Abstract
Humans have contributed to the increased frequency and severity of emerging infectious diseases, which pose a significant threat to wild and domestic species, as well as human health. This review examines major pathways by which humans influence parasitism by altering (co)evolutionary interactions between hosts and parasites on ecological timescales. There is still much to learn about these interactions, but a few well-studied cases show that humans influence disease emergence every step of the way. Human actions significantly increase dispersal of host, parasite and vector species, enabling greater frequency of infection in naive host populations and host switches. Very dense host populations resulting from urbanization and agriculture can drive the evolution of more virulent parasites and, in some cases, more resistant host populations. Human activities that reduce host genetic diversity or impose abiotic stress can impair the ability of hosts to adapt to disease threats. Further, evolutionary responses of hosts and parasites can thwart disease management and biocontrol efforts. Finally, in rare cases, humans influence evolution by eradicating an infectious disease. If we hope to fully understand the factors driving disease emergence and potentially control these epidemics we must consider the widespread influence of humans on host and parasite evolutionary trajectories.
This article is part of the themed issue ‘Human influences on evolution, and the ecological and societal consequences’.
Keywords: eco-evolutionary dynamics, emerging infectious diseases, land-use change, host–parasite coevolution, spillover, contemporary evolution
1. Introduction
Emerging infectious diseases (EIDs) have become increasingly common over the past several decades, and humans can be particularly important drivers of changes in parasite prevalence (box 1; [4]). Global travel and trade have facilitated unprecedented movement of species [2,5], and, when parasites successfully establish in a new location, the ensuing disease outbreaks can strongly impact humans as well as wild and domestic hosts [4]. In addition to directly influencing disease by moving hosts, parasites and vectors, human activities such as agriculture, urbanization, hunting, habitat fragmentation and climate change resulting from human activity can affect ecosystems in ways that impact infectious diseases (e.g. [1,5–8]). Human actions can also influence the subsequent evolutionary dynamics in these EID systems, though we are only starting to appreciate these influences [1,7–9].
Box 1. Terminology.
In this review, we use EIDs to refer to infectious diseases that are increasing significantly in prevalence, expanding in geographical range and/or infecting new host species [1]. We use ‘parasite’ and ‘pathogen’ to refer to infectious disease agents. ‘Spillover’ is when disease dynamics in one host species are driven by infectious propagules from another host species (the ‘reservoir’ host) [2]. ‘Virulence’ is a negative effect of a parasite on host fitness; in most cases discussed in this review, virulence refers to parasite-induced host mortality. ‘Resistance’ is used to refer to a host's ability to limit infection or parasite growth, whereas ‘tolerance’ is used to refer to host strategies that reduce the negative fitness effects of the parasite without limiting infection or growth [3]. It is important to remember that traits such as virulence, resistance and tolerance vary according to parasite and host species (and genotype) identity.
Hosts and parasites may impose strong selective pressures on one another, and both ecological and (co)evolutionary dynamics can play an important role in infectious disease emergence [6,10]. Over the course of an outbreak, selection on a parasite and its host is not constant: both virulence and resistance can evolve, often simultaneously [11] (box 1). Thus, in these systems, ecology and evolution will likely interact, and both ecological and evolutionary dynamics will occur on contemporary timeframes [12]. These eco-evolutionary dynamics can be difficult to observe in the field [13,14] and may only be beginning to unfold in the cases of recently emerging diseases. Thus, for many EID systems we are just beginning to understand the eco-evolutionary drivers at play. However, a few well-studied systems (e.g. box 2) illustrate that evolution can act on both hosts and parasites on ecological timescales [22], that there can be rapid coevolution of hosts and parasites, and that humans can strongly influence eco-evolutionary host–parasite dynamics.
Box 2. Case study: human impacts on eco-evolutionary disease dynamics in the poultry-house finch-Mycoplasma system.
An interesting case study demonstrating the impacts of humans on an EID system comes from the avian bacterial parasite Mycoplasma gallisepticum in eastern North America. This pathogen, which causes severe conjunctivitis, originally circulated in poultry, but then spread to house finches (Haemorhous mexicanus) in eastern North America in the 1990s [15]; backyard bird feeders are an important avenue of disease transmission in house finches [16] (figure 1).
Figure 1.

Mycoplasma gallisepticum infection of house finches is partly supported by high densities of finches maintained by bird feeders [17]. These bird feeders also facilitate parasite transmission [16]. Figure drawn by John Megahan. (Online version in colour.)
While this bacterium has made the shift from poultry to house finches multiple times, only one of these events was successful over the long term, founding the lineage that has spread throughout North America [15]. This lineage has evolved rapidly, increasing in virulence, likely owing to selection on transmission [18]. In addition to this phenotypic change, exceptionally rapid genomic evolution has been documented in the bacterium [19]. House finches have also evolved rapidly in response to this novel parasite. Finches from populations in eastern North America have evolved increased resistance and tolerance of the disease, and have altered expression of immune genes [19–21].
This system is exceptional in the wealth of information we have on it, and provides clear evidence for the potential for humans to influence evolution in nature via effects on emerging diseases. Humans created the agricultural system in which the pathogen originally circulated. It then spread from poultry to house finches after the latter were introduced to eastern North America by humans. Moreover, the high densities of house finch populations were maintained by bird feeders [17], which then also served as avenues of disease transmission [16]. Overall, humans clearly set the stage for the emergence of this infectious disease, an event that was then followed by very rapid evolution of both host and pathogen.
In this review, we investigate several ways in which humans drive evolution in host–parasite systems. In many cases this influence is indirect, via ecological impacts such as facilitating novel species interactions, increasing host population density or diversity, and/or altering abiotic environmental conditions. We also discuss cases where humans more directly affect evolution by changing host or parasite genetic diversity, or through management activities such as selective culling programmes and vaccination. Finally, we touch upon the rare instances where parasites have been eradicated and the impacts of this for evolution of hosts and parasites.
2. Human impacts on emerging infectious diseases via novel species associations
Humans can strongly impact EIDs by creating novel species associations. In many cases, this leads to evolution of the parasite and host in response. Often, these novel associations of hosts and parasites are the direct result of human activities; in particular, global travel and trade have created a world where species are more connected than ever before [5]. Humans also indirectly alter associations of hosts, parasites and vectors, primarily via anthropogenic climate change and land-use change. For example, increased temperatures at higher latitudes and elevations allow parasites and their vectors to expand their range into communities where hosts are naive [23].
Anthropogenic modifications of land use can also facilitate novel species associations, allowing a parasite to infect a novel host. Parasites associated with agriculture and animal husbandry can spill over into wild species as well as humans [8]. Domestic hosts can also serve as ‘stepping stones’ for parasites, which can move from wild to domestic species (e.g. [2,24]). Often, the stepping stones act ecologically (increasing parasite population sizes and/or transmission opportunities), allowing a parasite to move into a new species without evolutionary change. However, these systems also make it more likely that a genetic mutation will arise in the parasite that will allow it to successfully spill over into a novel host [9].
A complete accounting of the different ways in which humans create novel associations of hosts, parasites and vectors is beyond the scope of this review. Instead, we will focus on the potential role of evolution in facilitating changes in disease prevalence and the potential evolutionary consequences of altered prevalence.
In many cases, novel species associations are unlikely to result in sustained transmission (R0 > 1) [14]. However, parasites may evolve to better navigate barriers to infection, reproduction and transmission, making disease emergence more likely [1]. By dramatically increasing the dispersal of potential host and parasite species, humans provide the genetic diversity needed for evolutionary changes to take place [25]. The likelihood that a parasite species will switch successfully to a new host may depend on how much genetic change is needed to enable infection or transmission; in some cases, only a few nucleotide substitutions are needed, but in others, much larger changes are required [1].
Once a parasite has become established in a new host, that host might evolve rapidly in response. For example, house sparrows (Passer domesticus), a particularly widespread and competent host of West Nile virus, evolved increased resistance to NY99, the original strain of the virus that emerged in New York [26]. Similarly, recent studies have found evidence of evolution in bats after the emergence of white-nose syndrome (WNS) [27] and in amphibians after the emergence of the chytrid fungus (Batrachochytrium dendrobatidis) [28].
3. Human impacts on emerging infectious diseases mediated by changes in host and/or vector density and diversity
Human activities associated with urbanization and agriculture can also have significant impacts on species abundances [29]. Such impacts on host and vector population densities can play an important role in both ecological and evolutionary dynamics affecting parasite prevalence.
Extremely dense host populations increase the likelihood of parasite transmission, contributing to higher infection prevalence and increasing the potential for an epidemic [30]. Developed land can support particularly high densities of some host species, which appears to have contributed to epidemics in several instances. For example, prevalence of West Nile virus is strongly associated with both urban and agricultural land use in North America [31], likely as a result of elevated densities of host (bird) and vector (mosquito) populations in these habitats [32]. Conversely, land-use change could potentially decrease disease risk in some cases if it reduces host or vector density, or if it leads to habitat fragmentation that hinders vector or parasite dispersal.
One mechanism driving the association between host density and disease outbreaks is the evolution of increased parasite virulence. Parasite virulence is often correlated with transmission ability, as parasites that co-opt more host resources produce more transmission stages and are also more virulent [33]. Theory predicts that when parasite virulence and transmission rate are low, an increase in these traits can increase the likelihood of infecting additional hosts (i.e. it can increase parasite fitness); however, at very high levels of virulence, earlier host death may limit transmission [14]. When host population densities increase, this reduces the cost of earlier host mortality, selecting for parasites that are more virulent than might have been otherwise supported [7,14].
Much of our understanding of the relationship between host population density and parasite virulence comes from laboratory studies and modelling. However, there is some evidence from field populations suggesting that anthropogenic impacts on host density contribute to disease outbreaks, at least partly driven by evolution of increased virulence in the parasite. For example, it appears that chytrid fungus infection in amphibian populations occurring at the invasion front in Central America are especially virulent, possibly owing to the relatively high densities of susceptible host individuals present [34]. In addition, agriculture, animal husbandry and fish farming support host densities well above those found in natural populations, which also can promote the evolution of more virulent parasites [7,8]. The resulting extremely virulent parasites can become a major threat to susceptible wild host species when spillover occurs [7]. At the same time, in some systems (e.g. salmon; [35]), adaptation in wild host populations exposed to repeated parasite spillover can be limited by high gene flow.
In addition to host density, humans impact species diversity, which can alter disease dynamics and possibly host–parasite (co)evolution. By changing competent host density and/or the likelihood of transmission, higher biodiversity may decrease (dilute) or increase (amplify) parasitism [36]. Although there is still vigorous debate, growing evidence suggests that anthropogenic reductions in biodiversity generally increase disease, and that the strength of this dilution is driven by the change in a focal host species' frequency (relative abundance), rather than its density [36]. Such biodiversity-driven changes in disease incidence may alter selection for host resistance or tolerance. Additionally, because higher host diversity often correlates with higher parasite diversity [37], altered biodiversity might also influence cross-species transmission. While the exact evolutionary consequences are not yet known, future research would do well to investigate the influence of biodiversity on parasite spillover and host specialization.
4. Human impacts on emerging infectious diseases via changes to the abiotic environment
Coevolutionary dynamics between hosts and parasites are complex, particularly when other stressors come into play. Humans have altered abiotic conditions around the globe, changing the ecology of hosts and parasites and their evolutionary responses to each other [38]. Coping with altered conditions puts stress on plants and animals and can divert resources from fighting parasites [39,40]. Costly traits such as resistance may involve evolutionary trade-offs that can depend on life-history traits such as host lifespan and the ability to have acquired immunity (versus innate immunity; e.g. [41]). In addition, the source of host mortality may determine evolutionary responses of parasites [42]. Responses of hosts and parasites to abiotic changes associated with global climate change and increased chemical use have been particularly well studied, and we discuss these findings here. Less well studied, but equally important, is how hosts and parasites may evolve in response to changes in other interacting species owing to abiotic change [43,44]. This will be a crucial area for future research.
Global climate change is expected to lead to increased average temperatures and temperature variability [45]. Temperature affects the physiology of organisms, impacting a parasite's ability to develop and a host's ability to resist or tolerate parasites [46]. In cases where parasites have higher temperature optima than hosts, virulence can be higher at higher temperatures, imposing temperature-dependent selection on hosts [47]. When selection depends on temperature, environmental variability may act to maintain diversity in host and parasite populations as different genotypes of each may be selected for under different conditions [48].
Environmental exposure to pesticides can also impact the ecology and evolution of host–parasite interactions. For example, pesticide exposure increases honeybee susceptibility to parasites [49], and parasite exposure may decrease their ability to detoxify chemicals [50]. Moreover, genetic trade-offs between resistance to pesticides and parasites might impede evolution [51], though in some cases evolving resistance to pesticides also increases parasite resistance [52].
5. Human impacts on emerging infectious diseases via impacts on genetic diversity
Humans can also impact evolution in EID systems by altering genetic diversity of hosts and parasites. Genetic diversity of hosts plays an important role in their ability to adapt to parasites [6,53]. Thus, losses of diversity should increase host vulnerability to parasite outbreaks (though this may only happen after a certain diversity threshold is reached; [40]). Humans can strongly impact host genetic diversity through different processes, including habitat fragmentation, overharvesting and growing monocultures. Habitat fragmentation reduces population sizes and limits gene flow, both of which are linked to reductions in genetic diversity [54–56]. Roads, a major source of habitat fragmentation, also cause significant mortality, which can further reduce genetic diversity [57]. Similarly, harvesting natural populations (via hunting, fishing, logging or other natural resource use) can alter genetic diversity by reducing population sizes, altering gene flow, changing the dynamics of sexual selection and imposing selection directly via selective harvesting [58,59]. Finally, modern agriculture relies heavily on monocultures of crops and animals that harbour exceptionally low genetic diversity, leaving them extremely vulnerable to evolving parasites (e.g. [60]).
A current example of a monoculture that is vulnerable to disease is commercial bananas [61]. The main varieties sold are triploid, and all propagation is by cloning. A strain of the wilt fungus, Fusarium oxysporum f. sp. cubens, adapted to the widely grown variety of bananas known as Gros Michel in the 1960s. A resistant variety of banana, Cavendish, replaced Gros Michel in banana production regions. Soon, however, a virulent strain of the Fusarium wilt known as race 4 emerged on Cavendish bananas in Malaysia, and again, banana production is threatened [61].
Human activities also alter the genetic diversity of parasites. While humans can increase or decrease parasite diversity, evolution of parasite populations will be more rapid when they harbour high diversity. One way humans can increase diversity in parasite populations is by transporting parasites to new geographical locations (see above). Multiple introductions of a parasite to a new range can create introduced populations that are more diverse than their native counterparts [24]. In addition, with some parasites, hybridization between species or horizontal gene transfer can increase diversity. Again, this is facilitated by anthropogenic introductions of organisms around the world. For example, one newly emerged pathogen with a broad host range on multiple horticultural plant species has apparently arisen owing to hybridization between two species of Phytopthora (an oomycete), one native to the region of disease emergence and one recently introduced [62].
6. Human impacts on emerging infectious diseases via intentional interventions
Humans often directly intervene when parasites threaten our health or that of the ecosystems around us, and such interventions can have evolutionary consequences (e.g. [63,64]). One way that humans can impact parasite evolutionary trajectories is via vaccination. Vaccination is an extremely successful means by which humans intervene in disease systems, especially when vaccination induces perfect lifelong immunity in the host. However, there are circumstances where vaccination impacts evolution in ways that counter our efforts. Imperfect or ‘leaky’ vaccines reduce or eliminate the negative effects of the parasite, but do not entirely prevent the parasite from replicating within the host and transmitting to other individuals [65]. Because the parasite can still replicate in vaccinated hosts, leaky vaccines may actually select for increased virulence by altering the trade-off between virulence and transmission [65]. A highly virulent strain may not be able to persist in an unvaccinated host population because of the high level of parasite-induced host mortality; imperfect vaccination alleviates this cost by artificially increasing the length of the infectious period [66]. For example, imperfect vaccination against Marek's disease virus in poultry increases the infected host's lifespan, allowing for transmission of highly virulent parasite strains [67]. The successful transmission of more aggressive viruses in vaccinated hosts may partly explain the repeated need for new and improved vaccines against Marek's disease [67]. Thus, in the case of imperfect vaccination, our efforts to reduce the burden of disease in the host may actually select for a more harmful parasite.
Culling of hosts is another common intervention in host–parasite systems. In addition to potentially impacting host genetic diversity, culling can decrease the incidence of density-dependent transmitted diseases both by removing infected individuals and by reducing host population size [68]. Despite the short-term benefits of culling, it places evolutionary pressure on host and parasite populations. For hosts, culling may reduce selection for resistance, although selectively culling only infected individuals reduces this selection pressure [69]. For parasites, the evolutionary consequences of culling are more nuanced. On the one hand, theory predicts that increasing background mortality of hosts should select for increased virulence of parasites because of selection for increased host exploitation [70]. In support of this theory, an increase in virulence owing to culling has been suggested in avian influenza [69], and there is experimental evidence from serial passage experiments in other systems [70]. On the other hand, parasites may also experience selection for lower virulence in the face of culling because of a reduction in host population size, as appears to be the case in classical swine fever in wild boar [68]. Additionally, higher background mortality of hosts may reduce within-host competition between parasite strains, limiting selection for increased virulence [71,72]. Taken together, host and parasite evolution may depend on a combination of host population size and the culling strategy.
Humans also use parasites to their advantage, as biological control agents (e.g. [73]), and here parasite evolution can either be an asset or a complication. Parasites used as biological control agents are often analogous to an emerging disease, as they are introduced to naive host populations. In the case of biological control, high virulence is often desired, as this leads to more effective control of pest populations. Scientists can select for higher virulence in the laboratory or genetically modify parasites to increase virulence, and then introduce these deadly parasites into the wild [74]. However, once introduced to the wild, the optimal level of virulence for the parasite may not be the same level of virulence that makes the parasite an optimal biological control agent (e.g. [73]).
In short, human interventions to manipulate EIDs can drive unintended evolutionary responses in parasites and hosts that may result in undesirable outcomes. Thus, it would be prudent to gather as much information as possible about a disease system before intervening. As interventions may be more likely when diseases threaten human health or economic stability (e.g. agricultural parasites), such caution is particularly warranted.
7. Human impacts on emerging infectious diseases via infectious disease eradication
While the sections above have largely focused on EIDs, humans can also influence host evolution via disease eradication. Full eradication of infectious diseases remains rare, with smallpox and rinderpest being the only two infectious diseases that have been fully eradicated from nature [75]. However, eradication efforts are underway for several other parasites [75], and, even in cases where a parasite cannot be fully eradicated, it might be possible to eliminate it from a particular geographical area (e.g. [76]).
The infectious diseases targeted for eradication strongly impact host populations; thus, eradication is likely to dramatically alter the selective environment for the host. In cases where the host experiences trade-offs associated with disease resistance, this might lead to rapid evolution of the host population following eradication if resistance bears substantial costs. While the most straightforward prediction would be for evolution of decreased resistance or tolerance in response to the removal of a parasite, the reality is not always so simple. In a guppy–macroparasite system, removal of the parasite led to the evolution of increased resistance, most likely owing to changes in life history [77]. Furthermore, if the eradicated parasites interacted with other parasites, this might lead to dramatic changes in their infection prevalence, which might increase the strength of selection from those parasites.
An interesting example of these potential interactions comes from African buffalo (Syncerus caffer), which are infected by helminth worms and microparasites [78]. Because macroparasites and microparasites are targeted by different subsets of the vertebrate immune system, not having to fight off helminths might be expected to increase the immune response against microparasites, also reducing their prevalence. However, experimental treatment with anthelminthics substantially increased the lifespan of buffalo without altering their susceptibility to bovine tuberculosis. The net effect was to drive a large increase in the basic reproduction number, R0, of bovine tuberculosis, because buffalo infected with tuberculosis lived longer, spreading the pathogen to more individuals [78]. Thus, in this system, eradicating helminth infections would be expected to increase the strength of selection from bovine tuberculosis, potentially influencing host evolution.
In addition to influencing evolution of the host, eradication might influence the evolution of other parasites. Eradicating an infectious disease can alter competitive interactions and result in a vacated niche that can drive selection on other parasites [79]. For example, the eradication of smallpox has left an empty niche that might now be filled by a close relative, monkeypox. After the eradication of smallpox and cessation of vaccination campaigns (the smallpox vaccine provides considerable cross-immunity to monkeypox), monkeypox prevalence has increased in the Democratic Republic of Congo [80]. This means that there is now increased opportunity for selection on monkeypox to evolve increased transmission from human to human [9,80]. Overall, eradication of an infectious disease is likely to alter the selective environment for both the host and other infectious diseases in the system.
8. Knowledge gaps and future directions
We have shown that human actions can drive evolutionary dynamics that contribute to parasite emergence, yet we are just beginning to understand the details of these interactions. We know that EIDs drive evolution of both hosts and parasites, but at this point it is difficult to predict how evolutionary processes will unfold and how important evolution might be in shaping disease trajectories. One way to address this knowledge gap is to focus on key traits of host and parasite species that may contribute to their role in disease epidemics. Key questions include the following (i) Which traits make it likely that a parasite will be able to evolve in a way that allows it to emerge? (ii) Are there traits of hosts that make them more likely to be impacted by an EID? (iii) What host traits, parasite traits and ecological circumstances are important for (co)evolution in EID systems?
Existing research makes some interesting predictions regarding parasite traits that may facilitate evolutionary changes that contribute to disease emergence. For example, fungal pathogens frequently cause emerging diseases in plants and have led to high profile wildlife diseases in recent years [81]. These outbreaks may be supported by the ability of fungi to use many host species and to undergo rapid evolutionary change through processes such as hybridization and recombination [81]. In addition, long-lived resting stages enable long-distance dispersal, enabling humans to (unintentionally) transport fungal pathogens into areas with naive hosts. RNA viruses are prone to causing epidemics in humans and domestic mammals, and traits such as particularly fast generation times, high mutation rates, poor correction of mutations, and therefore, high capacity to adapt to a new host are thought to be important (e.g. [4,82]). Learning more about the traits that make parasites successful and likely to cause outbreaks could aid in our efforts to prevent, control or even eradicate EIDs of concern.
Interestingly, there may be a link between resilience to human disturbances, susceptibility to disease and the ability to rapidly adapt to changing conditions. ‘Weedy’ host species that invest more resources into reproduction and growth are often better suited to colonize habitats disturbed by human activities [83]. These species also may invest less into immunity and disease resistance, making them more susceptible to emerging diseases. However, weedy traits such as a fast generation time and high reproductive rate may also facilitate rapid evolutionary change in response to infectious diseases. Several well-studied examples of hosts that have evolved in response to EIDs feature ‘weedy’ host species (e.g. West Nile virus in house sparrows [26]; Mycoplasma infection of house finches [20]; and the classic example of Australian rabbit (Oryctolagus cuniculus) pest populations adapting to myxoma virus introduced as a biological control agent [73]).
One major reason that we care about evolution in the context of EIDs is when trying to predict the trajectory of a given EID. In particular, under what circumstances will a host population experiencing a devastating outbreak evolve and be able to persist? A current example is that of the fungal pathogen (Pseudogymnoascus destructans) causing WNS in bats in eastern North America. Since it was first observed in the US in 2006, WNS has caused devastating mortality in several bat species, including the formerly abundant little brown bat (Myotis lucifugus) [84,85]. However, in the regions of the US where WNS has been present the longest, little brown bats appear to be persisting and are resistant to the disease [27]. Whether other populations of the little brown bat will be able to adapt to this EID threat remains to be seen.
An important question that eco-evolutionary dynamics studies try to address is how rapid evolution can feedback to influence ecology [86]. The ecological consequences of rapid evolution by hosts and EIDs are likely to be significant. For example, WNS has led to major declines in North American bat populations, threatening some with regional extinction [85]. Bats play an important role in ecosystems as voracious consumers of insects, including agricultural and forest pests [87]. If bats evolve resistance or tolerance to this fungus, as appears to be happening in at least one species [27], the persistence of bats in this region could significantly dampen the ecological consequences of this epidemic.
9. Conclusion
We have shown that several common human environmental impacts can contribute to disease emergence, and that both ecological and evolutionary dynamics are occurring on contemporary timescales. Through global travel and trade, land-use change and climate change, humans facilitate movement of host, parasite and vector species, enabling infection of naive host populations as well as host switches. Urbanization and agriculture can support particularly high population densities of hosts, leading to the evolution of virulent parasites but also more resistant hosts. Human impacts on the abiotic environment, especially temperature and chemical pollution, can favour parasite emergence through both ecological and evolutionary pathways. Habitat fragmentation, hunting and agriculture can reduce host genetic diversity, limiting the potential for an evolutionary response in that host. In most of these cases, humans impact disease emergence unintentionally. Meanwhile, actions implemented to control disease, such as culling and vaccination programmes, can lead to unintended evolutionary consequences in both hosts and parasites, but rarely result in parasite disappearance. We have shown that all of these actions can drive rapid evolution of both parasites and hosts, highlighting the importance of considering evolutionary processes to fully understand why parasites emerge.
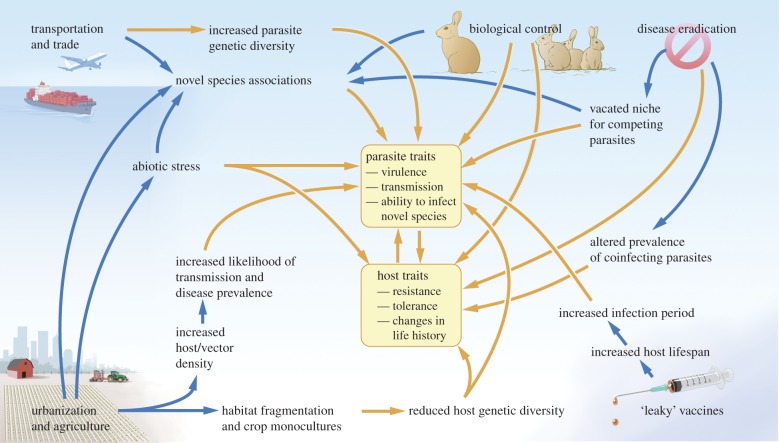
Additionally, while we have discussed these mechanisms and drivers separately, multiple human impacts often operate together to drive parasite emergence (figure 2). For example, intensive agriculture may drive evolution of increasing parasite virulence owing to both high host density and low host genetic diversity effects. Application of chemicals to control disease may further select for increasing virulence in remaining parasites. Spillover into closely related wild species may ensue, and transport of these virulent parasites by humans could spread the parasite to new naive host populations.
Figure 2.
Schematic illustrating some of the ways humans can influence ecological and evolutionary host–parasite dynamics in emerging disease systems. Interactions involving primarily ecological or evolutionary changes are drawn with blue (dark grey in print) and orange (light grey in print) arrows, respectively. Figure drawn by John Megahan. (Online version in colour.)
EIDs are a substantial threat to biodiversity, human health and economic well-being. Understanding the ecological and evolutionary mechanisms involved, particularly the phenotypic traits that enable parasites and hosts to (co)evolve, will help not only to improve our understanding of human influences on EIDs, but also aid in controlling or even eradicating parasites.
Acknowledgements
We thank Max Lambert, Kate Langwig, John Marino, Jonathan Richardson and two anonymous reviewers for comments on an earlier draft of this manuscript.
Authors' contributions
All authors contributed to the writing and editing of this review.
Competing interests
The authors state that they have no competing interests.
Funding
We acknowledge support from NSF DEB-1305836 (to M.A.D.) and from the USDA via the Colorado Agricultural Experiment Station (to R.A.H.).
References
- 1.Woolhouse MEJ, Haydon DT, Antia R. 2005. Emerging pathogens: the epidemiology and evolution of species jumps. Trends Ecol. Evol. 20, 238–244. ( 10.1016/j.tree.2005.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 3.Baucom RS, De Roode JC. 2011. Ecological immunology and tolerance in plants and animals. Funct. Ecol. 25, 18–28. ( 10.1111/j.1365-2435.2010.01742.x) [DOI] [Google Scholar]
- 4.Jones K, Patel N, Levy M, Storeygard A, Balk D, Gittleman J, Daszak P. 2008. Global trends in emerging infectious diseases. Nature 451, 990–993. ( 10.1038/nature06536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilpatrick AM, Randolph SE. 2012. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 380, 1946–1955. ( 10.1016/S0140-6736(12)61151-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altizer S, Harvell D, Friedle E. 2003. Rapid evolutionary dynamics and disease threats to biodiversity. Trends Ecol. Evol. 18, 589–596. ( 10.1016/j.tree.2003.08.013) [DOI] [Google Scholar]
- 7.Kennedy DA, Kurath G, Brito IL, Purcell MK, Read AF, Winton JR, Wargo AR. 2015. Potential drivers of virulence evolution in aquaculture. Evol. Appl. 9, 344–354. ( 10.1111/eva.12342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones BA, et al. 2013. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl Acad. Sci. USA 110, 8399–8404. ( 10.1073/pnas.1208059110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antia R, Regoes RR, Koella JC, Bergstrom CT. 2003. The role of evolution in the emergence of infectious diseases. Nature 426, 658–661. ( 10.1038/nature02177.1.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RM, May RM. 1982. Coevolution of hosts and parasites. Parasitology 85, 411–426. ( 10.1017/S0031182000055360) [DOI] [PubMed] [Google Scholar]
- 11.Bolker BM, Nanda A, Shah D. 2010. Transient virulence of emerging pathogens. J. R. Soc. Interface 7, 811–822. ( 10.1098/rsif.2009.0384) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendry AP. 2016. Eco-evolutionary. Princeton, NJ: Princeton University Press. [Google Scholar]
- 13.Fussmann GF, Loreau M, Abrams PA. 2007. Eco-evolutionary dynamics of communities and ecosystems. Funct. Ecol. 21, 465–477. ( 10.1111/j.1365-2435.2007.01275.x) [DOI] [Google Scholar]
- 14.Ebert D, Bull JJ. 2008. The evolution and expression of virulence, 2nd edn New York, NY: Oxford University Press, Inc; ( 10.1093/acprof:oso/9780199207466.003.0012) [DOI] [Google Scholar]
- 15.Hochachka WM, Dhondt AA, Dobson A, Hawley DM, Ley DH, Lovette IJ. 2013. Multiple host transfers, but only one successful lineage in a continent-spanning emergent pathogen. Proc. R. Soc. B 280, 20131068 ( 10.1098/rspb.2013.1068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adelman JS, Moyers SC, Farine DR, Hawley DM. 2015. Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proc. R. Soc. B 282, 20151429 ( 10.1098/rspb.2015.1429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer JD, Miller JR. 2015. Direct and indirect effects of anthropogenic bird food on population dynamics of a songbird. Acta Oecol. 69, 46–51. ( 10.1016/j.actao.2015.08.006) [DOI] [Google Scholar]
- 18.Hawley DM, Osnas EE, Dobson AP, Hochachka WM, Ley DH, Dhondt AA. 2013. Parallel patterns of increased virulence in a recently emerged wildlife pathogen. PLoS Biol. 11, e1001570 ( 10.1371/journal.pbio.1001570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delaney NF, Balenger SL, Bonneaud C, Marx CJ, Hill GE, Ferguson-Noel N, Tsai P, Rodrigo A, Edwards SV. 2012. Ultrafast evolution and loss of CRISPRs following a host shift in a novel wildlife pathogen, Mycoplasma gallisepticum. PLoS Genet. 8, e1002511 ( 10.1371/journal.pgen.1002511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonneaud C, Balenger SL, Russell AF, Zhang J, Hill GE, Edwards SV. 2011. Rapid evolution of disease resistance is accompanied by functional changes in gene expression in a wild bird. Proc. Natl. Acad. Sci. USA 108, 7866–7871. ( 10.1073/pnas.1018580108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adelman JS, Kirkpatrick L, Grodio JL, Hawley DM. 2013. House finch populations differ in early inflammatory signaling and pathogen tolerance at the peak of Mycoplasma gallisepticum infection. Am. Nat. 181, 674–689. ( 10.1086/670024) [DOI] [PubMed] [Google Scholar]
- 22.Hairston NG, Ellner SP, Geber MA, Yoshida T, Fox JA. 2005. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127. ( 10.1111/j.1461-0248.2005.00812.x) [DOI] [Google Scholar]
- 23.Freed LA, Cann RL, Goff ML, Kuntz WA, Gustav R. 2005. Increase in avian malaria at upper elevation in Hawai'i. Condor 107, 753–764. ( 10.1650/7820.1) [DOI] [Google Scholar]
- 24.Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 19, 535–544. ( 10.1016/j.tree.2004.07.021) [DOI] [PubMed] [Google Scholar]
- 25.Hendry AP, Gotanda KM, Svensson EI. 2017. Human influences on evolution, and the ecological and societal consequences. Phil. Trans. R. Soc. B 372, 20160028 ( 10.1098/rstb.2016.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duggal NK, et al. 2014. Evidence for co-evolution of West Nile virus and house sparrows in North America. PLoS Negl. Trop. Dis. 8, e3262 ( 10.1371/journal.pntd.0003262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langwig KE, Hoyt JR, Parise KL, Frick WF, Foster JT, Kilpatrick AM. 2017. Resistance in persisting bat populations after white-nose syndrome invasion. Phil. Trans. R. Soc. B 372, 20160044 ( 10.1098/rstb.2016.0044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savage AE, Zamudio KR. 2016. Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc. R. Soc. B 283, 20153115 ( 10.1098/rspb.2015.3115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turcotte MM, Araki H, Karp DS, Poveda K, Whitehead SR. 2017. The eco-evolutionary impacts of domestication and agricultural practices on wild species. Phil. Trans. R. Soc. B 372, 20160033 ( 10.1098/rstb.2016.0033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcox BA, Gubler DJ. 2005. Disease ecology and the global emergence of zoonotic pathogens. Environ. Health Prev. Med. 10, 263–272. ( 10.1007/BF02897701) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowden SE, Magori K, Drake JM. 2011. Regional differences in the association between land cover and West Nile virus disease incidence in humans in the United States. Am. J. Trop. Med. Hyg. 84, 234–238. ( 10.4269/ajtmh.2011.10-0134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilpatrick AM. 2011. Globalization, land use, and the invasion of West Nile virus. Science 334, 323–327. ( 10.1126/science.1201010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Roode JC, Yates AJ, Altizer S, De Roode JC, Yates AJ, Altizer S, de Roode JC, Yates AJ, Altizer S. 2008. Virulence–transmission trade-offs and population divergence in virulence in a naturally occurring butterfly parasite. Proc. Natl Acad. Sci. USA 105, 7489–7494. ( 10.1073/pnas.0710909105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips BL, Puschendorf R. 2013. Do pathogens become more virulent as they spread? Evidence from the amphibian declines in Central America. Proc. R. Soc. B 280, 20131290 ( 10.1098/rspb.2013.1290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krkosek M, Ford JS, Morton A, Lele S, Myers RA, Lewis MA. 2007. Declining wild salmon populations in relation to parasites in farm salmon. Science 318, 1772–1775. ( 10.1126/science.1148744) [DOI] [PubMed] [Google Scholar]
- 36.Civitello DJ, et al. 2015. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl Acad. Sci. USA 112, 8667–8671. ( 10.1073/pnas.1506279112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hechinger RF, Lafferty KD. 2005. Host diversity begets parasite diversity: bird final hosts and trematodes in snail intermediate hosts. Proc. R. Soc. B 272, 1059–1066. ( 10.1121/1.4929899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. 2013. Climate change and infectious diseases: from evidence to a predictive framework. Science 341, 514–519. ( 10.1126/science.1239401) [DOI] [PubMed] [Google Scholar]
- 39.Bostock RM, Pye MF, Roubtsova TV. 2014. Predisposition in plant disease: exploiting the nexus in abiotic and biotic stress perception and response. Annu. Rev. Phytopathol. 52, 517–549. ( 10.1146/annurev-phyto-081211-172902) [DOI] [PubMed] [Google Scholar]
- 40.Morley NJ, Lewis JW. 2014. Temperature stress and parasitism of endothermic hosts under climate change. Trends Parasitol. 30, 221–227. ( 10.1016/j.pt.2014.01.007) [DOI] [PubMed] [Google Scholar]
- 41.Miller MR, White A, Boots M. 2007. Host life span and the evolution of resistance characteristics. Evolution 61, 2–14. ( 10.1111/j.1558-5646.2007.00001.x) [DOI] [PubMed] [Google Scholar]
- 42.Williams PD, Day T. 2001. Interactions between sources of mortality and the evolution of parasite virulence. Proc. R. Soc. Lond. B 268, 2331–2337. ( 10.1098/rspb.2001.1795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genung MA, Schweitzer JA, Úbeda F, Fitzpatrick BM, Pregitzer CC, Felker-Quinn E, Bailey JK. 2011. Genetic variation and community change—selection, evolution, and feedbacks. Funct. Ecol. 25, 408–419. ( 10.1111/j.1365-2435.2010.01797.x) [DOI] [Google Scholar]
- 44.Lancaster LT, Morrison G, Fitt RN. 2017. Life history trade-offs, the intensity of competition, and coexistence in novel and evolving communities under climate change. Phil. Trans. R. Soc. B 372, 20160046 ( 10.1098/rstb.2016.0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.IPCC. 2013. Summary for Policymakers. In Climate change 2013: the physical science basis (eds Stocker T, et al.), pp. 1–27. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 46.Rohr JR, Raffel TR, Blaustein AR, Johnson PTJ, Paull SH, Young S. 2013. Using physiology to understand climate-driven changes in disease and their implications for conservation. Conserv. Physiol. 1, 1–15. ( 10.1093/conphys/cot022.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell SE, Rogers ES, Little TJ, Read AF, Mitchell SE, Rogers ES, Little TJ, Read AF. 2005. Host–parasite and genotype-by-environment interactions: temperature modifies potential for selection by a sterilizing pathogen. Evolution 59, 70–80. ( 10.1111/j.0014-3820.2005.tb00895.x) [DOI] [PubMed] [Google Scholar]
- 48.Wolinska J, King KC. 2009. Environment can alter selection in host–parasite interactions. Trends Parasitol. 25, 236–244. ( 10.1016/j.pt.2009.02.004) [DOI] [PubMed] [Google Scholar]
- 49.Pettis JS, Lichtenberg EM, Andree M, Stitzinger J, Rose R, VanEngelsdorp D. 2013. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 8, e70182 ( 10.1371/journal.pone.0070182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.VanEngelsdorp D, et al. 2010. Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J. Econ. Entomol. 103, 1517–1523. ( 10.1603/EC09429) [DOI] [PubMed] [Google Scholar]
- 51.Jansen M, Stoks R, Coors A, van Doorslaer W, de Meester L. 2011. Collateral damage: rapid exposure-induced evolution of pesticide resistance leads to increased susceptibility to parasites. Evolution 65, 2681–2691. ( 10.2307/41240852) [DOI] [PubMed] [Google Scholar]
- 52.McCarroll L, Paton MG, Karunaratne SHPP, Jayasuryia HTR, Kalpage KSP, Hemingway J. 2000. Insecticides and mosquito-borne disease. Nature 407, 961–962. ( 10.1038/35039671) [DOI] [PubMed] [Google Scholar]
- 53.King KC, Lively CM. 2012. Does genetic diversity limit disease spread in natural host populations? Heredity (Edinb) 109, 199–203. ( 10.1038/hdy.2012.33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dixo M, Metzger JP, Morgante JS, Zamudio KR. 2009. Habitat fragmentation reduces genetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biol. Conserv. 142, 1560–1569. ( 10.1016/j.biocon.2008.11.016) [DOI] [Google Scholar]
- 55.Dubois J, Cheptou P-O. 2017. Effects of fragmentation on plant adaptation to urban environments. Phil. Trans. R. Soc. B 372, 20160038 ( 10.1098/rstb.2016.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheptou P-O, Hargreaves AL, Bonte D, Jacquemyn H. 2017. Adaptation to fragmentation: evolutionary dynamics driven by human influences. Phil. Trans. R. Soc. B 372, 20160037 ( 10.1098/rstb.2016.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson ND, Fahrig L. 2011. Relative effects of road mortality and decreased connectivity on population genetic diversity. Biol. Conserv. 144, 3143–3148. ( 10.1016/j.biocon.2011.09.010) [DOI] [Google Scholar]
- 58.Allendorf FW, Hard JJ. 2009. Human-induced evolution caused by unnatural selection through harvest of wild animals. Proc. Natl Acad. Sci. USA 106(Suppl), 9987–9994. ( 10.1073/pnas.0901069106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuparinen A, Festa-Bianchet M. 2017. Harvest-induced evolution: insights from aquatic and terrestrial systems. Phil. Trans. R. Soc. B 372, 20160036 ( 10.1098/rstb.2016.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu Y, et al. 2000. Genetic diversity and disease control in rice. Nature 406, 718–722. ( 10.1038/35021046) [DOI] [PubMed] [Google Scholar]
- 61.Ploetz RC. 2006. Fusarium wilt of banana is caused by several pathogens referred to as Fusarium oxysporum f. sp. cubense. Phytopathology 96, 653–656. ( 10.1094/PHYTO-96-0653) [DOI] [PubMed] [Google Scholar]
- 62.Man In ‘T Veld WA, De Cock AWAM, Summerbell RC. 2007. Natural hybrids of resident and introduced Phytophthora species proliferating on multiple new hosts. Eur. J. Plant Pathol. 117, 25–33. ( 10.1007/s10658-006-9065-9) [DOI] [Google Scholar]
- 63.Cairns J, Becks L, Jalasvuori M, Hiltunen T. 2017. Sublethal streptomycin concentrations and lytic bacteriophage together promote resistance evolution. Phil. Trans. R. Soc. B 372, 20160040 ( 10.1098/rstb.2016.0040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hiltunen T, Virta M, Laine A-L. 2017. Antibiotic resistance in the wild: an eco-evolutionary perspective. Phil. Trans. R. Soc. B 372, 20160039 ( 10.1098/rstb.2016.0039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gandon S, Mackinnon MJ, Nee S, Read AF. 2001. Imperfect vaccines and the evolution of pathogen virulence. Nature 414, 751–756. ( 10.1038/414751a) [DOI] [PubMed] [Google Scholar]
- 66.Mackinnon MJ, Gandon S, Read AF. 2008. Virulence evolution in response to vaccination: the case of malaria. Vaccine 26, C42–C52. ( 10.1016/j.vaccine.2008.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Read AF, Baigent SJ, Powers C, Kgosana LB, Blackwell L, Smith LP, Kennedy DA, Walkden-Brown SW, Nair VK. 2015. Imperfect vaccination can enhance the transmission of highly virulent pathogens. PLoS Biol. 13, 1–18. ( 10.1371/journal.pbio.1002198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolzoni L, De Leo GA. 2013. Unexpected consequences of culling on the eradication of wildlife diseases: the role of virulence evolution. Am. Nat. 181, 301–313. ( 10.1086/669154) [DOI] [PubMed] [Google Scholar]
- 69.Shim E, Galvani AP. 2009. Evolutionary repercussions of avian culling on host resistance and influenza virulence. PLoS ONE 4, 1–8. ( 10.1371/journal.pone.0005503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cressler CE, McLeod DV, Rozins C, Van Den Hoogen J, Day T. 2015. The adaptive evolution of virulence: a review of theoretical predictions and empirical tests. Parasitology 143, 915–930. ( 10.1017/S003118201500092X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ebert D, Mangin KL. 1997. The influence of host demography on the evolution of virulence of a microsporidian gut parasite. Evolution 51, 1828–1837. ( 10.2307/2411005) [DOI] [PubMed] [Google Scholar]
- 72.Gandon S, Jansen VAA, van Baalen M. 2001. Host life history and the evolution of parasite virulence. Evolution 55, 1056–1062. ( 10.1111/j.1095-8649.2006.01157.x) [DOI] [PubMed] [Google Scholar]
- 73.Fenner F, Fantini B. 1999. Biological control of vertebrate pests: the history of myxomatosis—an experiment in evolution, 2nd edn London, UK: CABI Publishing. [Google Scholar]
- 74.Roderick GK, Navajas M. 2003. Genes in new environments: genetics and evolution in biological control. Nat. Rev. Genet. 4, 889–899. ( 10.1038/nrg1201) [DOI] [PubMed] [Google Scholar]
- 75.Klepac P, Metcalf CJE, McLean AR, Hampson K. 2013. Towards the endgame and beyond: complexities and challenges for the elimination of infectious diseases. Phil. Trans. R. Soc. B 368, 20120137 ( 10.1098/rstb.2012.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bosch J, Sanchez-Tome E, Fernandez-Loras A, Oliver JA, Fisher MC, Garner TWJ. 2015. Successful elimination of a lethal wildlife infectious disease in nature. Biol. Lett. 11, 20150874 ( 10.1098/rsbl.2015.0874) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dargent F, Scott ME, Hendry AP, Fussmann GF. 2013. Experimental elimination of parasites in nature leads to the evolution of increased resistance in hosts. Proc. R. Soc. B 280, 20132371 ( 10.1098/rspb.2013.2371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ezenwa VO, Jolles AE. 2015. Opposite effects of anthelmintic treatment on microbial infection at individual versus population scales. Science 347, 175–177. ( 10.1126/science.1261714) [DOI] [PubMed] [Google Scholar]
- 79.Lloyd-Smith JO. 2013. Vacated niches, competitive release and the community ecology of pathogen eradication. Phil. Trans. R. Soc. B 368, 20120150 ( 10.1098/rstb.2012.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rimoin AW, et al. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl Acad. Sci. USA 107, 16 262–16 267. ( 10.1073/pnas.1005769107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–194. ( 10.1038/nature10947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cleaveland S, Laurenson MK, Taylor LH. 2001. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Phil. Trans. R. Soc. Lond. B 356, 991–999. ( 10.1098/rstb.2001.0889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keesing F, et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. ( 10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blehert DS, et al. 2009. Bat white-nose syndrome: an emerging fungal pathogen? Science 323, 227 ( 10.1126/science.1163874) [DOI] [PubMed] [Google Scholar]
- 85.Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GG, Butchkoski CM, Kunz TH. 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682. ( 10.1126/science.1188594) [DOI] [PubMed] [Google Scholar]
- 86.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 87.Boyles JG, Cryan PM, McCracken GF, Kunz TH. 2011. Economic importance of bats in agriculture. Science 332, 41–42. ( 10.1126/science.1201366) [DOI] [PubMed] [Google Scholar]