Abstract
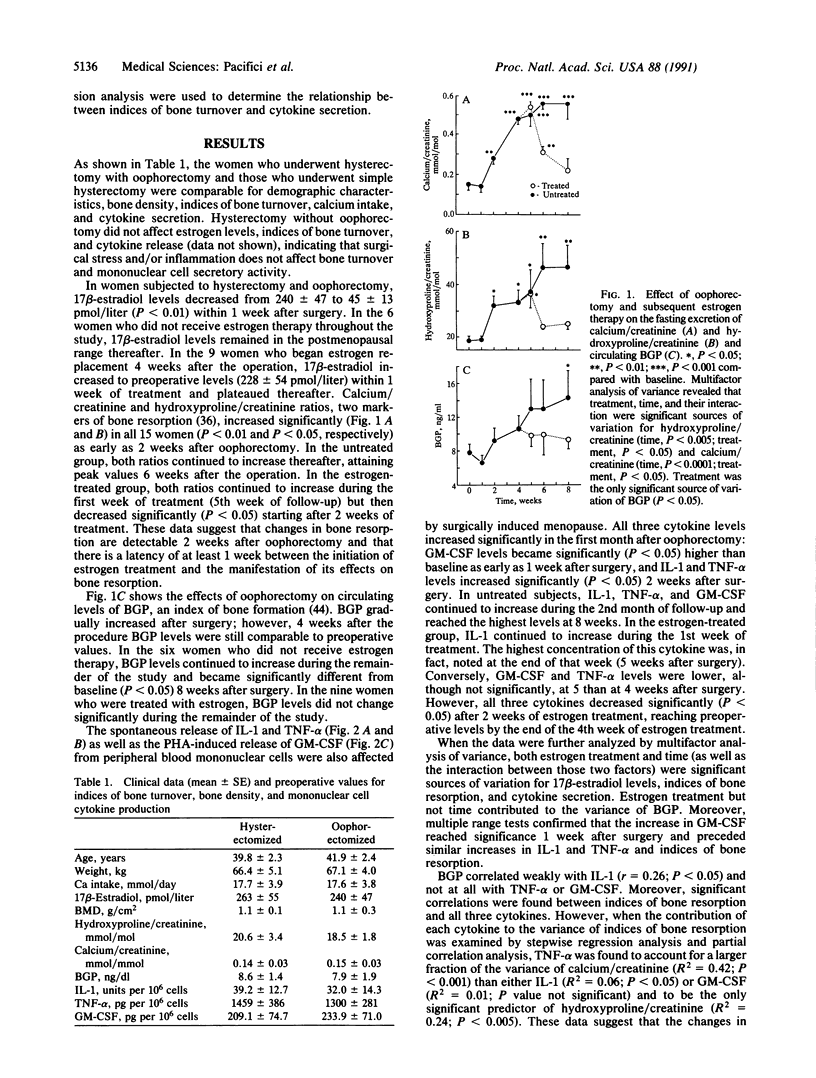
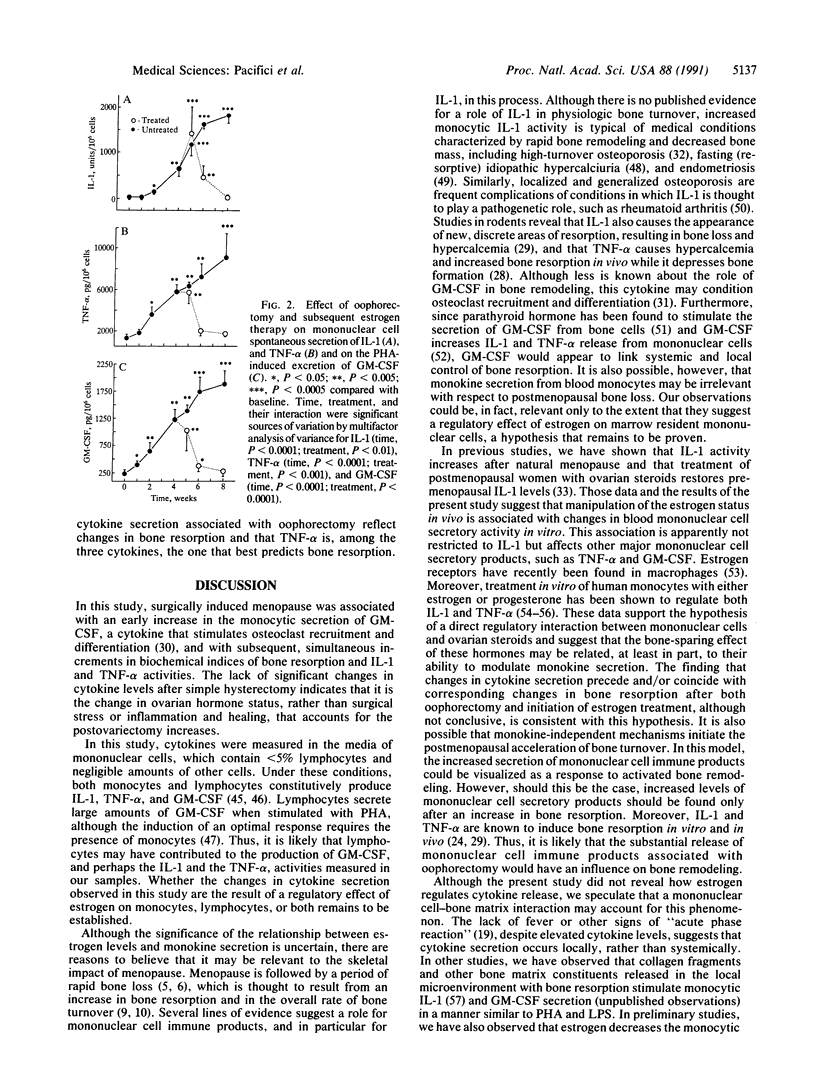
To determine whether mononuclear cell secretory products contribute to the changes in bone turnover that characterize the development of postmenopausal osteoporosis, we evaluated the effects of oophorectomy and subsequent estrogen replacement on the spontaneous secretion of interleukin 1 (IL-1) and tumor necrosis factor alpha (TNF-alpha) and on the phytohemagglutinin A-induced secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) from peripheral blood mononuclear cells. In 15 healthy premenopausal women who underwent oophorectomy, increases in GM-CSF activity were observed as early as 1 week after surgery, whereas elevations in IL-1 and TNF-alpha and in hydroxyproline/creatinine and calcium/creatinine ratios, two urinary indices of bone resorption, were detectable 2 weeks after the surgical procedure. Six of the oophorectomized women received no estrogen therapy after surgery and in these subjects hydroxyproline/creatinine and calcium/creatinine ratios plateaued 6 weeks postoperatively, and all three cytokines reached the highest levels 8 weeks after oophorectomy, when the study ended. In the remaining 9 women, who were started on estrogen replacement therapy 4 weeks after oophorectomy, decreases in the indices of bone resorption paralleled decreases in the secretion of the cytokines, with lower levels detected after 2 weeks of therapy. In the women who did not receive estrogen therapy, circulating osteocalcin, a marker of bone formation, increased beyond preoperative levels 8 weeks after oophorectomy, whereas in the estrogen-treated subjects osteocalcin remained unchanged in the entire study period. In 9 female controls who underwent simple hysterectomy, cytokine release and biochemical indices of bone turnover did not change after surgery. These data indicate that changes in estrogen status in vivo are associated with the secretion of mononuclear cell immune factors in vitro and suggest that alterations in the local production of bone-acting cytokines may underlie changes in bone turnover caused by surgically induced menopause and estrogen replacement.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beksaç M. S., Kişnişci H. A., Cakar A. N., Beksaç M. The endocrinological evaluation of bilateral and unilateral oophorectomy in premenopausal women. Int J Fertil. 1983;28(4):219–224. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Boyce B. F., Aufdemorte T. B., Garrett I. R., Yates A. J., Mundy G. R. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology. 1989 Sep;125(3):1142–1150. doi: 10.1210/endo-125-3-1142. [DOI] [PubMed] [Google Scholar]
- Brown C. B., Hart C. E., Curtis D. M., Bailey M. C., Kaushansky K. Two neutralizing monoclonal antibodies against human granulocyte-macrophage colony-stimulating factor recognize the receptor binding domain of the molecule. J Immunol. 1990 Mar 15;144(6):2184–2189. [PubMed] [Google Scholar]
- Brown J. P., Delmas P. D., Malaval L., Edouard C., Chapuy M. C., Meunier P. J. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet. 1984 May 19;1(8386):1091–1093. doi: 10.1016/s0140-6736(84)92506-6. [DOI] [PubMed] [Google Scholar]
- Canalis E. Interleukin-1 has independent effects on deoxyribonucleic acid and collagen synthesis in cultures of rat calvariae. Endocrinology. 1986 Jan;118(1):74–81. doi: 10.1210/endo-118-1-74. [DOI] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Dinarello C. A., Mier J. W. Lymphokines. N Engl J Med. 1987 Oct 8;317(15):940–945. doi: 10.1056/NEJM198710083171506. [DOI] [PubMed] [Google Scholar]
- Eriksen E. F., Colvard D. S., Berg N. J., Graham M. L., Mann K. G., Spelsberg T. C., Riggs B. L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988 Jul 1;241(4861):84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Ettinger B., Genant H. K., Cann C. E. Long-term estrogen replacement therapy prevents bone loss and fractures. Ann Intern Med. 1985 Mar;102(3):319–324. doi: 10.7326/0003-4819-102-3-319. [DOI] [PubMed] [Google Scholar]
- Fakih H., Baggett B., Holtz G., Tsang K. Y., Lee J. C., Williamson H. O. Interleukin-1: a possible role in the infertility associated with endometriosis. Fertil Steril. 1987 Feb;47(2):213–217. [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genant H. K., Cann C. E., Ettinger B., Gordan G. S. Quantitative computed tomography of vertebral spongiosa: a sensitive method for detecting early bone loss after oophorectomy. Ann Intern Med. 1982 Nov;97(5):699–705. doi: 10.7326/0003-4819-97-5-699. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Russell R. G. Stimulation of the proliferation of human bone cells in vitro by human monocyte products with interleukin-1 activity. J Clin Invest. 1985 Apr;75(4):1223–1229. doi: 10.1172/JCI111819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulshan S., McCruden A. B., Stimson W. H. Oestrogen receptors in macrophages. Scand J Immunol. 1990 Jun;31(6):691–697. doi: 10.1111/j.1365-3083.1990.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Hesketh P. J., Sullivan R., Valeri C. R., McCarroll L. A. The production of granulocyte-monocyte colony-stimulating activity by isolated human T lymphocyte subpopulations. Blood. 1984 May;63(5):1141–1146. [PubMed] [Google Scholar]
- Horowitz M. C., Coleman D. L., Flood P. M., Kupper T. S., Jilka R. L. Parathyroid hormone and lipopolysaccharide induce murine osteoblast-like cells to secrete a cytokine indistinguishable from granulocyte-macrophage colony-stimulating factor. J Clin Invest. 1989 Jan;83(1):149–157. doi: 10.1172/JCI113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komm B. S., Terpening C. M., Benz D. J., Graeme K. A., Gallegos A., Korc M., Greene G. L., O'Malley B. W., Haussler M. R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988 Jul 1;241(4861):81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Forrest C., Baird C. Prevention of spinal osteoporosis in oophorectomised women. Lancet. 1980 Nov 29;2(8205):1151–1154. doi: 10.1016/s0140-6736(80)92592-1. [DOI] [PubMed] [Google Scholar]
- MacDonald B. R., Mundy G. R., Clark S., Wang E. A., Kuehl T. J., Stanley E. R., Roodman G. D. Effects of human recombinant CSF-GM and highly purified CSF-1 on the formation of multinucleated cells with osteoclast characteristics in long-term bone marrow cultures. J Bone Miner Res. 1986 Apr;1(2):227–233. doi: 10.1002/jbmr.5650010210. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Morel G., Boivin G., David L., Dubois P. M., Meunier P. J. Immunocytochemical evidence for endogenous calcitonin and parathyroid hormone in osteoblasts from the calvaria of neonatal mice. Absence of endogenous estradiol and estradiol receptors. Cell Tissue Res. 1985;240(1):89–93. doi: 10.1007/BF00217561. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nilas L., Christiansen C. Bone mass and its relationship to age and the menopause. J Clin Endocrinol Metab. 1987 Oct;65(4):697–702. doi: 10.1210/jcem-65-4-697. [DOI] [PubMed] [Google Scholar]
- Nilas L., Gotfredsen A., Hadberg A., Christiansen C. Age-related bone loss in women evaluated by the single and dual photon technique. Bone Miner. 1988 Apr;4(1):95–103. [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Carano A., Santoro S. A., Rifas L., Jeffrey J. J., Malone J. D., McCracken R., Avioli L. V. Bone matrix constituents stimulate interleukin-1 release from human blood mononuclear cells. J Clin Invest. 1991 Jan;87(1):221–228. doi: 10.1172/JCI114975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., McCracken R., Vered I., McMurtry C., Avioli L. V., Peck W. A. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2398–2402. doi: 10.1073/pnas.86.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R., Rifas L., Teitelbaum S., Slatopolsky E., McCracken R., Bergfeld M., Lee W., Avioli L. V., Peck W. A. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4616–4620. doi: 10.1073/pnas.84.13.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici R., Rothstein M., Rifas L., Lau K. H., Baylink D. J., Avioli L. V., Hruska K. Increased monocyte interleukin-1 activity and decreased vertebral bone density in patients with fasting idiopathic hypercalciuria. J Clin Endocrinol Metab. 1990 Jul;71(1):138–145. doi: 10.1210/jcem-71-1-138. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Rupich R., Griffin M., Chines A., Susman N., Avioli L. V. Dual energy radiography versus quantitative computer tomography for the diagnosis of osteoporosis. J Clin Endocrinol Metab. 1990 Mar;70(3):705–710. doi: 10.1210/jcem-70-3-705. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Rupich R., Vered I., Fischer K. C., Griffin M., Susman N., Avioli L. V. Dual energy radiography (DER): a preliminary comparative study. Calcif Tissue Int. 1988 Sep;43(3):189–191. doi: 10.1007/BF02571319. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Chenu C., Bird A., Mundy G. R., Roodman G. D. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J Bone Miner Res. 1989 Feb;4(1):113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Daniele A., Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988 Jun;49(6):964–968. [PubMed] [Google Scholar]
- Polan M. L., Loukides J., Nelson P., Carding S., Diamond M., Walsh A., Bottomly K. Progesterone and estradiol modulate interleukin-1 beta messenger ribonucleic acid levels in cultured human peripheral monocytes. J Clin Endocrinol Metab. 1989 Dec;69(6):1200–1206. doi: 10.1210/jcem-69-6-1200. [DOI] [PubMed] [Google Scholar]
- Price P. A., Nishimoto S. K. Radioimmunoassay for the vitamin K-dependent protein of bone and its discovery in plasma. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2234–2238. doi: 10.1073/pnas.77.4.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisz L. G. Local and systemic factors in the pathogenesis of osteoporosis. N Engl J Med. 1988 Mar 31;318(13):818–828. doi: 10.1056/NEJM198803313181305. [DOI] [PubMed] [Google Scholar]
- Ralston S. H., Russell R. G., Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990 Sep;5(9):983–988. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- Richelson L. S., Wahner H. W., Melton L. J., 3rd, Riggs B. L. Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. N Engl J Med. 1984 Nov 15;311(20):1273–1275. doi: 10.1056/NEJM198411153112002. [DOI] [PubMed] [Google Scholar]
- Riggs B. L., Melton L. J., 3rd Evidence for two distinct syndromes of involutional osteoporosis. Am J Med. 1983 Dec;75(6):899–901. doi: 10.1016/0002-9343(83)90860-4. [DOI] [PubMed] [Google Scholar]
- Sambrook P. N., Reeve J. Bone disease in rheumatoid arthritis. Clin Sci (Lond) 1988 Mar;74(3):225–230. doi: 10.1042/cs0740225. [DOI] [PubMed] [Google Scholar]
- Selby P. L., Peacock M., Barkworth S. A., Brown W. B., Taylor G. A. Early effects of ethinyloestradiol and norethisterone treatment in post-menopausal women on bone resorption and calcium regulating hormones. Clin Sci (Lond) 1985 Sep;69(3):265–271. doi: 10.1042/cs0690265. [DOI] [PubMed] [Google Scholar]
- Sieff C. A. Hematopoietic growth factors. J Clin Invest. 1987 Jun;79(6):1549–1557. doi: 10.1172/JCI112988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson S. D., Dinarello C. A. Production of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood. 1988 Oct;72(4):1368–1374. [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R., Gans P. J., McCarroll L. A. The synthesis and secretion of granulocyte-monocyte colony-stimulating activity (CSA) by isolated human monocytes: kinetics of the response to bacterial endotoxin. J Immunol. 1983 Feb;130(2):800–807. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Voelkel E. F., Lazzaro M., Goad D., Bosma T., Levine L. Tumor necrosis factor-alpha (cachectin) stimulates bone resorption in mouse calvaria via a prostaglandin-mediated mechanism. Endocrinology. 1987 May;120(5):2029–2036. doi: 10.1210/endo-120-5-2029. [DOI] [PubMed] [Google Scholar]
- Thomson B. M., Mundy G. R., Chambers T. J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987 Feb 1;138(3):775–779. [PubMed] [Google Scholar]
- Thomson B. M., Saklatvala J., Chambers T. J. Osteoblasts mediate interleukin 1 stimulation of bone resorption by rat osteoclasts. J Exp Med. 1986 Jul 1;164(1):104–112. doi: 10.1084/jem.164.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. D., Cameron P. M. The relationship between bacterial endotoxin and human B cell-activating factor. J Immunol. 1978 Jul;121(1):53–60. [PubMed] [Google Scholar]


