Abstract
Life-history traits are generally assumed to be inherited quantitatively. Fishing that targets large, old individuals is expected to decrease age at maturity. In Atlantic salmon (Salmo salar), it has recently been discovered that sea age at maturity is under strong control by a single locus with sexually dimorphic expression of heterozygotes, which makes it less intuitive to predict how life histories respond to selective fishing. We explore evolutionary responses to fishing in Atlantic salmon, using eco-evolutionary simulations with two alternative scenarios for the genetic architecture of age at maturity: (i) control by multiple loci with additive effects and (ii) control by one locus with sexually dimorphic expression. We show that multi-locus control leads to unidirectional evolution towards earlier maturation, whereas single-locus control causes largely divergent and disruptive evolution of age at maturity without a clear phenotypic trend but a wide range of alternative evolutionary trajectories and greater trait variability within trajectories. Our results indicate that the range of evolutionary responses to selective fishing can be wider than previously thought and that a lack of phenotypic trend need not imply that evolution has not occurred. These findings underscore the role of genetic architecture of life-history traits in understanding how human-induced selection can shape target populations.
This article is part of the themed issue ‘Human influences on evolution, and the ecological and societal consequences’.
Keywords: age at maturation, Atlantic salmon, dimorphism, fisheries-induced evolution, Salmo salar, Vgll3TOP
1. Introduction
Fitness-related life-history traits are typically assumed to be coded by many loci with small additive effects [1] and to be moderately heritable [2]. In the context of fisheries-induced evolution, predictions of fish life-history change are based on the assumption that life-history traits are inherited quantitatively, such that juvenile trait values are expected to be equal to the average of parental trait values. For example, virtually all eco-evolutionary simulations either model the inheritance of life-history traits through a large number of loci with additive effects [3,4] or through continuous trait values [5,6], and assuming that the heritability of the quantitative traits is approximately 0.2–0.3 [7]. These assumptions are sound and in accordance with general knowledge of the genetic basis of life-history traits [1,2]. Nonetheless, the occurrence and the nature of fisheries-induced evolution might substantially differ if the genetic architecture of the traits under selection differs from that which is typically assumed.
Atlantic salmon (Salmo salar) age at maturity, based on data available for Norwegian and Finnish populations, was recently discovered to be largely controlled by one locus with two alleles, such that the locus explained about 40% of variation in the age at maturity [8]. Homozygotes for the early maturity allele tend to mature at the age of one sea winter (1 SW), whereas homozygotes for the late maturity allele often mature only at the age of 3 SW. The expression of the locus was also found to be sexually dimorphic for heterozygotes, such that female heterozygotes tended to postpone maturity, whereas male heterozygotes matured early [8]. These results present an interesting insight into the mechanisms by which natural populations maintain genetic and phenotypic diversity. However, they also make it difficult to intuitively predict whether and how life histories might respond to selective fishing mortality.
Atlantic salmon is an anadromous species for which many males (depending on the population), and almost all females, spend a juvenile phase in fresh water, a feeding migration to the sea and a spawning migration back to the natal river [9,10]. Based on studies of another salmonid, Chinook salmon (Oncorhynchus tshawytscha), coastal fishing that targets only the returning mature adults is generally not expected to have substantial evolutionary consequences, whereas a fishery on the feeding grounds which targets large individuals should favour early maturity, thus increasing the probability of reproduction before being caught by the fishery [11]. Using Atlantic salmon as a study system, the objective of the present study is to investigate how the evolutionary consequences of selective fishing can depend on the genetic architecture of a key viability trait under selection: age at maturity. To this end, we simulate eco-evolutionary dynamics of a salmon population exposed to selective fishing with two alternative scenarios for the genetic basis of age at maturity: (i) single-locus control, based on the maturation probability estimates by Barson et al. [8] and shown in table 1; and (ii) control by additive effects of 10 independent loci, mimicking traditionally assumed inheritance of quantitative traits.
Table 1.
The probabilities of postponing maturity beyond threshold ages for Vgll3TOP genotype × sex, as estimated by Barson et al. [8]. These probabilities were used in our model, where the age at maturity was coded by one locus having two alleles (0 and 1).
| sex | homozygote (11) | heterozygote (10 or 01) | homozygote (00) |
|---|---|---|---|
| female | |||
| 2 SW → 3 SW | 0.754 | 0.949 | 0.983 |
| 1 SW → 2 SW | 0.101 | 0.404 | 0.665 |
| male | |||
| 2 SW → 3 SW | 0.266 | 0.277 | 0.835 |
| 1 SW → 2 SW | 0.058 | 0.061 | 0.467 |
2. Material and methods
We parametrize an eco-evolutionary simulation model for Atlantic salmon, such that the genetic basis of age at maturity is coded in two alternative ways. The eco-evolutionary dynamics of a salmon population is simulated through time, allowing for dynamic equilibrium conditions to be attained, followed by a period of fishing and then a period of recovery in the absence of fishing. Through the simulations, populations were allowed to evolve, following basic Mendelian inheritance.
(a). Genetic basis for age at maturity
Single-locus control of age at maturity followed the gene expression pattern detected for Atlantic salmon by Barson et al. [8]. To this end, we coded maturity to depend on one locus having two alleles (0, 1). Of females and males, the probabilities for postponing maturity from 1 to 2 SW, and from 2 to 3 SW, differed between homozygote genotypes for allele 0, heterozygotes, and homozygotes for allele 1, according to the values given in table 1. Sexual dimorphism was manifest by heterozygotes, such that male heterozygotes generally matured early, whereas female heterozygotes tended to postpone maturity [8].
The alternative scenario, in which age at maturity was coded by multiple loci with small additive affects, was modelled through the use of 10 independent loci having two alleles each (again coded by 0 and 1). The choice of the number of loci is purely technical, as this number is sufficient to produce a pattern smooth enough to be interpretable. The genetic trait value was obtained through a sum of the alleles across the 10 loci (0–20); a random number was added to this (generated from a normal distribution with mean 0 and the standard deviation of 2) to generate phenotypic variation about the genotypes. The phenotypic trait value obtained in this manner was then linearly translated to maturity at the age of 1 SW, 2 SW or 3 SW, respectively (trait values 0–6.7 → 1 SW, 6.7–13.3 → 2 SW or 13.3–20 → 3 SW). The amount of phenotypic variation added to the generic trait value was scaled to yield a heritability of approximately 0.4 to roughly match the finding by Barson et al. [8] that one locus accounted for approximately 40% of the variation in age at maturity in salmon.
(b). Demographic rates
Salmon population dynamics were simulated through time at annual time steps. At each step, the processes of mortality, maturation and offspring production were simulated on an individual basis. Survival of eggs to the smolt, seaward-migrating stage was set to 0.015 and smolt-to-grilse survival to 0.07 [10]. After the age of 1 SW, annual survival depended on maturity, such that survival was 0.7 for immature fish and 0.2 for mature fish [10,12,13]. As these same survival parameters lead to different adaptive optima between single- and multi-locus scenarios, for the latter scenario we tested two alternative survival parameter sets (immature survival 0.8, mature survival 0.1 or 0.2) under which age at maturity evolved in the multi-locus scenario to levels similar to those under the single-locus scenario in accordance with the original mortality parameters (see the electronic supplementary material for further details). No sexual selection was assumed, such that for each mature female a mature male was assigned randomly. Female fecundity depended on the age of the fish. Maximum egg production was set to 3040, 7560 and 10 200 for 1 SW, 2 SW and 3SW and older, respectively [14]. Density-dependence in fecundity was accounted for by scaling the above egg production rates by a logistic equation (e5–10×N/K)/(1 + e5–10×N/K) consistent with stock : recruitment (parent : offspring) relationships for this species (e.g. [15]). While the exact shape of the equation is rather arbitrary, our key objective was simply to have an empirically defensible means of limiting fecundity at high population abundance.
(c). Simulation design
Simulated populations were first allowed to settle into dynamic equilibrium and to adapt, such that genetic stability was reached. A set of 100 adapted populations was recorded for each of the two maturity control scenarios. Main simulations were initiated by randomly sampling one of these pre-adapted populations and simulating its dynamics for another 100 years to ensure stability. After this, the population was exposed to fishing for a period of 100 years, followed by a recovery period of 100 years in the absence of fishing. Although all 1 SW and older individuals were vulnerable to fishing, fishing selectively targeted older individuals, such that the relative selectivity was set to 15%, 50% and 100% for 1 SW, 2 SW and 3 SW, respectively. The chosen fishing selectivities were not intended to mimic the full complexity of salmon fishing selectivities, but to simply create a selection pressure towards earlier age at maturity, such that we could focus on investigating evolutionary responses in this trait. Overall fishing mortality scenarios corresponded to the removal of 35%, 40% or 45% of the targeted fishes (1 SW and older). Each combination of fishing duration and mortality was replicated 50 times for both the maturity control scenarios.
3. Results
The life-history response to fisheries-induced selection depended on the underlying genetic architecture of age at maturity. In the multi-locus scenarios, age at maturity exhibited a clear decline with a comparatively low variability among the simulated runs (figure 1). By contrast, when maturation was under single-locus control, the responses in mean trait value were smaller but differentiation among replicated simulation runs was substantial.
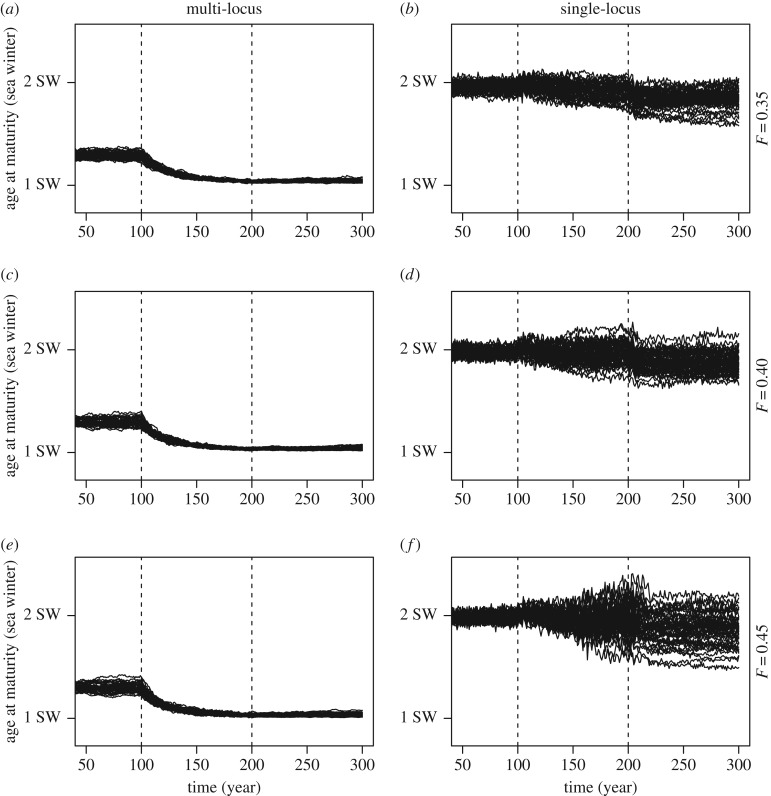
Figure 1.
The development of age at maturity in Atlantic salmon under two contrasted scenarios (multi- and single-locus) for the genetic architecture of the trait. Alternative fishing mortalities (F) are indicated on the right of each pair of graphs; the beginning and end of the fishing period are indicated by dashed vertical lines. Each individual line represents one replicated simulation line (N = 50).
In the multi-locus scenario, the average age at maturity was 1.29 SW at the onset of fishing (1 SW: 73.3%, 2 SW: 26.2%, 3 SW: 0.4%), declining by approximately 20% to 1.04 SW, 1.03 SW and 1.03 SW for fishing pressures F = 0.35, F = 0.40 and F = 0.45, respectively (figure 1a,c,e). In the single-locus scenarios, age at maturity declined marginally from an initial value of 1.97 SW (1 SW: 30.4%, 2 SW: 48.9%, 3 SW: 20.7%) to 1.92 SW, 1.94 SW and 1.93 SW (figure 1b,d,f). The width of the group of replicated simulations was always much wider in the single-locus scenario when compared with that of the multi-locus scenario (figure 1), suggesting that changes in age at maturity under the multi-locus scenario, as a selection response to fishing, is much more predictable than that under the single-locus scenario, for which replicated runs could lead to an increase, no change, or a decrease in age at maturity by chance alone. The evolutionary response to fishing under the single-locus scenario was largely disruptive, i.e. reflecting trait diversification, whereas in the multi-locus scenario it was unidirectional. (Results derived using survival parameters that lead to similar initial levels of age at maturity in the multi-locus scenario as in the single-locus scenario were analogous to those in the original multi-locus simulations. This underscores the point that the difference in start conditions for age at maturity between the original single- and multi-locus simulations was not responsible for the differences in the evolutionary responses to harvesting; electronic supplementary material, figure S1.)
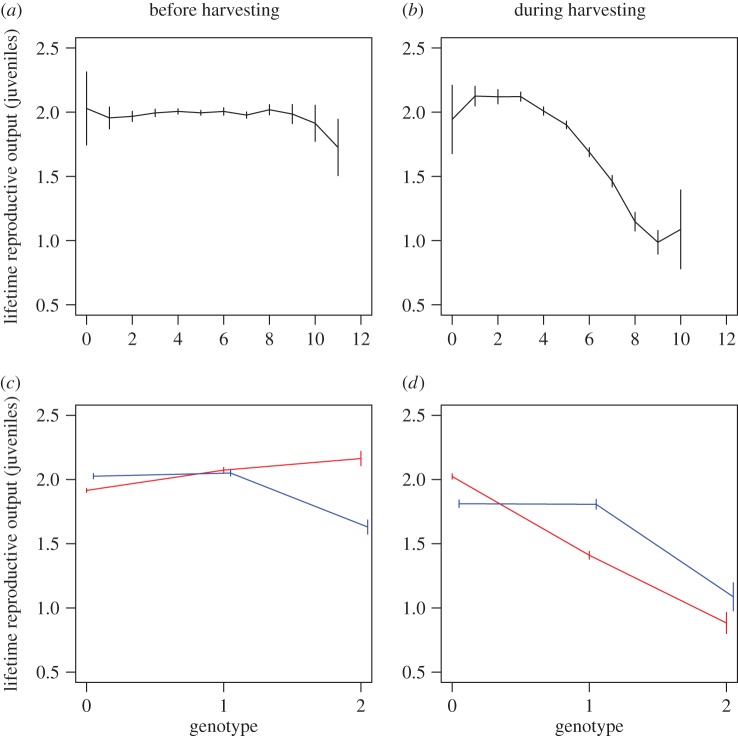
Disparate responses to fisheries-induced selection are illustrated by the fitness functions (figure 2). In the multi-locus scenario, in the absence of fishing lifetime reproductive output is markedly flat across a range of genotypes, but when exposed to fishing, lifetime fitness of late-maturing genotypes declines considerably. By contrast, in the single-locus scenario, in the absence of fishing, fitness functions are fairly flat across the three alternative genotypes and relatively similar between sexes (except for late-maturing males), whereas when exposed to selective fishing, the patterns of fitness differ between sexes and the fitness of late-maturing genotypes is reduced compared with the other genotypes (figure 2).
Figure 2.
Fitness estimated through lifetime reproductive output for the multi- (a,b) and single-locus scenarios (c,d) as a function of genotype across 50 replicated simulation runs at fishing mortality F = 0.4. Fitness functions are shown separately for the non-harvested population at its dynamic and adaptive equilibrium (a,c) and for the first 10 years of fishing (b,d). For the multi-locus scenario, genotypes are expressed by the sum of allelic values (0 or 1) across 10 diploid loci. For the single-locus scenario, genotype is 0, 1 (heterozygote) or 2, and the genotype expression depends on the sex. 95% CIs are indicated by vertical lines. In the single-locus scenario, females are indicated by red and males by blue. (Online version in colour.)
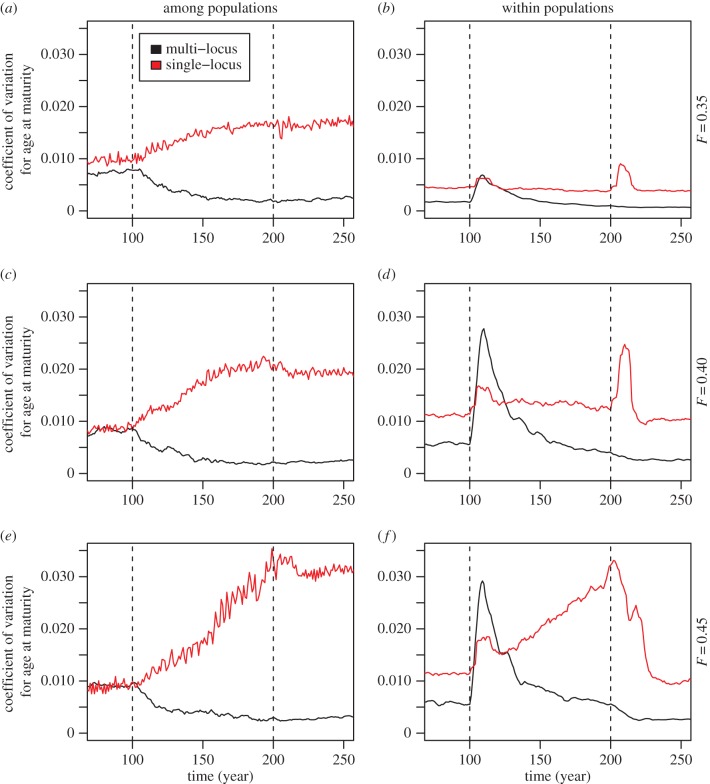
To standardize for differences in average trait values between the two scenarios, coefficients of variation (CV) for age at maturity were calculated for each simulation time point (year) across the replicated runs. During the fishing period, CVs markedly increased in the single-locus scenario when compared with the decline in CVs in the multi-locus scenario (figure 3a,c,e). The increases in CV for the single-locus scenario were further increased by fishing mortality, such that the higher the fishing mortality, the higher the rate of divergent evolution among replicated simulation runs (i.e. simulated populations), as reflected by the relative changes in single-locus scenario CVs during fishing (+64%, +113%, +245% for F = 0.35, F = 0.40, F = 0.45, respectively) as compared to the corresponding changes in CVs in the multi-locus scenario (−74%, −69%, −58%). Variability within individual simulations runs was estimated by calculating, at each simulation step, a running CV across the 10 previous years. Phenotypic variability within individual simulation runs was already higher for the single-locus scenario than for the multi-locus scenario under the dynamic equilibrium conditions (cf. CVs prior to and including year 100, figure 3b,d,f), and this difference was further increased by fishing mortalities F = 0.40 and F = 0.45. Notably, disruptive evolution within evolutionary trajectories also increased in the multi-locus scenario with increased fishing mortality, although the increase was smaller than in the single-locus scenario, except for the very beginning of the fishing period (figure 3).
Figure 3.
Coefficients of variation (CV) for age at maturity across the progress of simulations. CVs are calculated both among simulated populations (i.e. simulation runs; a,c,e) and within populations (i.e. runs; b,d,f). Within-run CV is calculated at each time point across the previous 10 years. Simulations illustrating single-locus control of the trait are in red and those illustrating multi-locus control in black. Alternative fishing mortalities (F) are indicated at the right of each row; the beginning and end of the fishing period are indicated by dashed vertical lines. (Online version in colour.)
After fishing had ended, age at maturity remained fairly stable throughout the 100-year recovery period. Also, the differences in phenotypic variability induced by fishing among populations (i.e. simulation runs) in the single-locus scenario persisted in the absence of fishing (figure 3). Thus, natural selection was not able to reverse the divergent evolutionary effect of fishing during the simulated century in the absence of fishing that followed the fishing period. Ecological recovery in turn was fast: the populations re-built their abundances within about 10 years after the end of fishing. After the end of fishing, within-population phenotypic variabilities returned to their pre-fishing levels, and variability in the single-locus scenario remained at a higher level than in the multi-locus scenario.
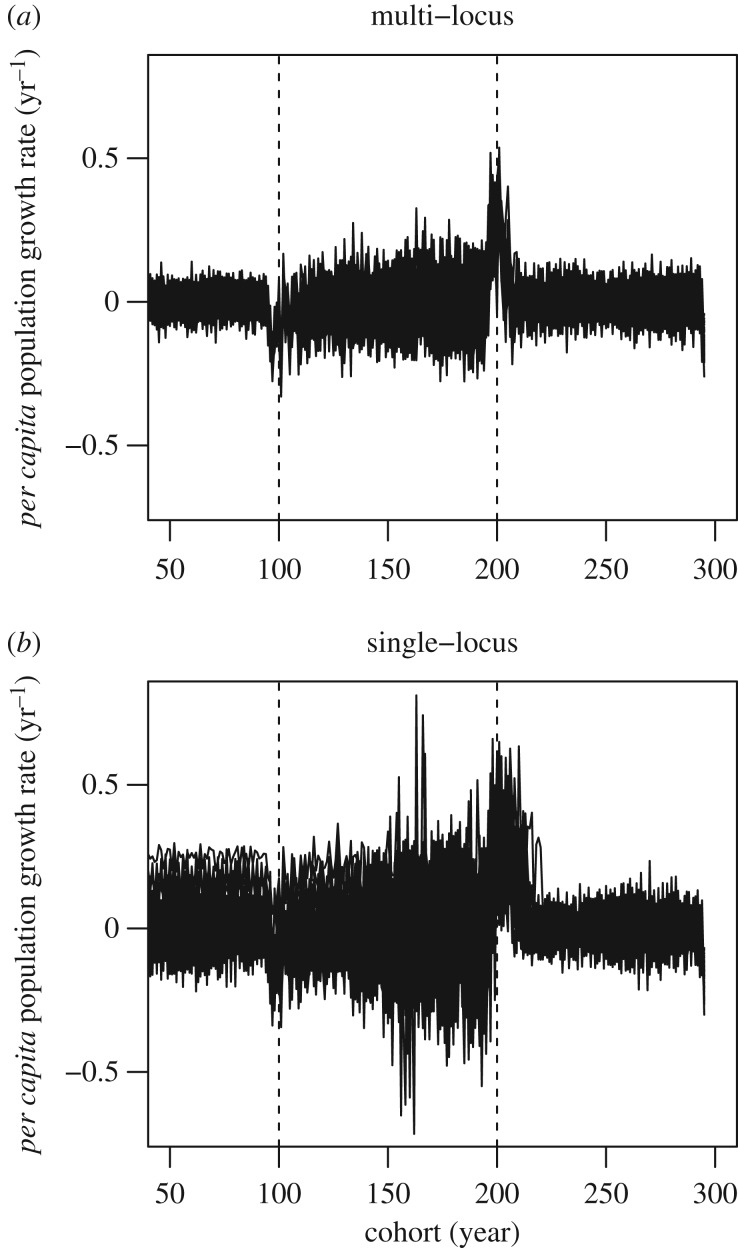
Ecological differences between the maturation scenarios were mostly manifest at the highest fishing mortality (F = 0.45), such that in multi-locus scenarios populations tolerated the 100-year fishing periods by declining to about 25% of the pre-fishing abundances, whereas in the single-locus scenario, populations that survived the fishing period declined very close to collapse by the end of fishing (typically at abundances 1–3% of pre-fishing levels) and several of the runs could not tolerate fishing without going extinct. The extinction rate was about 16%. The above presented evolutionary results are derived only from runs that did not go extinct, as the extinct ones would not have provided comparable information about the evolutionary dynamics under the chosen fishing pressure. Lower recovery ability under the single-locus control scenario was further reflected by per capita population growth rates that generally varied more during fishing in the single-locus scenario than in the multi-locus scenario (figure 4).
Figure 4.
Per capita population growth rates (r) plotted against cohort (x-axis) illustrated for (a) multi- and (b) single-locus control for age at maturity at a fishing mortality of F = 0.45. The beginning and end of the fishing period are indicated by dashed vertical lines.
4. Discussion
The present study suggests that the nature of fisheries-induced evolution depends on the underlying genetic architecture of the trait under selection. If Atlantic salmon age at maturity was under the control of one sexually dimorphically expressed locus, then evolutionary responses to selective fishing were largely divergent and also disruptive. These findings are in contrast with the typical expectation that selective harvesting targeting large individuals should shift life histories directionally towards earlier age at maturity [7,16], which here was shown to be the case only if the trait under selection was controlled by several loci with small additive effects. The study of fisheries-induced evolution is strongly driven by empirically documented trends for decreasing trends in the age at maturity in many commercially exploited fish stocks [17–19]. Interestingly, according to several reviews [20–24], evidence of a link between reductions in age at maturity and fisheries-induced evolution has not been forthcoming in Atlantic salmon. In light of our findings and the recently detected pattern of gene expression for Atlantic salmon age at maturity [8], the present study suggests that the range of evolutionary responses to fisheries-induced selection can be wider than previously thought and that a lack of phenotypic trend need not imply that no evolution caused by fishing has occurred.
As explained above, the majority of studies of fisheries-induced evolution deal with erosion of trait variability through directional changes in fish life histories towards earlier maturation and, subsequently, smaller adult body sizes [17–19]. As opposed to directional trait changes, in our simulations the single-locus control scenario lead to an increased ‘scattering’ of the ages at maturity both within and among populations, suggesting that fisheries-induced evolution was increasingly characterized by patterns approaching that typical of disruptive and divergent selection, respectively [1]. A rare empirical observation of fisheries-induced disruptive selection has been made in a 50-year time series of Windermere pike (Esox lucius) [25]. Pike phenotypic trait variance was seen to increase along with intensive fishing, and this was speculated to arise as a combination of selection for, on the one hand, late entry to fisheries but, on the other hand, fast growth of fish vulnerable to fisheries [25]. Similar indications of fisheries selectivity being disruptive have also been provided by theoretical analyses of adaptive fish population dynamics [26]. While these studies suggest that fisheries-induced evolution can indeed be disruptive due to counteracting components of fisheries selection [27,28], our study goes further by demonstrating that fisheries-induced disruptive evolution can arise also as a consequence of the genetic architecture of the trait targeted by fishing.
From a practical point of view, divergent evolution driven by harvesting suggests that the evolutionary outcome of a specific harvesting strategy can be highly uncertain. As illustrated by replicated simulation runs in figure 1, populations can be driven to alternative evolutionary trajectories just by chance. In other words, completely similar populations can respond to fishing very differently and life-history differences among populations need not imply that the populations were exposed to different mortality regimes. While unpredictability of responses to fishing can be disadvantageous for fisheries management and planning of sustainable fishing strategies, generally phenotypic variability is considered advantageous by harbouring a broad range of phenotypes and, thus, increasing capacity to adapt to new directional selection regimes or variable environmental conditions [1,8,25]. Notably, however, the present study also demonstrated how fish population resilience to fishing was negatively associated with the genetic control leading to divergent and disruptive evolution (figure 4). While these evolutionary patterns might be beneficial in order to preserve or even diversify trait variability within and among populations, benefits associated with it are less clear when viewed from an ecological perspective.
5. Limitations and future directions
Being primarily a simplified simulation approach contrasting two extreme scenarios, our study involves many limiting assumptions. Our single-locus scenario for the genetic control of age at maturity was based on conclusions drawn about the impacts of one gene [8]. It cannot, therefore, be ruled out that Atlantic salmon maturity is also affected by other loci with smaller effects. However, the detected single locus explained about 40% of variation in Atlantic salmon age at maturity and heritability of fitness-related life-history traits is unlikely to be much higher [23,24], such that the existence of other genes with substantial effects seems unlikely. Assuming one-locus control was, therefore, feasible for the purpose of the present study to contrast two qualitatively different scenarios and explore the evolutionary consequences arising therefrom. In reality, however, even if maturity was largely affected by just one gene, this observation does not rule out the possibility of indirect effects of other genes via, for example, body size or growth. Ongoing research on the salmon genome is likely to provide a more coherent picture of the nature of genetic control of key life-history traits in Atlantic salmon. Our study provides one example of the upscaling of such observations to an exploration of their feedbacks on eco-evolutionary dynamics.
Similarly, salmon life-history dynamics and salmon fisheries were in many ways simplified in this study. In reality, sexual selection is likely to modify natural selection of life histories, and this might also affect the evolutionary responses to fishing [27]. Similarly, the contribution of precociously mature males at the parr stage [28] on juvenile production or genetic variability was not considered. Depending on whether and how precocious maturation correlates with other life-history properties, the genetic composition of the juvenile cohort might be affected, yet comparatively little about this is known. Fisheries were assumed to take place at feeding grounds, yet salmon are often also harvested during their spawning migration. All these simplifications were made in order to narrow the focus of the study to a comparison of two alternative scenarios of the genetic architecture of age at maturity in the presence of selection towards earlier maturity. As such, the results can be readily contrasted to eco-gen simulations predicting evolutionary responses to fishing [3–6]. The focus of the study and its comparability would have been substantially obscured had we included additional components of selection and a more complicated fishery model. However, because of these limitations, this study by no means aims to mimic salmon eco-evolutionary dynamics in its full realistic complexity. Instead, the present study serves as a demonstration of qualitative differences in the evolution of a trait coded by one dimorphic locus as compared to a trait coded by many loci with small additive effects.
Our findings, combined with evidence of a strong influence of one locus on maturity in Atlantic salmon [8], challenge the common expectation that fisheries that selectively target larger, older individuals should lead to a reduction in age at maturity in the exploited populations [20–22]. Instead, depending on the genetic control of the traits under selection, evolutionary responses to harvesting can be difficult to predict, thus limiting the conclusions that can be firmly drawn about the drivers of evolution based solely on the evolutionary trajectory of a population.
Our findings underscore the importance of understanding the genetic basis of life-history traits when assessing how harvesting might affect targeted populations. Simple detection of life-history trends is clearly insufficient to draw meaningful conclusions for or against the existence of harvest-induced evolution. Rigorous attempts to combine and link genomics, life-history traits (including potential genetic correlations between maturity and growth), and population dynamics are necessary to obtain a deeper understanding of eco-evolutionary dynamics in natural populations.
6. Conclusion
The present study highlights the need to understand the genetic architecture of life-history traits in order to understand evolutionary processes driven by natural and anthropogenic selection. The strong, sexually dimorphic effect of a single locus on Atlantic salmon maturity [8] serves as a pilot study to challenge traditional quantitative-genetic perspectives in animal breeding and adaptation of natural populations. In the context of harvest-induced evolution, this study demonstrates that knowledge of the genetic control underlying the traits under selection is imperative [29] to understand and predict how alternative harvesting strategies might shape the target populations. Our study broadens the concept of harvesting-induced evolution by showing that, in addition to directional phenotypic evolution, harvesting can also lead to evolutionarily divergent and disruptive responses. Thus, the nature of harvest-induced evolution is more complicated than previously thought.
Supplementary Material
Acknowledgement
We thank Tutku Aykanat for providing us with the probability estimates presented in table 1.
Authors' contributions
The manuscript was jointly written by A.K. and J.A.H. Simulations and analyses were carried out by A.K.
Competing interests
We have no competing interests.
Funding
A.K.: Academy of Finland; Natural Sciences and Engineering Research Council of Canada (NSERC). J.A.H.: Natural Sciences and Engineering Research Council of Canada (NSERC).
References
- 1.Roff DA. 2002. Life history evolution. Sunderland, MA: Sinauer. [Google Scholar]
- 2.Mousseau TA, Roff DA. 1987. Natural selection and the heritability of fitness components. Heredity 59, 181–197. ( 10.1038/hdy.1987.113) [DOI] [PubMed] [Google Scholar]
- 3.Wang H-Y, Höök TO. 2010. Eco-genetic model to explore fishing-induced ecological and evolutionary effects on growth and maturation schedules. Evol. Appl. 2, 438–455. ( 10.1111/j.1752-4571.2009.00088.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuparinen A, Hutchings JA. 2012. Consequences of fisheries-induced evolution for population productivity and recovery potential. Proc. R. Soc. B 279, 2571–2579. ( 10.1098/rspb.2012.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlop ES, Heino M, Dieckmann U. 2009. Eco-genetic modeling of contemporary life-history evolution. Ecol. Appl. 19, 1815–1834. ( 10.1890/08-1404.1) [DOI] [PubMed] [Google Scholar]
- 6.Enberg K, Jørgensen C, Dunlop ES, Heino M, Dieckmann U. 2009. Implications of fisheries-induced evolution for stock rebuilding and recovery. Evol. Appl. 2, 394–414. ( 10.1111/j.1752-4571.2009.00077.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law R. 2007. Fisheries-induced evolution: present status and future directions. Mar. Ecol. Prog. Ser. 335, 271–277. ( 10.3354/meps335271) [DOI] [Google Scholar]
- 8.Barson NJ, et al. 2015. Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature 528, 405–408. ( 10.1038/nature16062) [DOI] [PubMed] [Google Scholar]
- 9.Marschall EA, Quinn TP, Roff DA, Hutchings JA, Metcalfe NB, Bakke TA, Saunders RL, Poff NL. 1998. A framework for understanding Atlantic salmon (Salmo salar) life history. Can. J. Fish. Aquat. Sci. 55, 48–58. ( 10.1139/d98-007) [DOI] [Google Scholar]
- 10.Hutchings JA, Jones MEB. 1998. Life history variation and growth rate thresholds for maturity in Atlantic salmon, Salmo salar. Can. J. Fish. Aquat. Sci. 55, 22–47. ( 10.1139/d98-004) [DOI] [Google Scholar]
- 11.Eldridge WH, Hard JJ, Naish KA. 2010. Simulating fishery-induced evolution in chinook salmon: the role of gear, location, and genetic correlation among traits. Ecol. Appl. 20, 1936–1948. ( 10.1890/09-1186.1) [DOI] [PubMed] [Google Scholar]
- 12.Williams JG, Zabel RW, Waples RS, Hutchings JA, Connor WP. 2008. Potential for anthropogenic disturbances to influence evolutionary change in the life history of a threatened salmonid. Evol. Appl. 1, 271–285. ( 10.1111/j.1752-4571.2008.00027.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempson JB, O'Connell MF, Schwarz CJ. 2004. Spatial and temporal trends in abundance of Atlantic salmon, Salmo salar, in Newfoundland with emphasis on impacts of the 1992 closure of the commercial fishery. Fish. Man. Ecol. 11, 387–402. ( 10.1111/j.1365-2400.2004.00407.x) [DOI] [Google Scholar]
- 14.Legault CM. 2004. Salmon PVA: a population viability analysis model for Atlantic salmon in the Maine distinct population segment, Northeast Fisheries Science Center Reference Document 04-02 Woods Hole, MA: US. Department of Commerce. [Google Scholar]
- 15.Hindar K, Hutchings JA, Diserud OH, Fiske P. 2011. Stock, recruitment and exploitation. In Atlantic salmon ecology (eds Aas O, Klemetsen A, Einum S, Skurdal J), pp. 299–331. London, UK: Wiley-Blackwell. [Google Scholar]
- 16.Kuparinen A, Festa-Bianchet M. 2017. Harvest-induced evolution: insights from aquatic and terrestrial systems. Phil. Trans. R. Soc. B 372, 20160036 ( 10.1098/rstb.2016.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharpe DMT, Hendry AP. 2009. Life history change in commercially exploited fish stocks: an analysis of trends across studies. Evol. Appl. 2, 260–275. ( 10.1111/j.1752-4571.2009.00080.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devine JA, Wright PJ, Pardoe HE, Heino M. 2012. Comparing rates of contemporary evolution in life-history traits for exploited fish stocks. Can. J. Fish. Aquat. Sci. 69, 1105–1120. ( 10.1139/f2012-047) [DOI] [Google Scholar]
- 19.Audzijonyte A, Kuparinen A, Fulton EA. 2013. How fast is fisheries-induced evolution? Quantitative analysis of modelling and empirical studies. Evol. Appl. 6, 585–595. ( 10.1111/eva.12044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieckmann U, Heino M. 2007. Probabilistic maturation reaction norms: their history, strengths, and limitations. Mar. Ecol. Prog. Ser. 335, 253–269. ( 10.3354/meps335253) [DOI] [Google Scholar]
- 21.Hutchings JA, Fraser DJ. 2008. The nature of fisheries- and farming-induced evolution. Mol. Ecol. 17, 294–313. ( 10.1111/j.1365-294X.2007.03485.x) [DOI] [PubMed] [Google Scholar]
- 22.Heino M, Diaz Pauli B, Dieckmann U. 2015. Fisheries-induced evolution. Ann. Rev. Ecol. Evol. Syst. 46, 461–480. ( 10.1146/annurev-ecolsys-112414-054339) [DOI] [Google Scholar]
- 23.Gjerde B. 1994. Response to individual selection for age at sexual maturity in Atlantic salmon. Aquaculture 38, 229–240. ( 10.1016/0044-8486(84)90147-9) [DOI] [Google Scholar]
- 24.García de Leániz C, et al. 2007. Local adaptation. In The Atlantic Salmon: Genetics, Conservation and Management (eds Verspoor E, Stradmeyer L, Nielsen JL), pp. 195–236. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 25.Edeline E, Le Rouzic A, Winfield IJ, Fletcher JM, James JB, Stenseth NC, Vøllestad LA. 2009. Harvest-induced disruptive selection increases variance in fitness-related traits. Proc. R. Soc. B 276, 4163–4171. ( 10.1098/rspb.2009.1106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landi P, Hui C, Dieckmann U. 2015. Fisheries-induced disruptive selection. J. Theor. Biol. 365, 204–216. ( 10.1016/j.jtbi.2014.10.017) [DOI] [PubMed] [Google Scholar]
- 27.Hutchings JA, Rowe S. 2008. Consequences of sexual selection for fisheries-induced evolution: an exploratory analysis. Evol. Appl. 1, 129–136. ( 10.1111/j.1752-4571.2007.00009.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchings JA, Myers RA. 1994. The evolution of alternative mating strategies in variable environments. Evol. Ecol. 8, 256–268. ( 10.1007/BF01238277) [DOI] [Google Scholar]
- 29.Kuparinen A, Merilä J. 2007. Detecting and managing fisheries-induced evolution. Trends. Ecol. Evol. 22, 652–659. ( 10.1016/j.tree.2007.08.011) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.