore, the pattern of reduced herbivore resistance in domesticated crops has broad implications for the ecology of agroecosystems, probably playing a key role in shaping herbivore communities and affecting interactions between herbivores and natural enemies [36]. However, in contrast to the clear effects of domestication on herbivore resistance, there were no overall effects of domestication on putative plant defence traits (figures 1 and 2). This was unexpected considering the conventional wisdom that plant traits such as secondary metabolites are the main drivers of herbivore resistance, and the strong theoretical support for the idea that domestication should reduce plant investment in defence production [3,5–7,11].
In interpreting our results for the effects of domestication on plant traits, it is important to consider that the effect sizes were highly variable both among and within crops (figure 1; electronic supplementary material, table S1). Of 632 wild/domestic comparisons included in our dataset, 293 showed a negative effect of domestication, 165 showed a positive effect of domestication and 174 showed no effect (based on 95% CIs for individual effect sizes). Although the direction of the effect was variable, the absolute magnitude of the effect sizes in crop–wild comparisons was larger by approximately 51% than similar comparisons between closely related wild taxa (see the electronic supplementary material for additional analysis description and figure S3). Clearly, plant traits do change during domestication, but not in a consistent manner. Research in this area should continue to explore both the causes of this variation and its consequences for plant–herbivore interactions.
The lack of concurrence between the results for herbivore resistance and plant traits also suggests that traits other than those that were the focus of our analysis could be driving the changes in herbivore resistance during domestication. Most of the studies that measured secondary metabolites focus on reporting total quantities of particular compound classes or the quantities of the most abundant individual metabolites. However, herbivores may not respond to total quantities, but instead only to certain metabolites or subsets of metabolites that may or may not be the most abundant metabolites detected. Furthermore, interactions among chemical traits or among chemical and other traits may obscure the relationships between secondary metabolites and resistance. In a meta-analysis by Carmona et al. [37], the authors found no overall correlation between secondary metabolites and herbivore resistance. Instead, resistance was best predicted by life history or other gross morphological traits [37], many of which may be affected by domestication [27]. Domestication can also have a strong impact on plant nutrient status [15,16], and insects may prefer and perform better on domesticated crops because they are of higher nutritional quality [38]. Although not typically considered as defence traits, plant nutritional and gross morphological traits may be important components of a defence strategy [22] and their importance for understanding plant–herbivore interactions in crops deserves further attention.
(b). Factors driving variation in the effects of domestication
Across studies that measured herbivore resistance, the negative effects of domestication were, as predicted, largest when resistance was assessed as plant herbivory or when it was measured in seeds (figure 2a). The stronger effects on plant herbivory relative to herbivore preference and performance were not surprising considering that both herbivore preference and herbivore performance were affected individually, and plant herbivory levels result from the combined effects of these two components of resistance. In addition, while measurements of herbivore preference and performance were almost always collected in laboratory or greenhouse settings, measurements of plant herbivory were often collected in the field, where plant damage provided an integrated estimate of the preference and performance of multiple herbivores simultaneously. The stronger effects of domestication on resistance in seeds relative to leaves also fit our predictions, which were primarily based on the idea that resource-allocation trade-offs should be strongest in costly reproductive structures [3,18]. This hypothesis is further supported by the results for plant traits, where fruits and seeds were the only organs where we detected a negative effect of domestication on defence traits.
Across studies that measured putative plant defence traits, we detected negative effects of domestication only when traits were measured in harvested organs or in fruits or seeds. Interpretation of these results is limited by the fact that the two variables were confounded: rarely were fruit and seed traits measured if those organs were not the ones harvested by humans. Thus, it is not possible to conclude with certainty whether these effects are due to differences among organs per se, differences based on which organ is harvested, or a combination of the two. Many studies did measure leaf traits regardless of whether leaves were harvested, and an examination of this data subset showed that defence traits were reduced in leaves only when leaves were the harvested organ (d = −2.04, 95% CI: −0.44 to −3.65; data not shown). This suggests that at least the differential effects of domestication on harvested and non-harvested organs probably hold across plant organs. An improved understanding of these patterns could come with more studies that measure multiple harvested and non-harvested organs in a single crop. This was rare among the studies in our database, but one excellent example is a study by Parker et al. [39] that examined phenolics in leaves, pulp and seeds of caimito (Chrysophyllum cainito), which is cultivated for its edible fruit. They found that, compared with wild types, cultivated caimito had lower concentrations of phenolics in fruit pulp and seeds, but higher concentrations in mature leaves. Clearly, domestication can have differential effects across different plant organs, but we need more evidence to understand how these effects are determined both by the physiology of the plant and the selective pressures imposed by humans and the environment.
Interestingly, several of the factors that have been previously hypothesized to affect the trajectory of plant evolution under domestication, including the domestication extent, crop use and plant life history [3,6,28], did not explain variation in the domestication effect size for either herbivore resistance or plant defence traits. It is possible that these factors are still important for certain crops, or may interact with other variables that we did not examine, making it difficult to detect their effects in our analysis. However, our results suggest that they are not the most important drivers of variation in the domestication effect.
(c). Evolutionary mechanisms: how important are resource-allocation trade-offs?
The hypothesis of resource-allocation trade-offs between crop yield and defence is strongly rooted in plant defence theory [18] and provides a compelling framework for understanding how domestication affects plant–herbivore interactions [3,10,20,21]. However, a number of evolutionary mechanisms may explain differences between crops and wild relatives. Although our meta-analysis was not designed to distinguish among these different mechanisms, several patterns do provide insight. First, the different patterns we saw for herbivore resistance and plant defence traits suggest that reduced allocation to defences is not the main driver of differences in herbivore resistance between crops and wild relatives. Furthermore, the finding that putative defence traits were reduced in harvested organs (figure 2b), suggests that direct selection for increased palatability or ease of handling may be more important than resource-allocation trade-offs in causing a reduction in plant defence during domestication. Thus, while resource-allocation trade-offs may occur and affect overall plant defence strategies in crops, our results suggest that other evolutionary mechanisms, such as direct selective pressure for increased palatability [26] or selection leading to increased plant nutritional quality for herbivores [5,15], may be even more important and warrant further attention.
(d). Caveats to interpretation and future directions
Our results can be considered robust to common sources of bias in meta-analyses, such as publication bias (electronic supplementary material, figures S1 and S2) or phylogenetic non-independence among species [30]. However, the residual heterogeneity in effect sizes was highly significant in all models (electronic supplementary material, table S1), suggesting that important explanatory variables were not included in our analysis. Clearly, there are many factors that may influence the trajectory of plant evolution under domestication that we did not test, some of which could confound the interpretation of the variables we did test. Furthermore, the vast majority of studies included in our analysis were not specifically designed to test how domestication influences plant defence. The crops and wild plants studied have variable evolutionary relationships (i.e. direct ancestors or close relatives) and may have been subject to highly variable environmental and geographical conditions, including completely different herbivore communities. Thus, the patterns we report regarding factors that influence the domestication effect should be viewed as promising starting points for future experimental or comparative studies using carefully chosen pairs of crops and wild ancestors.
The effects of domestication on both herbivore resistance and plant traits were highly variable, and we tested only a few possible factors that may explain this variation. Other potentially important explanatory factors that should be explored in future work may relate to: (i) plant traits, such as the mating system [13] or type of defence strategy employed [22]; (ii) herbivore traits, such as the feeding guild or level of specialization; (iii) the evolutionary history of plant–herbivore interactions [6]; (iv) the types of selective pressures imposed by humans, including those imposed for various food or horticultural uses; or (v) the environment in which domestication takes place, including climate, nutrient availability and the presence of other organisms, such as pollinators or plant-associated microbes. Furthermore, future research should broaden the scope of plant defences examined. We limited our review to focus on physical defences and non-volatile secondary metabolites, because these types of defences had the most available data. However, plant defences include many other strategies. In particular, our analysis did not include indirect plant defences, such as volatile organic compounds that defend plants by recruiting natural enemies of herbivores [40], or induced defences, which are produced in response to herbivore damage [41]. There are now a number of studies that have explored indirect or induced defences in crops (e.g. [5,42,43]), and a recent meta-analysis by Rowen & Kaplan [44] found that volatiles were more strongly induced by herbivory in crops compared with wild plants, suggesting that many crops may rely on induced volatiles and recruitment of natural enemies as part of their defence strategy. A fruitful area for future research is in understanding how domestication impacts the relative importance of different types of plant defence, including constitutive versus induced and direct versus indirect defences.
5. Conclusion
Crop domestication has long been recognized as a valuable model for understanding evolution. Humans have imposed strong directional selective pressures on crops, providing a unique opportunity to understand how those selective pressures can influence other aspects of plant ecology, such as interactions with herbivores. However, the effects of domestication on plant–herbivore interactions can be complex, and understanding this complexity requires an integrative view of the evolutionary ecology of interactions among plants, humans, herbivores and the surrounding environment. The results we have presented here have helped to clarify broad trends across crop plants, clearly demonstrating an overall negative effect of domestication on herbivore resistance, but also highlighting the need for more experimental and comparative studies aimed at an improved understanding of the mechanisms underlying these effects. Recent years have seen considerable advances in technologies such as high-throughput plant phenotyping [45] and comprehensive metabolomics [46] that hold considerable promise for addressing which plant traits are most affected by domestication and how they are related to herbivore resistance. Continued work in this area can provide theoretical insight into the complex dynamics of evolutionary processes involving humans and other organisms as well as a practical understanding of how evolutionary ecology shapes the food systems that sustain our lives.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Sascha Hernandez, Anna Wilson and Trey Ramsey for assistance with data extraction from published literature and database assembly.
Data accessibility
The assembled database and analysis code supporting this article are included as part of the electronic supplementary material.
Authors' contributions
S.R.W., K.P. and M.M.T. conceived of the study; S.R.W. and K.P. coordinated the database assembly; M.M.T. led the statistical analysis; S.R.W. drafted the manuscript and all authors contributed substantially to revisions.
Competing interests
We declare we have no competing interests.
Funding
S.R.W. received support from a USDA NIFA postdoctoral fellowship (Project No. NYC-76022). M.M.T. was supported by a postdoctoral fellowship from the Adaptation to a Changing Environment (ACE) center at ETH Zürich.
References
- 1.Turcotte MM, Araki H, Karp DS, Poveda K, Whitehead SR. 2017. The eco-evolutionary impacts of domestication and agricultural practices on wild species. Phil. Trans. R. Soc. B 372, 20160033 ( 10.1098/rstb.2016.0033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oerke EC. 2006. Crop losses to pests. J. Agric. Sci. 144, 31–43. ( 10.1017/S0021859605005708) [DOI] [Google Scholar]
- 3.Rosenthal J, Dirzo R. 1997. Effects of life history, domestication and agronomic selection on plant defence against insects: evidence from maizes and wild relatives. Evol. Ecol. 11, 337–355. ( 10.1023/A:1018420504439) [DOI] [Google Scholar]
- 4.Evans LT. 1993. Crop evolution, adaptation and yield. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Benrey B, Callejas A, Rios L, Oyama K, Denno RF. 1998. The effects of domestication of Brassica and Phaseolus on the interaction between phytophagous insects and parasitoids. Biol. Control 11, 130–140. ( 10.1006/bcon.1997.0590) [DOI] [Google Scholar]
- 6.Chen YH, Gols R, Benrey B. 2015. Crop domestication and its impact on naturally selected trophic interactions. Annu. Rev. Entomol. 60, 35–58. ( 10.1146/annurev-ento-010814-020601) [DOI] [PubMed] [Google Scholar]
- 7.Macfadyen S, Bohan DA. 2010. Crop domestication and the disruption of species interactions. Basic Appl. Ecol. 11, 116–125. ( 10.1016/j.baae.2009.11.008) [DOI] [Google Scholar]
- 8.Leiss KA, Cristofori G, van Steenis R, Verpoorte R, Klinkhamer PGL. 2013. An eco-metabolomic study of host plant resistance to Western flower thrips in cultivated, biofortified and wild carrots. Phytochemistry 93, 63–70. ( 10.1016/j.phytochem.2013.03.011) [DOI] [PubMed] [Google Scholar]
- 9.Chacon-Fuentes M, Parra L, Rodriguez-Saona C, Seguel I, Ceballos R, Quiroz A. 2015. Domestication in murtilla (Ugni molinae) reduced defensive flavonol levels but increased resistance against a native herbivorous insect. Environ. Entomol. 44, 627–637. ( 10.1093/ee/nvv040) [DOI] [PubMed] [Google Scholar]
- 10.Turcotte MM, Turley NE, Johnson MTJ. 2014. The impact of domestication on resistance to two generalist herbivores across 29 independent domestication events. New Phytol. 204, 671–681. ( 10.1111/nph.12935) [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary B. 2013. Plant domestication and resistance to herbivory. Int. J. Plant Genomics 2013, 1–14. ( 10.1155/2013/572784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olsen KM, Wendel JF. 2013. A bountiful harvest: genomic insights into crop domestication phenotypes. Annu. Rev. Plant Biol. 64, 47–70. ( 10.1146/annurev-arplant-050312-120048) [DOI] [PubMed] [Google Scholar]
- 13.Campbell SA. 2015. Ecological mechanisms for the coevolution of mating systems and defence. New Phytol. 205, 1047–1053. ( 10.1111/nph.13212) [DOI] [PubMed] [Google Scholar]
- 14.Wittkop B, Snowdon RJ, Friedt W. 2009. Status and perspectives of breeding for enhanced yield and quality of oilseed crops for Europe. Euphytica 170, 131–140. ( 10.1007/s10681-009-9940-5) [DOI] [Google Scholar]
- 15.Delgado-Baquerizo M, Reich PB, García-Palacios P, Milla R. 2016. Biogeographic bases for a shift in crop C:N:P stoichiometries during domestication. Ecol. Lett. 19, 564–575. ( 10.1111/ele.12593) [DOI] [PubMed] [Google Scholar]
- 16.Newell-McGloughlin M. 2008. Nutritionally improved agricultural crops. Plant Physiol. 147, 939–953. ( 10.1104/pp.108.121947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milla R, Osborne CP, Turcotte MM, Violle C. 2015. Plant domestication through an ecological lens. Trends Ecol. Evol. 30, 463–469. ( 10.1016/j.tree.2015.06.006) [DOI] [PubMed] [Google Scholar]
- 18.Herms DA, Mattson WJ. 1992. The dilemma of plants—to grow or defend. Q. Rev. Biol. 67, 283–335. ( 10.1086/417659) [DOI] [Google Scholar]
- 19.Brown JKM. 2002. Yield penalties of disease resistance in crops. Curr. Opin. Plant Biol. 5, 339–344. ( 10.1016/S1369-5266(02)00270-4) [DOI] [PubMed] [Google Scholar]
- 20.Mondolot L, Marlas A, Barbeau D, Gargadennec A, Pujol B, McKey D. 2008. Domestication and defence: foliar tannins and C/N ratios in cassava and a close wild relative. Acta Oecol. Int. J. Ecol. 34, 147–154. ( 10.1016/j.actao.2008.05.009) [DOI] [Google Scholar]
- 21.Massei G, Hartley SE. 2000. Disarmed by domestication? Induced responses to browsing in wild and cultivated olive. Oecologia 122, 225–231. ( 10.1007/PL00008850) [DOI] [PubMed] [Google Scholar]
- 22.Agrawal AA, Fishbein M. 2006. Plant defense syndromes. Ecology 87, S132–S149. ( 10.1890/0012-9658(2006)87%5B132:PDS%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 23.Skogsmyr I, Fagerström T. 1992. The cost of anti-herbivory defence: an evaluation of some ecological and physiological factors. Oikos 64, 451–457. ( 10.2307/3545160) [DOI] [Google Scholar]
- 24.Koricheva J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83, 176–190. ( 10.1890/0012-9658(2002)083%5B0176:MAOSOV%5D2.0.CO;2) [DOI] [Google Scholar]
- 25.McCall AC, Fordyce JA. 2010. Can optimal defence theory be used to predict the distribution of plant chemical defences? J. Ecol. 98, 985–992. ( 10.1111/j.1365-2745.2010.01693.x) [DOI] [Google Scholar]
- 26.Dicenta F, Martínez-Gómez P, Grané N, Martín ML, León A, Cánovas JA, Berenguer V. 2002. Relationship between cyanogenic compounds in kernels, leaves, and roots of sweet and bitter kernelled almonds. J. Agric. Food Chem. 50, 2149–2152. ( 10.1021/jf0113070) [DOI] [PubMed] [Google Scholar]
- 27.Meyer RS, Duval AE, Jensen HR. 2012. Patterns and processes in crop domestication : an historical review and quantitative analysis of 203 global food crops. New Phytol. 196, 29–48. ( 10.1111/j.1469-8137.2012.04253.x) [DOI] [PubMed] [Google Scholar]
- 28.McKey D, Elias M. 2012. Ecological approaches to crop domestication. In Biodiversity in agriculture: domestication, evolution and sustainability (eds Gepts P, Famula TR, Bettinger RL, Brush SB, Damania AB, McGuire PE, Qualset CO), pp. 377–406. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Cox CM, Garrett KA, Bockus WW. 2005. Meeting the challenge of disease management in perennial grain cropping systems. Renew. Agric. Food Syst. 20, 15–24. ( 10.1079/RAF200495) [DOI] [Google Scholar]
- 30.Koricheva J, Gurevitch J, Mengersen K. 2013. Handbook of meta-analysis in ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- 31.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 32.Webb CO, Donoghue MJ. 2005. Phylomatic: tree assembly for applied phylogenetics. Mol. Ecol. Notes 5, 181–183. ( 10.1111/j.1471-8286.2004.00829.x) [DOI] [Google Scholar]
- 33.Revell LJ.2016. ‘phytools’: Phylogenetic tools for comparative biology (and other things). https://cran.r-project.org/web/packages/phytools/index.html .
- 34.Paradis E, et al. 2015. ‘ape’: analyses of phylogenetics and evolution. https://cran.r-project.org/web/packages/phytools/index.html .
- 35.Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A. 2013. multcomp: simultaneous inference in general parametric models. Version 1.3-0. http://cran.r-project.org/web/packages/multcomp/index.html. [DOI] [PubMed] [Google Scholar]
- 36.Chen YH, Gols R, Stratton CA, Brevik KA, Benrey B. 2015. Complex tritrophic interactions in response to crop domestication: predictions from the wild. Entomol. Exp. Appl. 157, 40–59. ( 10.1111/eea.12344) [DOI] [Google Scholar]
- 37.Carmona D, Lajeunesse MJ, Johnson MTJ. 2011. Plant traits that predict resistance to herbivores. Funct. Ecol. 25, 358–367. ( 10.1111/j.1365-2435.2010.01794.x) [DOI] [Google Scholar]
- 38.Behmer ST. 2009. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 54, 165–187. ( 10.1146/annurev.ento.54.110807.090537) [DOI] [PubMed] [Google Scholar]
- 39.Parker IM, López I, Petersen JJ, Anaya N, Cubilla-Rios L, Potter D. 2010. Domestication syndrome in caimito (Chrysophyllum cainito L.): fruit and seed characteristics. Econ. Bot. 64, 161–175. ( 10.1007/s12231-010-9121-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dudareva N, Negre F, Nagegowda DA, Orlova I. 2006. Plant volatiles: recent advances and future perspectives. CRC. Crit. Rev. Plant Sci. 25, 417–440. ( 10.1080/07352680600899973) [DOI] [Google Scholar]
- 41.Karban R, Baldwin IT. 1997. Induced responses to herbivory. Chicago, IL: University of Chicago Press. [Google Scholar]
- 42.Gols R, Bukovinszky T, Van Dam NM, Dicke M, Bullock JM, Harvey JA. 2008. Performance of generalist and specialist herbivores and their endoparasitoids differs on cultivated and wild Brassica populations. J. Chem. Ecol. 34, 132–143. ( 10.1007/s10886-008-9429-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen YH, Welter SC. 2007. Crop domestication creates a refuge from parasitism for a native moth. J. Appl. Ecol. 44, 238–245. ( 10.1111/j.1365-2664.2006.01255.x) [DOI] [Google Scholar]
- 44.Rowen E, Kaplan I, Rowen E. 2016. Eco-evolutionary factors drive induced plant volatiles: a meta-analysis. New Phytol. 210, 284–294. ( 10.1111/nph.13804) [DOI] [PubMed] [Google Scholar]
- 45.Furbank RT, Tester M. 2011. Phenomics— technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 16, 635–644. ( 10.1016/j.tplants.2011.09.005) [DOI] [PubMed] [Google Scholar]
- 46.Hall RD. 2006. Plant metabolomics: from holistic hope, to hype, to hot topic. New Phytol. 169, 453–468. ( 10.1111/j.1469-8137.2005.01632.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The assembled database and analysis code supporting this article are included as part of the electronic supplementary material.