Abstract
The consequences of climate change for local biodiversity are little understood in process or mechanism, but these changes are likely to reflect both changing regional species pools and changing competitive interactions. Previous empirical work largely supports the idea that competition will intensify under climate change, promoting competitive exclusions and local extinctions, while theory and conceptual work indicate that relaxed competition may in fact buffer communities from biodiversity losses that are typically witnessed at broader spatial scales. In this review, we apply life history theory to understand the conditions under which these alternative scenarios may play out in the context of a range-shifting biota undergoing rapid evolutionary and environmental change, and at both leading-edge and trailing-edge communities. We conclude that, in general, warming temperatures are likely to reduce life history variation among competitors, intensifying competition in both established and novel communities. However, longer growing seasons, severe environmental stress and increased climatic variability associated with climate change may buffer these communities against intensified competition. The role of life history plasticity and evolution has been previously underappreciated in community ecology, but may hold the key to understanding changing species interactions and local biodiversity under changing climates.
This article is part of the themed issue ‘Human influences on evolution, and the ecological and societal consequences’.
Keywords: range shifts, no-analogue communities, global warming, trophic interactions and competition, community assembly, ecological niche
1. Introduction
Anthropogenic climate change is a primary risk to global biodiversity [1–3]. While most projections for future changes in biodiversity indicate that global warming will contribute significantly to biodiversity loss over the next decades (e.g. [2,4]), such projections often have wide error margins [5], particularly because we still have little insight into the roles of biotic interactions and evolutionary change. Multiple studies demonstrate that many species possess some capacity to persist under climate change, through a combination of phenotypic plasticity and evolutionary adaptation [6]. However, it is still largely unknown how rates and modes of phenotypic change and adaptation will vary between interacting species, and how these evolutionary responses will affect biotic interactions, communities and ecosystems. Much work in this area has focused on the potential for mismatches between species across trophic levels [7,8]. Potentially equally or more important to future community diversity are effects of warming on changes in the outcomes of competitive interactions, but these have received much less attention to date. Like trophic interactions, competitive interactions may intensify or diminish under climate change [9,10], with important consequences for local biodiversity and ecosystem services.
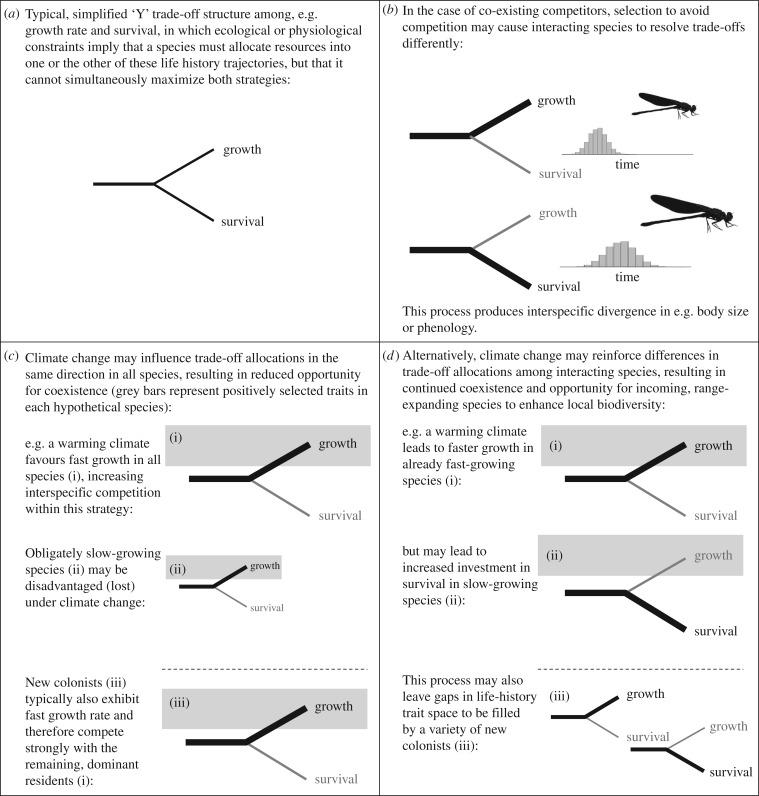
Here we propose a novel framework to predict effects of climate change on competition intensities, based on general life history theory. The framework rests on the observation that the strength of competition among species with similar ecological niches (such as within a guild) is often mediated by their degree of similarity in their evolved life histories, including strategies for survival, reproduction and dispersal [11,12]. Life history traits are fundamentally limited in nature by evolutionary and ecological trade-offs [13], and differences among species in how these trade-offs are resolved evolutionarily can allow fine partitioning of shared niches [14] and promote coexistence of competitors [15]. Moreover, the particular way that an individual or species resolves this trade-off (i.e. which dimensions are favoured over others) often reflects the species' historical legacy of biotic interactions and its colonization history [16,17]. By identifying patterns of coevolved life history trade-off strategies within communities that facilitate or limit coexistence of competing species, now and under future conditions [11,18,19], we can derive more general principles of coexistence under rapid environmental change and in evolving species assemblages (figure 1).
Figure 1.
A conceptual model of how life history shifts within communities may affect the future of coexistence under ongoing climate change, in both existing species assemblages and novel assemblages resulting from colonization events and range shifts.
2. Climate change and competitive interactions
Competition is a well-known driver of local (alpha) biodiversity [20], and thus effects of climate change on competitive interactions are likely to be critical mediators of biodiversity responses. While numerous studies have provided evidence of changing competitive interactions in response to climate change [10,21,22], it is more difficult to identify how climatic warming affects the overall importance of competition for shaping communities [23]. In general, changes in the intensity of interspecific competition under climate change are likely to be driven by several distinct, but sometimes synergistic, processes: first, changes in the extent and diversity of favourable habitats may alter patterns of competition for remaining space and resources [10,24–27]. Second, climate change may directly affect the competitive abilities of interacting species, via plastic or evolved effects on growth rates, body sizes and phenologies, and these changes can alter patterns of competitive dominance within or competitive exclusion from communities [21]. Third, competitive interactions may be influenced by the direct effects of climate on numerical abundances of each species [28]. Finally, range shifts mediated by climate change can directly impact the strength of competition within communities by changing the biodiversity of the regional species pool, altering the total potential number and identity of species interacting at each site [29,30]. Here I review the evidence for how these factors may interactively contribute to changing competitive interactions and biodiversity in different environments, and suggest how life history theory can help inform predictions for future loss of community diversity and ecosystem services.
3. Life history trade-offs and coexistence
All species face the problem of being limited in their ability to simultaneously maximize all components of fitness [31]. Such trade-offs are often mediated by extrinsic limits to time or energy, so that, for example, energy allocated to reproduction detracts from energy available for survival and maintenance. Individuals solve this dilemma by investing in some traits, which are related to primary energy allocation and which are closely linked to fitness (such as growth rate, body size, stress response, timing of reproduction, offspring quantity and quality, longevity and dispersal), at the expense of other such traits. The nature of trade-off functions among these traits can be quite complex, involving multidimensional allocation decisions or higher order properties of trait values [32]. Despite these complexities, a simplistic ‘Y’ model is often a useful heuristic to depict the essence of individual allocation decisions, with the caveat that many trade-offs do not conform to this simple structure [13] (figure 1a). Life history trade-offs are often strong determinants of competitive abilities under both stable and changing ecological conditions [21,33,34], and thus climate change-induced shifts in life history traits may be partially responsible for changing competitive interactions under climate change (see below).
Traditional community assembly theory suggests that species that occupy similar niche space will compete most intensely and will exclude each other from communities unless each can increase when rare [35,36]. Central to this theory is the tenet that the structuring of resource availability, and the strategies used to garner resources, are critical predictors of interspecific competition and coexistence within communities [15,37]. Also implicit in coexistence theory is the role of demographic trade-offs; i.e. coexistence occurs within communities because no species can simultaneously optimize all strategies for monopolizing the shared resource(s) [15]. This theory bears striking resemblance to life history theory in evolutionary biology, however, these parallels have rarely been acknowledged (but see [11,15]), in part, because classic community ecology often ignores the evolutionary processes occurring within communities (but see [38]).
Recent theory suggests that coexistence of competitors can occur on the basis of evolved differences in life history strategies [11]. Interspecific life history divergence can support coexistence for a variety of reasons. First, an oft-reported trade-off between competitive ability and longevity will favour the coexistence of species able to achieve quick numerical abundance, and species with slower growth but better persistence through time [15,39]. This mechanism is particularly salient when the environment fluctuates over time [19], but intransitive dynamics mediated by interspecific differences in density-dependent regulation can also maintain the coexistence of competitors with divergent life history strategies along the slow–fast continuum within communities, even in the absence of environmental fluctuations [40]. Note that this coexistence mechanism does not depend on competitors diverging in their resource requirements or resource acquisition abilities. Second, trade-offs between survival and growth rate (and contributing, for example, to variation in age and size at maturity, longevity and lifetime reproductive success) may be resolved differently among competing species, leading to phenological divergence of feeding and breeding times between competitors. This occurs because individuals of each species experience some measure of stabilizing selection to converge on particular strategies that ensure synchronization or overlap between mates or other cooperative strategies. When members of each species group together in life history trait space to ensure beneficial intraspecific interactions, temporal niches are made available for other species to fill. Once filled, each species' temporal niche may also be maintained by selection to avoid competition (character displacement). This mechanism is particularly relevant when there are external limits or guides on the life cycle imposed by seasonality, such that fast strategies automatically correlate to earlier emergence/peak feeding times, whereas slower strategies may monopolize later opportunities [17]. Under this mechanism, divergent life histories within communities facilitate temporal partitioning of a shared resource niche among competing species [14,41,42].
Empirical evidence from damselflies, plants, parasitoids, fig wasps, fish and Drosophila all indicate that life history divergence is often a critical coexistence mechanism within guilds, where species overlap very closely in specific resource requirement and acquisition traits [14,15,18,19,39,40,43]. Life history traits are also well-characterized mediators of competition and coexistence even among more diffuse competitors [37,44]. Despite this, little consideration has been given to the evolution of life history strategies (in other words, towards optimal allocation of resources across different, competing components of fitness) in the context of interspecific competitive interactions (but see [11]).
Life history traits are typically highly sensitive to climatic temperature changes, exhibiting typically high levels of thermal plasticity (for example, thermal dependence of growth rates in ectotherms and plants, and thermal reaction norms for survival and immune response across many species [45,46]). Furthermore, most of the best-documented examples of evolutionary responses to climate change also primarily involve shifts in life history traits, rather than shifts in environmental tolerances or resource requirements [47]. Thus, an important but largely unasked question is how ongoing climate change will affect competitive intensities and local biodiversity via its rapid effects on life histories of constituent species. If climate change drives convergence of life histories among competitors, competition for shared resources may intensify and competitive exclusion and local extirpations are likely to ensue (figure 1c). However, if climate change leads to life history divergence among competing species within communities, this may buffer communities from biodiversity loss but potentially leave them vulnerable to invasion (figure 1d). These alternative scenarios are discussed below.
4. Climate warming and life history convergence within communities
There are good reasons to expect that climate change will lead many species to adopt a ‘faster’ life history strategy, whether through evolutionary change or phenotypic plasticity [48]. This occurs for a number of reasons: first, climate change is in many cases resulting in earlier or shortened growing seasons in many biotas, selecting for both plastic and evolved responses towards earlier and faster growth, maturation and reproduction [7,8]. Evolutionary change under selection for faster life histories may occur under climate change to adaptively allow species to capitalize on earlier opportunities for reproduction, or to track changes in timing of resource abundances [49]. Depending on the degree of reliability of thermal cues to predict peak breeding or feeding opportunities, selection for faster life histories under warming climates may act on the trait values, or on the thermal dependence of the underlying traits (reaction norm evolution [50]), in either case producing evolutionary change. Second, many life history traits are inherently thermally dependent, and thus increasing temperatures will automatically result in acceleration of these processes purely via plasticity, which may or may not be adaptive [51] and may or may not affect future evolutionary change [52]. For example, the growth rate of ectotherms relates directly to temperature, e.g. via Dyer's Law [53], so higher temperatures automatically equate to faster growth rates and changes, typically but not always resulting in shifts to smaller adult body sizes [45]. This shift towards faster life histories, whether through plasticity or evolutionary change, often invokes life history trade-offs with survival or dispersal abilities [54]. A recent meta-analysis suggests that plants, fish, birds, mammals and terrestrial ectotherms have all tended to shift towards faster life histories and smaller body sizes under climate change [55].
The pressures of climate change to ‘speed up’ the life histories of most species may result in convergence of life history strategies among species within communities, increasing the opportunity for competition. This can occur for several, often simultaneous, reasons. First, coexistence is commonly maintained in part by selection to promote life history divergence among competing species (as reviewed above). Thus, advancing or accelerating life histories caused by climate change could result in more convergent strategies on average, particularly if life history shifts are purely plastic, and if the plasticity of a trait is negatively correlated with its mean value (as is often reported for life history and thermally sensitive traits [56,57]). In this scenario, all species are shifted to faster life history strategies, but species exhibiting slower values for a particular trait experience greatest rates of plastic change, resulting in life history convergence and increased opportunities for competition. If shifts towards faster life histories are evolutionary, however, then adaptive patterns of life history divergence may be preserved among interacting species under ongoing selection for reduced interspecific competition, providing that selection for faster life histories under climate change is very weak in comparison with ongoing selection to preserve life history divergence from interspecific competitors. Second, life history convergence within communities is likely to be even more pronounced if competitors share similar hard physiological or genetic constraints on the extent to which they can undergo climate change-driven changes in trait values [58]. Several previous studies have indicated that individuals or populations with more extreme life history trait values are less likely to exhibit sufficient genetic variation for or ability to plastically increase these traits [59,60]. Thus, warming-induced shifts in life histories of interacting species will potentially promote convergence on the most extreme strategy observed in the community under current conditions. Finally, given that shifts towards faster life histories are often adaptive for tracking advancing conditions under climate change, species best able to rapidly respond to this novel source of selection on life history may thus gain an increased evolutionary advantage over more obligately slow-developing species, as climates continue to change. This process could result in life history convergence within communities via species-level selection imposed by changing abiotic conditions. In other words, only species able to alter their life histories to match changing conditions will persist within communities. The loss of competitors with more divergent life histories frees up resources to allow numerical increases in the remaining species. These remaining species now compete more intensely because they are now dominating the resource and they overlap in their life history strategies [61,62].
If convergence of life histories occurs via any of the above mechanisms, interspecific competition will be more intense within these replacement communities, where the strength of competition is not alleviated by among-species life history diversification (figure 1c). It is worth noting that both plastic and evolutionary changes in life history allocations under climate change can produce changes in the variances as well as mean life history strategies within each population, and changes in intrapopulational variances may temper outcomes for predicted competitive intensities based on trait means. For instance, increased life history variability within species (such as via stress-induced expression of crypic genetic variation for life history traits) could temper increases in interspecific competition. By contrast, reduced life history variability within populations (such as under strong selection for extreme values) could compound effects of convergence to produce even greater intensities of interspecific competition [63].
5. Life history convergence and increased competition in the context of changing regional species pools
Within the Anthropocene, we are witnessing a mass reorganization of global, regional and local biotas [64]. Range expansions of many terrestrial and marine taxa are currently underway as climates warm to surpass minimally suitable temperatures just beyond their historical poleward or peak-ward range margins [65]; however, the relationship between these range movements and local (alpha) biodiversity is poorly understood. Increased competitive interactions under climate change may at least temporarily strengthen communities against colonization from lower latitudes [64] because it is more difficult for species to establish in novel habitats where existing competitors are already monopolizing the niche [66]. This protective feature of native competition could benefit native species by preventing interaction with novel competitors, but may have detrimental effects on range-shifting species by preventing their poleward movement [67]. However, it has been well documented that range expansions select for faster life history strategies [16,68,69], and the same traits associated with a successful range expansion are also favoured under competition during periods of rapid environmental change [21,34,70,71]. Thus competition between native species and novel competitors that have already gathered momentum in their poleward shifts will likely be fierce, because all are experiencing faster (converged) life histories (figure 1c). In other words, due to the special, evolved properties of range-shifting species combined with detrimental effects of warming on native species, priority effects may be insufficient to protect native species from novel competitive interactions created by widespread range expansions.
While intensified competition does not always lead to competitive exclusion [72], most empirical evidence suggests that incoming species will dominate in novel competitive interactions driven by climate change-induced range shifts [34,70], and competition between native and range-shifting species will contribute to native species population declines [26]. Thus, life history convergence and novel competitive interactions may play a significant role in the erosion of native biodiversity at high latitudes and contribute to biotic homogenization of the global flora and fauna [3]. Changes in the competitive outcomes between native and range-shifting species may also take time to resolve—a range-expanding species of damselfly in Scotland (Ischnura elegans) does not produce notable impacts on native community composition until it has naturalized for several generations in the new part of its range [73]. Thus, life history convergence among historic and novel competitors, resulting in intensified competitive interactions in the wake of climate change-mediated range expansions, could represent a major contribution to lingering extinction debt at high latitudes [30].
6. Climatic change and life history divergence within communities
Previous work has largely focused on the role of intensified competition under climate change [24–26,67], and relatively less effort has been expended to investigate the possibility that climate change reduces the intensity of competitive interactions (but see [29,74,75]). Competitive intensity may be reduced within communities if changing climates produce diverse effects on the life histories of constituent species, resulting in life history divergence among close competitors (figure 1d). Divergence in life histories within communities may occur as a by-product of longer growing seasons (particularly at high latitudes), or increased climate variability, both of which are important features of ongoing climate change [76,77]. For instance, at high latitudes where growing seasons are relatively short (with historically limited opportunities for temporal niche partitioning), climate change-mediated increases in growing season length may provide greater opportunity for a variety of growth rate and body size strategies to exist and coexist [78]. Similarly, increased climatic variability associated with global warming may support more diverse life history strategies, particularly because more variable climates allow persistence of species with life histories characterized by flexibility and opportunism, which can then coexist with species that exhibit intrinsically high growth rates [39]. Note that either intra- or interspecific life history variability can alleviate interspecific competition and support coexistence in principle [63], although there is little empirical evidence for the former as a coexistence mechanism.
Life history divergence might also be related to the numerical abundance of each species at starting conditions—species at relatively low abundance with lower levels of intraspecific competition may have a greater opportunity to respond to climate warming by increasing growth rates or decreasing adult body sizes (via rapid evolutionary change during periods of demographic growth, or because soft selection against plastic life history shifts is weaker), while more abundant species may be limited in this response due to higher levels of intraspecific competition and higher levels of soft selection against life history shifts [79]. Effects of different relative abundances on populations' potential responses to climate change may reinforce pre-existing interspecific variation in life history strategies.
Finally, climate change may promote life history divergence within communities by magnifying interspecific differences along pre-existing thermal reaction norms for underlying life history traits. Nearly all species have very slow growth rates or low survival at very low temperatures, but thermal optima may be more variable among competing species [80]. Given that many temperate species inhabit environmental temperatures which are cooler than their thermal optima [81], warming temperatures may move each species closer to its own, evolved thermal optimum. Differences among competing species in the degree of phenotypic plasticity or evolutionary potential to respond to climate may reinforce life history variation as climates warm [74]; such divergence can support continued coexistence within communities facing environmental change, provided that there remain opportunities for multiple life history strategies to obtain high fitness under the new conditions (in other words, opportunities provided by longer growing seasons or increased climatic fluctuations).
7. Life history divergence and decreased competition in the context of changing regional species pools
Because, globally, range expansions in response to climate change are more prevalent than contractions, the large-scale movement of species currently underway is generally resulting in an increase in regional biodiversity, particularly at high latitudes, even while global biodiversity declines [1,2,82,83]. Life history divergence under climate change may allow high-latitude communities to be more open to accepting these regional colonizers, if the process of life history divergence leaves gaps in life history trait space that can be filled by previously excluded species (figure 1d). If this occurs, then the regional increase in biodiversity currently witnessed at high latitudes will be supported at the community level as well, as climates continue to warm, and we can expect to see net benefits to high-latitude biodiversity over time at multiple scales [2,83]. This process may critically depend on range-expanding species possessing life history traits that fill the developing gaps within communities as climates change. If incoming species overlap strongly with native species in life history traits and outcompete them for shared resources [26], then species replacement and biotic homogenization may occur instead of enhanced biodiversity (figure 1c,d).
Life history divergence and associated relaxation of competition intensities may also occur under increasing drought or heat stress at lower latitudes and at the trailing edge of many species' ranges. However, the role of life history divergence to buffer these exceptionally stressed communities from biodiversity loss remains to be formally tested. A recent meta-analysis of plant communities along stress gradients indicated a role for life history in this process: the switch from competition to facilitation was more often observed under stressful conditions for adult life stages than juveniles within perennial plant communities, and more often in perennial than annual communities [75]. However, the role of life history shifts within these communities was not investigated for its effects on changing competitive interactions. Recent conceptual work suggests that high stress does not in fact alleviate competitive intensities when competing species overlap strongly in their life history strategies [12], and future studies should, therefore, focus on the role of evolutionarily diverging life histories as drivers of relaxed competition in stressed communities.
8. Taking the framework forward: a case study
While identifying the community-level consequences of evolved life history responses to climate change will require detailed, long-term, demographic and quantitative genetic studies in wild communities or mesocosm experiments, the effects of thermal plasticity in life history strategies on competition intensities in novel or threatened communities may be evaluated more readily, using laboratory temperature manipulations. We conducted a preliminary investigation of these effects using three damselfly species that commonly interact and compete within local guilds of ecologically similar species across NE Scotland. One of our study species (Ischnura elegans, Odonata: Coenagrionidae) is a recent immigrant to the region, and warming climates have facilitated its colonization of northern Britain [84]. The other two species are historic residents of the area [85] (Enallagma cyathigerum, Odonata: Coenagrionidae, and Lestes sponsa, Odonata: Lestidae).
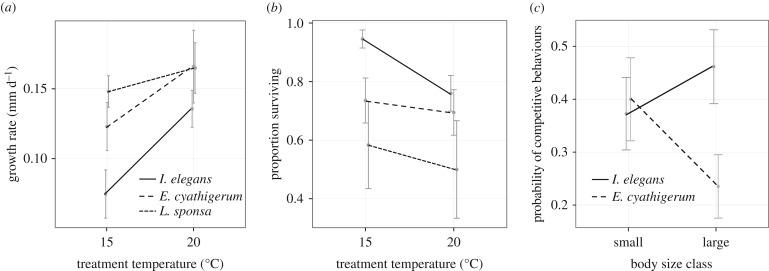
We reared larvae of these species under present and projected freshwater temperatures, and identified changes in the phenotypic responses to the trade-off between survival and growth rate, and cascading effects of these changes on competitive interactions. Rearing at current freshwater temperatures for the Scottish Highlands (15°C, [86]) revealed divergent life histories among both resident and colonizing, coexisting competitors along the growth rate versus survival trade-off axis (effect of species on growth rates at 15°C = −0.07 ± 0.002, t = 2.79, p = 0.008, figure 2a; effect of species on survival at 15°C = 2.52 ± 0.83, z = 3.02, p = 0.003, figure 2b). Rearing under temperatures projected for 2080 under a warming scenario (20°C, [86]), however, resulted in reduced interspecific variability in both growth rates (effect of species on growth rate at 20°C = −0.03 ± 0.03, t = 1.17, p = 0.25, figure 2a) and survival (effect of species on survival at 20°C = 1.15 ± 0.72, z = 1.62, p = 0.11, figure 2b), suggestive of life history convergence between colonizing and resident species as climates continue to warm.
Figure 2.
Effects of warming on life history and competitive outcomes in Scottish damselfly communities. (a) Larval growth rates under current (15°C) and projected (20°C) Scottish freshwater temperatures, for range-shifting species (Ischnura elegans) and resident species (Enallagma cyathigerum and Lestes sponsa). (b) Survival rates for larvae of these species when reared under current and projected conditions. (c) Effects of body size (positively correlated with growth rates, see text) on competitive outcomes. Error bars represent standard errors. Data reproduced from LT Lancaster, G Morrison and RN Fitt 2015, unpublished data.
Faster growth at higher temperatures led to larger body sizes for all species (effect of growth rate on adult body size = 16.76 ± 5.49, t = 3.05, p = 0.003), and this affected larval competitive outcomes. At smaller sizes, resident and colonizing species are competitively equivalent. However, the larger larval size classes produced by warmer temperatures resulted in competitive asymmetry between resident (E. cyathigerum) and colonizing species (I. elegans) (effect of species × body size on aggressive behaviours = 0.86 ± 0.31, z = 2.80, p = 0.005; figure 2c), which was resolved in favour of the colonizing species.
Together, these results suggestively illustrate one potential mechanism by which climate change may influence interspecific competition via thermal plasticity in life history strategies, such that life history divergence between colonizing and native species initially supports coexistence, but further climatic warming may promote life history convergence via shared physiological limits among all interacting species, or via inherently low thermal plasticity of non-range expanding, high-latitude populations. Converged life histories increased opportunity for competition, and also increased the asymmetry in competitive outcomes among these species, with the range-shifting species exhibiting the greatest increases in growth rate, adult body size and competitive advantage under a warming scenario (figure 2). Greater thermal plasticity observed in I. elegans in comparison with its local competitors is likely to contribute to its advantage under future warming conditions. Such plasticity may have adaptively evolved during the course of this species' range expansion [69,70,87], however, more detailed investigations and comparative work are needed to draw firmer conclusions.
This preliminary study suggests one way that warming climates may directly affect life history trade-offs and competitive abilities. Future efforts to identify links between climate change, life history and competition in a laboratory context should incorporate more species, a broader range of experimental temperatures, and larger sample sizes to investigate the generality and robustness of these preliminary effects. Furthermore, all such temperature manipulation experiments should be integrated with detailed ecological data or mesocosm studies to identify reciprocal demographic effects of each species' changing life history strategy under environmental change. This approach can generate short-term predictions for the effects of life history plasticity on community interactions as climates change, and combined with further breeding studies, may also reveal the genetic potential for rapid evolutionary change in the distribution of life histories within communities.
9. Knowledge gaps and future directions
This paper provides a conceptual framework for identifying and predicting changing strengths of competition and patterns of coexistence within communities under ongoing climate change. The framework is based on the role of life history trade-offs to enforce resource allocation decisions, and the predictable consequences of divergence or convergence of these allocation decisions within communities. The framework is relevant both within existing communities and in the context of ‘no-analogue communities’ created by shifting regional biodiversity pools [64]. The strength of this framework lies in its solid reliance on a well-developed set of principles for time and energy allocation [31,88], and the clear, predictable relationship between the directions of evolved or plastic life history shifts and the resulting intensities of competition within communities. However, before this framework can be effectively implemented in a predictive context to understand the links between climate change and local biodiversity, a number of critical issues must be addressed.
Primarily, more work is needed to understand how changing intensities of competition derive from contributions of climate change to life history syndrome evolution and development, versus contributions of climate change to changes in other, non-life-history traits such as environmental tolerances, nutritional requirements or feeding preferences. In general, little work has been done to understand how different coexistence mechanisms interact—for example, how do predators affect the limiting similarity in resource use (e.g. [89]), and how do these affect limiting similarities of life history? To what extent is coexistence mediated by trait divergence along any of these axes [72,90]? Within close-knit guilds of plants, insects and parasites, life history variation has proved to be a critical coexistence mechanism (as reviewed above), and evolved variation in life history strategies is also likely to be an important driver of local diversity in other communities. Life history traits are often highly responsive to changing climates [7,49,55,65,68], and have predictable properties of trade-offs and underlying constraints that facilitate predicting links between environmental variation, life history shifts and community change—this is the potential promise of the proposed framework.
Second, better evidence is needed to describe interspecific variation in effects of climate change on life history traits. Most previous studies support the hypothesis that warming will influence or select for ‘faster’ life histories in most species, and thus promote convergence and intensified competition, but this conclusion may reflect research and reporting biases [74]. Understanding the conditions and support for this pattern will help refine further predictions for changing community interactions. More work is also needed to discover how often physiological limits (i.e. the total extent to which climate change can shift life history traits towards extremes) are similar for closely interacting species. Finally, and potentially most critically, more work is needed to track plastic and evolving life histories in wild populations [7], how these changes respond to different features of environmental change (for example, climatic warming versus increased climate variability) and how these life history shifts affect local interaction strengths and community diversity.
So far, the observed changes in community diversity under changing climates remain poorly understood in terms of both process and mechanism [1,3]. It is hoped that applying life history theory to understand changing and evolving competitive interactions can pave the way to a more general understanding of community assembly under changing environmental conditions. These research efforts will facilitate the development of effective climate change adaptation strategies for preservation of local biodiversity and to protect the wide array of valuable ecosystem services that rely on particular patterns of local biodiversity for their effective delivery [91].
Acknowledgements
Thank you to Andrew Hendry, Kiyoko Gotanda and Erik Svensson for the opportunity to contribute to this special issue and for all their help along the way. Thanks also to Damaris Zurell, Heike Lischke, Bob O'Hara and most especially Julien Martin for helpful discussions during the development of these ideas. Thank you to Mike Logan and an anonymous reviewer for comments on a previous draft of this article.
Data accessibility
The data used to create figure 2 can be provided by the authors upon request.
Authors' contributions
L.T.L. conceived the idea for the paper. G.M. devised the experiment and collected the data, with assistance and input from L.T.L. and R.N.F. L.T.L. analysed the data and drafted the paper, which all three authors edited.
Competing interests
We have no competing interests.
Funding
University of Aberdeen School of Biological Sciences provided funds to support this study in the form of a MSc project allowance to G.M. and a start-up grant to L.T.L. R.N.F.'s salary is funded by a UK Natural Environment Research Council (NERC) PhD-ship awarded to the University of Aberdeen.
References
- 1.Vellend M, Baeten L, Myers-Smith IH, Elmendorf SC, Beauséjour R, Brown CD, De Frenne P, Verheyen K, Wipf S. 2013. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proc. Natl Acad. Sci. USA 110, 19 456–19 459. ( 10.1073/pnas.1312779110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warren R, et al. 2013. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Change 3, 678–682. ( 10.1038/nclimate1887) [DOI] [Google Scholar]
- 3.Dornelas M, Gotelli NJ, McGill B, Shimadzu H, Moyes F, Sievers C, Magurran AE. 2014. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299. ( 10.1126/science.1248484) [DOI] [PubMed] [Google Scholar]
- 4.Urban MC. 2015. Accelerating extinction risk from climate change. Science 348, 571–573. ( 10.1126/science.aaa4984) [DOI] [PubMed] [Google Scholar]
- 5.Pereira HM, et al. 2010. Scenarios for global biodiversity in the 21st century. Science 330, 1496–1501. ( 10.1126/science.1196624) [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470, 479–485. ( 10.1038/nature09670) [DOI] [PubMed] [Google Scholar]
- 7.Visser ME, Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569. ( 10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aberle N, Bauer B, Lewandowska A, Gaedke U, Sommer U. 2012. Warming induces shifts in microzooplankton phenology and reduces time-lags between phytoplankton and protozoan production. Mar. Biol. 159, 2441–2453. ( 10.1007/s00227-012-1947-0) [DOI] [Google Scholar]
- 9.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 10.Milazzo M, Mirto S, Domenici P, Gristina M. 2013. Climate change exacerbates interspecific interactions in sympatric coastal fishes. J. Anim. Ecol. 82, 468–477. ( 10.1111/j.1365-2656.2012.02034.x) [DOI] [PubMed] [Google Scholar]
- 11.Bonsall MB, Jansen VAA, Hassell MP. 2004. Life history trade-offs assemble ecological guilds. Science 306, 111–114. ( 10.1126/science.1100680) [DOI] [PubMed] [Google Scholar]
- 12.Maestre FT, Callaway RM, Valladares F, Lortie CJ. 2009. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 97, 199–205. ( 10.1111/j.1365-2745.2008.01476.x) [DOI] [Google Scholar]
- 13.Van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 14.Wissinger SA. 1988. Life history and size structure of larval dragonfly populations. J. N. Am. Benthol. Soc. 7, 13–28. ( 10.2307/1467827) [DOI] [Google Scholar]
- 15.Bonsall M, Hassell M, Asefa G. 2002. Ecological trade-offs, resource partitioning, and coexistence in a host-parasitoid assemblage. Ecology 83, 925–934. [Google Scholar]
- 16.Burton OJ, Phillips BL, Travis JMJ. 2010. Trade-offs and the evolution of life-histories during range expansion. Ecol. Lett. 13, 1210–1220. ( 10.1111/j.1461-0248.2010.01505.x) [DOI] [PubMed] [Google Scholar]
- 17.Qvarnström A, Wiley C, Svedin N, Vallin N. 2009. Life-history divergence facilitates regional coexistence of competing Ficedula flycatchers. Ecology 90, 1948–1957. ( 10.1890/08-0494.1) [DOI] [PubMed] [Google Scholar]
- 18.Leishman MR. 2001. Does the seed size/number trade-off model determine plant community structure? An assessment of the model mechanisms and their generality. Oikos 93, 294–302. ( 10.1034/j.1600-0706.2001.930212.x) [DOI] [Google Scholar]
- 19.Sevenster JG, Van Alphen JM. 1993. A life history trade-off in Drosophila species and community structure in variable environments. J. Anim. Ecol. 62, 720–736. ( 10.2307/5392) [DOI] [Google Scholar]
- 20.Macarthur R, Levins R. 1967. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 101, 377–385. ( 10.1086/282505) [DOI] [Google Scholar]
- 21.Sorte CJB, White JW. 2013. Competitive and demographic leverage points of community shifts under climate warming. Proc. R. Soc. B 280, 20130572 ( 10.1098/rspb.2013.0572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCoy SJ, Pfister CA. 2014. Historical comparisons reveal altered competitive interactions in a guild of crustose coralline algae. Ecol. Lett. 17, 475–483. ( 10.1111/ele.12247) [DOI] [PubMed] [Google Scholar]
- 23.Seifert LI, Weithoff G, Gaedke U, Vos M. 2015. Warming-induced changes in predation, extinction and invasion in an ectotherm food web. Oecologia 178, 485–496. ( 10.1007/s00442-014-3211-4) [DOI] [PubMed] [Google Scholar]
- 24.Huey RB, Deutsch CA, Tewksbury JJ, Vitt LJ, Hertz PE, Alvarez Pérez HJ, Garland T. 2009. Why tropical forest lizards are vulnerable to climate warming. Proc. R. Soc. B 276, 1939–1948. ( 10.1098/rspb.2008.1957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerry JT, Bellwood DR. 2015. Competition for shelter in a high-diversity system: structure use by large reef fishes. Coral Reefs 35, 245–252. ( 10.1007/s00338-015-1362-3) [DOI] [Google Scholar]
- 26.Alexander JM, Diez JM, Levine JM. 2015. Novel competitors shape species’ responses to climate change. Nature 525, 515–518. ( 10.1038/nature14952) [DOI] [PubMed] [Google Scholar]
- 27.Cheptou P-O, Hargreaves AL, Bonte D, Jacquemyn H. 2017. Adaptation to fragmentation: evolutionary dynamics driven by human influences. Phil. Trans. R. Soc. B 372, 20160037 ( 10.1098/rstb.2016.0037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poloczanska ES, Hawkins SJ, Southward AJ, Burrows MT. 2008. Modeling the response of populations of competing species to climate change. Ecology 89, 3138–3149. ( 10.1890/07-1169.1) [DOI] [PubMed] [Google Scholar]
- 29.Tilman D, Lehman C. 2001. Human-caused environmental change: impacts on plant diversity and evolution. Proc. Natl Acad. Sci. USA 98, 5433–5440. ( 10.1073/pnas.091093198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson ST, Sax DF. 2010. Balancing biodiversity in a changing environment: extinction debt, immigration credit and species turnover. Trends Ecol. Evol. 25, 153–160. ( 10.1016/j.tree.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 31.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 32.Lancaster LT, Hazard LC, Clobert J, Sinervo BR. 2008. Corticosterone manipulation reveals differences in hierarchical organization of multidimensional reproductive trade-offs in r-strategist and K-strategist females. J. Evol. Biol. 21, 556–565. ( 10.1111/j.1420-9101.2007.01478.x) [DOI] [PubMed] [Google Scholar]
- 33.Kawecki TJ. 1993. Age and size at maturity in a patchy environment: fitness maximization versus evolutionary stability. Oikos 66, 309–317. ( 10.2307/3544819) [DOI] [Google Scholar]
- 34.Penk MR, Jeschke JM, Minchin D, Donohue I. 2016. Warming can enhance invasion success through asymmetries in energetic performance. J. Anim. Ecol. 85, 419–426. ( 10.1111/1365-2656.12480) [DOI] [PubMed] [Google Scholar]
- 35.Gause GF. 1934. The struggle for existence. Baltimore, MD: Williams and Wilkins. [Google Scholar]
- 36.Volterra V. 1926. Fluctuations in the abundance of a species considered mathematically. Nature 118, 558–560. ( 10.1038/118558a0) [DOI] [Google Scholar]
- 37.Chesson P. 2003. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 38.Van Doorslaer W, Stoks R, Swillen I, Feuchtmayr H, Atkinson D, Moss B, De Meester L. 2010. Experimental thermal microevolution in community-embedded Daphnia populations. Clim. Res. 43, 81–89. ( 10.3354/cr00894) [DOI] [Google Scholar]
- 39.Bakun A. 2014. Active opportunist species as potential diagnostic markers for comparative tracking of complex marine ecosystem responses to global trends. ICES J. Mar. Sci. 71, 2281–2292. ( 10.1093/icesjms/fst242) [DOI] [Google Scholar]
- 40.Ghara M, Borges RM. 2010. Comparative life-history traits in a fig wasp community: implications for community structure. Ecol. Entomol. 35, 139–148. ( 10.1111/j.1365-2311.2010.01176.x) [DOI] [Google Scholar]
- 41.Harabiš F, Dolný A, Šipoš J. 2012. Enigmatic adult overwintering in damselflies: coexistence as weaker intraguild competitors due to niche separation in time. Popul. Ecol. 54, 549–556. ( 10.1007/s10144-012-0331-8) [DOI] [Google Scholar]
- 42.Freshwater C, Ghalambor CK, Martin PR. 2014. Repeated patterns of trait divergence between closely related dominant and subordinate bird species. Ecology 95, 2334–2345. ( 10.1890/13-2016.1) [DOI] [PubMed] [Google Scholar]
- 43.Nakano M, Kinoshita E, Ueda K. 2004. Life history traits and coexistence of an amphidiploid, Drosera tokaiensis, and its parental species, D. rotundifolia and D. spatulata (Droseraceae). Plant Species Biol. 19, 59–72. ( 10.1111/j.1442-1984.2004.00102.x) [DOI] [Google Scholar]
- 44.Kraft NJB, Godoy O, Levine JM. 2015. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl Acad. Sci. USA 112, 498–802. ( 10.1073/pnas.1413650112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atkinson D. 1995. Effects of temperature on the size of aquatic ectotherms: exceptions to the general rule. J. Therm. Biol. 20, 61–74. ( 10.1016/0306-4565(94)00028-H) [DOI] [Google Scholar]
- 46.Lochmiller RL, Deerenberg C. 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98. ( 10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- 47.Bradshaw WE, Holzapfel CM. 2006. Climate change. Evolutionary response to rapid climate change. Science 312, 1477–1478. ( 10.1126/science.1127000) [DOI] [PubMed] [Google Scholar]
- 48.Parmesan C. 2007. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Change Biol. 13, 1860–1872. ( 10.1111/j.1365-2486.2007.01404.x) [DOI] [Google Scholar]
- 49.Nussey DH. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306. ( 10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 50.Gavrilets S, Scheiner SM. 1993. The genetics of phenotypic plasticity. V. Evolution of reaction norm shape. J. Evol. Biol. 6, 31–48. ( 10.1046/j.1420-9101.1993.6010031.x) [DOI] [Google Scholar]
- 51.Ghalambor CK, Mckay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 52.Hendry AP. 2016. Key questions on the role of phenotypic plasticity in eco-evolutionary dynamics. J. Hered. 107, 25–41. ( 10.1093/jhered/esv060) [DOI] [PubMed] [Google Scholar]
- 53.Elliott JM. 2013. Contrasting dynamics from egg to adult in the life cycle of summer and overwintering generations of Baetis rhodani in a small stream. Freshw. Biol. 58, 866–879. ( 10.1111/fwb.12093) [DOI] [Google Scholar]
- 54.Abrams PA, Leimar O, Nylin S, Wiklund C. 1996. The effect of flexible growth rates on optimal sizes and development times in a seasonal environment. Am. Nat. 147, 381–395. ( 10.1086/285857) [DOI] [Google Scholar]
- 55.Sheridan JA, Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406. ( 10.1038/nclimate1259) [DOI] [Google Scholar]
- 56.Rocha F, Medeiros HF, Klaczko LB. 2009. The reaction norm for abdominal pigmentation and its curve in Drosophila mediopunctata depend on the mean phenotypic value. Evolution 63, 280–287. ( 10.1111/j.1558-5646.2008.00503.x) [DOI] [PubMed] [Google Scholar]
- 57.Somero GN. 2010. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920. ( 10.1242/jeb.037473) [DOI] [PubMed] [Google Scholar]
- 58.Chevin L-M, Gallet R, Gomulkiewicz R, Holt RD, Fellous S. 2013. Phenotypic plasticity in evolutionary rescue experiments. Phil. Trans. R. Soc. B 368, 20120089 ( 10.1098/rstb.2012.0089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills SC, Hazard L, Lancaster L, Mappes T, Miles D, Oksanen TA, Sinervo B. 2008. Gonadotropin hormone modulation of testosterone, immune function, performance, and behavioral trade-offs among male morphs of the lizard Uta stansburiana. Am. Nat. 171, 339–357. ( 10.1086/527520) [DOI] [PubMed] [Google Scholar]
- 60.Sniegula S, Golab MJ, Drobniak SM, Johansson F. 2016. Seasonal time constraints reduce genetic variation in life-history traits along a latitudinal gradient. J. Anim. Ecol. 85, 187–198. ( 10.1111/1365-2656.12442) [DOI] [PubMed] [Google Scholar]
- 61.Darling ES, McClanahan TR, Côté IM. 2013. Life histories predict coral community disassembly under multiple stressors. Glob. Change Biol. 19, 1930–1940. ( 10.1111/gcb.12191) [DOI] [PubMed] [Google Scholar]
- 62.De Mazancourt C, Johnson E, Barraclough TG. 2008. Biodiversity inhibits species’ evolutionary responses to changing environments. Ecol. Lett. 11, 380–388. ( 10.1111/j.1461-0248.2008.01152.x) [DOI] [PubMed] [Google Scholar]
- 63.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urban MC, Tewksbury JJ, Sheldon KS. 2012. On a collision course: competition and dispersal differences create no-analogue communities and cause extinctions during climate change. Proc. R. Soc. B 279, 2072–2080. ( 10.1098/rspb.2011.2367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 66.De Meester L, Vanoverbeke J, Kilsdonk LJ, Urban MC. 2016. Evolving perspectives on monopolization and priority effects. Trends Ecol. Evol. 31, 136–146. ( 10.1016/j.tree.2015.12.009) [DOI] [PubMed] [Google Scholar]
- 67.Bocedi G, Atkins KE, Liao J, Henry RC, Travis JMJ, Hellmann JJ. 2013. Effects of local adaptation and interspecific competition on species’ responses to climate change. Ann. N. Y. Acad. Sci. 1297, 83–97. ( 10.1111/nyas.12211) [DOI] [PubMed] [Google Scholar]
- 68.Phillips BL, Brown GP, Shine R. 2010. Life-history evolution in range-shifting populations. Ecology 91, 1617–1627. ( 10.1890/09-0910.1) [DOI] [PubMed] [Google Scholar]
- 69.Colautti RI, Alexander JM, Dlugosch KM, Keller SR, Sultan SE. 2017. Invasions and extinctions through the looking glass of evolutionary ecology. Phil. Trans. R. Soc. B 372, 20160031 ( 10.1098/rstb.2016.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chown SL, Slabber S, McGeouch M, Janion C, Leinaas HP. 2007. Phenotypic plasticity mediates climate change responses among invasive and indigenous arthropods. Proc. R. Soc. B 274, 2531–2537. ( 10.1098/rspb.2007.0772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lancaster LT. 2016. Widespread range expansions shape latitudinal variation in insect thermal limits. Nat. Clim. Change 6, 618–621. ( 10.1038/nclimate2945) [DOI] [Google Scholar]
- 72.Fox JW, Vasseur DA. 2015. Character convergence under competition for nutritionally essential resources. Am. Nat. 172, 667–680. ( 10.1086/591689) [DOI] [PubMed] [Google Scholar]
- 73.Fitt RN, Lancaster LT. Submitted. Range shifting species reduce phylogenetic diversity in high latitude damselfly assemblages via competition. [DOI] [PubMed]
- 74.Logan ML, Huynh RK, Precious RA, Calsbeek RG. 2013. The impact of climate change measured at relevant spatial scales: new hope for tropical lizards. Glob. Change Biol. 19, 3093–3102. ( 10.1111/gcb.12253) [DOI] [PubMed] [Google Scholar]
- 75.He Q, Bertness MD, Altieri AH. 2013. Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 16, 695–706. ( 10.1111/ele.12080) [DOI] [PubMed] [Google Scholar]
- 76.Schär C, Vidale PL, Lüthi D, Frei C, Häberli C, Liniger MA, Appenzeller C. 2004. The role of increasing temperature variability in European summer heatwaves. Nature 427, 332–336. ( 10.1038/nature02300) [DOI] [PubMed] [Google Scholar]
- 77.Menzel A, Fabian P. 1999. Growing season extended in Europe. Nature 397, 659 ( 10.1038/17709) [DOI] [Google Scholar]
- 78.Altermatt F. 2010. Climatic warming increases voltinism in European butterflies and moths. Proc. R. Soc. B 277, 1281–1287. ( 10.1098/rspb.2009.1910) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Köster FW, Trippel EA, Tomkiewicz J. 2013. Linking size and age at sexual maturation to body growth, productivity and recruitment of Atlantic cod stocks spanning the North Atlantic. Fish. Res. 138, 52–61. ( 10.1016/j.fishres.2012.07.002) [DOI] [Google Scholar]
- 80.Knies JL, Kingsolver JG, Burch CL. 2009. Hotter is better and broader: thermal sensitivity of fitness in a population of bacteriophages. Am. Nat. 173, 419–430. ( 10.1086/597224) [DOI] [PubMed] [Google Scholar]
- 81.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sax DF, Gaines SD. 2003. Species diversity: from global decreases to local increases. Trends Ecol. Evol. 18, 561–566. ( 10.1016/S0169-5347(03)00224-6) [DOI] [Google Scholar]
- 83.Thuiller W, Lavergne S, Roquet C, Boulangeat I, Lafourcade B, Araujo MB. 2011. Consequences of climate change on the tree of life in Europe. Nature 470, 531–534. ( 10.1038/nature09705) [DOI] [PubMed] [Google Scholar]
- 84.Hickling R, Roy DB, Hill JK, Thomas CD. 2005. A northward shift of range margins in British Odonata. Glob. Change Biol. 11, 502–506. ( 10.1111/j.1365-2486.2005.00904.x) [DOI] [Google Scholar]
- 85.Cham S, Nelson B, Parr A, Prentice S, Smallshire D, Taylor P. 2014. Atlas of dragonflies in Britain and Ireland. Telford, UK: The Field Studies Council for the Biological Records Centre, Centre for Ecology & Hydrology with the British Dragonfly Society. [Google Scholar]
- 86.Hrachowitz M, Soulsby C, Imholt C, Malcolm I, Tetzlaff D. 2010. Thermal regimes in a large upland salmon river: a simple model to identify the influence of landscape controls and climate change on maximum temperatures. Hydrol. Process. 24, 3374–3391. ( 10.1002/hyp.7756) [DOI] [Google Scholar]
- 87.Lancaster LT, Dudaniec RY, Hansson B, Svensson EI. 2015. Latitudinal shift in thermal niche breadth results from thermal release during a climate-mediated range expansion. J. Biogeogr. 42, 1953–1963. ( 10.1111/jbi.12553) [DOI] [Google Scholar]
- 88.Zera A, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 89.Chesson P, Kuang JJ. 2008. The interaction between predation and competition. Nature 456, 235–238. ( 10.1038/nature07248) [DOI] [PubMed] [Google Scholar]
- 90.Mayfield MM, Levine JM. 2010. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 13, 1085–1093. ( 10.1111/j.1461-0248.2010.01509.x) [DOI] [PubMed] [Google Scholar]
- 91.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to create figure 2 can be provided by the authors upon request.