Summary
Lck is a T cell lineage-specific tyrosine kinase critical for T cell development and activation through its role in pre-TCR and TCR signaling. Expression of identical Lck protein can be mediated by either proximal or distal lck promoter, but the function of these promoters in T cell development has not been assessed. We generated BAC transgenic mice in which either BAC lck promoter was deleted and bred these transgenes to an Lck knockout background. Lck-PROX mice, in which only the proximal promoter is functional, had maximal Lck protein and normal thymic development through CD4-CD8- double negative (DN) and CD4+CD8+ double positive (DP) stages, but undetectable Lck later in development and reduced mature single positive thymocytes. In contrast, Lck-DIST mice, in which only distal promoter was functional, were deficient in Lck protein in DN and DP thymocytes and severely defective in early T cell development, with a block at the DN3-DN4 beta checkpoint equivalent to complete Lck knockouts. The ability of the proximal lck promoter to support thymic development was independent of Fyn; while, in contrast, the distal lck promoter alone was completely unable to support development in the absence of Fyn. Notably, normal thymocyte development was restored by presence of both proximal and distal promoters, even when independently expressed on different lck genes. These results define distinct and complementary requirements for proximal and distal lck promoters during T cell development.
Keywords: T cell, thymus, lck, promoter, development
Introduction
Approximately 50% of mouse and human genes are estimated to have two or more promoters, but little is known about the functional role of alternative promoter usage in regulating expression of a single protein product during differentiation (1, 2). The protein tyrosine kinase lck gene, which is specifically expressed in T cells and is critical for T cell development, has two promoters (3), the proximal and distal promoters separated by approximately 15 kb (4). Early studies in both human and mouse concluded that proximal promoter transcripts are present in thymus but not peripheral T cells, while distal promoter transcripts are present in both thymus and peripheral T cells (5). Using reporter transgenic mice, about 600bp of DNA in the region of the proximal promoter was initially identified to be necessary and sufficient for T cell specific expression of lck (6). Studies of transgenic reporter mice expressing GFP under control of this lck proximal promoter sequence suggested that the proximal promoter is able to drive lck expression in all developmental stages of T cells, in DN, DP, and single positive (SP) thymocytes, and in mature peripheral T cells (7). A reporter transgene under control of a 3 kb fragment of the distal promoter demonstrated reporter expression that correlated with endogenous CD3ε, which is undetectable in the most immature thymocytes, low to intermediate during thymocyte development, and high in mature thymocytes and peripheral T cells (8). However, no studies have directly assessed the functional requirements for each of the lck promoters during T cell development. It was therefore of interest, both in understanding Lck regulation during T cell development and in informing our general understanding of the role of multiple promoters for individual genes, to carry out such an analysis.
In the present study, we used genetic manipulation to generate lck promoter-deleted mice which were utilized to study the physiological functions of each lck promoter. We found that both proximal and distal promoters are essential for normal T development, and that the two promoters have distinct functional roles. Only the proximal lck promoter contributes to early DN stage development, while the distal lck promoter provides partial rescue of later stage DP and SP thymocytes, a pattern distinct from that seen with proximal promoter reconstitution. The presence of both proximal and distal promoters, even when expressed on different lck genes, was necessary and sufficient for normal thymocyte development.
Results
Developmental phenotypes resulting from deletion of individual lck promoters
The lck gene is expressed specifically in T cells and is controlled by two promoters, the proximal and distal promoters (5). When measured by RT-PCR in fractionated wild type thymocytes, expression from the lck proximal promoter was detected in DN thymocytes and reached maximum levels in DP cells, followed by significant reduction in thymic SP and splenic T cells; conversely, expression from the distal promoter was low in DN cells, and increased significantly between DP and SP stages of development. Little or no expression from either proximal or distal promoter was detected in splenic B cells (Fig 1A). Lck protein was expressed at all stages during T cell development in wild-type mice, with comparable levels in thymic DN, thymic DP, thymic CD4 SP, thymic CD8 SP, splenic CD4 and splenic CD8 populations as normalized to actin controls, while no Lck protein was detected in B cells (Fig. 1B).
Figure 1.
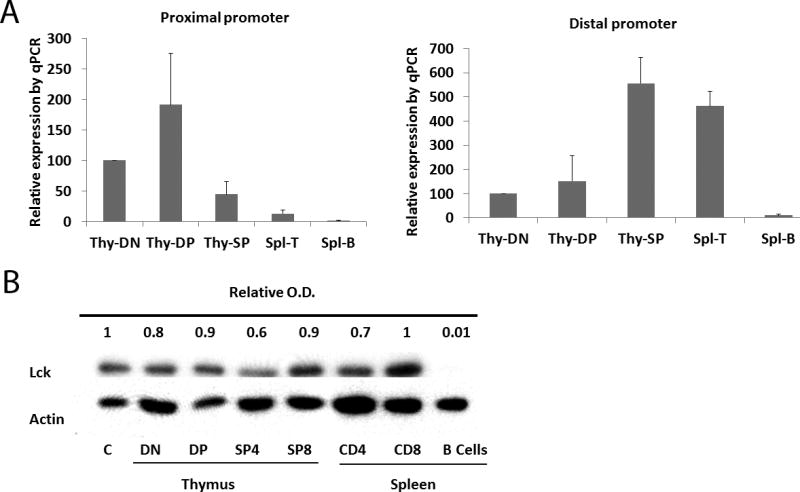
Expression of proximal and distal lck promoters. A) Lck mRNA expression is regulated by its proximal and distal promoters during T cell development. DN (Thy-DN), DP (Thy-DP) and SP (Thy-SP) cells were purified from wild-type mouse thymus; peripheral T cells (Spl-T) and B cells (Spl-B) were from wild-type mouse spleen. The purified cells were used for isolation of total RNA which was used for reverse transcription and real-time PCR. Results are representative of 3 independent experiments. B) Lck protein expressed during T cell development. DN, DP, CD4 SP, and CD8 SP cells were purified from wild-type mouse thymus; peripheral CD4 cells, CD8, and B cells were from wild-type mouse spleen. Lysates from purified cells were used for Western blot analysis. C represents a reference control lysate prepared from total thymocytes. Results are representative of 3 independent experiments.
The functional role of the two lck promoters has not been directly assessed. To directly address this question, we mutated either the proximal or distal lck promoter and observed effects on T cell development and function. The distal promoter including non-coding exon 1.1 was deleted in mouse lck bacterial artificial chromosome (BAC) DNA which was then used to make transgenic mice (Fig. 2A). These BAC transgenic mice were bred to the lck knockout (9) background to generate Lck-PROX mice, in which only the lck proximal promoter is active. By a similar strategy, we generated Lck-DIST mice that express lck only from the distal promoter. Two independent lines each of Lck-PROX and Lck-DIST mice were bred and analyzed, and replicate founders gave equivalent results.
Figure 2.
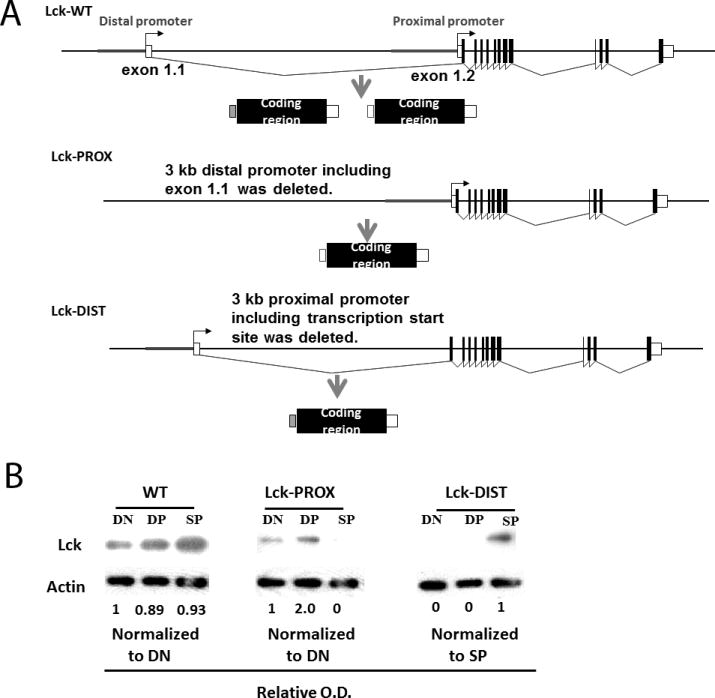
Generation of lck BAC transgenic mice. A) Strategy for generation of lck BAC transgenic mice. B) DN, DP and SP thymocytes were sorted from WT, Lck-PROX and Lck-DIST mouse thymus by flow cytometry, protein lysates prepared and used for Western blot analysis. Results are representative of 3 independent experiments.
In wild-type mice, Lck protein was found in DN, DP, and SP thymocytes at approximately equal levels (Fig. 1B and Fig. 2B). In BAC transgenic mice expressing only the proximal or distal lck promoter, total Lck protein was lower than in wild type; and notably the pattern of Lck protein expression during development differed markedly between mice expressing either proximal or distal promoter. In Lck-PROX mice, in which only the proximal lck promoter was intact, Lck protein was maximally expressed in DN and DP cells and was much reduced or undetectable in SP cells. In contrast, in Lck-DIST mice, Lck protein was abundant in SP cells, while little or no Lck protein was detected in DN and DP cells (Fig. 2B). It was not possible to assess promoter-specific lck mRNA expression in Lck-PROX and Lck-DIST mice due to expression of endogenous lck mRNA in lck knockout mice which do not express Lck protein (10). Inactivation of proximal or distal lck promoter thus resulted in distinct developmental stage-specific reductions in Lck protein, demonstrating the differential importance of the proximal promoter for Lck protein expression early in T cell development and of the distal promoter for Lck expression in later development. We therefore next assessed the effect of promoter inactivation on thymic T cell development.
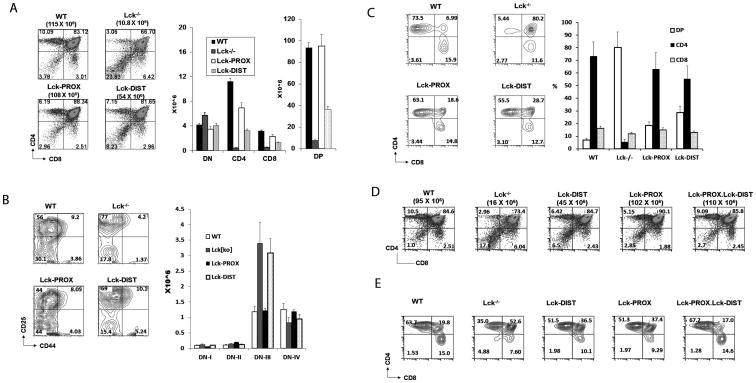
The total thymic cellularity, as well as proportions and numbers of SP and DP cells, was dramatically reduced in lck-/- mice (Fig. 3A) consistent with previous reports (9). In addition, development of DN cells was impaired, with normal total numbers of DN cells, but a developmental arrest reflected in increased DN3 and decreased DN4 numbers (Fig. 3B). Staining of thymocytes for surface expression of TCRβ revealed that TCRβ (H57)hi thymocytes contained a dramatically increased proportion of DP cells in lck-/- thymus, indicating a failure of TCRβ (H57)hi DP thymocytes to mature to SP cells, a positive selection event that is dependent upon signaling through the mature TCRαβ receptor (Fig. 3C).
Figure 3.
Effect of lck promoter deletion on thymocytes development. A) Thymocytes from WT, lck-/-, Lck-PROX and Lck-DIST mice were stained with anti-CD4 and anti-CD8, and assessed by flow cytometry. Histograms represent mean +/- SE number of thymocytes of each thymic subpopulation. Statistical analysis is presented in Supporting Figure 1. B) Thymocytes from WT, lck-/-, Lck-PROX and Lck-DIST mice were stained with anti-CD25 and anti-CD44, gated to exclude cells expressing CD4, CD8, NK1.1, Mac-1, Gr1, CD3, or B220, and assessed by flow cytometry. Histograms represent number of thymocytes of each thymic subpopulation. Statistical analysis is presented in Supporting Figure 1. C) Thymocytes from WT, lck-/-, Lck-PROX and Lck-DIST mice were gated on TCR(H57)hi cells and analyzed for CD4 and CD8 expression to identify CD4, CD8, and DP cells (Gating strategy presented in Supporting Fig. 6). Histograms represent number of thymocytes of each thymic subpopulation as mean +/- SE from 3 independent experiments, in each of which single age-matched mice of each genotype were tested. D) Thymocytes from WT, lck-/-, Lck-PROX, Lck-DIST, and double transgenic Lck-PROX.Lck-DIST mice were stained with anti-CD4 and anti-CD8, and assessed by flow cytometry. Results are representative of 3 independent experiments, in each of which single age-matched mice were tested. Statistical analysis is presented in Supporting Figure 3. E) Thymocytes from WT, lck-/-, Lck-PROX, Lck-DIST, and double transgenic Lck-PROX.Lck-DIST mice were gated on TCR(H57)hi cells and analyzed for CD4 and CD8. Results are representative of 3 independent experiments, in each of which single age-matched mice were tested.
Proximal lck promoter activity in Lck-PROX mice restored the numbers of total thymocytes and DP thymocytes to wild-type levels (Fig. 3A and Supp. Fig. 1C). The development of DN subsets was similarly restored to wild-type levels in Lck-PROX thymus, consistent with restored signaling through the pre-TCR in the DN3-DN4 transition or beta checkpoint (Fig. 3B, Supp. Fig. 1D,E). The number of SP thymocytes was increased relative to lck-/- mice, but was not restored to wild-type levels in Lck-PROX mice (Fig. 3A, Supp. Fig. 1A,B). In Lck-PROX thymus, the proportion of DP cells in the TCRβ (H57)hi population was increased in comparison to wild-type thymus, but to a lesser degree than lck-/- thymus (Fig. 3C). Lck driven by the proximal promoter is thus sufficient to restore development through the stage of DP thymocytes, but not to fully restore SP differentiation.
In contrast to the developmental phenotype of Lck-PROX thymus, Lck expression from the distal promoter in Lck-DIST mice was significantly less effective in restoring thymic cellularity, including numbers of DP and SP cells (Fig. 3A and Supp. Fig. 1 A,B,C). Additionally, the developmental block in DN3 to DN4 transition that is observed in lck-/- was not normalized in Lck-DIST mice, in contrast to restoration of this early defect in Lck-PROX thymocytes (Fig. 3B and Supp. Fig. 1 D,E). TCRβhi thymocytes in Lck-DIST thymus had an increased proportion of DP cells relative to wild-type thymus, as was seen in Lck-PROX thymocytes (Fig. 3C). Distal promoter-driven Lck expression thus completely failed to restore the defect in early DN3-DN4 development, while providing partial rescue of later stage DP and SP thymocytes, a pattern distinct from that seen with proximal promoter reconstitution.
Normal thymic development supported by proximal and distal promoters expressed on different lck genes
It is not known whether the proximal and distal lck promoters function independently, or whether interaction between the two promoters plays a role in thymocyte development. We assessed whether expression of both proximal and distal lck promoters in trans, by distinct BAC transgenes, would fully reconstitute thymocyte development We crossed Lck-PROX and Lck-DIST mice and examined the phenotype of offspring expressing both transgenes. The thymic developmental phenotype of Lck-PROX.Lck-DIST double transgenic mice fully recapitulated that of mice expressing a wild-type BAC transgene, including numbers of DP and SP cells and the efficient transition from DP to SP TCRβhi thymocytes (Fig. 3D,E and Supp. Fig. 2). Expression of the two lck promoters, even when on distinct BAC transgenes was thus sufficient to support normal thymocyte development.
Effect of fyn on requirements for promoter-specific lck expression
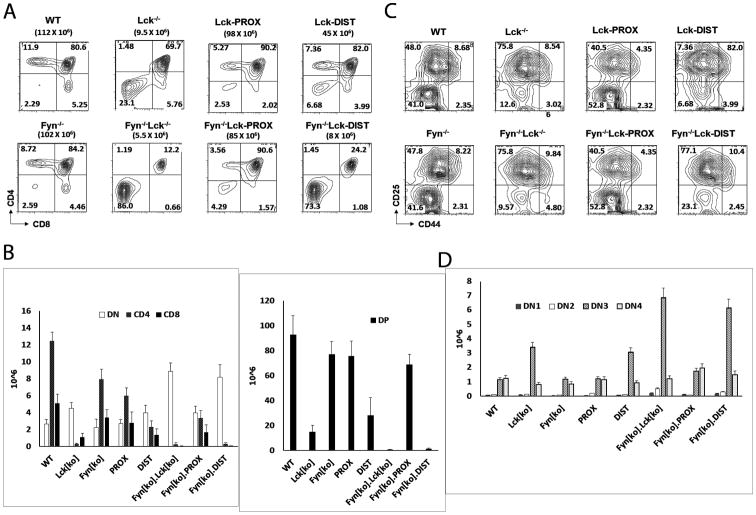
The developmental defect in lck-/- mice, although substantial, is not complete. It has previously been reported that inactivation of fyn, a related Src kinase, has only a modest effect on thymocyte development, but that combined inactivation of lck and fyn results in a defect that is more profound than that seen in single lck-deficient mice, with an essentially complete block at the DN3-DN4 developmental transition (11). To further characterize the functional roles of proximal and distal lck promoters in thymic development, we therefore generated mice deficient in fyn in addition to having promoter-specific restriction in lck expression. Consistent with previous studies, we found very low numbers of SP and DP cells in fyn-/-lck-/- thymus, substantially reduced from the numbers in lck single knockouts (Fig. 4A, B).
Figure 4.
Effect of fyn on requirements for promoter-specific lck expression. A, B) Thymocytes from WT, lck-/-, Lck-PROX, Lck-DIST, fyn-/-, fyn-/-Lck-PROX, and fyn-/- Lck-DIST mice were stained with anti-CD4 and anti-CD8, and assessed by flow cytometry. Histograms represent mean +/- SE number of thymocytes of each thymic subpopulation. Statistical analysis is presented in Supporting Figure 3. C,D) Thymocytes from WT, lck-/-, Lck-PROX, Lck-DIST, fyn-/-, fyn-/-Lck-PROX, and fyn-/-Lck-DIST mice were stained with anti-CD25 and anti-CD44, gated to exclude cells expressing CD4, CD8, NK1.1, Mac-1, Gr1, CD3, or B220, and assessed by flow cytometry. Histograms represent mean +/- SE number of thymocytes of each thymic subpopulation. Statistical analysis is presented in Supporting Figure 3.
We examined the effect of fyn deletion on the developmental phenotypes of mice with Lck promoter defects. Lck-DIST mice that were also deficient in fyn (fyn-/-Lck-DIST) had extremely low numbers of SP and DP thymocytes that were not increased above those in fyn-/-lck-/- double knockouts; and there was similarly no rescue from the DN3 block observed in fyn-/-lck-/- thymocytes (Fig. 4C, D and Supp. Fig. 3). The lck distal promoter thus had no effect on the developmental defects in fyn-/-lck-/-thymus development. In contrast, fyn-/-Lck-PROX thymus showed substantially increased numbers of both SP and DP thymocytes relative to fyn-/-lck-/- double knockouts, with a phenotype similar to that of Lck-PROX thymus (Fig. 4A, B and Supp. Fig. 3), as well as rescue of DN3-DN4 transition to the essentially wild-type level seen in fyn-/- singly deficient thymocytes (Fig. 4C, D and Supp. Fig. 3). These findings indicate that the proximal lck promoter is essential and largely sufficient to support early T cell development, even in the absence of Fyn, whereas the distal lck promoter has little or no ability to support T cell development in the absence of both Fyn and the lck proximal promoter.
Effect of proximal or distal lck promoter expression on peripheral T cells
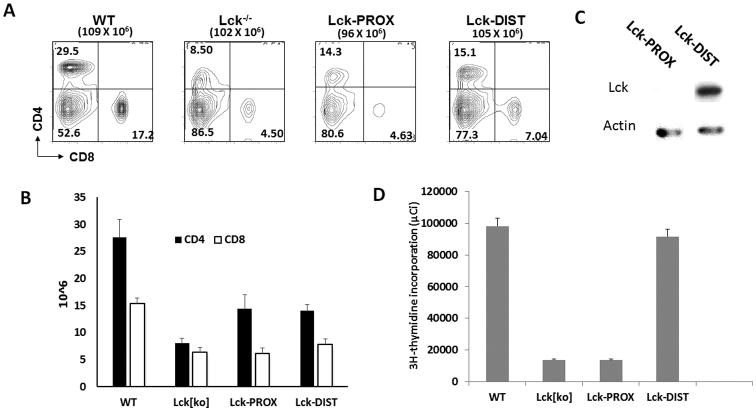
Peripheral CD4 and CD8 cell numbers were much reduced in lck-/- mice, with reduced cell surface expression of CD4 on CD4+ cells, consistent with previous (Figure 1 of van Oers at al. (11) and Figure 1 of Seddon et al. (12)) (Fig. 5A, B). Lck-PROX and Lck-DIST mice had similar numbers of peripheral CD4 T cells, which were greater than those in lck-/- mice, but significantly less than those in wild-type mice (Fig. 5A,B and Supp. Fig. 4). Lck-DIST CD4 T cells had easily detected levels of Lck protein; while, in contrast, Lck protein was undetectable in Lck-PROX T cells. Lck expression in peripheral T cells thus did not correlate with the number of peripheral T cells supported at homeostatic levels in these mice.
Figure 5.
Effect of proximal or distal lck promoter expression on peripheral T cells. A, B) Spleen cells from WT, lck-/-, Lck-PROX and Lck-DIST mice were stained with anti-CD4 and anti-CD8 antibodies, and analyzed by flow cytometry. Histograms represent mean +/- SE number of spleen CD4 and CD8 cells. Statistical analysis is presented in Supporting Figure 4. C) Spleen CD 4 cells were sorted from Lck-PROX and Lck-DIST mouse spleens by flow cytometry, protein lysates prepared and used for Western blot analysis. Results are representative of 3 independent experiments. D) T cell proliferation assays were carried with spleen CD 4 cells which were isolated by MAC cell isolation kit from WT, lck-/-, Lck-PROX and Lck-DIST mouse spleens. Results are representative of 3 independent experiments.
To test the capacity of T cells to proliferate in response to TCR-mediated stimulation, CD4 cells were purified from wild-type, lck-/-, Lck-DIST, and Lck-PROX spleen and lymph nodes and stimulated with anti-CD3 antibody. Proliferation of lck-/- CD4 cells was dramatically reduced in comparison to wild type T cells (Fig. 5D). Distal lck promoter activity restored proliferation in peripheral Lck-DIST CD4 T cells to a level comparable to wild type T cells; while, in contrast, Lck-PROX T cells were as defective as lck-/- thymocytes to TCR stimulation (Fig. 5D). Proliferative responses to anti-CD3 stimulation thus correlated with Lck protein in peripheral T cells.
Discussion
Lck is a Src family kinase that plays a critical role in signal transduction through the pre-TCR and TCR and is therefore critical to thymic T cell development (9, 13). Although it is known that expression of Lck is determined by two distinct promoters, the proximal and distal lck promoters (3), the functional roles of these promoters have not previously been analyzed. We directly analyzed promoter function by ablating either proximal or distal promoter and measuring effects on T cell development, and have demonstrated distinct and complementary functional roles of the two lck promoters. The proximal but not distal promoter is sufficient to support efficient early thymic differentiation through the pre-TCR-dependent DN3-DN4 transition to the DP stage, but is not able to support efficient TCR-dependent SP T cell development. In contrast, the distal lck promoter does not support early thymic development, but provides partial rescue of late stages of thymic development. These observed phenotypes correlate with Lck protein expression, the lck proximal promoter supporting highest Lck protein levels in DN and DP cells, while the distal promoter supports detectable Lck protein only in SP cells. The reconstitution of normal thymocyte development in double transgenic mice expressing proximal and distal lck promoters on distinct BAC transgenes indicates that the coordinated activity of these two promoters can function efficiently in trans, and potentially independently, to achieve efficient T cell development. These findings do not exclude effects of long-range genomic interactions in the regulation of lck expression, but they suggest that major aspects of developmental regulation are mediated within the domains of the BAC constructs used in these studies.
There may be functional advantage to differentially regulating the strength of signaling through the pre-TCR and TCR at distinct stages of development. The pivotal role of Lck in this signaling makes it a candidate for involvement in differential signaling. At the DN3-DN4 transition or “beta checkpoint”, Lck-dependent transduction of pre-TCR signaling is essential for commitment of developing T cells to the TCRαβ lineage (9). The studies reported here indicate that these events are highly dependent upon expression of the proximal but not distal lck promoter. Lck is also critical to signaling through the antigen-specific TCR that functions at later stages of development, in positive and negative selection of DP and SP thymocytes, as well as in activation of mature peripheral T cells (14). In DP thymocytes, Lck-dependent TCRαβ signaling contributes to determination of CD4 or CD8 SP lineage (15). Positive and negative selection of a TCR repertoire reactive with foreign but not self antigens is similarly dependent upon differential consequences of TCR signaling. Our findings indicate that activity of both proximal and distal lck promoters is required for optimal transition from DP to SP stage, likely a reflection of a quantitative Lck function in TCR signaling. It is notable that evolution has selected regulation of lck expression through two promoters, which we have shown to function differentially during development, rather than by differential regulation through a single promoter. Our efforts at analysis of the evolutionary relationships between proximal and distal lck promoters and the fyn promoter were uninformative due to the poor conservation of these promoter sequences, in contrast to coding sequences, during evolution.
In addition to Lck, a second Src kinase, Fyn, plays an important and partially redundant role in supporting T cell development. As previously reported, the T cell developmental defect in fyn-/-lck-/- mice was even more extreme than the lck single knockout (11). Fyn is expressed in early DN thymocytes, but not in later stages of DP and SP development. It was thus of interest to determine the combined effects of Fyn deletion and lck promoter disruption. The ability of the proximal lck promoter to support thymic development was largely independent of Fyn, consistent with a redundancy of Fyn and Lck in early thymic development. In contrast, the distal lck promoter alone was completely unable to support development of DP and SP thymocytes in the absence of Fyn, demonstrating that either Fyn or Lck driven from the proximal promoter is essential for early thymic T cell development.
As previously reported, lck-/- mice generate small numbers of SP thymocytes and peripheral CD4 and CD8 T cells. We found that Lck protein was expressed in Lck-DIST peripheral CD4 T cells but was undetectable in Lck-PROX peripheral T cells. Nevertheless, both Lck-PROX and Lck-DIST mice had similar peripheral T cell numbers, intermediate between those in complete lck KO mice and wild-type mice, suggesting that Lck protein levels are not critical for maintenance of peripheral CD4 T cell numbers, consistent with previous publication (12). Lck-DIST T cells proliferated normally in response to in vitro TCR stimulation, while Lck-PROX T cells had much reduced proliferation, suggesting that the distal lck promoter was required for T cell proliferation in response to TCR stimulation. Taken together, these findings indicate a discordance between maintenance of T cell numbers in vivo, which are not affected by the differences in Lck protein levels that are supported by proximal and distal Lck promoters, versus responses to TCR stimulation in vitro, which are highly affected by reduction in Lck protein.
The findings reported here identify integrated and complementary roles of two lck promoters, whose coordinated regulation of Lck supports signaling necessary for early pre-TCR-dependent and later TCR-dependent stages of thymic T cell differentiation. Characterizing the role of differential epigenetic changes and transcription factor activity at these loci during T cell development will provide further insight into the mechanisms underlying complex developmental regulation. The BAC transgenic model employed here, while highly effective in characterizing the functional role of the two lck promoters during T cell development, is not optimal for studying the regulation of expression at a molecular level. The presence of endogenous lck genes with intact promoters and aborted transcripts in the lck-/- knockout background complicates any attempt to analyze the consequences of promoter engineering. Future studies will be facilitated by the emergence of CRISPR-Cas9 technologies that will allow direct targeted genomic manipulation and analysis of lck promoter structure and molecular regulation, in parallel with analysis of functional effects.
Methods and Materials
Generation of promoter mutated lck transgenic mice
An lck C57BL/6 mouse BAC clone was purchased from Chiri (Oakland, CA). Mutant lck BAC clones were generated by the protocol of Yu et al. (16). Primer pair, Lck-P2-F (ATTCCTTCTGCT CCGTCTTCTCG ATTTCCTTCA CCGCTGTATACC ATCCGTAG CATATTATCATTA TTATTATT ACTG Gaa ttc gta aaa cga cgg cca g) and Lck-P2-R (TAGAGAGGCCT GAAGGAGCTGCCC CTAAGCCACTCGC CCCAACATCAGA CATCCTAGAGCCCT GTTCTGG AAGTTACAAAct g gcc gtc gtt tta c), were used to make the lck-PROX[del] BAC clone in which 2kb of proximal promoter region including part of the non-coding exon 2.1 was deleted (Fig. 2A). Primer pair, Lck-P1-F(TAGGGATTCCA CAACAGGAGACAA GATGGCACCAGGTGT CCCCAACAGCTC CCGATCTATTTC TCTAACTTTATC AAATTctgc aggaaacagctatg c) and Lck-P1-R (AAGGCATTTCTG AAAGGTTTCTGTAC ACCTAAGGAGCCT AGGCCTCCACAG ACGCAGTGTTCT GCTGTCTGGTTC CCACCgtcat agctgtttcctg), were used to make the lck-DIST[del] BAC clone in which 2 kb of the lck distal promoter region including non-coding exon 1.1 was deleted (Fig. 2A). Transgenic mice were generated by standard protocol (17) with BAC DNA microinjected into C57BL/6 mouse oocytes.
Mice
lck knockout (9) and fyn knockout (18) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Protocols for animal care and use were conducted consistent with the Guide for the Animal Care and Use of Laboratory Animals of National Institutes of Health and were approved by the Committee of the Animal Care and Use of Laboratory Animals of National Institutes of Health (IACUC protocol number: EIB-029). No surgery was performed, and humane endpoints were followed per IACUC guidelines. All animals were housed at the Frederick Cancer Research Facility of the National Cancer Institute (NCI).
Antibodies
Anti-CD69-FITC, anti-CD4-FITC, anti-CD8-FITC, anti-NK1.1-FITC, anti-Mac-1-FITC, anti-Gr1-FITC, anti-CD3-FITC, anti-B22-FITC, anti-CD44-PE, anti-CD25-Biotin anti-CD4-PE-Cy7, anti-CD8-PB, anti-CD5-biotin, anti-HSA-PE, anti-TCR-β−-Alex-647 monoclonal antibodies, avidin-594 were purchased from Biolegend (San Diego, CA).
RT-PCR analysis
DN thymocytes, DP thymocytes, SP thymocytes and splenic T cells were purified by flow cytometry from mouse thymus and spleen, and used to extract total RNA using NucleoSpin kits (BD Biosciences Clontech, Palo Alto, CA). One μg of total RNA was reverse-transcribed with random hexamers by using the SuperScript first-strand synthesis system for RT-PCR (Invitrogen, Carsbad, CA). Each PCR reaction contained first-strand cDNA corresponding to 25 ng of RNA, TaqMan universal PCR master mix (Invitrogen, Carsbad, CA), a set of primers for detecting Lck-PROX (5′taa ggg gta ctg gga aca aca-3′, 5′-ctc tga gct caa gga act gga-3′), or Lck-DIST (5′-atg ttg ggg cga gtg gct tag g-3′, 5′-atg gga tag tgg cag ttt tca-3′) or actin (5′-atg cca aca cag tgc tgt ctg gtg g-3′, 5′-ctg atc cac atc tgc tgg aag gtg-3′). Real-time detection of PCR products was performed by a PRISM 7700 sequence detector (Invitrogen, Carsbad, CA). RT-PCR amplified lck product was normalized to actin product for each sample. Reactions were in triplicate for each sample.
Western blot analysis
DN thymocytes, DP thymocytes, SP thymocytes and splenic T cells were isolated by flow cytometry from mouse thymus and spleen. The purified cells were lysed in buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1 mM Na2VO4, 1% NP-40 and protease inhibitor cocktail. Protein lysates were used for biochemical analysis (19).
T cell proliferation
CD4 T cells were prepared from spleen and lymph nodes with MAC cell isolation kit (Miltenyi Biotech, Auburn, CA). One million cells per ml were plated in dishes coated with 1 μg/ml anti-mouse CD3 antibody and 1 μg/ml anti-mouse CD28 antibody. Thymidine incorporation was measured after two days of culture at 37°C (20).
Statistical analysis
Statistical comparisons were made using one-way non-parametric ANOVA followed by Dunnett's method for multiple comparisons.
Supplementary Material
Acknowledgments
We are grateful to Rhonda Anderson and staff at the National Cancer Institute-Frederick animal facility for expert animal care and breeding. We thank flow cytometry facility staff of the Experimental Immunology Branch for help in cytometric analysis, and thank Jeffrey Hammer for isolation of mouse tail DNA. We thank Alfred Singer and Dinah Singer for critical reading and helpful comments.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Abbreviations
- BAC
bacterial artificial chromosomes
- DN
double negative
- DP
Double positive
- SP
single positive
References
- 1.Davuluri RV, Suzuki Y, Sugano S, Plass C, Huang TH. The functional consequences of alternative promoter use in mammalian genomes. Trends in genetics : TIG. 2008;24:167–177. doi: 10.1016/j.tig.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Landry JR, Mager DL, Wilhelm BT. Complex controls: the role of alternative promoters in mammalian genomes. Trends in genetics : TIG. 2003;19:640–648. doi: 10.1016/j.tig.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Voronova AF, Adler HT, Sefton BM. Two lck transcripts containing different 5′ untranslated regions are present in T cells. Mol Cell Biol. 1987;7:4407–4413. doi: 10.1128/mcb.7.12.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garvin AM, Pawar S, Marth JD, Perlmutter RM. Structure of the murine lck gene and its rearrangement in a murine lymphoma cell line. Mol Cell Biol. 1988;8:3058–3064. doi: 10.1128/mcb.8.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildin RS, Garvin AM, Pawar S, Lewis DB, Abraham KM, Forbush KA, Ziegler SF, Allen JM, Perlmutter RM. Developmental regulation of lck gene expression in T lymphocytes. J Exp Med. 1991;173:383–393. doi: 10.1084/jem.173.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen JM, Forbush KA, Perlmutter RM. Functional dissection of the lck proximal promoter. Mol Cell Biol. 1992;12:2758–2768. doi: 10.1128/mcb.12.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu C, Kawamoto H, Yamashita M, Kimura M, Kondou E, Kaneko Y, Okada S, Tokuhisa T, Yokoyama M, Taniguchi M, Katsura Y, Nakayama T. Progression of T cell lineage restriction in the earliest subpopulation of murine adult thymus visualized by the expression of lck proximal promoter activity. Int Immunol. 2001;13:105–117. doi: 10.1093/intimm/13.1.105. [DOI] [PubMed] [Google Scholar]
- 8.Wildin RS, Wang HU, Forbush KA, Perlmutter RM. Functional dissection of the murine lck distal promoter. J Immunol. 1995;155:1286–1295. [PubMed] [Google Scholar]
- 9.Penninger J, Kishihara K, Molina T, Wallace VA, Timms E, Hedrick SM, Mak TW. Requirement for tyrosine kinase p56lck for thymic development of transgenic gamma delta T cells. Science. 1993;260:358–361. doi: 10.1126/science.8469988. [DOI] [PubMed] [Google Scholar]
- 10.Legname G, Seddon B, Lovatt M, Tomlinson P, Sarner N, Tolaini M, Williams K, Norton T, Kioussis D, Zamoyska R. Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity. 2000;12:537–546. doi: 10.1016/s1074-7613(00)80205-8. [DOI] [PubMed] [Google Scholar]
- 11.van Oers NS, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. alpha beta T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 12.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival but impaired homeostatic proliferation of Naive T cells in the absence of p56lck. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 13.Samelson LE. Adaptor proteins and T-cell antigen receptor signaling. Progress in biophysics and molecular biology. 1999;71:393–403. doi: 10.1016/s0079-6107(98)00050-9. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A. The right team at the right time to go for a home run: tyrosine kinase activation by the TCR. Nat Immunol. 2010;11:101–104. doi: 10.1038/ni0210-101. [DOI] [PubMed] [Google Scholar]
- 15.Van Laethem F, Tikhonova AN, Pobezinsky LA, Tai X, Kimura MY, Le Saout C, Guinter TI, Adams A, Sharrow SO, Bernhardt G, Feigenbaum L, Singer A. Lck availability during thymic selection determines the recognition specificity of the T cell repertoire. Cell. 2013;154:1326–1341. doi: 10.1016/j.cell.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci U S A. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang XW, Gong S. An overview on the generation of BAC transgenic mice for neuroscience research. Current protocols in neuroscience / editorial board, Jacqueline N Crawley … [et al] 2005;Chapter 5 doi: 10.1002/0471142301.ns0520s31. Unit 5 20. [DOI] [PubMed] [Google Scholar]
- 18.Stein PL, Lee HM, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 19.Chiang YJ, Hodes RJ. Regulation of T cell development by c-Cbl: essential role of Lck. Int Immunol. 2015;27:245–251. doi: 10.1093/intimm/dxu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiang YJ, Kole HK, Brown K, Naramura M, Fukuhara S, Hu RJ, Jang IK, Gutkind JS, Shevach E, Gu H. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–220. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.