Abstract
Objective
To determine the long-term outcomes of patients with locally advanced esophageal cancer (LAEC) who underwent esophagectomy and survived at least 5 years, and the predictors of disease-free survival (DFS) beyond 5 years.
Methods
This was a retrospective review of a prospective database to identify patients with clinical stage T2N0M0 or higher LAEC. Medical records were reviewed to obtain demographic, clinical, and pathological characteristics, as well as data on recurrence and survival. Multivariable analysis of predictors of DFS beyond 5 years was performed using a Cox regression model.
Results
Between 1988 and 2009, 355 of 500 patients underwent esophagectomy for cT2N0M0 or higher disease. Of these 355 patients, 126 were alive and disease-free at the 5-year follow-up, for an actuarial 5-year DFS of 33%. Recurrent esophageal cancer developed in 8 patients after 5 years. Among the 126 surviving patients, the actuarial overall survival was 94% at 7 years and 80% at 10 years. On multivariable analysis, the sole significant predictor of DFS after the 5-year time point was non–en bloc resection at the original operation (P = .006). Pulmonary-related deaths accounted for 10 out of 22 noncancer deaths. A second primary cancer developed in 23 of the 126 surviving patients.
Conclusions
Prolonged survival can be obtained in one-third of patients with LAEC. An en bloc resection at the original operation is the most significant predictor of prolonged survival. Survivors experience a high rate of second primary cancer and an apparently high rate of deaths from pulmonary disease. Careful follow-up is necessary for these patients, even after the 5-year mark.
Keywords: esophageal cancer, disease-free survival, en bloc resection, recurrence
Graphical Abstract
Overall survival for disease-free patients after 5 years of follow-up.
Despite advances in surgical techniques and neoadjuvant treatment strategies, locally advanced esophageal cancer (LAEC) remains a virulent malignancy associated with poor survival.1,2 Advances in surgical techniques, perioperative care, and preoperative treatment strategies have improved outcomes somewhat; however, long-term survival beyond 5 years is considered uncommon. There is little published information on the outcome of patients with LAEC treated by esophagectomy with or without induction therapy beyond the 5-year mark, and whether these patients are truly cured remains unclear. The objective of the present study was to determine the outcomes of patients with LAEC clinically staged as T2N0M0 or higher who had survived at least 5 years after esophagectomy and were without disease at that time point.
PATIENTS AND METHODS
Study Design
We conducted a retrospective review of a prospectively assembled thoracic surgery database. The Institutional Review Board of Weill Cornell Medical College and the New York–Presbyterian Hospital approved the database and the study design. Patient consent was waived. Patients were considered eligible for this review who had locally advanced carcinoma of the esophagus or gastroesophageal junction (Siewert type I or II) clinically staged as cT2N0M0 or higher and were treated by esophagectomy, with or without preoperative induction therapy, between January 1988 and September 2009. Patients undergoing esophagectomy after September 2009 were excluded, to obtain a minimum of 5 years of follow-up. Patients with clinical T1 disease were also excluded, because they were considered to have early-stage disease with a favorable long-term outcome.
A total of 355 patients met the selection criteria, of whom 126 patients were alive and disease-free at the 5-year time point. The hospital records of these 126 patients were reviewed for demographic and clinical characteristics, including age, sex, clinical stage, and use of neoadjuvant therapy. Performance status of all patients was graded according to Eastern Cooperative Oncology Group guidelines, and comorbidities were graded using the Charlson Comorbidity Index (CCI).3 Records were also reviewed for perioperative and pathological data, including surgical approach, extent of resection, fields of lymph nodes resected, pathological stage, histological cell type, and completeness of resection (R status).
Preoperative Evaluation
All patients were evaluated by history, physical examination, upper gastrointestinal endoscopy with biopsy and computed tomography (CT) of the chest and upper abdomen. Endoscopic ultrasonography and positron emission tomography (PET) were routinely obtained preoperatively only in the latter half of the cohort, from 1999 to 2009. Pulmonary function tests were routinely obtained, and cardiac stress testing was done if risk factors were present. Patients were considered for surgical resection if preoperative evaluation revealed no evidence of distant visceral metastases or clear evidence of direct tumor invasion of the airway or major vascular structures. The presence of extensive nodal disease was not considered a contraindication to resection unless it clearly extended beyond the proposed fields of dissection.
Neoadjuvant Therapy
The majority of patients received neoadjuvant chemotherapy alone. Neoadjuvant chemotherapy included 2 to 4 cycles of a platinum-based regimen with either a taxane or epirubicin and capecitibine. Preoperative chemoradiotherapy was provided using either cisplatin/5-flourouracil or, more commonly, carboplatin/paclitaxel delivered concurrently with radiotherapy. Esophagectomy was performed at 3 to 6 weeks after the conclusion of preoperative therapy.
Surgical Treatment
Esophagectomy was performed with either a transthoracic en bloc approach or a transhiatal approach. En bloc esophagectomy with 2- or 3-field lymphadenectomy was performed in medically fit patients aged <80 years. The basic technique of en bloc esophagectomy has been described previously.4 Alternatively, resection was done by either a non–en bloc conventional (Ivor-Lewis) transthoracic approach or a transhiatal approach. Regardless of surgical approach, upper abdominal and retroperitoneal node dissection was performed in all patients. Reconstruction with a greater curvature gastric tube was done in most of cases. Pathological staging was done according to the TNM classification of the seventh edition of the American Joint Committee for Cancer staging manual.5
Follow-up
After hospital discharge, patients were seen at 3-month intervals for the first 12 months, then every 6 months up to year 3, and annually thereafter. Patients from distant geographic locations were followed by contacting their treating physician and/or by direct telephone contact. CT scans of the chest and upper abdomen were done postoperatively every 6 months for 3 years and annually thereafter. Other studies, such as endoscopy and PET scanning, were done only if clinically indicated. All data were collected, entered into a prospective database, and updated at regular intervals until the patient’s last follow-up or death.
Recurrence
Local recurrence was defined as recurrence within the wall or the lumen at or close to the anastomotic site. Regional recurrence was defined as nodal recurrence at any site within the operative field. Distant recurrence was defined as any tumor recurrence in nodal basins outside the field of surgical dissection or in distant organs. The operating surgeon reported recurrence data based on review of findings on CT, PET, endoscopy, or relevant biopsy analyses whenever possible.
Statistical Analysis
The database was queried to identify relevant clinical and pathological characteristics, which were represented by descriptive statistics (frequency and percentage, median and interquartile range). Cox proportional hazards regression analysis was performed to identify predictors of disease-free survival (DFS) at 5 or more years after esophagectomy. Univariate analyses examined age, sex, performance status, pulmonary comorbidity, neoadjuvant therapy, extent of resection, pathological T classification, pathological N classification, angiolymphatic invasion, histological cell type, and postoperative complications. To avoid overdetermination, the multivariable model included only highly significant (P < .10) univariate factors: pulmonary comorbidity, extent of resection, pathological N classification, and angiolymphatic invasion. Collinearity between potential predictors was examined (using the κ statistic) before the final multivariable model was formulated. In the final multivariable model, predictors with a P value ± .05 were deemed significant predictors.
Overall survival (OS) was defined as the time from surgical resection until the date of death or last follow-up. DFS was defined as the time from surgical resection to EC recurrence or death from any cause or last follow-up. Survival probabilities were estimated using Kaplan-Meier survival analysis. Median follow-up time for the cohort was based on surviving patients. All analyses were performed using SPSS version 22.0 (IBM, Armonk, NY).
RESULTS
Clinicopathological Characteristics
Among the 355 patients with LAEC, 126 were disease-free at 5 years, for an actuarial 5-year DFS of 33% (95% confidence interval [CI], 28.1%–37.9%). Patient characteristics are reported in Table 1. The majority were men who had adenocarcinoma of the lower-third of the esophagus and gastroesophageal junction. Clinical staging was accomplished using CT scanning in all 126 patients, along with endoscopic ultrasonography in 70 patients (55.6%) and PET scanning in 80 patients (63.5%). Slightly more than one-half of the patients (51.6 %) received induction therapy (58 patients with chemotherapy only and 7 with chemoradiotherapy). En bloc esophagectomy (2 or 3 fields) was performed in 104 patients (82.5%), and an R-0 resection was accomplished in all patients. Patients treated with an en bloc resection were generally younger (P = .165), less likely to have pulmonary comorbidity (P = .051), and more likely to have a higher tumor burden (pN1-3: 53% vs 33%; P = .141) compared with patients treated with non–en bloc resection (data not shown).
TABLE 1.
Demographic, clinical, and pathological characteristics for patients who were disease-free after 5-years of follow-up (n = 126)
| Characteristic | Value |
|---|---|
| Age at esophagectomy, years, median (IQR), years | 63 (55–70) |
| Male sex, n (%) | 97 (77) |
| Charlson Comorbidity Index = 0, n (%) | 65 (52) |
| Normal activity (performance status = 0), n (%) | 81 (64) |
| Absence of pulmonary comorbidities, n (%) | 95 (75) |
| Tumor location, n (%) | |
| Upper third | 7 (6) |
| Middle third | 22 (17) |
| Lower third/GEJ | 97 (77) |
| Treatment modality, n (%) | |
| Neoadjuvant therapy | 65 (52) |
| Surgery ± adjuvant therapy | 61 (48) |
| En bloc resection, n (%) | 104 (82) |
| Two-field | 37 (29) |
| Three-field | 67 (53) |
| Total lymph nodes harvested, n, median (IQR) | 30 (21–41) |
| Histological type, n (%) | |
| Adenocarcinoma | 78 (62) |
| Squamous cell carcinoma | 48 (38) |
| Histological grade of differentiation, n (%) | |
| Well differentiated | 14 (11) |
| Moderately differentiated | 59 (47) |
| Poorly differentiated/undifferentiated | 53 (42) |
| Complications, n (%) | 55 (44) |
| Pulmonary | 17 (14) |
| Cardiac | 7 (6) |
| Anastomotic leakage | 13 (10) |
| Recurrent laryngeal nerve injury | 3 (2.5) |
| Chylothorax | 3 (2.5) |
| Wound infection | 8 (6) |
| Other complications | 4 (3) |
IQR, Interquartile range; GEJ, gastroesophageal junction.
Incidence and Patterns of Recurrence
The 126 patients who are the subject of this report included 10 patients who had developed EC recurrence before the 5-year mark and were treated for recurrence and rendered disease-free. One patient had local recurrence, 6 patients had regional recurrence, and 3 patients had distant recurrence (4 treated by surgery with chemoradiation, 5 treated by definitive chemoradiation, and 1 treated by chemotherapy). Eight patients (6.3%) developed first recurrence more than 5 years after esophagectomy; among those 8 patients, recurrent EC was local only in 1 patient (unresectable and treated by chemotherapy alone), regional only in 1 patient (treated by surgical resection of recurrent laryngeal lymph nodes, followed by chemoradiation), and distant in 6 patients (treated by chemotherapy with or without radiation). Overall, 7 of 18 patients survived at least 5 years after treatment of recurrence (2 treated surgically, 3 treated by definitive chemoradiation, and 2 treated by chemotherapy alone).
Survival
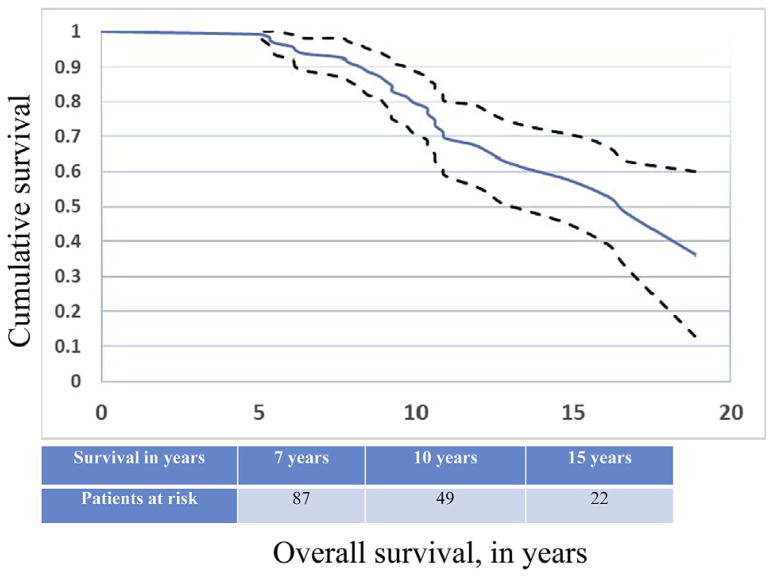
Median follow-up for the 126 surviving patients was 106 months (range, 60–270 months) from the date of surgery and 46 months from the onset of the landmark observation period. Thirty-three patients died after 5 years from either recurrent EC or non–EC- related causes. Kaplan-Meier OS was 94% (95% CI, 89.1%–98.1%) at 7 years, 80% (95% CI, 71.0%–88.6%) at 10 years, and 59% (95% CI, 45.6%–71.4%) at 15 years (Figure 1).
FIGURE 1.
OS for the group of patients who were disease-free after 5 years of follow-up (n = 126).
Univariate analysis identified the presence of any pulmonary comorbidity, non–en bloc resection, and angiolymphatic invasion as factors predictives of DFS (Table 2). Pulmonary comorbidity was entered into the multivariable model rather than the CCI, because it was collinear with the CCI, which in turn was not significantly associated with mortality in the univariate analyses (data not shown). In the multivariable model, only non–en bloc resection (hazard ratio, 4.36; 95% CI, 1.52–12.50; P = .006) was a significant independent predictor of DFS after the 5-year time point. The results of the multivariable model were unchanged after excluding 13 patients with pT1N0 treated by esophagectomy alone (data not shown).
TABLE 2.
Predictors of DFS [recurrence or death (n = 34)] among patients who were disease-free after 5 years of follow-up (n = 126), Cox proportional hazards model
| Independent variable | Events, n/N (%) | Univariate predictors
|
Multivariable predictors*
|
||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at surgery | |||||
| <63 y | 15/64 (23) | Reference | |||
| >63 y | 19/62 (31) | 1.28 (0.64–2.56) | .489 | ||
| Sex | |||||
| Female | 8/29 (28) | Reference | |||
| Male | 26/97 (27) | 1.12 (0.50–2.49) | .782 | ||
| Performance status | |||||
| 0 | 18/81 (22) | Reference | |||
| ≥1 | 16/45 (36) | 0.67 (0.31–1.47) | .318 | ||
| Pulmonary comorbidity | |||||
| Absence | 22/95 (23) | Reference | |||
| Presence | 12/31 (39) | 2.74 (1.32–5.68) | .007 | 2.19 (0.99–4.83) | .051 |
| Neoadjuvant therapy | |||||
| Neoadjuvant | 15/65 (23) | Reference | |||
| Surgery alone | 19/61 (31) | 0.67 (0.32–1.38) | .278 | ||
| Extent of resection | |||||
| En bloc | 27/104 (26) | Reference | |||
| Non–en bloc | 7/22 (32) | 4.80 (1.72–3.41) | .003 | 4.36 (1.52–2.50) | .00 |
| Pathological T classification | |||||
| Tis, T0, T1 | 6/42 (14) | Reference | |||
| T2, T3, T4 | 28/84 (33) | 1.97 (0.75–5.16) | .167 | ||
| Pathological N classification | |||||
| N0 | 13/68 (19) | Reference | |||
| N1, N2, N3 | 21/58 (36) | 1.93 (0.96–3.90) | .065 | 1.87 (0.86–4.05) | .111 |
| Angiolymphatic invasion | |||||
| Absence | 21/86 (24) | Reference | |||
| Presence | 13/40 (33) | 2.35 (1.14–4.85) | .021 | 1.53 (0.67–3.48) | .315 |
| Histological cell type | |||||
| Squamous cell carcinoma | 14/48 (29) | Reference | |||
| Adenocarcinoma | 20/78 (26) | 0.92 (0.46–1.85) | .818 | ||
| Postoperative complications | |||||
| Uncomplicated | 19/71 (27) | Reference | |||
| Complicated | 15/55 (27) | 0.89 (0.44–1.79) | .748 | ||
HR, Hazard ratio; CI, confidence interval.
Only variables with P<.10 were included in the multivariable analysis.
Other Primary Cancers
Twenty-three patients developed a second primary cancer, including 13 who developed second primary cancer after 5-years of follow-up. The types of second primary cancers are listed in Table 3. Prostate cancer was the most frequent type, occurring in 6 patients, followed by lung cancer, occurring in 3 patients.
TABLE 3.
Types of second primary cancers (n = 23)
| Second primary cancer | n (%) |
|---|---|
| Prostate cancer | 6 (4.8) |
| Lung cancer | 3 (2.4) |
| Gastric cancer | 2 (1.6) |
| Head and neck cancers | 2 (1.6) |
| Breast cancer | 2 (1.6) |
| Ovarian carcinoma | 2 (1.6) |
| Skin cancers (melanoma and SCC) | 2 (1.6) |
| Urinary bladder cancer | 2 (1.6) |
| Colon cancer | 1 (0.8) |
| Meningioma | 1 (0.8) |
SCC, Squamous cell carcinoma.
Causes of Death
There were a total of 33 deaths among the 126 patients, for a 10-year Kaplan-Meier projected mortality rate of 20% (95% CI, 11.2%–28.8%). Seven deaths were due to recurrent EC, 4 were from a second primary cancer (gastric, ovarian, urinary bladder, and skin), and 22 were from a non–cancer-related cause. Pulmonary disease was the most common cause of non–cancer-related death (10 of 33 deaths), followed by cardiovascular disease. The causes of deaths are listed in Table 4.
TABLE 4.
Causes of death (n = 33)
| Cause | n (%) |
|---|---|
| Recurrence of esophageal cancer | 7 (21.2) |
| Pulmonary diseases | 10 (30.3) |
| Cardiac diseases | 6 (18.2) |
| Second primary cancer | 4 (12.1) |
| Stroke | 2 (6.1) |
| Renal failure | 1 (3) |
| Unknown cause | 3 (9.1) |
DISCUSSION
The majority of previous studies investigating the long-term outcomes after esophagectomy for EC focused on patients’ quality of life, alimentary satisfaction, and gastrointestinal symptoms.6,7 Few if any studies have evaluated oncologic outcomes in patients surviving at least 5 years after esophagectomy for cancer. In this sense, it is difficult to know whether these patients can be considered “cured” of their disease.8,9 Here we report data on survival and recurrence in patients with LAEC surviving at least 5 years after resection, as well as other causes of death in this highly selected patient population. Because we assumed that patients with early-stage disease are highly likely to be cured of EC,10 we focused on patients with locally advanced disease treated with either esophagectomy alone or preoperative therapy followed by resection.
Our data permit several important observations. The 33% actuarial 5-year DFS of this cohort of patients with LAEC is promising. These excellent results are likely the result of accurate preoperative staging, standardization of surgical and perioperative management, and the use of multimodality treatment strategies. We did not investigate the relative contribution of each of these variables in improving survival, but rather focused on what occurs at and beyond the 5-year mark.
Despite being free of disease at 5 years, patients with LAEC may remain at risk for disease recurrence. In addition, patients who are disease-free after treatment of initial recurrence before 5 years continue to be at risk for further disease progression. These data suggest that, given the risk of recurrence, continued surveillance of patients with EC may be necessary perhaps as long as 10 years after resection. Importantly, surveillance does not appear to be futile, given that 7 of 18 patients treated for recurrent EC survived at least 5 years after this treatment. Our results do not permit us to advocate for a specific treatment strategy once recurrence occurs, but aggressive local therapy with curative intent should be pursued whenever possible.11
In the present study, we identified en bloc resection as the sole independent predictor of DFS in our patients who had reached the 5-year time point. It is perhaps surprising that a surgical technique should remain predictive of survival after 5 years. It is possible that patients undergoing en bloc resection were selected for that surgical procedure because of better performance status, fewer comorbidities, or less frail appearance. Such a group might be expected to have a longer OS on the basis of those differences alone. Nonetheless, we also believe that improved local control translates into less disease recurrence and improved survival. In a previous report, we identified en bloc resection as an independent predictor of freedom from recurrence in a larger cohort of 465 patients.2 The small number of patients in the present study who experienced recurrence after 5 years make it difficult to draw statistically meaningful conclusions in this cohort. A recent study suggested that T classification and age may be important predictors of survival after the 5-year time point10; however, with a similar number of patients, we did not find those 2 factors to be significant.
Second primary cancers developed in 23 patients who were disease-free at the onset of the observation period. This suggests continued the need for careful follow-up of these long-term survivors.
Finally, chronic pulmonary disease was responsible for nearly one-third of all deaths in our cohort. Multiple quality of life studies have identified reflux-related chronic nocturnal cough and possible aspiration as significant long-term consequences of esophagectomy. Greene and colleagues12 reported that chronic respiratory symptoms such as cough were present in >25% of their patients and that aspiration requiring hospitalization occurred in 15%. Similarly, Derogar and Lagegren13 reported a prospective nationwide quality of life study among Swedish patients surviving 5 years after esophagectomy. Trouble with cough persisted in as many as 50% of patients at 5 years. These potentially modifiable adverse events of esophagectomy may be mitigated by proper counseling of patients to avoid meals for 2 to 3 hours before bedtime and to sleep with the head of the bed elevated. Certainly recurrent microaspiration occurs in some patients and can lead to chronic pulmonary damage. Regardless of the oncologic issues, postesophagectomy patients need to be followed by physicians familiar with the long-term sequelae of esophagectomy and their management.
Although the present study is limited by its retrospective design, the data were prospectively collected in our database by a dedicated data manager and updated at regular intervals. The patients evaluated were operated on over a 20-year period. It is possible that changes in treatment modalities could have had variable effects on patient outcomes; however, these factors are accounted for in the multivariable analysis. Another limitation is the lack of a consistent method of preoperative staging, with 55% to 63% of patients staged by endoscopic ultrasonography and PET scanning. Despite this limitation, only 13 patients (10%) were eventually found to have pT1N0 disease, and the results remained the same after those patients were excluded from the analysis.
We conclude that despite being alive and disease-free at 5 years after esophagectomy for LAEC, a subset of patients remains at risk for recurrence of EC, for another primary cancer, and for pulmonary disease possibly related to the sequelae of esophagectomy. These results suggest the need for continued careful follow-up of these patients.
Central Message.
In LAEC patients who survived at least 5 years after esophagectomy, actuarial overall survival at 10 years and 15 years is 80% and 59%, respectively. Although recurrent EC is uncommon, patients remain at risk for another primary cancers and pulmonary morbidities.
Perspective.
Among 5-year survivors, non–en bloc resection was the sole significant predictor of disease-free survival. Recurrent esophageal cancer, another primary cancers, and pulmonary diseases were the most common causes of death beyond 5 years postesophagectomy. Careful follow-up is mandatory for patients with locally advanced esophageal cancer even after 5 years of follow-up.
Acknowledgments
P.J.C. was partially supported by the Clinical and Translational Science Center at Weill Cornell Medical College (Grant UL1-TR000457-06).
Abbreviations and Acronyms
- CCI
Charlson Comorbidity Index
- CI
confidence interval
- CT
computed tomography
- DFS
disease-free survival
- LAEC
locally advanced esophageal cancer
- OS
overall survival
- PET
positron emission tomography
Footnotes
Read at the 95th Annual Meeting of The American Association for Thoracic Surgery, Seattle, Washington, April 25–29, 2015.
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
You can watch a Webcast of this AATS meeting presentation by going to: http://webcast.aats.org/2015/Video/Wednesday/04-29-15_608_0908_Ghaly.mp4.
References
- 1.Stein HJ, von Rahden BH, Siewert JR. Survival after oesophagectomy for cancer of the oesophagus. Langenbecks Arch Surg. 2005;390:280–5. doi: 10.1007/s00423-004-0504-9. [DOI] [PubMed] [Google Scholar]
- 2.Lee PC, Mirza FM, Port JL, Stiles BM, Paul S, Christos P, et al. Predictors of recurrence and disease-free survival in patients with completely resected esophageal carcinoma. J Thorac Cardiovasc Surg. 2011;141:1196–206. doi: 10.1016/j.jtcvs.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 4.Altorki N, Skinner D. Should en bloc esophagectomy be the standard of care for esophageal carcinoma? Ann Surg. 2001;234:581–7. doi: 10.1097/00000658-200111000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 6.McLarty AJ, Deschamps C, Trastek VF, Allen MS, Pairolero PC, Harmsen WS. Esophageal resection for cancer of the esophagus: long-term function and quality of life. Ann Thorac Surg. 1997;63:1568–72. doi: 10.1016/s0003-4975(97)00125-2. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs M, Macefield RC, Elbers RG, Sitnikova K, Korfage IJ, Smets EM, et al. Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery. Qual Life Res. 2014;23:1097–115. doi: 10.1007/s11136-013-0545-z. [DOI] [PubMed] [Google Scholar]
- 8.Fahn HJ, Wang LS, Huang BS, Huang MH, Chien KY. Tumor recurrence in long-term survivors after treatment of carcinoma of the esophagus. Ann Thorac Surg. 1994;57:677–81. doi: 10.1016/0003-4975(94)90566-5. [DOI] [PubMed] [Google Scholar]
- 9.Kakuta T, Kosugi S, Kanda T, Ishikawa T, Hanyu T, Suzuki T, et al. Prognostic factors and causes of death in patients cured of esophageal cancer. Ann Surg Oncol. 2014;21:1749–55. doi: 10.1245/s10434-014-3499-7. [DOI] [PubMed] [Google Scholar]
- 10.Hirst J, Smithers BM, Gotley DC, Thomas J, Barbour A. Defining cure for esophageal cancer: analysis of actual 5-year survivors following esophagectomy. Ann Surg Oncol. 2011;18:1766–74. doi: 10.1245/s10434-010-1508-z. [DOI] [PubMed] [Google Scholar]
- 11.Port JL, Nasar A, Lee PC, Paul S, Stiles BM, Andrews W, et al. Definitive therapy for isolated esophageal metastases prolongs survival. Ann Thorac Surg. 2012;94:413–9. doi: 10.1016/j.athoracsur.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 12.Greene CL, DeMeester SR, Worrell SG, Oh DS, Hagen JA, DeMeester TR. Alimentary satisfaction, gastrointestinal symptoms, and quality of life 10 or more years after esophagectomy with gastric pull-up. J Thorac Cardiovasc Surg. 2014;147:909–14. doi: 10.1016/j.jtcvs.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Derogar M, Lagergren P. Health-related quality of life among 5-year survivors of esophageal cancer surgery: a prospective population-based study. J Clin Oncol. 2012;30:413–8. doi: 10.1200/JCO.2011.38.9791. [DOI] [PubMed] [Google Scholar]