Abstract
The Mitochondrial Lon protease, also called LonP1 is a product of the nuclear gene LONP1. Lon is a major regulator of mitochondrial metabolism and response to free radical damage, as well as an essential factor for the maintenance and repair of mitochondrial DNA. Lon is an ATP-stimulated protease that cycles between being bound (at the inner surface of the inner mitochondrial membrane) to the mitochondrial genome, and being released into the mitochondrial matrix where it can degrade matrix proteins. At least three different roles or functions have been ascribed to Lon: 1) Proteolytic digestion of oxidized proteins and the turnover of specific essential mitochondrial enzymes such as aconitase, TFAM, and StAR; 2) Mitochondrial (mt)DNA-binding protein, involved in mtDNA replication and mitogenesis; and 3) Protein chaperone, interacting with the Hsp60–mtHsp70 complex. LONP1 orthologs have been studied in bacteria, yeast, flies, worms, and mammals, evincing the widespread importance of the gene, as well as its remarkable evolutionary conservation. In recent years, we have witnessed a significant increase in knowledge regarding Lon's involvement in physiological functions, as well as in an expanding array of human disorders, including cancer, neurodegeneration, heart disease, and stroke. In addition, Lon appears to have a significant role in the aging process. A number of mitochondrial diseases have now been identified whose mechanisms involve various degrees of Lon dysfunction. In this paper we review current knowledge of Lon's function, under normal conditions, and we propose a new classification of human diseases characterized by a either over-expression or decline or loss of function of Lon. Lon has also been implicated in human aging, and we review the data currently available as well as speculating about possible interactions of aging and disease. Finally, we also discuss Lon as potential therapeutic target in human disease.
Keywords: Lon, Proteolysis, Mitochondria, Mitochondrial DNA, Aging, Cancer, Mitochondrial diseases, Cancer, Neurodegeneration
1. Introduction
Mitochondria are essential components of cellular metabolism, and rapid changes in the mitochondrial protein pool are required for adaptation to a variety of conditions, from increased demand for oxidative energy production to hypoxic adaptation. Mitochondrial homeostasis is crucial for cell survival, and mitochondrial dysfunction leads to apoptotic cell death in normal cells and to aberrant adaptation and selection of hypoxic phenotypes in pathologic conditions such as cancer.
The turnover of mitochondrial proteins was once believed to be a function of lysosomes which can carry out autophagy of the whole organelle [1]. Mitochondrial autophagy varies widely in different metabolic conditions, with an estimated median half-life for liver mitochondria of 1.83 days in control conditions, which can decrease to 1.16 days following 3 months of caloric restriction [2]. Lysosomal autophagy, however, cannot explain wide differences in the individual turnover rates of various mitochondrial proteins; for example, the two isoforms of mitochondrial acetyl-CoA acetyl-transferase (acetoacetyl-CoA thiolase, EC 2.3.1.9) A1 and A2 have half-lives of 24.5 h and 7.2 h respectively [3]. Similarly, the uncoupling proteins (UCPs) – a very important subset of the mitochondrial solute carrier family - have very different half-lives [4]. UCP2 has a remarkably short half-life of ~1 h in cells [5], while UCP1 has a much longer half-life, of ~1–4 days [6] and UCP3 has a short half-life of 0.5–4 h [4].
In order to maintain an active, balanced production of energy, mitochondria need to expeditiously remove and replace any modified or damaged proteins. This is absolutely crucial, due to the fact that oxidative phosphorylation (OX-PHOS) constantly induces oxidative damage to the respiratory subunits as well as to other essential enzymes involved in mitochondrial metabolism [7]. Mitochondrial proteases demonstrate substrate preferences, which suggest possible recognition based on protein modifications [100]. As the vast majority of mitochondrial proteins are encoded in the nucleus, an imbalance between the production of mitochondrial subunits – which is inducible – and mitochondrial degradation could lead to serious mitochondrial disorders of homeostasis and (finally) to cellular disease and death [8,9].
These findings suggest that mitochondria possess a complex proteolytic pathway which modulates the selective and precisely-timed degradation of internal proteins [10]. At least five different proteases are responsible for targeted protein quality control (UPRmt, the mitochondrial unfolded protein response): most prominently represented being the Lon protease and the other AAA + family proteases (ATPases associated with a variety of cellular activities) [11,116]. The mitochondrial ability to respond to organelle- specific stressors is essential for the degradation of unfolded proteins in the mitochondrial matrix [117].
The human Lon protease is highly conserved through multiple levels of evolution, and is the homolog of the bacterial La [12] and the yeast PIM1/Lon [13]. Lon expression is essential for survival in mammals, and homozygous deletion of LONP1 causes early embryonic lethality [14]. In recent years, an explosion in our understanding of Lon function has occurred. As such, Lon dysfunctions/mutations have now been associated with multiple physiologic and pathologic conditions in humans, from aging to cancer and neurodegeneration [15]. This review attempts to provide a systematic approach to Lon involvement in health, disease, and aging, and also to provide an etiologic classification of Lon-related disorders.
2. Lon discovery, structure, and regulation
2.1. Lon discovery
The presence of an ATP stimulated proteolytic activity has been described in the matrix of mitochondria from a variety of mammalian organs and systems since 1982, including bovine adrenal cortex [16–18], rat liver [19,20] and human heart, brain, liver [21] and placenta [22].
The mitochondrial matrix protease responsible for this activity was initially purified by two different groups as a 600–650 kDa hexameric homoprotein complex (subunit mass 106 kDa) [23–25]. The human enzyme has a subunit size of 100 kDa in the mature form [26] and is widely expressed in multiple human tissues – with the highest levels in the most metabolically active organs – heart, brain, liver and skeletal muscle [21]. The nuclear gene encoding mitochondrial Lon is LONP1 (PRSS15), situated on chromosome 19. There is also a peroxisomal form of the Lon protease, called LonP2; despite apparent similarities to mitochondrial LonP1 in proteolytic functions, the peroxisomal LonP2 is encoded by a completely different nuclear gene, and is regulated independently of LonP1 [27].
2.2. Lon protein structure
Lon's protein sequence was initially solved for the human protein. The cloned nuclear gene encoded a protein of 963 amino acids with a calculated molecular mass of 106 kDa [21], which is then processed to a mature 100 kDa mitochondrial matrix protein kDa [21]. There are three distinctive, highly conserved structural sequences that have been evolutionarily conserved: 1) a mitochondrial matrix targeting sequence [21], 2) a serine protease domain, [21] and 3) a Walker-type ATP binding motif (ATPase functional domain, AAA+ module) that is involved both with nucleotide binding and ATP hydrolysis. [28]
The complete crystal structure of Lon was resolved in Thermococcus onnurineus [29] and in the Escherichia coli [30] bacterium. The published 2.0-Å resolution crystal structure of Thermococcus onnurineus NA1 Lon (TonLon) reveals a three-tiered hexagonal cylinder with a large, central, unfolding and degradation chamber [29]. However, E. coli Lon analysis by size-exclusion chromatography (SEC) and multiangle laser light scattering (MALS) identified both hexamers (calculated MR 525 kDa) and dodecamers (calculated MR 1050 kDa) [29]. Formation of the dodecamer inhibits the proteolysis of large but not small protein substrates, suggesting that the repertoire of Lon substrates depends on its state of assembly [29]. The resolved structure of human mitochondrial Lon proteolytic domain is resembles those of the previously determined Lon proteolytic domains from Escherichia coli, with six protomers in the asymmetric unit. The active site contains a 310 helix attached to the N-terminal end of α-helix 2, which leads to the insertion of Asp852 into the active site [31]. The full –sequence human Lon protease is yet to be crystalized – but human Lon displays significant homology the bacterial protease La (lon gene) and the yeast form of Lon, which is called Pim-1 (PIM1 gene) [13,32]. As such, the homology model of human Lon suggests a hexameric complex with an open-ring arrangement similar to that of yeast (Pim-1) Lon [33].
2.3. Lon regulation
The mitochondrial Lon protease is a one of the most important cellular stress-responsive proteins [34]. Acute stressors, such as heat shock [34], serum starvation, and oxidative stress [35,36] lead to Lon upregulation. However, regulation of Lon is biphasic, and chronic and severe stress conditions – such as aging [15], extensive hypoxia [35] and prolonged oxidative stress [37] cause Lon down-regulation, suggesting that Lon is a significant factor in aging and degenerative diseases.
The LONP1 promoter region −623/+1 contains a Nuclear Respiratory Factor 2 (NRF-2) consensus binding site and is essential for response to reactive oxygen species [36]. Additional proximal NRF-2/GABP consensus sites were recently found to be essential for growth-related transcriptional activation of LONP1 [89]. The NRF-2 consensus binding site of LONP1 should not be confused with the ARE or EPRE consensus binding sites on many other genes, for another well-known (and similarly named) transcription factor, the nuclear factor erythroid 2–related factor 2 (abbreviated as Nrf2) which plays a major role in cellular adaptation to various perturbations such as oxidative stress [38].
The ability of NRF2 to regulate Lon expression is a key factor in its ability to promote the expression of key components of the mtDNA transcription and replication machinery, as well as of genes encoding respiratory subunits [39]. Another very important transcriptional site is the region −2023/−1230 – containing a putative binding site for NF-kB [36]. The presence of an NF-kB binding site further consolidate Lon's role as a stress protein [34], as well as its involvement in oncogenesis [40] – a process which significantly involves NF-kB regulated pathways [41].
Lon expression is also significantly induced by Hypoxia-inducible factor 1 (HIF-1) [42]. The Lon gene promoter also contains of five potential HIF-1-binding sites within 0.6 kb of the transcription start site, and ChIP assays have demonstrated the binding of protein complexes containing HIF-1α and HIF-2α to the Lon promoter [42]. Another regulator of Lon expression is the Epidermal Growth Factor (EGF) [43]. EGF is one of the main genes involved in tumorigenic transformation, and disruption of mitochondrial homeostasis is an important characteristic of cancer. EGF regulation of Lon expression is mediated by two important oncogenic pathways: the Extracellular signal-regulated protein kinase (ERK) pathway and the Phosphoinositide 3-kinase (PI3K) pathway [42].
Lon is also regulated at a post-translational level. The NAD+ -dependent mitochondrial deacetylase sirtuin 3 (SIRT3) mediates Lon deacetylation, and SIRT3 silencing causes an increase of Lon protein levels but not of Lon mRNA [44].
3. Normal functions of Lon in healthy individuals
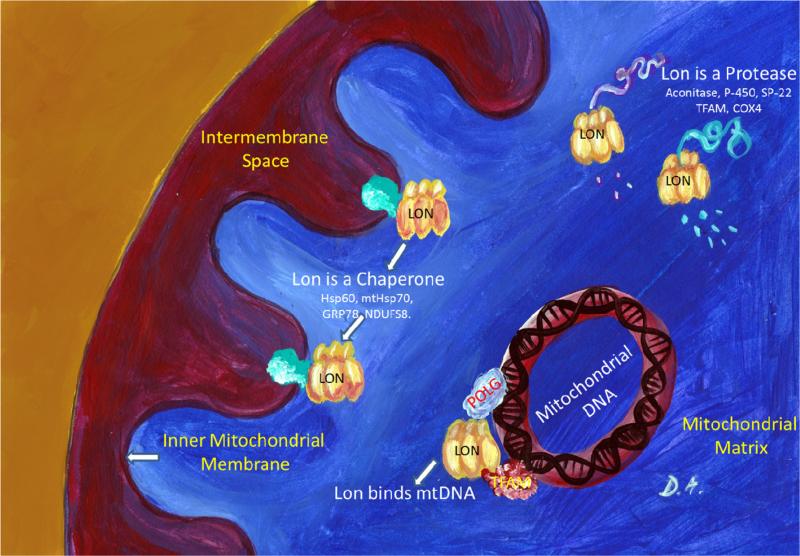
Lon can function as a protease, as a chaperone, and as a mtDNA–binding protein. These various functions are discussed below, and the spectrum of Lon functions described is also reviewed in Table 1.
Table 1.
Lon functions in mammalian cells.
| Function | Partners/Substrates |
|---|---|
| Protease | adrenodoxin reductase [16] |
| cytochrome P-450 [16] 99 | |
| SP-22 [51] | |
| Aconitase [37,45,53] | |
| TM [56] | |
| StAR [89,90] | |
| COX4–1 [41] | |
| ALAS-1 [90] | |
| PINK1 [66] | |
| POLG [54] | |
| Chaperone | Hsp60 [57] |
| mtHsp70 [57] | |
| Myosin-9/10 [57] | |
| 78 kDa glucose-regulated protein (GRP78) [57] | |
| protocadherin-18 [57] | |
| NADH:ubiquinone oxidoreductase core subunit S8 (NDUFS8) [57]. | |
| mtDNA binding | mtDNA sequences containing at least 4 contiguous guanine residues - G-quadruplexes (four-stranded intra- or inter-molecular structures with a tetrad arrangement of guanines) [60,61] |
3.1. Lon as a protease
The initial function discovered for mitochondrial Lon was its proteolytic activity. Our original work in human cell WI-38 VA-13 lung fibroblasts identified an ATP-stimulated serine protease localized in the mitochondrial matrix which is responsible for the degradation of oxidized mitochondrial aconitase [45]. The proteolytic activity is maintained even in the absence of ATP, but protein degradation is stimulated by to up to nine-times by ATP availability [25]. Importantly, ATP hydrolysis is required for this stimulatory effect and non-hydrolyzable ATP analogs are ineffective. Interestingly, Lon can degrade its substrates both in a folded and in an unfolded state – preferring the degradation of unassembled proteins [46]. In the case of properly folded, functionally competent mitochondrial proteins, the first cleavage occurs preferentially between hydrophobic amino acids located within highly charged environments, while subsequent cleavages proceed sequentially along the primary polypeptide sequence [46].
The list of Lon substrates is growing every year – and includes both specific and non-specific targets. A very important function of Lon is to maintain mitochondrial homeostasis – by recognizing and degrading oxidatively modified proteins [45]. Mitochondrial proteins are constantly in contact with free radical species generated by the respiratory chain, and the oxidized proteins have to be quickly removed and replaced, before they accumulate and cause further toxicity [47]. In mammalian cells, Lon down-regulation by either genetic manipulation [48,49] or by physiologic conditions in intact organisms [37] (oxidative stress, aging) leads to accumulation of damaged proteins inside mitochondria. The proteolytic function is conserved from bacteria to yeast and to humans – as a yeast strain lacking a functional Lon gene also has a reduced ability to degrade mitochondrial matrix proteins, which accumulate as electron-dense inclusions [13]. As expected, the mutant yeast strain can be rescued by expressing bacterial (Lon) protease La [50].
The identified specific Lon targets are mitochondrial matrix proteins, involved either in mitochondrial bioenergetic processes, protection against oxidative damage, or in mitochondrial DNA maintenance. Some of the first targets identified were adrenodoxin reductase and cytochrome P-450 [16]. Another proposed protein substrate is SP-22 [51], a thioredoxin-dependent peroxide reductase which functions as a defense system against superoxide, hydrogen peroxide, and related reactive species in adrenocortical mitochondria [52].
We and others have published extensively on Lon protease's ability to selectively degrade oxidatively-modified aconitase [37,45,53]. The primary role of mitochondrial aconitase is to catalyze the stereo-specific isomerization of citrate to isocitrate, via cis-aconitate, in the mitochondrial matrix Krebs cycle [54]. Aconitase easily undergoes oxidative modification and its activity is widely used as a biomarker for oxidative stress. Aconitase inactivation in aging and certain oxidative stress-related disorders [37,53], and its accumulation, is a major pathologic event, which leads to cellular dysfunction and death [55].
Another important Lon substrate is the mitochondrial transcription factor A (TFAM) [56]. TFAM directly regulates the mtDNA copy number in mammals [57], and is one of the core mitochondrial nucleoid components, together with mitochondrial single-strand binding protein (mtSSB), polymerase gamma (POLG), and mtRNA polymerase (POLRMT). By controlling TFAM degradation, Lon regulates the DNA copy number and transcription [56]. TFAM is phosphorylated within its HMG box 1 (HMG1) by a cAMP-dependent protein kinase, and its phosphorylation impairs the ability of TFAM to bind DNA and to activate transcription. TFAM phosphorylation also promotes TFAM degradation, as Lon degrades only the phosphorylated TFAM [58].
Finally, Lon's proteolytic activity is also involved in hypoxic adaptation, through the Hypoxia-inducible factor 1 (HIF-1) pathway [42]. HIF-1 regulates COX4 subunit composition by increasing the transcription of both Lon and COX4-2. Lon overexpression then promotes the degradation of COX4-1. The change in COX4 subunit expression allows for the optimization of mitochondrial ATP production, O2 consumption, and reactive oxygen species generation at different O2 concentrations [42].
3.2. Lon as a chaperone
Co-immunoprecipitation experiments have identified 76 different mitochondrial proteins that bind Lon [59]. Some of these proteins are Hsp60, mtHsp70, Myosin-9/10, 78 kDa glucose-regulated protein (GRP78), [23] and protocadherin-18, and NADH: ubiquinone oxidoreductase core subunit S8 (NDUFS8). Lon contributes to the stability of Hsp60–mtHsp70 complex, and protects cell from apoptosis under environmental stress through interacting with and stabilizing Hsp60.[59]
3.3. Lon as a mtDNA–binding protein
Initial reports suggested Lon might be involved in controlling the number of mitochondria in cells, or the extent of the mitochondria reticulum. Lon over-expression and increased protease activity were reported in cells with an increased mitochondrial biogenesis, such as Zajdela hepatoma tumor [60]. We now know that human Lon can bind to mitochondrial promoters, and may control mtDNA transcription and translation by degrading multiple regulatory proteins involved in mtDNA maintenance (such as TFAM and POLG) [56,61]. Enzymatically active mouse Lon that hydrolyses ATP and degrades protein and peptide substrates in an ATP-stimulated manner also specifically binds to single-stranded but not to double-stranded DNA oligonucleotides [62]. Human Lon associates with mtDNA sequences containing at least 4 contiguous guanine residues - which can form G-quadruplexes (four-stranded intra- or inter- molecular structures with a tetrad arrangement of guanines) [63]. In human fibroblasts and in colon cancer cells, Lon down-regulation is associated with loss of mitochondrial DNA [14,48]. However, this is not an universal finding – other groups have reported that Lon downregulation in the LS174T cells no significant difference in mtDNA copy number was observed [64] (Fig. 1)
Fig. 1.
Schematic representation of Lon involvement in mitochondrial biology.
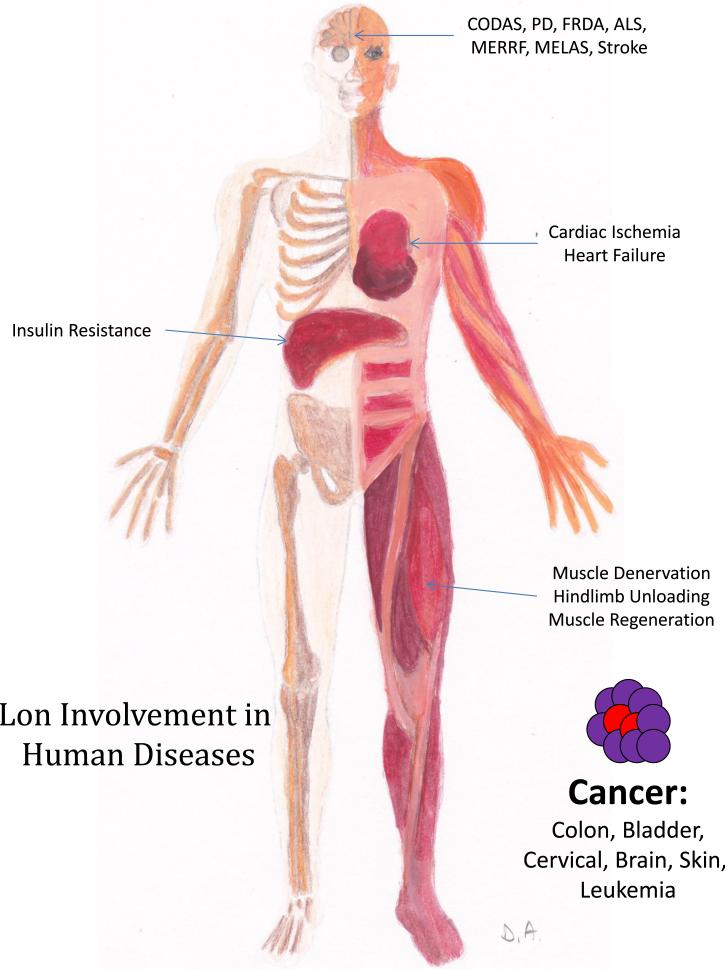
4. Lon involvement in human diseases
As mentioned above, Lon expression is highest in the most metabolically active organs – brain, heart, liver and skeletal muscle [21]. Therefore, these organs seem to be commonly involved in conditions caused by Lon dysregulation (see Fig. 2). In addition, malignant transformation and oncogenic growth are highly dependent on adaptation to new sources of energy and to hypoxia – which explains the important role Lon plays in a variety of malignancies. As Lon is upregulated by acute oxidative stress, it is also involved in a number of conditions caused by the ingestion of toxins and drugs which cause damage via generation of reactive oxygen or nitrogen species such superoxide, hydrogen peroxide, and peroxynitrite, as in different tissues involved in detoxification such as the kidney and adipose tissues.
Fig. 2.
Schematic representation of Lon involvement in human diseases.
4.1. Lon involvement in neurological disorders
4.1.1. Lon and cerebral, ocular, dental, auricular, skeletal anomalies (CODAS) syndrome
Cerebral, ocular, dental, auricular, skeletal anomalies (CODAS) syndrome is a rare, multi-system developmental disorder originally described in 1991 [65]. Less than 20 cases have been reported to this day [65–67]. Two different groups reported in 2015 that the genetic abnormalities causing the CODAS Syndrome are compound heterozygous or homozygous mutations in LONP1 (either missense or nonsense point mutations or small in-frame deletions) [66,67]. The CODAS pattern of inheritance is autosomal recessive [66,67]. The amino acid substitutions cluster within the ATP-binding (AAA) and proteolytic domains of Lon [66,67]. Lymphoblastoid cell lines generated from CODAS patients have swollen mitochondria with electron-dense inclusions and abnormal inner-membrane morphology, aggregated mtDNA-encoded subunit II of cytochrome c oxidase; and altered respiratory capacity, leading to impaired mitochondrial energy production [67].
A number of children identified from the Old Order Amish community of Pennsylvania were homozygous for LONP1 c.2161C>G [67]. The homozygous LONP1 phenotype is very severe – confirming the very important role that LONP1 regulation of mitochondrial activity has in human development [67]. All the affected children were very severely impacted by their disease – they either died of laryngeal obstruction in the first days of life or required tracheostomies to survive infancy. Another case was identified during pregnancy – and was a stillborn at 39 weeks of gestation due to severe abnormalities. Less than half of the children survived their early years – and had paretic, atrophic vocal cords, glottis narrowing, chronic sialorrhea, and swallowing dysfunction. They required a gastrostomy tube for nutrition.
The CODAS phenotype included broad skull and flattened midface, dysmorphic ears and noses, and with advancing age, short stature, scoliosis, genu valgus, and pes valgus. Metaphyseal dysplasia (most evident at hip joints), and both hypoplasia and delayed ossification of epiphyses were present. Scoliosis was diagnosed within the first few years of life [67]. Teeth erupted late with cusp-tip extensions. Dense bilateral nuclear cataracts and hearing loss were present [67].
All the affected children had developmental and cognitive abnormalities – including hypotonia, developmental delay, and variable intellectual disability. Brain magnetic resonance imaging (MRI) studies were notable for revealing extensive brain atrophy, with prominent cortical sulci and ventriculomegaly as well as for subcortical hypomyelination, and a thin corpus callosum [67].
4.1.2. Lon and hereditary Parkinson disease
Loss-of-function mutations in the PARK6 gene coding for PTEN-induced kinase 1 (PINK1), which encodes a mitochondrially targeted serine/threonine kinase, cause an early-onset, autosomal recessive form of Parkinson's disease. PINK1 is constitutively degraded in healthy cells, but selectively accumulates on the surface of depolarized mitochondria, thereby initiating their autophagic degradation. Lon down-regulation causes dramatic accumulation of processed PINK1 species in the mitochondrial matrix. The processed forms of PINK1 that accumulate upon Lon inactivation are capable of activating the PINK1-Parkin pathway in vivo [68], and to target mitochondria for mitophagy [68].
4.1.3. Lon and Friedreich ataxia (FRDA)
Friedreich ataxia (FRDA) is a genetic neurodegenerative disease affecting the central and peripheral nervous systems, heart, skeleton, and endocrine pancreas [69]. The cause of the disease is a homozygous guanine-adenine-adenine (GAA) trinucleotide repeat expansion on chromosome 9q13 that causes a transcriptional defect of the frataxin gene, resulting in reduced expression of mitochondrial frataxin, an iron chaperone involved in the maturation of Fe-S cluster proteins [69]. In the FRDA mouse model, frataxin deficiency causes cardiodegeneration, deficiency of respiratory chain complexes I–III and aconitases, and mitochondrial iron accumulation [70]. The same animals show a clear progressive increase in Lon protein levels. Lon upregulation is also accompanied by an increase in proteolytic activity, and by decreased levels of mitochondrial Fe–S proteins. The effect of Lon upregulation on loss of mitochondrial Fe-S proteins during the progression of the disease suggests that Fe-S proteins may be targets of Lon in FRDA [71].
4.1.4. Lon and familial amyotrophic lateral sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS) is a severe neurodegenerative disease characterized by progressive motor neuron death, causing progressive paralysis and lethal respiratory failure [72]. Mutations in copper-zinc superoxide dismutase (SOD1) represent the most common defect seen in the familial forms of ALS (fALS) [73]. Lon expression is down-regulated in the NSC34 cells (a motor neuron-like cell line) expressing mutant G93A-SOD1 gene [74]. Increasing evidence supports the concept that mitochondrial dysfunction and activation of apoptosis play critical roles in the fALS etiology. Lon down-regulation causes apoptosis in multiple human cell lines [48,59] – raising the question of a potential direct relation between Lon downregulation and motor neuron apoptosis in fALS [74].
4.1.5. Lon and Myoclonic epilepsy and ragged-red fibers (MERRF) syndrome
Myoclonic epilepsy and ragged-red fibers (MERRF) syndrome is a rare mitochondrial disorder characterized by myoclonus, muscle weakness, cerebellar ataxia, heart conduction block, and dementia [75]. The increase in reactive oxygen or nitrogen species such superoxide, hydrogen peroxide, and peroxynitrite, caused by the respiratory chain dysfunction due to A8344G mutation in the tRNALys mitochondrial gene causes damage proteins containing Fe–S clusters such as mitochondrial aconitase [76] – an enzyme predominantly degraded by Lon [45]. In MERRF cybrids containing >90% mtDNA with the A8344G mutation, aconitase activity is significantly decreased while Lon protein expression is highly up-regulated. However, the activity of Lon protease in the MERRF cybrids was significantly lower than that of the wild-type cybrids, suggesting that the up-regulation of Lon protease in the MERRF cells may be a compensation for the decrease of its enzymatic activity caused by oxidative damage.
4.1.6. Lon and myopathy, encephalopathy, lactic acidosis, stroke-like episodes syndrome (MELAS)
The myopathy, encephalopathy, lactic acidosis, stroke-like episodes syndrome (MELAS) is a mitochondrial disorder most frequently associated with an A to G transition at position 3243 of the mitochondrial tRNA(Leu(UUR)) gene [77]. MELAS patient-derived cells have increased mitochondrial Lon protein expression, which accumulates preferentially in MELAS cells containing numerous mitochondria with mutated genomes. Enzymatic assays revealed that –in contrast with the MERRF cybrids–Lon protease activity is also increased in MELAS cell lysates [78].
4.1.7. Lon and brain ischemia/stroke
Lon mRNA and protein levels are induced by hypoxia in cultured astrocytes. In the rat model of ischemic stroke (middle cerebral artery occlusion), both Lon mRNA and protein expression are induced by ischemia, especially in neurons [79]. Cerebral hypoxia/ischemia is known to induce severe endoplasmic reticulum (ER) stress. Attenuation of protein synthesis in response to ER stress occurs to lessen the load of protein entering the ER, and this pathway requires an activation of the ER-resident protein kinase, PERK. Lon induction by ER stress is inhibited in PERK (−/−) cells, confirming Lon's involvement in the ER stress response [79].
4.2. Lon involvement in cardiac disorders
4.2.1. Lon, cardiac ischemia and heart failure
In ischemic conditions, cardiomyocytes are subjected to increased generation of reactive oxygen and nitrogen species, mitochondrial dysfunction and apoptosis [80]. Lon is upregulated in hypoxia-induced cardiomyocytes, while Lon downregulation attenuates hypoxia-induced cardiomyocyte apoptosis through decreased oxidant generation. Lon overexpression stimulated oxidant production and induced apoptosis under normoxic conditions in cardiomyocytes [81] – in contrast with data in other human cells where Lon over-expression stimulates cell proliferation [60], while Lon downregulation causes senescence [47] and/or apoptosis [48,49].
Lon carbonylation and tyrosine nitration are prominent in a mouse model of pressure overload heart failure after transverse aortic constriction (TAC) [82]. The measurement of ATP-dependent proteolytic activity in isolated mitochondria revealed marked reductions in mitochondrial protease activity. Oxidative post-translational modifications (OPTM) contribute to the proteolytic deficit of Lon and OPTM-mediated protease dysfunction and is closely associated with declines in electron transport chain (ETC) protein homeostasis, mitochondrial energetics, and left ventricular contractility. Infusion of mitoTEMPO, a mitochondria-targeted superoxide dismutase mimetic, improves mitochondrial respiration capacity and cardiac function at least partially through the improvement of Lon activity in heart failure [82].
4.3. Lon involvement in skeletal muscle pathology
4.3.1. Lon and muscle denervation
Muscle denervation is commonly seen in traumatic injuries. In the experimental model of sciatic nerve transection, Lon protease was down-regulated at day 30 after surgery, which correlated with a decreased number of functional mitochondria [83].
4.3.2. Lon and Hindlimb unloading (loss of gravitational pull in space travel)
Hindlimb unloading (HU) rodent models are frequently used to model the neuromuscular changes taking place in a real microgravity environment such as that experienced during spaceflights [84]. HU is characterized by muscle atrophy and dysfunction, and loss of mitochondria – with the loss of subsarcolemmal mitochondria being much greater than the loss of intermyofibrillar mitochondria [85]. Lon is down-regulated in HU exposed muscles, which might explain the decreased mitochondrial biogenesis, suggesting that Lon protease may play some role in mitochondrial adaptations to HU [85].
4.3.3. Lon and muscle regeneration
Skeletal muscles exhibit a high capacity to repair and regenerate after trauma or disease. Upon injury, satellite muscle cells can proliferate, and fuse with the existing myofibers or form new myofibers [86]. Myogenic differentiation is associated with an increase in mitochondrial biogenesis, and requires increased expression of mitochondrial biogenesis-related genes including Lon. These results suggest that mitochondrial biogenesis is activated early during muscle regeneration and that mitochondrial biogenesis plays a significant role in muscle regeneration. [87]
4.4. Lon involvement in steroid hormone synthesis (StAR protein degradation)
Steroidogenic acute regulatory protein (StAR) is required for steroid hormone production in the adrenal cortex and the gonads. StAR mediates the transport of cholesterol into the inner mitochondrial membranes where is converted to a steroid [88]. Lon protease is involved in StAR proteolysis [118], and gonadotropin administration to prepubertal rats stimulates ovarian follicular development and increases Lon expression. Furthermore, StAR expression stimulates LONP1 gene transcription by acting through the NRF-2 pathways on the proximal promoter of the LON gene proteases [89] (which contains the NRF-2 responsive elements)-suggesting a novel regulatory loop through which Lon and StAR regulate each other's availability [90].
4.5. Lon involvement in hepatic disorders
4.5.1. Lon and 5-aminolevulinic acid synthase protein metabolism
5-Aminolevulinic acid synthase (ALAS-1) is the first rate controlling enzyme that controls cellular heme biosynthesis in the liver and in bone marrow cells [91]. Negative regulation of ALAS-1 by the end product heme provides the foundation for heme treatment of acute porphyrias; these are a group of diseases caused by genetic defects in the heme biosynthesis pathway that are exacerbated by controlled up-regulation of ALAS-1. Heme and other metalloporphyrins decrease mitochondrial ALAS-1 protein levels by accelerating its proteolytic degradation by Lon protease. Conversely, down-regulation of Lon activity diminishes the negative effect of heme on mitochondrial ALAS-1 [92].
4.5.2. Lon and hepatic insulin resistance
Mitochondrial dysfunction is a cause of insulin resistance and type 2 diabetes [93]. In human liver cells, Lon downregulation causes impaired insulin signaling and increased levels of gluconeogenic enzymes, while Lon over-expression diminishes the insulin resistance induced by treatment with cholesterol and palmitate. In-vivo, Lon protein levels are significantly lower in the livers of diabetic db/db mice compared with their lean mice counterparts, suggesting that Lon down-regulation might be involved in the development of insulin resistance and type 2 diabetes [94].
4.6. Lon involvement in cancer
Mitochondrial dysfunction is a hallmark of cancer biology. Tumor mitochondrial metabolism is characterized by an abnormal ability to function in scarce oxygen conditions [95] by using glycolysis (the Warburg effect), and accumulation of mitochondrial DNA defects is present in both hereditary neoplasias and sporadic cancers [96]. Lon is highly expressed in aggressive tumors [97], which are characterized by extensive hypoxic areas caused by insufficient oxygen and nutrient supply which are the hallmarks of aggressive tumors [98]. Several key roles for Lon in cancer [99] include: 1) hypoxic survival, 2) invasion and metastasis, 3) resistance to apoptosis, and 4) treatment resistance.
4.6.1. Lon and malignant transformation
Lon is involved in Epidermal Growth Factor (EGF) and ErbB2/Her-2 induced tumorigenic transformation in both epidermal and mammary cancers. Irreversibly transformed mouse epidermal cells have higher Lon levels than the parent, non-transformed cells (JB6 P+). In addition the epidermal growth factor which induces tumorigenic transformation of the JB6 P+ cells also up-regulates Lon expression [43].
4.6.2. Lon and colon cancer
Lon silencing in RKO colon cancer cells, either by using either Lon shRNA or the triterpenoid Lon-inhibitor, 2-cyano-3,12-dioxooleana-1,9,-dien-28-oic acid, causes profound alterations in the mitochondrial proteome and function, and cell death. Lon-silenced cells display altered levels of mitochondrial proteins, low levels of mtDNA transcripts, and reduced levels of OX-PHOS complexes. Lon-deficient mitochondria have dilated cristae, vacuoles, and electron-dense inclusions [97]. Transgenic mice deficient in Lon protease are protected against the development of chemically-induced colon and skin tumors [14]. LONP1 is upregulated in colon adenomas and in colon cancer specimens, and high LONP1 expression correlated with decreased survival in colon cancer patients [14].
4.6.3. Lon and bladder cancer
Lon expression in bladder cancer tissues is significantly higher than in noncancerous tissues. Down-regulation of Lon in bladder cancer cells significantly blocked cancer cell proliferation and increased the sensitivity of bladder cancer cells to chemotherapeutic agents by promoting apoptosis. {Granot, 2007 #244} High expression of Lon is related to the TNM (tumor, nodes, metastasis) stage, as well as histological grade of bladder cancer patients, and is an independent, negative prognostic factor for the overall survival of bladder cancer patients {Granot, 2007 #244}.
4.6.4. Lon and cervical cancer (HeLa cells)
Lon appears to play less of an essential role in the HeLa cells derived from a cervical cancer patient. In these cells, Lon depletion causes no significant alteration of mitochondrial activities and dynamics. However, the Lon depleted HeLa cells do show an increase in mitochondrial superoxide and hydrogen peroxide generation, and accumulate oxidized proteins, which also eventually results in inhibition of cytoplasmic 20 S proteasome [101].
4.6.5. Lon and leukemia
Adaptation of leukemia cell to hypoxia requires a successful transition from OX-PHOS to glycolysis. The THP-1 cells, which largely depend on OX-PHOS in mitochondria under normoxia, are able to accelerate their growth under hypoxia by changing metabolism from OX-PHOS to glycolysis . This transition is mediated by substituting COX 4 subunit (from COX 4-1 to COX 4-2) through Lon upregulation [102].
4.6.6. Lon and brain cancer (glioblastoma)
Our group showed that Lon controls metabolic adaptation to hypoxia and to starvation in glioma cells in direct response to HIF1-α activation [103]. Also, Lon induction is used by glioma cells to increase resistance to radiation and chemotherapy by direct repair of mtDNA [103].
4.6.7. Lon as a therapeutic target for cancer treatment
The wealth of data showing a crucial role for Lon in cancer growth, invasion and metastasis has also lead to the development of novel Lon inhibitors. The first published Lon inhibitors are coumarinic derivatives - small, non-peptidic molecules, with no reported ability to inhibit other proteases (such as the proteasome) and with potential to cross the blood-brain barrier - an important requirement for drugs targeted to central nervous system malignancies [104].
Triterpenoids such as 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) and its derivatives promote B-lymphoid cell apoptosis by inhibiting Lon's proteolytic activity [105]. Lon protein levels are substantially elevated in malignant lymphoma cells and Lon knockdown leads to lymphoma cell death, providing ‘proof of concept’ for the hypothesis that mitochondrial Lon can be a novel target for new anticancer drugs [105]. Similar activity of triterpenoids was found also in RKO human colon cancer cells, in HepG2 hepatocarcinoma cells and in MCF7 breast carcinoma cells [106].
Obtusilactone A and (−)-sesamin, compounds derived from Cinnamomum kotoense are potent Lon protease inhibitors, which interact with Ser855 and Lys898 residues in the active site of the Lon protease [107]. Lon is upregulated in non-small-cell lung cancer (NSCLC) cell lines, and downregulation of Lon with either Obtusilactone A or (−)-sesamin triggers caspase-3 mediated apoptosis [107].
4.7. Lon and the response to acute exposure to environmental toxins and to medications
4.7.1. Lon prevents renal cytotoxicity caused by ochratoxin A (OTA)
OTA is a naturally occurring mycotoxin and food contaminant produced by several species of Aspergillus and Penicillium. It is very commonly found in a variety of foods and represents a serious threat to human health. OTA has multiple toxicological effects, including nephrotoxicity, hepatotoxicity, genotoxicity, immunotoxicity, teratogenicity, mutagenicity and carcinogenicity (classified as a possible human carcinogen (group 2B) [108]. OTA exposure causes Lon down-regulation in kidney cells [109], and the Lon-deficient cells are more sensitive to OTA injury. Lon confers protection to OTA-induced renal cytotoxicity via 4 processes: defensing against OTA-induced oxidative stress in the mitochondria; regulating protein synthesis, modification and repair; maintaining the balance of carbohydrate metabolism; and assisting in mtDNA maintenance. [110]
4.7.2. Lon and HIV therapy (HAART) induced lipodystrophy
The combination of antiretroviral medication generally known as HAART (highly active antiretroviral therapy) has revolutionized HIV treatment, by allowing the infected patients to survive for a very long period of time. However, Nucleosidic inhibitors of the reverse transcriptase (NRTI), main components of HAART, display relevant toxicity for mitochondria and can provoke metabolic changes and body fat redistribution, resulting in lipodystrophy, a side effect significantly involving mitochondrial function. The LONP1 gene is significantly upregulated in adipose tissue from lipodystrophy patients, and correlates with the high turnover of damaged proteins caused by increases in metabolic production of oxidants. However, increased Lon expression is not sufficient to eliminate all the oxidatively damaged proteins that accumulate and contribute to the development of lipodystrophy [111].
4.8. Proposed etiology of Lon involvement in human pathology and disease
Mitochondrial proteolytic mechanisms in mammals are complex, a feature required to maintain mitochondrial homeostasis. Potentially, any imbalance in the fragile equilibrium between components encoded by the mitochondrial genome and produced in the mitochondrial matrix, and components encoded by the nuclear genome and translocated from the cell cytoplasm can cause human pathology.
Though multiple proteolytic systems are described in mammalian mitochondria [10], the Lon protease seems to be the dominant enzyme involved in maintaining appropriate mitochondrial function, protecting the mtDNA against a variety of insults and controlling mitochondrial protein stoichiometry. Lon itself is a Janus-like protein, with tightly regulated levels, and with potential harm that can result from either downregulation or overexpression. In patients where either Lon expression or Lon function is significantly dysregulated, pathology inevitably seems to occur. Lon downregulation causes increased susceptibility to toxins, and is involved with a wide array of degenerative conditions, involving multiple organs. Lon upregulation promotes malignant transformation, increases the resistance of multiple malignancies to radiation and chemotherapy, and predicts worse outcomes in cancer patients.
In this paper we have reviewed existing knowledge of both the normal functions and the associated pathologies associated with Lon in humans. We would also like to propose a novel etiological classification of human disorders involving Lon. Our classification identifies four different categories of pathology, based on the presence or absence of a genetic mutation in the LONP1 gene, Lon protein level, and Lon protease activity (see below, and also Table 2).
Table 2.
Etiological classification of Lon-related human disorders.
| Category | Disease | LONP1 Gene | Lon Expression | Lon Proteolytic Function |
|---|---|---|---|---|
| 1) Decreased Lon Expression and Loss of Function Caused by Mutations or Deleons in the Encoding Gene | Cerebral, ocular, dental, auricular, skeletal anomalies (CODAS) syndrome [65–67] | mutated | decreased | decreased |
| 2) Decreased Lon Expression and Decreased Proteolytic Activity Causing/Contributing to a Pathologic State | Early-onset Parkinson's disease [68] | normal | decreased | decreased |
| Familial ALS [74] | normal | decreased | decreased | |
| MELAS [78] | normal | decreased | decreased | |
| Muscle denervation [83] | normal | decreased | decreased | |
| Hindlimb Unloading [85] | normal | decreased | decreased | |
| Insulin resistance and Type 2 diabetes 94] | normal | decreased | decreased | |
| Ochratoxin A Toxicity [109,110] | normal | decreased | decreased | |
| 3) Lon Overexpression and Increased Proteolytic Activity Causing/Contributing to a Pathologic State | Friedreich ataxia (FRDA) [71] | normal | increased | increased |
| Brain Ischemia/Stroke [79] | normal | increased | increased | |
| HAART Induced Lipodystrophy [111] | normal | increased | increased | |
| Multiple Malignancies [99] | normal | increased | increased | |
| 4) Lon Overexpression but Decreased Lon Proteolytic Activity Causing/Contributing to a Pathologic State | MERRF [76] | normal | increased | decreased |
| Cardiac Ischemia and Heart Failure [81] | normal | increased | decreased |
4.8.1. Proposed etiological classification of Lon-related disorders
- Decreased Lon Expression and Loss of Function Caused by Mutations or Deletions in the Encoding Gene (LONP1):
- –
- Cerebral, ocular, dental, auricular, skeletal anomalies (CODAS) syndrome.
- Decreased Lon Expression and Decreased Proteolytic Activity Causing/Contributing to a Pathologic State (no genetic defect in LONP1 gene):
- –
- Early-onset, autosomal recessive form of Parkinson's disease.
- –
- Familial Amyotrophic Lateral Sclerosis (ALS).
- –
- Myopathy, encephalopathy, lactic acidosis, stroke-like episodes syndrome (MELAS).
- –
- Muscle denervation.
- –
- Hindlimb Unloading.
- –
- Insulin resistance and Type 2 diabetes.
- –
- Ochratoxin A Toxicity.
- Lon Overexpression and Increased Proteolytic Activity Causing/Contributing to a Pathologic State (no genetic defect in LONP1 gene):
- –
- Friedreich ataxia (FRDA).
- –
- Brain Ischemia/Stroke.
- –
- HAART Induced Lipodystrophy.
- –
- Multiple Malignancies.
- Lon Overexpression but Decreased Lon Proteolytic Activity Causing/Contributing to a Pathologic State (no genetic defect in LONP1 gene):
- –
- Myoclonic epilepsy and ragged-red fibers (MERRF) syndrome (increased expression but loss of proteolytic activity).
- –
- Cardiac Ischemia and Heart Failure (increased expression but loss of proteolytic activity).
5. Lon involvement in aging
Levels of Lon mRNA transcripts were found to be about four-times lower in skeletal muscles from aged mice, than in young adult control animals, but this phenomenon was prevented by caloric restriction [112]. We next found that Lon protein levels, as well as total Lon proteolytic activity, were greatly reduced in the muscles of aged normal mice, and that this phenomenon was even more severe in mice burdened by a lifetime of chronic exposure to oxidative stress, caused by an approximate 50% reduction in manganese superoxide dismutase activity (manganese SOD heterozygote mice) [37]. Interesting, exercise can effectively minimize both the age-induced Lon decline and decreased mitochondrial biogenesis in aging muscle [113]. These results may actually suggest that declining Lon transcription, translation, and enzymatic activity in aging may result from a combination of both age and disuse, at least in skeletal muscles.
Age-related decline in Lon expression and/or its proteolytic activity might also be tissue-specific [114]. For example, a measurable decrease in Lon proteolytic activity occurs in parallel with the accumulation of damaged proteins in rat liver mitochondria isolated from aged animals [115]. However, Lon protease activity remains constant in the heart mitochondrial matrix isolated from the same aged rats, and the levels of expression of the Lon protease actually increased in the older animals in comparison with the younger ones [53]. This may, again, be evidence for a strong influence of tissue metabolic activity (mitochondrial respiration?) on Lon transcription, translation, and enzymatic activity, since heart muscle (unlike skeletal muscles) must be at least minimally active throughout life.
Clearly, the question of Lon regulation in aging remains largely unanswered and significant work will be needed to identify tissue-specific patterns of expression in aging, as well as the effects of exercise and other potential metabolic effectors. Finally, it will be very important to discover the effects of interaction of age and disease on Lon transcription, translation, and enzymatic activity.
6. Conclusions
A number of human diseases have been identified whose mechanisms involve Lon dysfunction. These diseases and a proposed etiological classification are summarized in Table 2. Many of the same mechanisms that govern Lon-related pathology are also involved in the aging process, and in diminished resistance to multiple stressors including oxidative stress. Extensive further study of human diseases involving mitochondrial Lon, including mechanistic studies of enzymatic function and elucidation of substrate recognition, is required in light of the number and the variety of severe mitochondrial conditions of unknown etiology. New therapies focused on Lon inhibition as a potential target for cancer therapies are currently underway. However, their development has to be approached with careful attention to possible severe central nervous system and cardiac toxicities – that are potentially associated with chronic Lon down-regulation. Finally, the interaction of Lon regulation in aging, with the effects of many age-related pathologies will be a significant challenge for researchers in the field to tackle.
Acknowledgements
KJAD was supported by grant # ES 003598 from the National Institute of Environmental Health Sciences of the US National Institutes of Health.
DAB was supported by the National Institute for Neurological Diseases and Stroke Award number NS072234, by the UCI Cancer Center Award Number P30CA062203 from the National Cancer Institute.
Abbreviations
- ALAS-1
5-Aminolevulinic acid synthase
- ALS
Amyotrophic Lateral Sclerosis
- CDDO
2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid
- CODAS
Cerebral, ocular, Dental, Auricular, Skeletal Anomalies Syndrome
- EGF
Epidermal Growth Factor
- ERK
Extracellular signal-regulated protein kinase
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FRDA
Friedreich ataxia
- GRP78
78 kDa glucose-regulated protein
- HAART
highly active antiretroviral therapy
- HIF-1
Hypoxia-inducible factor 1
- HU
Hindlimb unloading
- MELAS
Myopathy, Encephalopathy, Lactic Acidosis, Stroke-like Episodes Syndrome
- MERRF
Myoclonic Epilepsy and Ragged-red Fibers Syndrome
- mtSSB
mitochondrial single-strand binding protein
- NDUFS8
NADH:ubiquinone oxidoreductase core subunit S8
- NRF-2
Nuclear Respiratory Factor 2
- NRTI
Nucleosidic inhibitors of the reverse transcriptase
- NSCLC
non-small-cell lung cancer
- OPTM
Oxidative post-translational modifications
- OTA
Ochratoxin A
- OX-PHOS
Oxidative phosphorylation
- PI3K
Phosphoinositide 3-kinase
- PINK1
PTEN-induced kinase 1
- POLG
polymerase gamma
- POLRMT
mtRNA polymerase
- PERK
ER-resident protein kinase
- SOD
superoxide dismutase
- StAR
Steroidogenic acute regulatory protein
- TAC
transverse aortic constriction
- TFAM
mitochondrial transcription factor A
- TNM
tumor, nodes, metastasis stage
- UCP
Uncoupling proteins
References
- 1.Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miwa S, Lawless C, von Zglinicki T. Mitochondrial turnover in liver is fast in vivo and is accelerated by dietary restriction: application of a simple dynamic model. Aging Cell. 2008;7:920–923. doi: 10.1111/j.1474-9726.2008.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwerdt G, Huth W. Turnover and transformation of mitochondrial acetyl-CoA acetyltransferase into CoA-modified forms. Biochem. J. 1993;292:915–919. doi: 10.1042/bj2920915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzu V, Mookerjee Shona A, Brand Martin D. Rapid turnover of mitochondrial uncoupling protein 3. Biochem. J. 2010;426:13–17. doi: 10.1042/BJ20091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousset S, Mozo J, Dujardin G, et al. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett. 2007;581:479–482. doi: 10.1016/j.febslet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Moazed B, Desautels M. Differentiation-dependent expression of cathepsin D and importance of lysosomal proteolysis in the degradation of UCP1 in brown adipocytes. Can. J. Physiol. Pharmacol. 2002;80:515–525. doi: 10.1139/y02-067. [DOI] [PubMed] [Google Scholar]
- 7.Rego AC, Oliveira CR. Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem. Res. 2003;28:1563–1574. doi: 10.1023/a:1025682611389. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CY, Lee CF, Wei YH. Role of reactive oxygen species-elicited apoptosis in the pathophysiology of mitochondrial and neurodegenerative diseases associated with mitochondrial DNA mutations. J. Formos. Med. Assoc. 2009;108:599–611. doi: 10.1016/s0929-6646(09)60380-6. [DOI] [PubMed] [Google Scholar]
- 10.Bota DA, Davies KJ. Protein degradation in mitochondria: implications for oxidative stress, aging and disease: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion. 2001;1:33–49. doi: 10.1016/s1567-7249(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 11.Bender T, Lewrenz I, Franken S, Baitzel C, Voos W. Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim1/LON protease. Mol. Biol. Cell. 2011;22:541–554. doi: 10.1091/mbc.E10-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung CH, Goldberg AL. The product of the lon (capR) gene in Escherichia coli is the ATP-dependent protease, protease La. Proc. Natl. Acad. Sci. USA. 1981;78:4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki CK, Suda K, Wang N, Schatz G. Requirement for the yeast gene LON in intramitochondrial proteolysis and maintenance of respiration. Science. 1994;264:273–276. doi: 10.1126/science.8146662. [DOI] [PubMed] [Google Scholar]
- 14.Quiros PM, Espanol Y, Acin-Perez R, et al. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep. 2014;8:542–556. doi: 10.1016/j.celrep.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Ngo JK, Pomatto LC, Davies KJ. Upregulation of the mitochondrial Lon Protease allows adaptation to acute oxidative stress but dysregulation is associated with chronic stress, disease, and aging. Redox Biol. 2013;1:258–264. doi: 10.1016/j.redox.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watabe S, Hara T, Kohno H, Hiroi T, Yago N, Nakazawa T. In vitro degradation of mitochondrial proteins by ATP-dependent protease in bovine adrenal cortex. J. Biochem. 1993;113:672–676. doi: 10.1093/oxfordjournals.jbchem.a124101. [DOI] [PubMed] [Google Scholar]
- 17.Watabe S, Kimura T. ATP-dependent protease in bovine adrenal cortex. Tissue specificity, subcellular localization, and partial characterization. J. Biol. Chem. 1985;260:5511–5517. [PubMed] [Google Scholar]
- 18.Yamamoto M, Hiroi T, Kohno H, et al. Nucleotide sequence for cDNA of bovine mitochondrial ATP-dependent protease and determination of N-terminus of the mature enzyme from the adrenal cortex. DNA Seq. 2005;16:474–478. doi: 10.1080/10425170500289233. [DOI] [PubMed] [Google Scholar]
- 19.Desautels M, Goldberg AL. Demonstration of an ATP-dependent, vanadate-sensitive endoprotease in the matrix of rat liver mitochondria. J. Biol. Chem. 1982;257:11673–11679. [PubMed] [Google Scholar]
- 20.Desautels M, Goldberg AL. Liver mitochondria contain an ATP-dependent, vanadate-sensitive pathway for the degradation of proteins. Proc. Natl. Acad. Sci. USA. 1982;79:1869–1873. doi: 10.1073/pnas.79.6.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang N, Gottesman S, Willingham MC, Gottesman MM, Maurizi MR. A human mitochondrial ATP-dependent protease that is highly homologous to bacterial Lon protease. Proc. Natl. Acad. Sci. USA. 1993;90:11247–11251. doi: 10.1073/pnas.90.23.11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii A, Watabe S, Hamada H. Atp-dependent protease in human placenta. Placenta. 1992;13:343–347. doi: 10.1016/0143-4004(92)90058-2. [DOI] [PubMed] [Google Scholar]
- 23.Desautels M, Goldberg AL. Demonstration of an ATP-dependent, vanadate-sensitive endoprotease in the matrix of rat liver mitochondria. J. Biol. Chem. 1982;257:11673–11679. [PubMed] [Google Scholar]
- 24.Watabe S, Kimura T. ATP-dependent protease in bovine adrenal cortex. Tissue specificity, subcellular localization, and partial characterization. J. Biol. Chem. 1985;260:5511–5517. [PubMed] [Google Scholar]
- 25.Watabe S, Kimura T. Adrenal cortex mitochondrial enzyme with ATP-dependent protease and protein-dependent ATPase activities. Purification and properties. J. Biol. Chem. 1985;260:14498–14504. [PubMed] [Google Scholar]
- 26.Wang N, Maurizi MR, Emmert-Buck L, Gottesman MM. Synthesis, processing, and localization of human Lon protease. J. Biol. Chem. 1994;269:29308–29313. [PubMed] [Google Scholar]
- 27.Pomatto LC, Raynes R, Davies KJ. The peroxisomal Lon protease LonP2 in aging and disease: functions and comparisons with mitochondrial Lon protease LonP1. Biol. Rev. Camb. Philos. Soc. 2016 doi: 10.1111/brv.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 29.Cha SS, An YJ, Lee CR, et al. Crystal structure of Lon protease: molecular architecture of gated entry to a sequestered degradation chamber. EMBO J. 2010;29:3520–3530. doi: 10.1038/emboj.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vieux EF, Wohlever ML, Chen JZ, Sauer RT, Baker TA. Distinct quaternary structures of the AAA+ Lon protease control substrate degradation. Proc. Natl. Acad. Sci. USA. 2013;110:E2002–E2008. doi: 10.1073/pnas.1307066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-Nafría J, Ondrovičová G, Blagova E, et al. Structure of the catalytic domain of the human mitochondrial Lon protease: proposed relation of oligomer formation and activity. Protein Sci. 2010;19:987–999. doi: 10.1002/pro.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amerik A, Petukhova GV, Grigorenko VG, et al. Cloning and sequence analysis of cDNA for a human homolog of eubacterial ATP-dependent Lon proteases. FEBS Lett. 1994;340:25–28. doi: 10.1016/0014-5793(94)80166-5. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesh S, Lee J, Singh K, Lee I, Suzuki CK. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim. Biophys. Acta - Mol. Cell Res. 2012;1823:56–66. doi: 10.1016/j.bbamcr.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngo JK, Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic. Biol. Med. 2009;46:1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanyer L, Jorgensen W, Hori O, Clark JB, Heales SJR. Inactivation of brain mitochondrial Lon protease by peroxynitrite precedes electron transport chain dysfunction. Neurochem. Int. 2008;53:95–101. doi: 10.1016/j.neuint.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Pinti M, Gibellini L, De Biasi S, et al. Functional characterization of the promoter of the human Lon protease gene. Mitochondrion. 2011;11:200–206. doi: 10.1016/j.mito.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Bota DA, Van Remmen H, Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Davies KJ, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim. Biophys. Acta. 2002;1576:1–14. doi: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 40.Cheng CW, Kuo CY, Fan CC, et al. Overexpression of Lon contributes to survival and aggressive phenotype of cancer cells through mitochondrial complex I-mediated generation of reactive oxygen species. Cell Death Dis. 2013;4:e681. doi: 10.1038/cddis.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 42.Fukuda R, Zhang H, Kim J-w L, Shimoda CV, Dang L. Semenza Gregg, HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y, Wang M, Lin H, Huang C, Shi X, Luo J. Epidermal growth factor up-regulates the transcription of mouse lon homology ATP-dependent protease through extracellular signal-regulated protein kinase- and phosphatidylinositol-3-kinase-dependent pathways. Exp. Cell Res. 2002;280:97–106. doi: 10.1006/excr.2002.5621. [DOI] [PubMed] [Google Scholar]
- 44.Gibellini L, Pinti M, Beretti F, et al. Sirtuin 3 interacts with Lon protease and regulates its acetylation status. Mitochondrion. 2014;18:76–81. doi: 10.1016/j.mito.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat. Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 46.Ondrovičová G, Liu T, Singh K, et al. Cleavage site selection within a folded substrate by the ATP-dependent Lon protease. J. Biol. Chem. 2005;280:25103–25110. doi: 10.1074/jbc.M502796200. [DOI] [PubMed] [Google Scholar]
- 47.Ngo JK, Pomatto LC, Bota DA, Koop AL, Davies KJ. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:1178–1185. doi: 10.1093/gerona/glr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bota DA, Ngo JK, Davies KJ. Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic. Biol. Med. 2005;38:665–677. doi: 10.1016/j.freeradbiomed.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Levine RL. Bota DA, Ngo JK, Davies KJA, editors. Commentary on “Downregulation of the human Lon protease impairs mitochondrial structure and function and causes cell death. Free Radic. Biol. Med. 2005;38:1445–1446. doi: 10.1016/j.freeradbiomed.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 50.Teichmann U, van Dyck L, Guiard B, et al. Substitution of PIM1 protease in mitochondria by Escherichia coli Lon protease. J. Biol. Chem. 1996;271:10137–10142. doi: 10.1074/jbc.271.17.10137. [DOI] [PubMed] [Google Scholar]
- 51.Watabe S, Kohno H, Kouyama H, Hiroi T, Yago N, Nakazawa T. Purification and characterization of a substrate protein for mitochondrial ATP-dependent protease in bovine adrenal cortex. J. Biochem. 1994;115:648–654. doi: 10.1093/oxfordjournals.jbchem.a124390. [DOI] [PubMed] [Google Scholar]
- 52.Watabe S, Hiroi T, Yamamoto Y, et al. SP-22 is a thioredoxin-dependent peroxide reductase in mitochondria. Eur. J. Biochem. 1997;249:52–60. doi: 10.1111/j.1432-1033.1997.t01-1-00052.x. [DOI] [PubMed] [Google Scholar]
- 53.Delaval E, Perichon M, Friguet B. Age-related impairment of mitochondrial matrix aconitase and ATP-stimulated protease in rat liver and heart. Eur. J. Biochem. 2004;271:4559–4564. doi: 10.1111/j.1432-1033.2004.04422.x. [DOI] [PubMed] [Google Scholar]
- 54.Lushchak OV, Piroddi M, Galli F, Lushchak VI. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. 2014;19:8–15. doi: 10.1179/1351000213Y.0000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yan LJ, Levine RL, Sohal RS. Oxidative damage during aging targets mitochondrial aconitase. Proc. Natl. Acad. Sci. USA. 1997;94:11168–11172. doi: 10.1073/pnas.94.21.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsushima Y, Goto Y-I, Kaguni LS. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM) Proc. Natl. Acad. Sci. USA. 2010;107:18410–18415. doi: 10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekstrand MI, Falkenberg M, Rantanen A, et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 58.Lu B, Lee J, Nie X, et al. Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA+ lon protease. Mol. Cell. 2013;49:121–132. doi: 10.1016/j.molcel.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kao TY, Chiu YC, Fang WC, et al. Mitochondrial Lon regulates apoptosis through the association with Hsp60-mtHsp70 complex. Cell Death Dis. 2015;6:e1642. doi: 10.1038/cddis.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luciakova K, Sokolikova B, Chloupkova M, Nelson BD. Enhanced mitochondrial biogenesis is associated with increased expression of the mitochondrial ATP-dependent Lon protease. FEBS Lett. 1999;444:186–188. doi: 10.1016/s0014-5793(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 61.Fu GK, Markovitz DM. The human LON protease binds to mitochondrial promoters in a single-stranded, site-specific, strand-specific manner. Biochemistry. 1998;37:1905–1909. doi: 10.1021/bi970928c. [DOI] [PubMed] [Google Scholar]
- 62.Lu B, Liu T, Crosby JA, Thomas-Wohlever J, Lee I, Suzuki CK. The ATP-dependent Lon protease of Mus musculus is a DNA-binding protein that is functionally conserved between yeast and mammals. Gene. 2003;306:45–55. doi: 10.1016/s0378-1119(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 63.Chen S-H, Suzuki CK, Wu S-H. Thermodynamic characterization of specific interactions between the human Lon protease and G-quartet DNA. Nucleic Acids Res. 2008;36:1273–1287. doi: 10.1093/nar/gkm1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu B, Yadav S, Shah PG, et al. Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J. Biol. Chem. 2007;282:17363–17374. doi: 10.1074/jbc.M611540200. [DOI] [PubMed] [Google Scholar]
- 65.Shebib SM, Reed MH, Shuckett EP, Cross HG, Perry JB, Chudley AE. Newly recognized syndrome of cerebral, ocular, dental, auricular, skeletal anomalies: CODAS syndrome–a case report. Am. J. Med. Genet. 1991;40:88–93. doi: 10.1002/ajmg.1320400118. [DOI] [PubMed] [Google Scholar]
- 66.Dikoglu E, Alfaiz A, Gorna M, et al. Mutations in LONP1, a mitochondrial matrix protease, cause CODAS syndrome. Am. J. Med. Genet. A. 2015;167:1501–1509. doi: 10.1002/ajmg.a.37029. [DOI] [PubMed] [Google Scholar]
- 67.Strauss KA, Jinks RN, Puffenberger EG, et al. CODAS syndrome is associated with mutations of LONP1, encoding mitochondrial AAA+ Lon protease. Am. J. Hum. Genet. 2015;96:121–135. doi: 10.1016/j.ajhg.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas RE, Andrews LA, Burman JL, Lin W-Y, Pallanck LJ. PINK1-parkin pathway activity is regulated by degradation of PINK1 in the mitochondrial matrix. PLoS Genet. 2014;10:e1004279. doi: 10.1371/journal.pgen.1004279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koeppen AH. Friedreich's ataxia: pathology, pathogenesis, and molecular genetics. J. Neurol. Sci. 2011;303:1–12. doi: 10.1016/j.jns.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rotig A, de Lonlay P, Chretien D, et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat. Genet. 1997;17:215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- 71.Guillon B, Bulteau AL, Wattenhofer-Donze M, et al. Frataxin deficiency causes upregulation of mitochondrial Lon and ClpP proteases and severe loss of mitochondrial Fe-S proteins. FEBS J. 2009;276:1036–1047. doi: 10.1111/j.1742-4658.2008.06847.x. [DOI] [PubMed] [Google Scholar]
- 72.Julien JP. Amyotrophic lateral sclerosis. unfolding the toxicity of the misfolded. Cell. 2001;104:581–591. doi: 10.1016/s0092-8674(01)00244-6. [DOI] [PubMed] [Google Scholar]
- 73.Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 74.Fukada K, Zhang F, Vien A, Cashman NR, Zhu H. Mitochondrial proteomic analysis of a cell line model of familial amyotrophic lateral sclerosis. Mol. Cell. Proteom. 2004;3:1211–1223. doi: 10.1074/mcp.M400094-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Area-Gomez E, Schon EA. Mitochondrial genetics and disease. J. Child Neurol. 2014;29:1208–1215. doi: 10.1177/0883073814539561. [DOI] [PubMed] [Google Scholar]
- 76.Wu S-B, Ma Y-S, Wu Y-T, Chen Y-C, Wei Y-H, Mitochondrial DNA. Mutation-elicited oxidative stress, oxidative damage, and altered gene expression in cultured cells of patients with MERRF syndrome. Mol. Neurobiol. 2010;41:256–266. doi: 10.1007/s12035-010-8123-7. [DOI] [PubMed] [Google Scholar]
- 77.Wallace DC. Diseases of the mitochondrial DNA. Annu. Rev. Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- 78.Felk S, Ohrt S, Kussmaul L, Storch A, Gillardon F. Activation of the mitochondrial protein quality control system and actin cytoskeletal alterations in cells harbouring the MELAS mitochondrial DNA mutation. J. Neurol. Sci. 2010;295:46–52. doi: 10.1016/j.jns.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 79.Hori O, Ichinoda F, Tamatani T, et al. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J. Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santos CX, Anilkumar N, Zhang M, Brewer AC, Shah AM. Redox signaling in cardiac myocytes. Free Radic. Biol. Med. 2011;50:777–793. doi: 10.1016/j.freeradbiomed.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuo C-Y, Chiu Y-C, Lee AY-L, Hwang T-L. Mitochondrial Lon protease controls ROS-dependent apoptosis in cardiomyocyte under hypoxia. Mitochondrion. 2015;23:7–16. doi: 10.1016/j.mito.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Hoshino A, Okawa Y, Ariyoshi M, et al. Oxidative post-translational modifications develop LONP1 dysfunction in pressure overload heart failure. Circ.: Heart Fail. 2014;7:500–509. doi: 10.1161/CIRCHEARTFAILURE.113.001062. [DOI] [PubMed] [Google Scholar]
- 83.Wagatsuma A, Kotake N, Mabuchi K, Yamada S. Expression of nuclear-encoded genes involved in mitochondrial biogenesis and dynamics in experimentally denervated muscle. J. Physiol. Biochem. 2011;67:359–370. doi: 10.1007/s13105-011-0083-5. [DOI] [PubMed] [Google Scholar]
- 84.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J. Appl. Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. 1985. [DOI] [PubMed] [Google Scholar]
- 85.Wagatsuma A, Kotake N, Kawachi T, Shiozuka M, Yamada S, Matsuda R. Mitochondrial adaptations in skeletal muscle to hindlimb unloading. Mol. Cell. Biochem. 2011;350:1–11. doi: 10.1007/s11010-010-0677-1. [DOI] [PubMed] [Google Scholar]
- 86.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. 1985. [DOI] [PubMed] [Google Scholar]
- 87.Wagatsuma A, Kotake N, Yamada S. Muscle regeneration occurs to coincide with mitochondrial biogenesis. Mol. Cell. Biochem. 2011;349:139–147. doi: 10.1007/s11010-010-0668-2. [DOI] [PubMed] [Google Scholar]
- 88.Sher N, Yivgi-Ohana N, Orly J. Transcriptional regulation of the cholesterol side chain cleavage cytochrome P450 gene (CYP11A1) revisited: binding of GATA, cyclic adenosine 3′,5′-monophosphate response element-binding protein and activating protein (AP)-1 proteins to a distal novel cluster of cis-regulatory elements potentiates AP-2 and steroidogenic factor-1-dependent gene expression in the rodent placenta and ovary. Mol. Endocrinol. 2007;21:948–962. doi: 10.1210/me.2006-0226. [DOI] [PubMed] [Google Scholar]
- 89.Bahat A, Perlberg S, Melamed-Book N, et al. Transcriptional activation of LON Gene by a new form of mitochondrial stress: a role for the nuclear respiratory factor 2 in StAR overload response (SOR) Mol. Cell. Endocrinol. 2015;408:62–72. doi: 10.1016/j.mce.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 90.Bahat A, Perlberg S, Melamed-Book N, Lauria I, Langer T, Orly J. StAR enhances transcription of genes encoding the mitochondrial proteases involved in its own degradation. Mol. Endocrinol. 2014;28:208–224. doi: 10.1210/me.2013-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Podvinec M, Handschin C, Looser R, Meyer UA. Identification of the xenosensors regulating human 5-aminolevulinate synthase. Proc. Natl. Acad. Sci. USA. 2004;101:9127–9132. doi: 10.1073/pnas.0401845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tian Q, Li T, Hou W, Zheng J, Schrum LW, Bonkovsky HL. Lon peptidase 1 (LONP1)-dependent breakdown of mitochondrial 5-aminolevulinic acid synthase protein by heme in human liver cells. J. Biol. Chem. 2011;286:26424–26430. doi: 10.1074/jbc.M110.215772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 94.Lee HJ, Chung K, Lee H, Lee K, Lim JH, Song J. Downregulation of mitochondrial lon protease impairs mitochondrial function and causes hepatic insulin resistance in human liver SK-HEP-1 cells. Diabetologia. 2011;54:1437–1446. doi: 10.1007/s00125-011-2074-z. [DOI] [PubMed] [Google Scholar]
- 95.Kato K, Ogura T, Kishimoto A, et al. Critical roles of AMP-activated protein kinase in constitutive tolerance of cancer cells to nutrient deprivation and tumor formation. Oncogene. 2002;21:6082–6090. doi: 10.1038/sj.onc.1205737. [DOI] [PubMed] [Google Scholar]
- 96.Bayley JP, Devilee P. Warburg tumours and the mechanisms of mitochondrial tumour suppressor genes. Barking up the right tree? Curr. Opin. Genet. Dev. 20:324–329. doi: 10.1016/j.gde.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 97.Gibellini L, Pinti M, Boraldi F, et al. Silencing of mitochondrial Lon protease deeply impairs mitochondrial proteome and function in colon cancer cells. FASEB J. 2014;28:5122–5135. doi: 10.1096/fj.14-255869. [DOI] [PubMed] [Google Scholar]
- 98.Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J. Bioenerg. Biomembr. 2007;39:251–257. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- 99.Bulteau AL, Bayot A. Mitochondrial proteases and cancer. Biochim. Biophys. Acta. 2011;1807:595–601. doi: 10.1016/j.bbabio.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 100.Lau E, et al. Substrate- and isoform-specific proteome stability in normal and stressed cardiac mitochondria. Circ. Res. 2011;110:1174–1178. doi: 10.1161/CIRCRESAHA.112.268359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bayot A, Gareil M, Chavatte L, et al. Effect of Lon protease knockdown on mitochondrial function in HeLa cells. Biochimie. 2014;100:38–47. doi: 10.1016/j.biochi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Goto M, Miwa H, Suganuma K, et al. Adaptation of leukemia cells to hypoxic condition through switching the energy metabolism or avoiding the oxidative stress. BMC Cancer. 2014;14:1–9. doi: 10.1186/1471-2407-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gong X, Vuong Y, Bota DA. Mitochondrial LON is the first identified mitochondrial protein to mediate hypoxic adaptation, invasion, and treatment resistance to radiation and temozolomide in malignant glioma cell lines. Neuro-Oncology. 2010;12:iv7–iv25. [Google Scholar]
- 104.Bayot A, Basse N, Lee I, et al. Towards the control of intracellular protein turnover: mitochondrial Lon protease inhibitors versus proteasome inhibitors. Biochimie. 2008;90:260–269. doi: 10.1016/j.biochi.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 105.Bernstein SH, Venkatesh S, Li M, et al. The mitochondrial ATP-dependent Lon protease: a novel target in lymphoma death mediated by the synthetic triterpenoid CDDO and its derivatives. Blood. 2012;119:3321–3329. doi: 10.1182/blood-2011-02-340075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gibellini L, Pinti M, Bartolomeo R, et al. Inhibition of Lon protease by triterpenoids alters mitochondria and is associated to cell death in human cancer cells. Oncotarget. 2015;6:25466–25483. doi: 10.18632/oncotarget.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang H-M, Cheng K-C, Lin C-J, et al. Obtusilactone A and (−)-sesamin induce apoptosis in human lung cancer cells by inhibiting mitochondrial Lon protease and activating DNA damage checkpoints. Cancer Sci. 2010;101:2612–2620. doi: 10.1111/j.1349-7006.2010.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pfohl-Leszkowicz A, Manderville RA, Ochratoxin A. An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 109.Shen XL, Zhang Y, Xu W, et al. An iTRAQ-based mitoproteomics approach for profiling the nephrotoxicity mechanisms of ochratoxin A in HEK 293 cells. J. Proteom. 2013;78:398–415. doi: 10.1016/j.jprot.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 110.Zhang B, Shen XL, Liang R, et al. Protective role of the mitochondrial Lon protease 1 in ochratoxin A-induced cytotoxicity in HEK293 cells. J. Proteom. 2014;101:154–168. doi: 10.1016/j.jprot.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 111.Pinti M, Gibellini L, Guaraldi G, et al. Upregulation of nuclear-encoded mitochondrial LON protease in HAART-treated HIV-positive patients with lipodystrophy: implications for the pathogenesis of the disease. AIDS. 2010;24:841–850. doi: 10.1097/QAD.0b013e32833779a3. [DOI] [PubMed] [Google Scholar]
- 112.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 113.Koltai E, Hart N, Taylor AW, et al. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R127–R134. doi: 10.1152/ajpregu.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ngo JK, Davies KJ. Importance of the lon protease in mitochondrial maintenance and the significance of declining lon in aging. Ann. N.Y. Acad. Sci. 2007;1119:78–87. doi: 10.1196/annals.1404.015. [DOI] [PubMed] [Google Scholar]
- 115.Bakala H, Delaval E, Hamelin M, et al. Changes in rat liver mitochondria with aging. Eur. J. Biochem. 2003;270:2295–2302. doi: 10.1046/j.1432-1033.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- 116.Quiros PM, et al. ATP-dependent Lon protease controls tumor bioenergetics by reprogramming mitochondrial activity. Cell Rep. 2014;8(2):542–556. doi: 10.1016/j.celrep.2014.06.018. 24. [DOI] [PubMed] [Google Scholar]
- 117.Zhao Q, et al. A Mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21(17):4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Granot Z, et al. Turnover of mitochondrial steroidogenic acute regulatory (StAR) protein by lon protease: The unexpected effect of proteasome inhibitors. Molecular Endocrinol. 2007;21:2164–2177. doi: 10.1210/me.2005-0458. [DOI] [PubMed] [Google Scholar]