Abstract
Commercial and recreational harvests create selection pressures for fitness-related phenotypic traits that are partly under genetic control. Consequently, harvesting can drive evolution in targeted traits. However, the quantification of harvest-induced evolutionary life history and phenotypic changes is challenging, because both density-dependent feedback and environmental changes may also affect these changes through phenotypic plasticity. Here, we synthesize current knowledge and uncertainties on six key points: (i) whether or not harvest-induced evolution is happening, (ii) whether or not it is beneficial, (iii) how it shapes biological systems, (iv) how it could be avoided, (v) its importance relative to other drivers of phenotypic changes, and (vi) whether or not it should be explicitly accounted for in management. We do this by reviewing findings from aquatic systems exposed to fishing and terrestrial systems targeted by hunting. Evidence from aquatic systems emphasizes evolutionary effects on age and size at maturity, while in terrestrial systems changes are seen in weapon size and date of parturition. We suggest that while harvest-induced evolution is likely to occur and negatively affect populations, the rate of evolutionary changes and their ecological implications can be managed efficiently by simply reducing harvest intensity.
This article is part of the themed issue ‘Human influences on evolution, and the ecological and societal consequences'.
Keywords: contemporary evolution, eco-evolutionary dynamics, fisheries, hunting, selection, life histories
1. Introduction
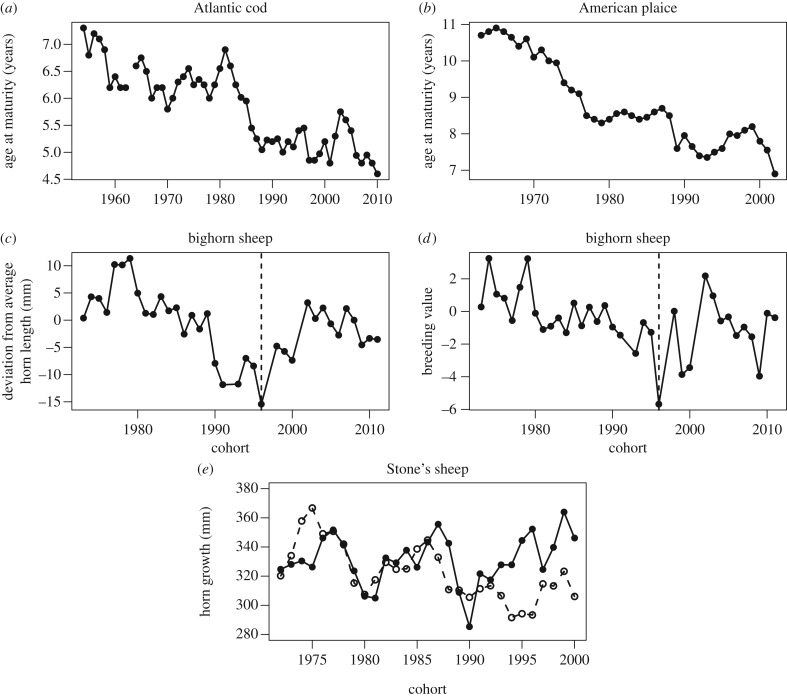
The study of harvest-induced evolution only began to attract substantial scientific attention in the 1990s, even though it had been recognized for a century that harvesting of wild animal populations often selects individuals in a manner opposite to the core principles of animal breeding [1]. Initially, studies focused on measuring the prerequisites of evolution, heritability and selection in wild populations where specific phenotypic traits were targeted by fisheries or hunting [2,3]. For example, commercial fisheries typically target fishes of large size (figure 1), and at times this phenotypic selection is mandated by regulations, including provisions for a minimum landing size and restrictions in fishing gears. Phenotypic traits in fish were known to have a genetic component, leading to predictions that intensive selection coupled with heritable variation should cause evolutionary shifts towards smaller size and earlier maturation in exploited fish stocks [3,8]. This theoretical prediction was supported by empirical observations provided by long time series of fisheries surveys, which confirmed that over time fish had indeed become smaller and an increasing proportion reached maturity at a younger age and smaller size [9].
Figure 1.
Examples of life-history trends in marine and terrestrial harvested populations. Fisheries: age at maturity of Atlantic cod ((a) Gadus morhua; unit 3PS) [4] and American plaice ((b) Hippoglossoides platessoides; unit 2J3 K) [5], plotted against the year the cohort was born. Hunting: changes in age-adjusted deviations from average horn length (c) and breeding value (d) for horn length in cohorts of bighorn sheep (Ovis canadensis) at Ram Mountain, Alberta, Canada [6]. End of harvesting of the population is indicated by the dashed vertical line. Cohort-specific horn growth during the second and third year of life of Stone's sheep (O. dalli) males (e) from the heavily harvested Peace area (dashed line) and lightly harvested Skeena area (solid line) of northern British Columbia, Canada [7].
In terrestrial systems, attention to possible harvest-induced evolution is even more recent, and has mostly focused on ungulates (figure 1), though a rare example of harvest-driven phenotypic changes in plants also exits [10]. Ungulates do not have indeterminate growth and compared with many fishes show much weaker phenotypic variation in the size-maturity relationship, or in the relationship between size and fecundity. Available long-term time series on harvested mammals tend to focus instead on body mass and on the size of male weapons such as horns, antlers and tusks (table 1) [7,11]. In addition, unlike in wild fish, a few long-term studies of terrestrial mammals have established deep pedigrees and measured both trait heritability and selection in the wild, although rarely in hunted populations [6,12].
Table 1.
Key traits underlying differences in harvest-induced evolution driven by commercial fisheries and hunting of terrestrial mammals.
| fisheries | terrestrial mammals | |
|---|---|---|
| growth | indeterminate | determinate |
| variability in age and size at maturity | very large | limited |
| correlation between female mass and fertility | strong | weak |
| harvest strategy | biomass based | number based, often with sex- and age-specific quotas |
| traits primarily targeted by harvest | allowable landing size | trophy traits |
| traits affected by artificial selection | age-size at maturity, body size | weapon size, birth timing |
| evidence of evolution | from common garden experiments, selection experiments | changes in breeding values, harvest records |
| harvest value | quantity | quality |
A key challenge in the interpretation of phenotypic trends in harvested populations is that the plastic changes in phenotype and vital rates expected from harvest-induced reduction in population density are often similar to the expected potential harvest-driven evolution, such that the relative contributions of these two pathways are difficult to disentangle [13–15]. For example, in ungulates, both high adult female mortality and greater resource abundance are expected to lead to earlier primiparity [16–18]. If hunting increases female mortality and lowers population density, its evolutionary and ecological effects will be confused [19].
Harvest-induced evolution has important applied implications, and is a controversial subject. Many fisheries and wildlife managers, decision makers and individual hunters and fishers are reluctant to accept that selective harvest may have evolutionary consequences. The burden of proof is typically with evolutionary ecologists, particularly when evidence of evolutionary effects implies that some management practices are unsustainable and require changes. Initial approaches included the development of probabilistic maturation reaction norms to establish how much of the variability in age-specific probability of maturity was explained by concomitant changes in growth. Growth rate was generally assumed to reflect variation in resource availability driven by changes in population density. On the other hand, changes in age-specific probability of maturity that were independent of changes in growth rate were suggested to reflect potential evolutionary shifts in maturity schedule [19]. One key caveat of this method is that it assumes that growth rate will not co-evolve with changes in age-specific maturity [14]. Other indirect ways to demonstrate the potential for harvest-induced evolution involved the reconstruction of selection differentials [20], analyses of fitness landscapes [2,21] and eco-evolutionary simulations [22].
The increasing theoretical expectation and empirical support for harvest-induced evolution, however, led to a debate about the need for direct evidence of evolution and what constitutes such evidence. Two schools of thought emerged. One advocated a precautionary approach to fisheries management, arguing fisheries-induced evolution to be the most parsimonious explanation for phenotypic trends in exploited populations despite the absence of data on the genetic background of observed changes [15]. A somewhat extreme opposing view demanded direct evidence of changes in the specific genes unambiguously responsible for the traits in question and a demonstration at the gene level that harvest had selected for a change in trait [13]. In fishes, genetic evidence of harvesting-induced changes is very recent and only derived from artificial selection experiments [23,24]. The genetics underlying phenotypic trends have rarely been studied in wild fish. In ungulates, changes in the frequency of alleles that affect antler development in red deer (Cervus elaphus) have been associated with different selective hunting regimes [25]. In the population of bighorn sheep (Ovis canadensis) at Ram Mountain in Canada, a deep pedigree allowed estimation of breeding values for horn length, which declined under intense selective hunting [26] and stopped declining when hunting pressure was first greatly relaxed, then ended (figure 1) [6].
The questions of what constitutes credible evidence for evolution have been extensively discussed elsewhere recently, for example in the context of possible adaptations to climate change [27], and we will not replicate that discussion. Furthermore, we argue that the debate about the genetic versus plastic basis of observed phenotypic changes in harvested species may at times distract from the urgent considerations of the strong ecological consequences that these life-history changes have on populations, communities and ecosystems [28,29]. Here, we update the current status of scientific knowledge of harvest-induced evolution, based upon a literature survey focused on six key questions. We explore areas where a general scientific consensus has been reached and examine some of the key sources of uncertainty in our theoretical understanding of harvest-induced evolution, and their implications for conservation and management. We provide insights from fishes and terrestrial mammals, expecting that the fundamental difference in body growth, indeterminate in most fishes and determinate in nearly all mammals, may lead to differences in how these groups respond to selective harvest, both in terms of phenotypic plasticity and evolutionary changes in size and reproductive patterns (table 1).
2. Does harvest-induced evolution happen?
Despite the debate surrounding the paucity of rigorous investigations linking observed phenotypic trends to changes in the genetic composition of harvested populations, generally scientists agree that the essential prerequisites for harvest-induced evolution are often present. Those prerequisites include demonstrated trait heritability and strong artificial selective pressures upon those traits. A recent meta-analysis across aquatic and terrestrial species revealed that human harvests can substantially exceed the mortality caused by other predators, especially among fishes and terrestrial carnivores [30]. High adult mortality alone should select for faster life histories and erode a population's reproductive capacity. These impacts are further amplified when harvests selectively target large and fast-growing individuals [14,31]. Disagreement persists, however, upon the exact rates of harvest-induced evolution and their relative role compared with plastic phenotypic changes.
Rates of evolution predicted by eco-evolutionary simulations are substantially lower than observed rates of phenotypic changes in fishes [32], suggesting that plastic responses to fishing may account for the majority of documented trends. Phenotypic plasticity, however, may also mask underlying evolutionary processes, as reduced population abundance accelerates individual growth and advances maturation and, consequently, leads to smaller adult body sizes, such that evolutionary responses to harvesting can go unnoted [13–15]. In bighorn sheep, a decline in breeding values appeared to account for nearly 20% of the overall decrease in horn size observed over 23 years (figure 1). The study population doubled in size during the study, leading to a large density-dependent decrease in horn size [6]. The same absolute evolutionary effect on horn size, extended over a longer period of intense selective hunting, however, may well explain most of the declines seen in other mountain sheep populations where density has not changed substantially [7,18,33].
Phenotypic trends in a population can have multiple sources other than harvesting and harvest-induced evolution. For example, immigration of phenotypically different individuals led to declining age at maturation and body size in a Eurasian perch (Perca fluviatilis) population in Estonia [34]. Similarly, a drastic decline in chamois (Rupicapra rupicapra) body mass was correlated with warmer spring temperatures and appeared more likely caused by climate change than by selective hunting [35].
Uncertainty about the occurrence and rate of harvest-induced evolution is closely linked to our limited knowledge of the fitness pay-offs of alternative life histories as age- and trait-specific mortality schedules change. While it is relatively easy to quantify how harvests target fitness-related traits, it is much more difficult to assess trade-offs between the targeted traits and other key fitness determinants. We also know little about the relative strengths of artificial and natural selective pressures, especially when they act antagonistically. For example, a key question in fisheries is how advanced maturation and smaller body size may affect lifetime reproductive output. In fishes, small juveniles produced by young, small spawners are typically strongly selected against [36], and this selection is the primary mechanism favouring large adults [37]. Earlier maturation in fishes is also associated with increased survival costs of reproduction [38]. Natural selection normally favours a later age and larger size at maturity to avoid these fitness costs, but intense fishing mortality increases the payoff of early reproduction, because few or no fish survive to the age where those fitness cost would be manifest [3]. Similar conflicts between natural and harvest-induced selection have also been documented in male bighorn sheep for which the negative selective pressure on fast-growing horns through the trophy hunt occurs at the ages of 4–5 years, whereas the benefits of large horns through sexual selection are minor unless a male survives to 7–8 years [26,39].
The rate and direction of evolution will depend on the relative strengths of natural selection, harvest intensity and selectivity [40,41]. Evolutionary changes are notoriously difficult to predict, even with strong directional selection at a particular age or life stage, and an assessment of fitness across the entire lifespan is required. In addition, the fitness function may not always be bell-shaped as expected for traits with a single and clear optimal peak value. If the fitness function is relatively flat across a range of phenotypes [42], the ecological impacts of harvest-induced evolution will be reduced, but so will the rate of evolutionary recovery after the artificial selective pressure of intense harvest stops [2,43].
3. Is harvest-induced evolution beneficial?
Evolutionary changes induced by harvesting are expected to maximize individual fitness under conditions of very high adult mortality. Nonetheless, the absolute fitness benefits depend on the baseline: harvest-induced evolution might increase fitness relative to a scenario where the same harvesting regime elicits no evolutionary changes, but absolute fitness may remain much lower than in the absence of harvesting [44]. If harvesting is relaxed, individual fitness would therefore be expected to be lower than it was before harvesting, constraining population growth. Owing to sharply lower population density, rapidly maturing phenotypes might have an initial advantage through shorter generation time and give the appearance of a faster initial recovery [45]. Eco-evolutionary simulations, however, suggest that fisheries-induced evolution is instead likely to have either negligible effects on population recovery rates at all abundances, due to flat fitness landscapes across alternative life histories [42,43,46], or negative effects through reduced juvenile production [47]. Notably, those fish populations that have shown phenotypic changes under intensive fishing have not recovered from overfishing even after a moratorium [48,49]. Terrestrial systems provide no evidence that harvest-induced evolution aids recovery. For example, intense hunting pressure (45% a year) selected for earlier primiparity in wild boar (Sus scrofa) [50], but the consequences of a subsequent relaxation of harvest are unclear. In moose (Alces alces), earlier birthdates selected for by sport hunting would probably be maladaptive should harvests cease [51]. In red deer, despite theoretical predictions to the contrary [17], age of primiparity was not affected by four decades of moderate (12–14%) harvest in Norway [52].
Multispecies modelling of marine ecosystems has provided further insights into the potential mechanisms affecting a species' ability to recover following fisheries-induced evolution. Decreases in fish body size increase vulnerability to predation and are predicted to increase natural mortality by an amount comparable to doubling the fishing pressure [53]. These predictions are confirmed by empirical observations. For example, several populations of Atlantic cod in the Western Atlantic that are considered to have experienced fishing-induced evolutionary decreases in body size and age at maturation (figure 1) [20,54] have suffered steeply increased predation by grey seals (Halichoerus grypus), which may drive some populations into extinction even after fishing is stopped [49].
Modelling studies and empirical observation, combined with biological theory, suggest that harvest-induced evolution is unlikely to have any positive effects on the mean fitness of affected populations. At the same time, the importance of negative fitness effects on population dynamics remains unclear. In the absence of harvesting, individual fitness functions can be markedly flat across a range of phenotypes [2,42,43], suggesting that the mean population fitness consequences of phenotypic shifts might be limited. On the other hand, selective pressures for evolutionary recovery following the cessation of harvesting are likely to be weak. Harvest-induced phenotypic changes are therefore likely to persist long after harvests cease. Selection experiments have confirmed this prediction for fishes [55]. This pattern is also clearly seen in wild bighorn sheep, as the breeding value for male horn size was steadily reduced by intense trophy hunting, but after hunting ceased it showed no measurable recovery [6]. Slow or negligible phenotypic recovery following harvest-induced evolution may reduce adaptability of populations to environmental changes.
The net population-level costs and benefits of harvest-induced evolution can be estimated through lifetime reproductive output R0 and per capita population growth rate r, the major correlates of extinction risk and population recovery ability [56]. Selection on one trait can cause disadvantageous selection on correlated traits [14] and these effects may interact across the lifespan. For example, even if harvest-induced selection enhanced early-life reproduction, lifetime reproductive output could be reduced through survival costs of reproduction and lower age-specific juvenile production because of reduced body size. In the context of fisheries, the conflict is evident, as fisheries managers estimate population renewal ability based on so-called stock-recruitment relationships that describe juvenile production as a function of mature population biomass. Fisheries-induced evolution might appear to have negligible or even positive effects on stock-recruitment relationships through earlier maturity, but at the same time R0 and r might be reduced by a shortened lifespan [44]. Among terrestrial mammals, genetic correlations among reproductive traits are well known [57]. In bighorn sheep, male horn length, the main target of selective hunting, is positively genetically correlated with several fitness traits in both sexes [39]. Therefore, selective hunting may have unwanted consequences on population dynamics through correlated selection favouring genes that reduce juvenile survival and female fecundity. Although those genetic correlations are clear, there are currently no population-level data available to test this prediction.
4. How does harvest-induced evolution modify biological dynamics?
Both theory and empirical studies suggest that harvest-induced evolution could lead to reduced body size and earlier maturation. These evolutionary changes are unlikely to be reversed rapidly when harvesting ceases, because artificial selective pressures are often much stronger than natural selection [14]. Permanent decreases in body size and in age of maturation, in turn, reduce population equilibrium biomass, or virgin biomass in the absence of harvesting, such that populations may not recover to pre-harvesting abundance [31,43,47,58] and future catches may therefore remain lower. For terrestrial mammals, although theoretical models suggest that even unselective harvests should affect evolution of reproductive strategies by favouring r-strategists [59], the evolutionary consequences of releasing a population from intense harvests have not been explored. It seems reasonable to speculate that populations that are r-selected by intense harvest-induced adult mortality would grow very rapidly if harvest was relaxed, also because of the high resource availability associated with low population density. A severe overshoot of carrying capacity may then ensue, especially in seasonal environments, presumably leading to a selective regime favouring a set of adaptations that may differ from those expected under both the pre-harvested and the heavily harvested regimes.
Apart from their direct consequences on biomass and population recovery, body size and growth rate are fundamental correlates of many behavioural, physiological and life-history traits with important consequences for individuals and populations [60,61]. In fishes, small body size is associated with lack of boldness [62]. Small fish might spend more time hiding and be less efficient foragers, and their mating success and ability to provide parental care might be lowered [63]. In terrestrial mammals, harvest-induced changes in behaviour, including shyness, exploratory tendency and habitat selection are to be expected as they affect vulnerability to hunting, as shown in elk (Cervus canadensis), where individual movement rates and habitat selection affected the probability of surviving the hunting season [64].
From an ecosystem perspective, phenotypic changes affect predator–prey interactions and the time that individuals spend at body sizes more vulnerable to predation. Mesocosm experiments with guppies (Poecilia reticulata) have demonstrated how contemporary life-history changes and alterations in population density can rapidly modify both ecosystem structure and function, through differences in diets of different guppy phenotypes [65]. The higher the trophic level of the affected species, the more likely that harvest-induced changes will have cascading effects [66]. For example, analyses of the Lake Constance food web suggested that fishing-induced changes in fish body sizes and age at maturation destabilize the plankton community and increase temporal variability in fish reproduction. These changes are primarily mediated through reductions in fish body size, which lower the ecosystem-level predator–prey body size ratio, a well-known correlate of food web stability [31]. Similarly, in the California Current increased fluctuations in larval abundance were linked to decreasing age at maturity of fishes [67]. Furthermore, in addition to intrinsically driven instability in species dynamics, harvest-induced life-history changes might also interact with external sources of variability: faster life histories can magnify population oscillations prompted by environmental drivers and reduce resilience to environmental changes and disturbances [68].
Apart from direct impacts on rebuilding population biomass and on average demographic rates, harvest-induced evolution, just like any directional evolution, may also reduce the phenotypic and genotypic diversity of a population [69,70]. This may reduce the ability of populations to adapt to future changes in environmental conditions. Modelling studies suggest that reductions in the effective population size Ne should be expected under heavy harvest [71], but the relative importance of evolution and direct reduction in abundance for changes in Ne remain unclear [72]. Atlantic cod populations that have experienced substantial fishing pressure and parallel life-history changes appear to maintain high levels of genetic diversity [73]. Harvest-induced evolution might actually buffer against losses in genetic diversity by increasing juvenile production by young age classes through earlier maturation [72]. In terrestrial systems, the consequences of selective harvesting for Ne remain largely unexplored. In some ungulates, selective removal of the largest males may increase Ne if it reduced mating skew among males, but the consequences of changes in age structure on male mating success are complex [74]. Removal of the largest males may also have unknown consequences in species where female mate choice is important [75].
5. Can harvest-induced evolution be avoided?
Managers should consider ‘evolutionary sustainable’ harvesting strategies to prevent the long-term negative impacts of harvest-induced evolution. Some level of harvest is usually essential because of economic reasons and the need to provide food for the growing human population. Similarly, recreational fishing and hunting are culturally important and generate substantial revenues that may be directed to conservation [76]. What harvesting strategies are likely to minimize evolutionary impacts?
Clearly, evolution is more likely when harvests are intense, very selective for a given trait, occur over a large area, persist over time and shift mortality schedules to younger adults. Regulations enforcing a minimum harvestable size (or minimum landing size in fisheries) are a key component of harvesting-induced selection favouring small body size and early reproduction. To counteract this trend, upper size limits are often suggested, to increase the survival of large and mature individuals [77]. While theoretically a sound idea, in practice it might be difficult to regulate harvest to completely avoid selection [78]. Secondly, the effectiveness of this method largely depends on the mortality experienced at intermediate sizes: if very few individuals survive to reach the upper size limit, the harvest restriction will have no impact [79]. In many fisheries, even if large fishes are released, their survival may be substantially impaired by injuries, stress during capture and interruptions in nest guarding [80]. In the case of trophy hunting, evolutionary changes are related to the intensity of harvest. For example, apparent evolutionary changes in the horn size of both bighorn and Stone's (O. dalli) sheep have occurred in areas with intense harvest but not where greater restrictions or difficult access reduce the artificial selective pressure [7,33]. In the pedigreed population of bighorn sheep at Ram Mountain, harvest-induced changes in breeding value for horn size ceased when harvest intensity was reduced (figure 1) [6].
While size limits alone might not be sufficient to mitigate harvest-induced evolution, they are likely to be effective if combined with reduced harvest mortality. Because harvest-induced selection typically opposes natural selection, the relative strengths of these selection components determine the direction of phenotypic changes [40]. Even if harvesting remained selective, if harvest mortality was moderate, natural selection is likely to remain stronger. This pattern was demonstrated in Northern pike (Esox lucius) fisheries in lake Windermere (UK): pike growth rates over 50 years were driven by natural selection when fishing intensity was low, but harvest-induced selection prevailed under intensive fishing [40,41]. Similarly, life-table based Atlantic cod fitness analyses suggest that fisheries-induced evolution can be mitigated by simply reducing fishing pressure to the reference points traditionally considered sustainable (but not necessarily followed) in fisheries management [81,82].
No-take reserves, such as marine protected areas, have also been suggested as a method to preserve genetic and phenotypic diversity of harvested populations, because they provide a potential source of unselected individuals [83]. While in theory protected areas may increase population reproductive rates and juvenile survival, their effectiveness to mitigate evolutionary effects of harvesting is heavily dependent on species mobility, the spatial extent of the protected areas [84] and the ability of dispersing individuals to survive and reproduce. For example, sharp increases in bighorn ram harvest late in the hunting season in Alberta suggest that many rams exiting the National Parks seeking breeding opportunity are shot, diminishing the potential for genetic rescue [85]. To prevent fisheries-induced evolution, upper size limits combined with reduced fishing mortality have been found equally or more effective than immigration from protected areas [86].
6. How strong is harvest-induced evolution compared with other drivers of life-history changes?
To quantify harvest-induced evolution, one must disentangle the genetic component of trait changes from phenotypic plasticity. That is a challenging task, because in exploited populations both density-dependent feedback and environmental drivers can cause shifts in phenotypes similar to those expected from harvest-induced evolution. First, harvesting reduces density and relaxes interspecific competition. Greater per capita resource abundance can lead to accelerated juvenile growth, earlier maturation and, in fishes, smaller adult body sizes as individuals start to allocate energy to reproduction at an early age [13,15]. Such density-dependent feedback can further magnify evolutionary changes in phenotypes [65]. One approach to disentangle plastic growth-mediated changes from evolutionary shifts in maturation schedule is to use probabilistic maturation reaction norms [8]. A few case studies suggest that trends in fish age and size at maturation are primarily attributable to accelerated growth and, therefore, likely to be plastic responses to reduced competition [13,15]. In temperate ungulates, sport harvesting in autumn reduces intraspecific competition in winter, at times leading to strong compensatory population responses, especially in juvenile production and survival but also in earlier primiparity [86].
Apart from intrinsic density-dependent feedback, harvested populations are also subject to numerous external environmental drivers, such as temperature. In a context of global warming, this presents an additional challenge to assessing harvest-induced evolution, as some environmental drivers may change directionally over time, making it difficult to separate climate effects from those due to harvest. For example, in chamois (Rupicapra rupicapra), temporal changes in mass appeared more likely to be caused by climate change than by sport harvest [87]. Projected increases in temperatures are also likely to affect individual growth rates and accelerate development. For example, in marine invertebrates, a 1°C increase in water temperature is predicted to decrease body sizes by 0.5–4.0% [88]. Therefore, global warming may drive some changes in species life histories similar to those expected from harvest-induced selection [58]. Therefore, to properly disentangle evolutionary and plastic changes attributable to harvesting, environmental drivers must be accounted for. Environment-driven phenotypic changes could easily be mistakenly considered as evidence of harvest-induced evolution or vice versa.
Two meta-analyses have shown positive correlations between the rate of phenotypic changes in fishes and fishing intensity [32,89], suggesting that fishing mortality affected the magnitude of changes in fish phenotype even under climate change. Nonetheless, observed phenotypic changes were much larger than those predicted by eco-evolutionary modelling [32], suggesting that a substantial component of life-history changes in fishes can be attributed to demographic or environmental drivers. On the other hand, this observation also suggests that harvested populations can undergo much faster life-history changes than those expected solely based on the prevailing drivers of harvesting-induced evolution.
One interesting aspect related to the interplay between harvesting and other environmental drivers is that harvest-induced selection typically leads to shorter generation time due to earlier maturation. This, in turn, might accelerate evolutionary processes in response to environmental drivers in populations that have previously undergone harvesting-induced evolution, provided that the population has maintained sufficient levels of genetic and phenotypic variation.
7. Should harvest-induced evolution be accounted for in management?
Our literature review suggests that harvest-induced evolution is likely to reduce the resilience and recovery ability of affected populations. Combined with uncertainties related to future environmental changes and species ability to adapt to those changes, sustainable management should consider potential evolutionary responses to harvesting and their costs in terms of population growth and harvest yields. Nonetheless, apart from academic debates about the evidence for evolution, perhaps the most pronounced disagreement surrounding harvest-induced evolution is the mismatch between the precautionary views of most scientists and the reluctance by some management authorities to accept new methods and theories. Fisheries management, for example, is traditionally very data- and evidence-driven and much of it has developed somewhat in isolation from other ecological and evolutionary research [90]. The sport hunting community, on the other hand, feels under pressure from public opinion, driven by media frenzies such as that over ‘Cecil the lion‘ [91], and tends to react defensively to any suggestion that hunting may have selective effects. Encouragingly, evolutionary impacts assessment has been suggested as a component of standard management strategies [92], to estimate the costs associated with ignoring life-history evolution in management, as opposed to the costs of considering the possibility of life-history evolution. Management plans that ignore the ecological feedback of life-history changes may overestimate sustainable yield and, therefore, lead to overharvest. Combined with potential increases in population fluctuations, overfishing can easily reduce populations to very low abundance. Bioeconomic analyses of alternative fishing strategies for Northeast Arctic cod, however, underline that if harvest was sustainable, then costs of ignoring evolution might remain negligible [81]. In the case of trophy hunting, evolutionary reductions in horn size are associated with declines in harvest as fewer males reach trophy size [7,18] and may have economic consequences when hunters' willingness to pay is partly related to the expectation of trophy size.
8. Knowledge gaps and future directions
There is increased awareness of the potential importance of human-driven evolution in natural populations [93]. While fisheries-induced evolution is currently not accounted for in management strategies and regulations, managers are becoming increasing aware of its possibility, through accumulating scientific literature published in journals read by fisheries scientists and managers, and theme symposia in key conferences within the field. In contrast with the wealth of studies in the context of fisheries, the scientific literature on the effects of hunting in terrestrial systems is substantially poorer. That is possibly because much of the evidence is published in academic journals that are rarely read by wildlife managers [6,59] or because fewer researchers have examined this possibility. It is also likely, however, that biological differences between fish and terrestrial mammals, and the similarity in ecological and evolutionary expected consequences of increased adult mortality, make it more difficult to explore the life-history consequences of intense harvests in mammals. So far, much of the debate has been animated by a few studies on horn size in mountain ungulates. Evidence of life-history effects, however, is beginning to emerge [50,51,94].
Despite differences in both the amount of scientific studies and management awareness of potential evolutionary consequences of harvesting, one stunningly similar conclusion emerges from both hunting and fishing perspectives. The easiest way to both avoid harvest-induced evolution and to manage costs related to it is to simply limit overall harvest mortality, especially when it is very selective and runs counter to the pattern of natural mortality [7,81,82]. The simplicity of this management measure, as opposed to complicated size-limit regulations, suggests that accounting for harvest-induced evolution in wildlife and fisheries management is achievable and not very difficult: it faces challenges that are more related to attitudes than to implementation.
Authors' contributions
The manuscript was jointly written by A.K. and M.F.-B.
Competing interests
We have no competing interests.
Funding
A.K. was funded by Academy of Finland and Natural Sciences and Engineering Research Council of Canada (NSERC). M.F.-B. was funded by Natural Sciences and Engineering Research Council of Canada (NSERC), Alberta Fish & Wildlife and Alberta Conservation Association.
References
- 1.Rutter C. 1902. Natural history of the Quinnat salmon: a report of investigations in the Sacramento River, 1886–1901. Bull. U. S. Fish Comm. 22, 65–141. [Google Scholar]
- 2.Law R, Grey DR. 1989. Evolution of yields from populations with age-specific cropping. Evol. Ecol. 3, 343–359. ( 10.1007/BF02285264) [DOI] [Google Scholar]
- 3.Law R. 2000. Fishing, selection and phenotypic evolution. ICES J. Mar. Sci. 57, 659–668. ( 10.1006/jmsc.2000.0731) [DOI] [Google Scholar]
- 4.Rideout RM, Ings DW, Healey BP, Brattey J, Morgan MJ, Maddock Parsons D, Koen-Alonso M, Vigneau J. 2016. Assessing the status of the cod (Gadus morhua) stock in NAFO Subdivision 3Ps in 2015. DFO Can. Sci. Advis. Sec. Res. Doc. 2016/048 vi+90p.
- 5.Morgan MJ, Bailey J, Healey BP, Maddock Parsons D, Rideout R. 2011. Recovery potential assessment of American Plaice (Hippoglossoides platessoides) in Newfoundland and Labrador. DFO Can. Sci. Advis. Sec. Res. Doc. 2011/047. iv+32p.
- 6.Pigeon G, Festa-Bianchet M, Coltman DW, Pelletier F. 2016. Intense selective hunting leads to artificial evolution in horn size. Evol. Appl. 9, 521–530. ( 10.1111/eva.12358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douhart M, Festa-Bianchet M, Pelletier F, Gaillard JM, Bonenfant C. 2016. Changes in horn size of Stone's sheep over four decades correlate with trophy hunting pressure. Ecol. Appl. 26, 309–321. ( 10.1890/14-1461) [DOI] [PubMed] [Google Scholar]
- 8.Heino M, Godø OR. 2002. Fisheries-induced selection pressures in the context of sustainable fisheries. Bull. Mar. Sci. 70, 639–656. [Google Scholar]
- 9.Sharpe DMT, Hendry AP. 2009. Life history change in commercially exploited fish stocks: an analysis of trends across studies. Evol. Appl. 2, 260–275. ( 10.1111/j.1752-4571.2009.00080.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law W, Salic J. 2005. Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae). Proc. Natl Acad. Sci USA 102, 10 218–10 220. ( 10.1073/pnas.0502931102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mysterud A, Solberg EJ, Yoccoz NG. 2005. Ageing and reproductive effort in male moose under variable levels of intrasexual competition. J. Anim. Ecol. 74, 742–754. ( 10.1111/j.1365-2656.2005.00965.x) [DOI] [Google Scholar]
- 12.Pemberton JM. 2008. Wild pedigrees: the way forward. Proc. R. Soc. B 275, 613–621. ( 10.1098/rspb.2007.1531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuparinen A, Merilä J. 2007. Detecting and managing fisheries-induced evolution. Trends Ecol. Evol. 22, 652–659. ( 10.1016/j.tree.2007.08.011) [DOI] [PubMed] [Google Scholar]
- 14.Law R. 2007. Fisheries-induced evolution: present status and future directions. Mar. Ecol. Prog. Ser. 335, 271–277. ( 10.3354/meps335271) [DOI] [Google Scholar]
- 15.Heino M, Dieckmann U. 2008. Detecting fisheries-induced life-history evolution: an overview of the reaction-norm approach. Bull. Mar. Sci. 83, 69–93. [Google Scholar]
- 16.Proaktor G, Coulson T, Milner-Gulland EJ. 2007. Evolutionary responses to harvesting in ungulates. J. Anim. Ecol. 76, 669–678. ( 10.1111/j.1365-2656.2007.01244.x) [DOI] [PubMed] [Google Scholar]
- 17.Proaktor G, Coulson T, Milner-Gulland EJ. 2008. The demographic consequences of the cost of reproduction in ungulates. Ecology 89, 2604–2611. ( 10.1890/07-0833.1) [DOI] [PubMed] [Google Scholar]
- 18.Festa-Bianchet M. 2003. Exploitative wildlife management as a selective pressure for the life-history evolution of large mammals. In Animal behavior and wildlife conservation (eds Festa-Bianchet M, Apollonio M), pp. 191–207. Washington, DC: Island Press. [Google Scholar]
- 19.Heino M, Godø OR, Dieckmann U. 2002. Measuring probabilistic reaction norms for age and size at maturation. Evolution 56, 669–678. ( 10.1111/j.0014-3820.2002.tb01378.x) [DOI] [PubMed] [Google Scholar]
- 20.Swain DP, Sinclair AF, Hanson JM. 2007. Evolutionary response to size-selective mortality in an exploited fish population. Proc. R. Soc. B 274, 1015–1022. ( 10.1098/rspb.2006.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchings JA. 2005. Life-history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 62, 824–832. ( 10.1139/f05-081) [DOI] [Google Scholar]
- 22.Dunlop ES, Heino M, Dieckmann U. 2009. Eco-genetic modeling of contemporary life-history evolution. Ecol. Appl. 19, 1815–1834. ( 10.1890/08-1404.1) [DOI] [PubMed] [Google Scholar]
- 23.van Wijk SJ, Taylor MI, Creer S, Dreyer C, Rodrigues FM, Ramnarine IW, van Oosterhout C, Carvalho GR. 2013. Experimental harvesting of fish populations drives genetically based shifts in body size and maturation. Front. Ecol. Environ. 11, 181–187. ( 10.1890/120229) [DOI] [Google Scholar]
- 24.Uusi-Heikkilä S, et al. 2015. The evolutionary legacy of size-selective harvest extends from genes to populations. Evol. Appl. 8, 597–620. ( 10.1111/eva.12268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartl GB, Lang G, Klein F, Willing R. 1991. Relationships between allozymes, heterozygosity and morphological characters in red deer (Cervus elaphus), and the influence of selective hunting on allele frequency distribution. Heredity 66, 343–350. ( 10.1038/hdy.1991.43) [DOI] [PubMed] [Google Scholar]
- 26.Coltman DW, O'Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M. 2003. Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658. ( 10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- 27.Merilä J, Hendry AP. 2014. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol. Appl. 7, 1–14. ( 10.1111/eva.12137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchings JA, Fraser DF. 2008. The nature of fisheries and farming induced evolution. Mol. Ecol. 17, 294–313. ( 10.1111/j.1365-294X.2007.03485.x) [DOI] [PubMed] [Google Scholar]
- 29.Palkovacs EP, Kinnison MT, Correa C, Dalton CM, Hendry AP. 2012. Fates beyond traits: ecological consequences of human-induced trait change. Evol. Appl. 5, 183–191. ( 10.1111/j.1752-4571.2011.00212.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darimont CT, Fox CH, Bryan HM, Reimchen TE. 2015. The unique ecology of human predators. Science 349, 858–860. ( 10.1126/science.aac4249) [DOI] [PubMed] [Google Scholar]
- 31.Kuparinen A, Boit A, Valdovinos FS, Lassaux H, Martinez ND. 2016. Fishing-induced life-history changes degrade and destabilize harvested ecosystems. Sci. Rep. 6, 22245 ( 10.1038/srep22245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audzijonyte A, Kuparinen A, Fulton EA. 2013. How fast is fisheries-induced evolution? Quantitative analysis of modelling and empirical studies. Evol. Appl. 6, 585–595. ( 10.1111/eva.12044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hengeveld P, Festa-Bianchet M. 2011. Harvest regulations and artificial selection on horn size in male bighorn sheep. J. Wildl. Manage. 75, 189–197. ( 10.1002/jwmg.14) [DOI] [Google Scholar]
- 34.Pukk L, Kuparinen A, Järv L, Gross R, Vasemägi A. 2013. Genetic and life-history changes associated with fisheries-induced population collapse. Evol. Appl. 6, 749–760. ( 10.1111/eva.12060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rughetti M, Festa-Bianchet R. 2010. Compensatory growth limits opportunities for artificial selection in Alpine chamois. J. Wildl. Manage. 74, 1024–1029. ( 10.2193/2009-335) [DOI] [Google Scholar]
- 36.Peres KO, Munch SB. 2010. Extreme selection on size in the early lives of fish. Evolution 64, 2450–2457. ( 10.1111/j.1558-5646.2010.00994.x) [DOI] [PubMed] [Google Scholar]
- 37.Rollinson N, Rowe L. 2015. Persistent directional selection on body size and a resolution to the paradox of stasis. Evolution 69, 2441–2451. ( 10.1111/evo.12753) [DOI] [PubMed] [Google Scholar]
- 38.Bell G. 1980. The costs of reproduction and their consequences. Am. Nat. 116, 45–76. ( 10.1086/283611) [DOI] [Google Scholar]
- 39.Coltman DW, O'Donoghue P, Jorgenson JT, Hogg JT, Festa-Bianchet M. 2005. Selection and genetic (co)variance in bighorn sheep. Evolution 59, 1372–1382. ( 10.1111/j.0014-3820.2005.tb01786.x) [DOI] [PubMed] [Google Scholar]
- 40.Edeline E, Carlson SM, Stige LC, Winfield IJ, Fletcher JM, James JB, Haugen TO, Vøllestad A, Stenseth NC. 2007. Trait changes in a harvested population are driven by a dynamic tug-of-war between natural and harvest selection. Proc. Natl Acad. Sci. USA 104, 15 799–15 804. ( 10.1073/pnas.0705908104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson SM, Edeline E, Vøllestad LA, Haugen TO, Winfield IJ, Fletcher JM, James JB, Stenseth NC. 2007. Four decades of opposing natural and human-induced artificial selection acting on Windermere pike (Esox lucius). Ecol. Lett. 10, 512–521. ( 10.1111/j.1461-0248.2007.01046.x) [DOI] [PubMed] [Google Scholar]
- 42.Haller BC, Hendry AP. 2014. Solving the paradox of stasis: squashed stabilizing selection and the limits of detection. Evolution 68, 483–500. ( 10.1111/evo.12275) [DOI] [PubMed] [Google Scholar]
- 43.Kuparinen A, Hutchings JA. 2012. Consequences of fisheries-induced evolution for population productivity and recovery potential. Proc. R. Soc. B 279, 2571–2579. ( 10.1098/rspb.2012.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuparinen A, Stenseth NC, Hutchings JA. 2014. Fundamental population-productivity relationships can be modified through density-dependent feedbacks of life-history evolution. Evol. Appl. 7, 1218–1225. ( 10.1111/eva.12217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heino M, et al. 2013. Can fisheries-induced evolution shift reference points for fisheries management? ICES J. Mar. Sci. 70, 707–721. ( 10.1093/icesjms/fst077) [DOI] [Google Scholar]
- 46.Audzijonyte A, Kuparinen A. 2016. The role of life histories and trophic interactions in population recovery. Conserv. Biol. 30, 734–743. ( 10.1111/cobi.12651) [DOI] [PubMed] [Google Scholar]
- 47.Enberg K, Jørgensen C, Dunlop ES, Heino M, Dieckmann U. 2009. Implications of fisheries-induced evolution for stock rebuilding and recovery. Evol. Appl. 2, 394–414. ( 10.1111/j.1752-4571.2009.00077.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchings JA, Reynolds JD. 2004. Marine fish population collapses: consequences for recovery and extinction risk. BioScience 54, 297–309. ( 10.1641/0006-3568(2004)054%5B0297:MFPCCF%5D2.0.CO;2) [DOI] [Google Scholar]
- 49.Swain DP. 2011. Life-history evolution and elevated natural mortality in a population of Atlantic cod (Gadus morhua). Evol. Appl. 4, 18–29. ( 10.1111/j.1752-4571.2010.00128.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gamelon M, Besnard A, Gaillard JM, Servanty S, Baubet E, Brandt S, Gimenez O. 2011. High hunting pressure selects for earlier birth date: wild boar as a case study. Evolution 65, 3100–3112. ( 10.1111/j.1558-5646.2011.01366.x) [DOI] [PubMed] [Google Scholar]
- 51.Kvalnes T, Saether B-E, Røed KH, Engen S, Solberg EJ. 2016. Harvest-induced phenotypic selection in an island population of moose, Alces alces. Evolution 70, 1486–1500. ( 10.1111/evo.12952) [DOI] [PubMed] [Google Scholar]
- 52.Mysterud A, Yoccoz NG, Langvatn R. 2009. Maturation trends in red deer females over 39 years in harvested populations. J. Anim. Ecol. 78, 595–599. ( 10.1111/j.1365-2656.2008.01514.x) [DOI] [PubMed] [Google Scholar]
- 53.Audzijonyte A, Kuparinen A, Gorton R, Fulton EA. 2013. Ecological consequences of body size decline in harvested fish species: positive feedback loops in trophic interactions amplify human impact. Biol. Lett. 9, 20121103 ( 10.1098/rsbl.2012.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U. 2004. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428, 932–935. ( 10.1038/nature02430) [DOI] [PubMed] [Google Scholar]
- 55.Conover DO, Munch SB, Arnott SA. 2009. Reversal of evolutionary downsizing caused by selective harvest of large fish. Proc. R. Soc. B 276, 2015–2020. ( 10.1098/rspb.2009.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dulvy NK, Ellis JR, Goodwin NB, Grant A, Reynolds JD, Jennings S. 2004. Methods of assessing extinction risk in marine fishes. Fish Fish. 5, 255–276. ( 10.1111/j.1467-2679.2004.00158.x) [DOI] [Google Scholar]
- 57.Morrissey MB, Walling CA, Wilson AJ, Pemberton JM, Clutton-Brock TH, Kruuk LEB. 2012. Genetic analysis of life-history constraint and evolution in a wild ungulate population. Am. Nat. 179, E97–E114. ( 10.1086/664686) [DOI] [PubMed] [Google Scholar]
- 58.Lancaster LT, Morrison G, Fitt RN. 2017. Life history trade-offs, the intensity of competition, and coexistence in novel and evolving communities under climate change. Phil. Trans. R. Soc. B 372, 20160046 ( 10.1098/rstb.2016.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engen S, Lande R, Saether B-E. 2014. Evolutionary consequences of nonselective harvesting in density-dependent populations. Am. Nat. 184, 714–726. ( 10.1086/678407) [DOI] [PubMed] [Google Scholar]
- 60.Peters RH. 1986. Ecological implications of body size. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 61.Charnov EL. 1993. Life-history invariants, 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 62.Biro PA, Post JR. 2008. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl Acad. Sci. USA 105, 2919–2922. ( 10.1073/pnas.0708159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uusi-Heikkilä S, Wolter C, Klefoth T, Arlinghaus R. 2008. A behavioral perspective on fishing-induced evolution. Trends Ecol. Evol. 23, 419–421. ( 10.1016/j.tree.2008.04.006) [DOI] [PubMed] [Google Scholar]
- 64.Ciuti S, Murphy TB, Paton DG, McDevitt AD, Musiani M, Boyce MS. 2012. Human selection of elk behavioural traits in a landscape of fear. Proc. R. Soc. B 279, 4407–4416. ( 10.1098/rspb.2012.1483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bassar RD, et al. 2010. Local adaptation in Trinidadian guppies alters ecosystem processes. Proc. Natl Acad. Sci. USA 107, 3616–3621. ( 10.1073/pnas.0908023107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scheffer M, Carpenter S, de Young B. 2005. Cascading effects of overfishing marine systems. Trends Ecol. Evol. 20, 579–581. ( 10.1016/j.tree.2005.08.018) [DOI] [PubMed] [Google Scholar]
- 67.Hsieh CH, Reiss CS, Hunder JR, Beggington JR, May RM, Sugihara G. 2006. Fishing elevates variability in the abundance of exploited species. Nature 443, 859–862. ( 10.1038/nature05232) [DOI] [PubMed] [Google Scholar]
- 68.Rouyer T, Sadykov A, Ohlberger J, Stenseth NC. 2012. Does increasing mortality change the response of fish populations to environmental fluctuations? Ecol. Lett. 15, 658–665. ( 10.1111/j.1461-0248.2012.01781.x) [DOI] [PubMed] [Google Scholar]
- 69.Olsen EM, Carlson SM, Gjosaeter J, Stenseth NC. 2009. Nine decades of decreasing phenotypic variability in Atlantic cod. Ecol. Lett. 12, 622–631. ( 10.1111/j.1461-0248.2009.01311.x) [DOI] [PubMed] [Google Scholar]
- 70.Edeline E, Le Rouzic A, Winfield IJ, Fletcher JM, James JB, Stenseth NC, Vøllestad LA. 2009. Harvest-induced disruptive selection increases variance in fitness-related traits. Proc. R. Soc. B 276, 4163–4171. ( 10.1098/rspb.2009.1106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marty L, Dieckmann U, Ernande B. 2015. Fisheries-induced neutral and adaptive evolution in exploited fish populations and consequences for their adaptive potential. Evol. Appl. 8, 47–63. ( 10.1111/eva.12220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuparinen A, Hutchings JA, Waples R. 2016. Harvest-induced evolution and effective population size. Evol. Appl. 9, 658–672. ( 10.1111/eva.12373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Therkildsen N, Nilsen EE, Swain DP, Pedersen JS. 2010. Large effective population size and temporal genetic stability in Atlantic cod (Gadus morhua) in the southern Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 67, 1585–1595. ( 10.1139/F10-084) [DOI] [Google Scholar]
- 74.Martin AM, Festa-Bianchet M, Coltman D, Pelletier F. 2016. Demographic drivers of age-dependent fluctuating sexual selection. J. Evol. Biol. 29, 1427–1446. ( 10.1111/jeb.12883) [DOI] [PubMed] [Google Scholar]
- 75.Byers JA, Moodie JD, Hall N. 1994. Pronghorn females choose vigorous mates. Anim. Behav. 47, 33–43. ( 10.1006/anbe.1994.1005) [DOI] [Google Scholar]
- 76.Naidoo R, Weaver LC, Diggle RW, Matongo G, Stuart-Hill G, Thouless C. 2016. Complementary benefits of tourism and hunting to communal conservancies in Namibia. Conserv. Biol. 30, 628–638. ( 10.1111/cobi.12643) [DOI] [PubMed] [Google Scholar]
- 77.Birkeland C, Dayton PK. 2005. The importance in fishery management of leaving the big ones. Trends Ecol. Evol. 20, 356–358. ( 10.1016/j.tree.2005.03.015) [DOI] [PubMed] [Google Scholar]
- 78.Arlinghaus R, Matsumura S, Dieckmann U. 2009. Quantifying selection differentials caused by recreational fishing: development of modeling framework and application to reproductive investment in pike (Esox lucius). Evol. Appl. 2, 335–355. ( 10.1111/j.1752-4571.2009.00081.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gårdmark A, Dieckmann U. 2006. Disparate maturation adaptations to size-dependent mortality. Proc. R. Soc. B 273, 2185–2192. ( 10.1098/rspb.2006.3562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sutter DAH, Suski CD, Philipp DP, Klefoth T, Wahl D, Kersten P, Cooke SJ, Arlinghaus R. 2012. Recreational fishing selectively captures individuals with the highest fitness potential. Proc. Natl Acad. Sci. USA 109, 20 960–20 965. ( 10.1073/pnas.1212536109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eikeset AM, Richter A, Dunlop E, Dieckmann U, Stenseth NC. 2013. Economic repercussions of fisheries-induced evolution. Proc. Natl Acad. Sci. USA 110, 12 259–12 264. ( 10.1073/pnas.1212593110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hutchings JA. 2009. Avoidance of fisheries-induced evolution: management implications for catch selectivity and limit reference points. Evol. Appl. 2, 324–334. ( 10.1111/j.1752-4571.2009.00085.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tenhumberg B, Tyre AJ, Pople AR, Possingham HP. 2004. Do harvest refuges buffer kangaroos against evolutionary responses to selective harvesting? Ecology 85, 2003–2017. ( 10.1890/03-4111) [DOI] [Google Scholar]
- 84.Baskett ML, Levin SA, Gaines SD, Dushoff J. 2005. Marine reserve design and the evolution of size at maturation in harvested fish. Ecol. Appl. 15, 882–901. ( 10.1890/04-0723) [DOI] [Google Scholar]
- 85.Pelletier F, Festa-Bianchet M, Jorgenson JT, Feder C, Hubbs A. 2014. Can phenotypic rescue from harvest refuges buffer wild sheep from selective hunting? Ecol. Evol. 4, 3375–3382. ( 10.1002/ece3.1185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boyce MS, Sinclair ARE, White GC. 1999. Seasonal compensation of predation and harvesting. Oikos 87, 419–426. ( 10.2307/3546808) [DOI] [Google Scholar]
- 87.Rughetti M, Festa-Bianchet M. 2012. Effects of spring-summer temperatures on body mass of chamois. J. Mamm. 93, 1301–1307. ( 10.1644/11-MAMM-A-402.1) [DOI] [Google Scholar]
- 88.Cheung WWL, Sarmiento JL, Dunne J, Froelicher TL, Lam V, Palomares MLD, Watson R, Pauly D. 2012. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 3, 254–258. ( 10.1038/nclimate1691) [DOI] [Google Scholar]
- 89.Devine JA, Wright PJ, Pardoe HE, Heino M. 2012. Comparing rates of contemporary evolution in life-history traits for exploited fish stocks. Can. J. Fish. Aquat. Sci. 69, 1105–1120. ( 10.1139/f2012-047) [DOI] [Google Scholar]
- 90.Kuparinen A, Mäntyniemi S, Hutchings JA, Kuikka S. 2012. Increasing biological realism of fisheries stock assessment: towards hierarchical Bayesian methods. Environ. Rev. 20, 135–151. ( 10.1139/a2012-006) [DOI] [Google Scholar]
- 91.Di Minin E, Leader-Williams N, Bradshaw CJA. 2016. Banning trophy hunting will exacerbate biodiversity loss. Trends Ecol. Evol. 31, 99–102. ( 10.1016/j.tree.2015.12.006) [DOI] [PubMed] [Google Scholar]
- 92.Laugen AT, et al. 2014. Evolutionary impact assessment: integrating evolutionary consequences of fishing into the ecosystem-approach to fisheries management. Fish Fish. 15, 65–96. ( 10.1111/faf.12007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hendry AP, Gotanda KM, Svensson EI. 2017. Human influences on evolution, and the ecological and societal consequences. Phil. Trans. R. Soc. B 372, 20160028 ( 10.1098/rstb.2016.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zedrosser A, Steyaert SMJG, Gossow H, Swenson JE. 2011. Brown bear conservation and the ghost of persecution past. Biol. Conserv. 144, 2163–2170. ( 10.1016/j.biocon.2011.05.005) [DOI] [Google Scholar]