Abstract
Objective
To compare health and growth outcomes in children infected with HIV, children exposed but uninfected with HIV, and children unexposed to HIV.
Study design
Our cohort was comprised of 3,554 Tanzanian children enrolled in two trials of micronutrient supplementation. Among infants born to mothers infected with HIV, 264 were infected with HIV and 2088 were exposed but uninfected at age 6 weeks. An additional 1202 infants were unexposed to HIV. Infants were followed until age 18 months, death or loss to follow-up. Morbidity and growth were assessed at monthly nurse visits.
Results
Compared with unexposed infants, hazard ratios (95% CI) for all-cause mortality in infants infected with HIV and infected exposed but uninfected with HIV were 28.99 (14.83, 56.66) and 2.79 (1.41, 5.53) respectively after adjusting for demographic and nutritional covariates. Infants infected with HIV also had higher risks of all morbidities, but HIV-EU infants were significantly more likely to have cough, fever, unscheduled outpatient visits, and hospitalizations. Infants infected with HIV also were more likely to experience stunting, wasting and underweight at baseline and during follow-up. Infants exposed but uninfected with HIV were more likely to be underweight at baseline [adjusted RR (95%CI): 2.05 (1.45, 2.89)], but on average, experienced slower declines in HAZ, WAZ and WHZ and a lower rate of stunting over follow-up, compared with unexposed infants.
Conclusion
In addition to preventing and treating HIV infection in infants, PMTCT and child health services should also target children exposed but uninfected with HIV to improve health outcomes in this vulnerable population.
Keywords: HIV, HIV-Exposed-Uninfected, child health, mortality, morbidity, growth
Globally, 35.2 million people are estimated to be living with the human immunodeficiency virus (HIV), 3.2 million of whom are children (1). One of the great health achievements in the past two decades has been the global expansion of prevention-of-mother-to-child-transmission of HIV (PMTCT) services. In 2014, coverage of antiretroviral treatment for PMTCT services reached 74% of pregnant women living with HIV, resulting in a 48% decline in new pediatric HIV infections between 2009 and 2014 (2). As a result of expanded PMTCT services, each year almost a quarter of infants born in several sub-Saharan African countries are HIV-uninfected infants born to HIV-infected mothers, the so-called HIV-exposed-uninfected (HIV-EU) children (3). Whether HIV-EU children have the potential for similar health outcomes as HIV-unexposed infants, or whether they have outcomes ‘between’ those of HIV-infected and HIV-uninfected infants, has not been determined (3).
There are several mechanisms through which maternal HIV infection can lead to suboptimal child health. HIV-infected children have a considerably higher risk of mortality, morbidity and growth failure compared with uninfected infants (4–7). Even if an infant avoids HIV infection, however, prenatal exposure to HIV may influence developmental programming (8). A handful of studies have documented impaired immune function (9–12) and an increased risk of morbidity and mortality (7, 13–15) among HIV-EU infants compared with HIV-unexposed infants. Additional studies also have documented an increased risk of low birthweight – due to both intrauterine growth restriction as well as prematurity compared with HIV-unexposed children (7, 16–19). Recent research also has indicated that maternal antiretroviral therapy (ART) during pregnancy may have independent negative effects on infant health and growth (20–22); however very few studies have assessed the effect of HIV and/or ART exposure on long-term health outcomes such as growth (5). Furthermore, much of the research on health outcomes in HIV-EU children comes from cohorts in wealthy nations where antiretroviral access and underlying risk factors for poor growth, morbidity and mortality are quite different than in the low-resource settings (5, 23). In order to better describe the unique health risks of HIV-infected and HIV-EU infants relative to unexposed infants in low-resource settings, we evaluated mortality, morbidity and growth outcomes in HIV-infected and HIV-EU children in Dar es Salaam, Tanzania, compared with unexposed infants from the same peri-urban community.
Methods
The study sample was comprised of 3,554 children in Dar es Salaam, Tanzania who participated in two micronutrient supplementation trials (Clinicaltrials.gov: NCT00197730 and NCT00421668). The first trial randomized infants born to HIV-infected mothers to either daily multivitamins (vitamins B-complex, C & E) or placebo at age 6 weeks (24). Children were tested for HIV infection at age 6 wk by using the Amplicor HIV-1 DNA assay version 1.5 (Roche Molecular Systems Inc). Tests at 18 mo of age were performed using HIV ELISAs followed by Enzygnost anti-HIV-1/2 Plus (Dade Behring); discordant results were resolved by using a Western blot test. Samples from children who tested positive at 18 mo were then back tested via polymerase chain reaction to estimate time of transmission. Infants confirmed to be HIV-infected at baseline (age 6 wk) serve as the HIV-infected sample for analyses, and infants who were uninfected at baseline serve as the HIV-EU sample. If HIV-EU infants tested positive for HIV at a later visit, they were censored after their last negative test.
The second trial was implemented with a 2×2 factorial design assessing zinc and multivitamins, zinc only, multivitamins only or placebo among 2400 infants born to HIV-uninfected women (25). In order to enhance comparability with the first study, we restricted our current analyses to infants who received multivitamins alone or placebo. Multivitamin supplements in both trials were identical in composition and dosage (24, 25). Infants randomized to multivitamins consumed one capsule per day containing 150–600% of the US Adequate Intake (AI) for children aged 0–6 mo, and two capsules per day after 6 months containing 200–400% of the AI for infants 6–12 months and 133–800% of the US RDA for children aged 1–3 y. The two studies were designed to allow for a pooled analysis – they were conducted in overlapping clinics with similar staff, identical inclusion/exclusion criteria (other than maternal HIV-status), and they collected the same demographic and clinical data on all mothers and children (24, 25). The first trial did not show an effect of multivitamin supplements on morbidity and mortality (24), nor on growth (26). The second trial, which was not powered to assess the effect of supplements on mortality in an HIV-unexposed population, also did not show substantial effects of multivitamins on morbidity (25) or growth (27).
Approval for both trials was granted by the Harvard T.H. Chan School of Public Health Human Subjects Committee, the Muhimbili University of Health and Allied Science Committee of Research and Publications, the Tanzanian National Institute of Medical Research and the Tanzanian Food and Drug Authority. All mothers provided written informed consent to enroll themselves and their infants in the studies.
In both trials, mothers were asked to bring children to the clinic for follow-up monthly. During follow-up visits, a trained study nurse performed a standardized assessment of child morbidity based on maternal recall aided by the mother’s symptom diary that she received at the previous visit. The symptom diary was a pictorial aid of illness symptoms (eg, diarrhea, vomiting) where mothers were asked to check off which days their child had from these symptoms. A trained nurse also measured child anthropometry using standard techniques (28). Weight was measured on a digital infant balance scale with 10-g precision (Tanita) and length with 1-mm precision using a rigid length board with an adjustable foot piece. For our analysis, we calculated age- and sex-specific z-scores for three anthropometric indexes: weight-for-height (WAZ), height-for-age (HAZ) and weight-for-age (WAZ) using the 2006 WHO growth standards (29). Children who missed their monthly follow-up appointment were visited at home by a study nurse, and their vital status was confirmed through contact with immediate family members.
Standard of Care
The medical care provided to mothers and children in both trials has been previously been described (24, 25). In brief, mothers and children received medical care in accordance with Tanzanian guidelines. On the basis of earlier findings of the benefits of prenatal multivitamins among HIV-infected women who were not receiving ARVs (29,30), all HIV-infected women received supplements containing high doses of vitamins B-complex, C, and E during pregnancy and lactation. When the first trial began, ARV medication was limited to nevirapine prophylaxis for maternal to child transmission (21). As the trial progressed, access to ARVs expanded rapidly. Beginning in July 2005, women and children were screened for ARV eligibility and treated according to national guidelines. For adults, eligibility was based on WHO stage IV disease, or CD4 cell count ≤200 cells/mL, or WHO stage III and CD4 cell count ≤350 cells/mL. For children, eligibility was based on CD4%<20 or Pediatric WHO Stage III. The standard first-line regimen was stavudine, lamivudine, and nevirapine for adults and zidovudine, lamivudine, and nevirapine for children; alternative drugs were available for specific circumstances. Also in accordance with the WHO and Tanzanian guidelines at the time, HIV-infected mothers were counseled on the risks and benefits of exclusive breastfeeding. HIV-uninfected mothers were encouraged to exclusively breastfeed for 6 months followed by complementary feeding and continued breastfeeding until 2 years. All children received growth monitoring, immunizations, high-dose vitamin A supplementation and routine medical care for illnesses. Infants born to HIV-infected mothers also received cotrimoxazole prophylaxis until age 6 months, and continued cotrimoxazole as long as children continued breastfeeding.
Statistical Analyses
Baseline characteristics of the three groups were compared using chi-squared and ANOVA tests for categorical and continuous variables respectively. Cox proportional hazards models were used to compare mortality rates with HIV-unexposed infants as the reference group. To select covariates for adjusted models, univariate models of common predictors of mortality were conducted and all variables significant at the p<0.20 level were retained in adjusted models. A priori, we determined infant sex and treatment group (multivitamin vs. placebo) would be retained in all models. Generalized estimating equations with the binomial distribution, log link, and exchangeable covariance structure were used to compare relative risks of common morbidities at each nurse visit across the three groups. We determined a priori to also include infant age (<26 wks, 26–52 wks and ≥52 wks) in all morbidity models. Covariate selection was similar to that for mortality models, but to enhance consistency and interpretability, we selected the same set of covariates for all multivariate morbidity models - any covariate significant at p<0.20 for at least two morbidity outcomes was retained in all adjusted morbidity models. Baseline relative risks of stunting, wasting and underweight were compared using generalized linear models with the logarithm as the link function, empirical variance and a binomial distribution; time to stunting, wasting and underweight over follow-up was compared using Cox proportional hazards models. Mixed effects models with restricted cubic splines (with set knots at 10 weeks, 3, 6, 9 and 15 months) were used to model mean height-for-age, weight-for-age and weight-for-height z-scores (HAZ, WAZ and WHZ) in infants from 6 weeks through 18 months. Covariates in the mixed effects models were identical to those in models for stunting, wasting and underweight.
Because the guidelines for the initiation of ARVs changed during the first trial, we were able to assess ARV use during pregnancy as an effect modifier of the relationship between HIV-exposure group and mortality. We first created three categories for all HIV-infected mothers: mothers who initiated ARVs during pregnancy, mothers with early-stage HIV (who would not qualify for ARVs before or after the change of guidelines) and mothers with late-stage HIV who did not receive ARVs during pregnancy. Late-stage HIV was defined based on criteria for ARV initiation at the time of the study (WHO stage IV disease, or CD4 ≤200 cells/mL, or WHO III and CD4 ≤350) during pregnancy or at baseline. Early-stage HIV was defined as the inverse of late-stage HIV. For infants whose mothers did not receive ARVs during pregnancy but initiated treatment after birth, we censored the children once the mothers initiated ARVs. To assess effect modification in the Cox proportional hazard models for mortality, we conducted likelihood ratio tests comparing full models containing infant HIV-exposure group as well as interaction terms for infant HIV-exposure group and maternal HIV-stage/ARV group versus reduced models that only contained infant HIV-exposure group. When overall test for effect modification yielded a p-value <0.05, maternal HIV-stage/ARV use was considered a significant effect modifier, and we repeated our primary analyses stratified by maternal HIV-stage/ARV group. Because infant feeding mode could be on the causal pathway between infant HIV-exposure group and all health outcomes, we repeated all analyses of adjusted models excluding infant feeding covariates to assess whether this substantially changed our primary results. All analyses were conducted in SAS version 9.3 (SAS Institute, Cary, NC USA).
Results
The first trial enrolled 2387 infants of HIV-infected mothers, randomized to multivitamins or placebo at 6 weeks of age. Of these, 264 infants were confirmed to be HIV-infected at baseline and 2088 were confirmed HIV-uninfected, and thereby served as the HIV-EU sample. Randomization occurred between August 2004 and November 2007; follow-up ended in May 2008. The second trial enrolled 2400 infants born to HIV-uninfected mothers, approximately half of whom were randomized to daily zinc supplementation. The remaining 1202 infants who received only multivitamins or placebo serve as the HIV-unexposed sample. Randomization occurred between September 2007 and December 2009; follow-up ended in July 2010.
With the exception of infant sex, all baseline variables were significantly different across the three HIV-exposure groups (Table I). On average, HIV-uninfected mothers were younger, taller, had larger middle-upper arm circumferences, were less likely to be a housewife without income, more likely to be married or cohabitating with their partner, and had fewer previous pregnancies. They also lived in households that were more likely to own refrigerators and/or televisions. Infants born to HIV-infected mothers were more likely to be low birth weight, particularly if they were HIV-infected themselves. HIV-infected infants also were significantly more likely to be born pre-term. Breastfeeding practices among HIV-infected and HIV-uninfected mothers were notably different. HIV-infected mothers exclusively breastfed for longer than uninfected mothers; however, HIV-infected mothers ceased breastfeeding much earlier than HIV-uninfected mothers (mean duration of 4.5 months versus 13.2 months).
Table 1.
Comparison of Maternal, Child and Sociodemographic Characteristics in HIV-unexposed, HIV-Exposed-Uninfected and HIV-Infected Children
| HIV-Unexposed n=1202 |
HIV-EU n=2088 |
HIV-Infected n=264 |
P value1 | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Age (y) | 26.4 ± 5.01 | 28.2 ± 5.0 | 28.5 ± 4.9 | <0.001 |
| Height (cm) | 156.7 ± 5.7 | 156.3 ± 6.1 | 155.6 ± 6.8 | 0.034 |
| Middle-upper arm circumference (cm) | 27.0 ± 3.1 | 26.0 ± 3.2 | 25.2 ± 3.3 | <0.001 |
| Formal education [n (%)] | <0.001 | |||
| None | 19 (1.6) | 143 (6.9) | 12 (4.6) | |
| 1–7 y3 | 869 (72.7) | 1475 (71.3) | 202 (77.1) | |
| ≥ 8 y | 307 (25.7) | 451 (21.8) | 48 (18.3) | |
| Employment [n (%)] | <0.001 | |||
| Housewife without income | 723 (60.6) | 1328 (66.0) | 180 (70.9) | |
| Housewife with income | 388 (32.5) | 439 (21.8) | 40 (15.8) | |
| Other | 82 (6.9) | 245 (12.2) | 34 (13.4) | |
| Marital status [n (%)] | ||||
| Married or cohabitating with partner | 1079 (90.7) | 1803 (87.2) | 220 (84.6) | 0.002 |
| Prior pregnancies [n (%)] | <.0001 | |||
| None | 392 (33.0) | 477 (23.1) | 56 (21.5) | |
| 1–4 | 725 (61.0) | 1424 (68.9) | 182 (69.7) | |
| ≥ 5 | 72 (6.1) | 166 (8.0) | 23 (8.8) | |
| Socioeconomic characteristics | ||||
| Household appliances4 [n (%)] | <.0001 | |||
| None | 407 (34.1) | 1143 (55.3) | 137 (52.5) | |
| Either a television or refrigerator | 384 (32.1) | 495 (24.0) | 63 (24.1) | |
| Both a television and refrigerator | 404 (33.8) | 429 (20.8) | 61 (23.4) | |
| Child characteristics | ||||
| Male sex [n (%)] | 627 (52.2) | 1141 (54.7) | 131 (49.6) | 0.169 |
| Age at baseline (weeks) | 5.9 ± 0.4 | 5.8 ± 0.5 | 5.9 ± 0.4 | <0.001 |
| Low birth weight, < 2500 g [n (%)] | 39 (3.3) | 114 (5.7) | 42 (17.0) | <0.001 |
| Born preterm, <37 wk [n (%)] | 143 (13.0) | 299 (14.5) | 50 (19.4) | 0.032 |
| Duration of exclusive breastfeeding (months) | 1.8 (1.5) | 3.6 (2.1) | 3.1 (2.1) | <0.001 |
| Duration of any breastfeeding (months) | 13.2 (5.9) | 4.4 (2.4) | 4.5 (3.2) | <0.001 |
Mean ± SD (all such values), unless otherwise shown. P-values for continuous variables are from ANOVA; p-values for categorical variables are from chi-squared tests
In Tanzania, 7 years is the duration of most primary schools.
From a list that includes television & refrigerator
Infants born to HIV-infected mothers experienced substantially higher rates of mortality in both crude and adjusted models compared with infants born to HIV-uninfected mothers, even when the infants remained uninfected (Table II). Compared with unexposed infants, HIV-infected infants experienced a nearly thirty-fold increase in mortality, and HIV-EU infants had a nearly three-fold increase in rate of mortality, even after adjusting for sociodemographic characteristics and infant feeding characteristics. In longitudinal analyses, there were 264 HIV-infected children at baseline, 187 at age 6 months, 133 at age 12 months and 104 at age 18 months. There were 2088 HIV-EU children at baseline, 1951 at 6 months, 1734 at 12 months and 1524 at 18 months; and 1200 HIV-unexposed children at baseline, 1051 at age 6 months, 869 at 12 months and 660 at 18 months.
Table 2.
Mortality Rates in HIV-Unexposed, HIV-Exposed-Uninfected and HIV-infected Infants
| HIV-Unexposed n=1202 |
HIV-EU n=2088 |
HIV-Infected n=264 |
|
|---|---|---|---|
| Observed # deaths before age 18 months (%) | 16 (1.3%) | 119 (5.7%) | 113 (42.8%) |
| Mortality rate (per 100 person-years)1 | 1.2 (0.8, 2.0) | 4.6 (3.9, 5.6) | 46.5 (38.7, 55.9) |
|
| |||
| Crude hazard ratio (95% CI)2 | ref | 3.76 (2.23, 6.33) | 37.97 (22.49, 64.10) |
| P-value | – | <0.001 | <0.001 |
|
| |||
| Adjusted hazard ratio (95%CI)4 | ref | 2.79 (1.41, 5.53) | 28.99 (14.83, 56.66) |
| P-value | – | 0.003 | <0.001 |
Mortality rate estimated from poisson regression models.
All hazard ratios, 95% confidence intervals and p-values are from cox proportional hazards models with the exact method for ties.
Adjusted model is adjusted for maternal education (0, 1–7 or 8+ years), occupation (housewife with income, housewife without income, or other), height (cm), number of household assets (0, 1 or 2 from a list that included television and refrigerator), infant sex and treatment group (multivitamin vs. placebo), and time-updating covariates for duration of exclusive breastfeeding and duration of any breastfeeding.
Infants born to HIV-infected mothers also had higher rates of most common infectious morbidities in crude and adjusted models compared with infants born to HIV-uninfected mothers (Table III). After adjusting for sociodemographic and infant feeding characteristics, HIV-infected infants had an increased risk of all morbidities assessed ranging from a 46% increase in the risk of diarrhea to an almost 13-fold increase in the risk of hospitalization in multivariate models. Compared with unexposed infants, HIV-EU infants experienced a significantly increased risk of cough [RR (95% CI): 1.28 (1.19, 1.37)], fever [RR (95% CI): 1.16 (1.03, 1.29)], unscheduled outpatient visits [RR (95% CI): 1.74 (1.35, 2.25)] and hospitalizations [RR (95% CI): 3.56 (1.80, 7.05)], although their risks remained substantially lower than those of HIV-infected infants.
Table 3.
Comparing common morbidities in HIV-infected, HIV-EU and HIV-unexposed infants
| HIV-unexposed (reference) | HIV-EU | HIV-Infected | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n=14,8231 | n=36,5611 | n=3,2141 | |||||
|
| |||||||
| # events (%)2 |
# events (%)2 |
Crude RR (95%CI)3 |
Adjusted RR (95% CI)4 |
# events (%)2 |
Crude RR (95%CI) |
Adjusted RR (95% CI)4 |
|
| Diarrhea | 566 (4.01) |
1,380 (4.81) |
1.21 (1.09, 1.35)**** |
1.18 (1.00, 1.39)* |
154 (5.86) |
1.50 (1.23, 1.81)**** |
1.46 (1.16, 1.84)** |
| Cough | 3322 (23.39) |
8,672 (30.26) |
1.31 (1.25, 1.37)**** |
1.28 (1.19, 1.37)**** |
942 (35.90) |
1.53 (1.41, 1.67)**** |
1.52 (1.38, 1.68)**** |
| Fever | 1471 (10.36) |
3348 (11.68) |
1.14 (1.07, 1.23)**** |
1.16 (1.03, 1.29)** |
543 (20.69) |
2.03 (1.82, 2.27)**** |
2.11 (1.83, 2.43)**** |
| Anorexia | 346 (2.44) |
800 (2.79) |
1.16 (1.01, 1.34)** |
1.02 (0.81, 1.29) |
118 (4.50) |
1.90 (1.50, 2.41)**** |
1.85 (1.35, 2.52)**** |
| Vomiting | 237 (1.67) |
502 (1.75) |
1.07 (0.90, 1.27) |
0.97 (0.74, 1.28) |
70 (2.67) |
1.64 (1.22, 2.21)** |
1.58 (1.11, 2.23)** |
| Ear infection | 71 (0.50) |
198 (0.69) |
1.45 (1.05, 2.01)** |
1.54 (0.94, 2.52)* |
75 (2.86) |
5.88 (3.99, 8.67)**** |
5.85 (3.37, 10.15)**** |
| Unscheduled outpatient visits | 223 (1.62) |
870 (3.03) |
1.87 (1.59, 2.21)**** |
1.74 (1.35, 2.25)**** |
113 (4.30) |
2.70 (2.13, 3.43)**** |
2.67 (1.96, 3.63)**** |
| Hospitalizations | 20 (0.14) |
176 (0.61) |
4.32 (2.66, 6.99)**** |
3.56 (1.80, 7.05)**** |
55 (2.09) |
15.07 (8.69, 26.13)**** |
12.96 (6.42, 26.17)**** |
n is the sum of the number of nurse visits for all children.
Number of events defined as maternal report of morbidity in the 28 days (4 weeks) prior to each nurse visit. Denominator for percentage calculations was the total number of nurse visits.
Crude RR, 95% CI, and corresponding P-values were obtained from generalized estimating equations with the binomial distribution, log link, and exchangeable covariance structure.
Adjusted RR, 95% CI, and corresponding P-values were obtained from generalized estimating equations with the binomial distribution, log link, and exchangeable covariance structure. Adjusted models were adjusted for child’s sex and age (<6 months, 6–12 months and >12 months), treatment group (multivitamin supplement vs. placebo) and maternal education (0, 1–7, or 8 years), occupation (housewife with no income, housewife with income, other), marital status (cohabitating with partner, yes vs. no), parity (0, 1–4, 5+), height (tertiles) and age (tertiles) and feeding mode at each nurse visit (exclusive breastfeeding, mixed feeding, or no breastfeeding).
p<0.10;
p<0.05;
p<0.01;
p<0.001
With regard to growth outcomes, HIV-infected infants experienced significantly impaired growth relative to their HIV-unexposed peers at all time periods in both crude and multivariate models (Figure and Table IV; Table IV available at www.jpeds.com). Of particular note, HIV-infected infants already had an increased risk of stunting [RR (95% CI): 2.59 (1.77, 3.78), p<0.001], wasting [RR (95% CI): 2.03 (1.32, 3.12), p=0.001] and underweight [RR (95% CI): 5.11 (3.40, 7.67), p<0.001] by study entry at 6 weeks of age in adjusted models. They also continued to have significantly higher rates of stunting, wasting and underweight for the duration of follow-up. At 6 weeks of age, HIV-EU infants had lower mean HAZ, WAZ and WHZ than HIV-unexposed infants; however only the difference in risk of underweight was significantly different at baseline [Adjusted RR (95%CI): 1.90 (1.33, 2.73), p<0.001]. The mixed effects models with restricted cubic splines show that HIV-EU infants experienced slower declines in HAZ, WAZ and WHZ than their unexposed peers; however the difference was most marked in HAZ where the curves crossed in the first few months of life. In the Cox proportional hazard models assessing rates of stunting, wasting and underweight from 6 week through 18 months, HIV-EU infants had significantly higher rates of wasting and underweight in the crude models; however neither remained significant in multivariate models. By contrast, HIV-EU infants had lower rates of stunting over follow-up compared with HIV-unexposed infants in both crude and adjusted models [Adjusted HR (95% CI): 0.64 (0.51, 0.81), p<0.001]. In models that did not include infant feeding as a covariate, estimates of the effect of infant HIV-exposure group on mortality, morbidity and growth were not substantially different than those from models that adjusted for infant feeding.
Figure 1.
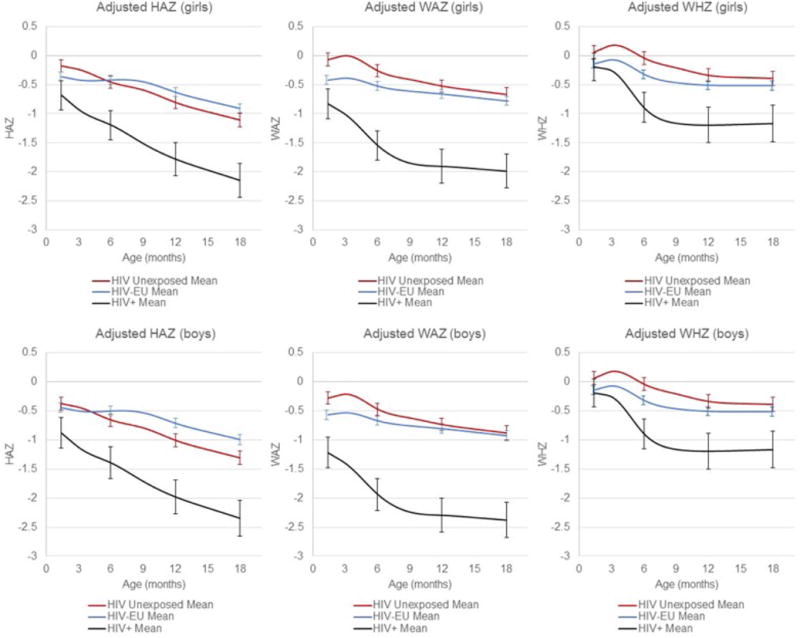
Height-for-age, Weight-for-age and Weight-for-height Z-scores over time in HIV-positive, HIV-exposed-uninfected and HIV-unexposed Infants (adjusted for baseline covariates). Curves were creating using mixed effects models with restricted cubic splines with knots at 10 weeks and 3, 6, 9 12 & 15 months of age. The models are adjusted for maternal height and education, as well as infant sex and treatment group. The adjusted means presented here are for children who received placebo in the parent trial, live in a household with 1–3 assets and whose mother is the median height (156cm), is a housewife without income, and has 1–7 years of education. Bars represent 95% confidence intervals for each of the three groups at age 6 weeks and 6, 12 and 18 months.
Table 4.
Comparing Rates of Stunting, Wasting and Underweight among HIV-unexposed, HIV-exposed and uninfected, and HIV-infected infants at Baseline and over Follow-up
| Relative Risks at Baseline (6 weeks)1 | ||||
|---|---|---|---|---|
|
| ||||
| HIV-Unexposed (reference) | HIV-EU | HIV-Infected | ||
| N | 1178 | 2052 | 258 | |
| Stunting | n (%) | 72 (6.1) | 149 (7.3) | 42 (16.3) |
| Crude RR (95% CI)1 | – | 1.19 (0.91, 1.56) | 2.66 (1.87, 3.80)**** | |
| Adjusted RR (95% CI)2 | 1.19 (0.88, 1.61) | 2.59 (1.77, 3.78)**** | ||
|
| ||||
| N | 1177 | 2043 | 256 | |
| Wasting | n (%) | 69 (5.9) | 139 (6.8) | 30 (11.7) |
| Crude RR (95% CI) | 1.16 (0.88,1.53) | 2.00 (1.33, 3.00)**** | ||
| Adjusted RR (95% CI) | 1.22 (0.89, 1.66) | 2.03 (1.32, 3.12)** | ||
|
| ||||
| N | 1182 | 2065 | 261 | |
| Underweight | n (%) | 46 (3.9) | 154 (7.5) | 54 (20.7) |
| Crude RR (95% CI) | – | 1.92 (1.39, 2.64)**** | 5.32 (3.67, 7.69)**** | |
| Adjusted RR (95% CI)3 | 1.90 (1.33, 2.73)**** | 5.11 (3.40, 7.67)**** | ||
|
| ||||
| Hazard Ratios from 6 weeks – 18 months | ||||
|
| ||||
| N | 1090 | 1871 | 205 | |
| Stunting | n (%) | 278 (25.5) | 456 (24.4) | 94 (45.9) |
| Crude HR (95% CI)3 | – | 0.80 (0.69, 0.93)*** | 2.21 (1.75, 2.80)**** | |
| Adjusted HR (95% CI)4 | – | 0.64 (0.51, 0.81)**** | 1.72 (1.31, 2.27)**** | |
|
| ||||
| N | 1092 | 1881 | 214 | |
| Wasting | n (%) | 180 (16.5) | 510 (27.1) | 104 (48.6) |
| Crude HR (95% CI) | – | 1.52 (1.28, 1.80)**** | 4.03 (3.16, 5.14)**** | |
| Adjusted HR (95% CI) | – | 1.16 (0.90, 1.50) | 3.21 (2.38, 4.34)**** | |
|
| ||||
| N | 1114 | 1869 | 197 | |
| Underweight | n (%) | 185 (16.6) | 441 (23.6) | 107 (54.3) |
| Crude HR (95% CI) | – | 1.29 (1.09, 1.54)*** | 4.46 (3.51, 5.67)**** | |
| Adjusted HR (95% CI) | – | 1.17 (0.90, 1.51) | 3.54 (2.63, 4.77)**** | |
All relative risk (RRs) and corresponding 95% CIs and p-values are from generalized estimating equations with the logarithm as the link function, empirical variance and a binomial distribution.
Adjusted risk ratio models are adjusted for treatment group (multivitamin vs. placebo), child’s sex, maternal education (0, 1–7 or 8+ yrs), maternal height (tertiles), household asset score (0, 1 or 2 from a list of television & refrigerator), as well as infant feeding mode at baseline (exclusively breastfeeding, mixed feeding, no breastfeeding).
Hazard ratios are from cox proportional hazards models with the exact method for ties.
Adjusted cox proportional hazards are adjusted for treatment group (multivitamin vs. placebo), child’s sex, maternal education (0, 1–7 or 8+ yrs), maternal height (tertiles), household asset score (0, 1 or 2 from a list of television & refrigerator), and the corresponding anthropometric z-score at baseline, as well as time-varying covariates for breastfeeding duration and exclusive breastfeeding duration.
p<0.10;
p<0.05;
p<0.01;
p<0.001
We found that maternal HIV disease progression/ARV use was a significant effect modifier of the relationship between HIV-exposure and mortality (Table V; available at www.jpeds.com). Specifically, HIV-EU infants born to mothers with late-stage HIV who did not receive ARVs during pregnancy had substantially higher rates of mortality than infants of mothers with early stage HIV or mothers who received ARVs during pregnancy. Adjusted hazard ratios (95% CIs) comparing HIV-EU infants of mothers with early-stage HIV, mothers with late-stage HIV (and no ARVs during pregnancy) and mothers who received ARVs during pregnancy to HIV-unexposed children were: 1.55 (0.63, 3.80), 4.96 (1.76, 14.04) and 1.92 (0.73, 5.05) respectively. The elevated rate of mortality in infants of mothers with late-stage HIV who did not receive ARVs during pregnancy was also replicated in models for HIV-infected infants.
Table 5.
Effect Modification of the Relationship between HIV-Exposure Group and Mortality by Maternal Disease Progression and ARV Us
| HIV-Unexposed | HIV-EU | HIV-Infected | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| (ref.) | Early-stage maternal HIV1 no ARVs | Late-stage maternal HIV2 no ARVs | Maternal ARVs during pregnancy | Early-stage maternal HIV1 no ARVs | Late-stage maternal HIV2 no ARVs | Maternal ARVs during pregnancy | |
| N | 1202 | 791 | 136 | 370 | 65 | 37 | 51 |
| Observed # deaths (%) | 16 (1.3%) | 24 (3.0%) | 10 (7.4%) | 15 (4.1%) | 18 (27.7%) | 15 (40.5%) | 17 (33.3%) |
| 1-year Mortality Rate3 | 1.2% (0.8, 2.0) | 2.3% (1.6, 3.5) | 7.4% (4.0, 13.8) | 3.1% (1.9, 5.1) | 24.1% (15.2, 38.2) | 54.2% (32.7, 89.8) | 29.8% (18.5, 47.9) |
|
| |||||||
|
Crude HR (95%CI)4 with HIV-unexposed as referent |
Ref | 1.96 (1.02, 3.76) | 6.05 (2.65, 13.82) | 2.47 (1.19, 5.11) | 20.06 (10.02, 40.17) | 44.77 (21.23, 94.42) | 24.13 (11.93, 48.84) |
| P-value | – | 0.043 | <0.001 | 0.015 | <0.001 | <0.001 | <0.001 |
|
Crude HR (95%CI) with infants whose mothers had early-stage HIV (no ARVs) as referent |
– | Ref. | 3.08 (1.43, 6.66) | 1.26 (0.65, 2.44) | Ref. | 2.23 (1.08, 4.62) | 1.20 (0.61, 2.38) |
| P-value | – | – | 0.004 | 0.500 | – | 0.030 | 0.596 |
|
Crude HR (95%CI) with infants whose mothers had late-stage HIV (no ARVs) as referent |
– | – | Ref. | 0.41 (0.18, 0.94) | 0.54 (0.26, 1.13) | ||
| P-value | – | – | – | 0.036 | – | – | 0.100 |
|
| |||||||
|
Adjusted HR (95%CI)5 with HIV-unexposed as referent |
Ref | 1.55 (0.63, 3.80) | 4.96 (1.76, 14.04) | 1.92 (0.73, 5.05) | 18.73 (7.52, 46.62) | 33.50 (12.55, 89.41) | 19.96 (8.00, 49.77) |
| P-value | – | 0.343 | 0.003 | 0.186 | <0.001 | <0.001 | <0.001 |
|
Adjusted HR (95%CI) with infants whose mothers had early-stage HIV (no ARVs) as referent |
– | Ref. | 3.21 (1.47, 7.01) | 1.24 (0.63, 2.44) | Ref. | 1.79 (0.84, 3.83) | 1.07 (0.52, 2.16) |
| P-value | – | – | 0.003 | 0.527 | – | 0.134 | 0.860 |
|
Adjusted HR (95%CI) with infants whose mothers had late-stage HIV (no ARVs) as referent |
– | – | Ref. | 0.39 (0.17, 0.90) | – | Ref. | 0.60 (0.28, 1.28) |
| P-value | – | – | – | 0.028 | – | – | 0.185 |
Early stage defined as WHO stage I or II and CD4 cell count >200 cells/mL, or WHO stage III and CD4 cell count >350 cells/mL.
Based on criteria for ARV initiation in Tanzania as of July 2005, late-stage defined as WHO stage IV disease, or CD4 count ≤200 cells/mL, or WHO stage III and CD4 count ≤350).
1-year mortality rate estimated from poisson regression models.
All hazard ratios, 95% confidence intervals and p-values are from cox proportional hazards models with the exact method for ties.
Adjusted Models were adjusted for maternal education (0, 1–7 or 8+ years), occupation (housewife with income, housewife without income, or other), height (cm), number of household assets (0, 1 or 2 from a list that included television and refrigerator), and infant sex and treatment group (multivitamin vs. placebo), and also time-varying covariates for breastfeeding duration and exclusive breastfeeding duration.
Discussion
In this longitudinal study of >3500 infants born to HIV-infected and HIV-uninfected mothers in Dar es Salaam, Tanzania, we found that HIV-infected infants had the highest rates of mortality, morbidity and all three types of growth failure from 6 weeks through 18 months of age. HIV-EU infants also had substantially higher rates of morbidity and mortality than HIV-unexposed infants, but mixed outcomes with regards to growth. HIV-EU infants were more likely to be underweight at 6 weeks of age; however, they experienced slower declines in HAZ, WAZ and WHZ over follow-up, and by 18 months they actually exhibited a higher mean HAZ score than their HIV-unexposed counterparts.
Our findings on the increased likelihood of mortality (4, 6), morbidity (30) and poor growth (31) among HIV-infected infants are consistent with the literature on pediatric HIV-infection in sub-Saharan Africa. Research comparing health outcomes in HIV-EU children to HIV-infected or unexposed infants in low-resource settings is limited; however, our findings are consistent with a handful of other studies which found that morbidity (32, 33) and mortality (34, 35) among HIV-EU children is greater than that of HIV-unexposed children. One explanation for the increased risk of mortality and morbidity in HIV-EU children compared with unexposed infants is prenatal alterations in immune function. Studies have documented immune abnormalities in HIV-EU children compared with unexposed infants including reduced thymic output - measured with cord blood mononuclear cells to determine T-lymphocyte receptor excision circles, lower naive CD4 counts (11), as well as reductions in CD4/CD8 ratios and CD41, CD81 and naive T-lymphocyte percentages (12). In addition, HIV-EU children are also more likely to be exposed to infections due to maternal HIV status (3). In particular, studies from sub-Saharan Africa have documented higher rates of tuberculosis (13) and Pneumonocystis jirovecii (15) in HIV-EU children compared with their unexposed peers.
Reduced breastfeeding may also be an important cause of increased morbidity and mortality in HIV-EU infants. In our study, we found that on average, HIV-infected mothers exclusively breastfed their infants longer than HIV-uninfected mothers, but discontinued breastfeeding much earlier than HIV-uninfected mothers. This is consistent with the counselling that HIV-infected mothers received focusing on the benefits of exclusively breastfeeding over mixed feeding, but also on the continued risk of transmitting the virus to their children with prolonged breastfeeding. Comparing our analyses from models including and excluding infant feeding covariates, we found that adjusting for infant feeding in our analyses explained only a small amount of the difference in mortality and growth outcomes, but did not fully explain the effect of HIV-exposure group on morbidity and mortality.
Our finding that infants of mothers with late-stage HIV who did not receive ARVs had the highest rates of mortality is consistent with other studies that have found maternal HIV disease progression is inversely related to health outcomes in their children (36, 37). Interestingly, we also found that infants of mothers who received ARVs had lower rates of mortality than infants of mothers with late-stage HIV who did not receive ARVs; though, our power to compare these two groups was limited. Although there is strong evidence that maternal ARV use during pregnancy and lactation can lower the risk of HIV-transmission to her child (38), our findings add to the growing body of evidence that maternal ARVs may confer health benefits to their children beyond HIV-transmission (39–42). It is worth noting that although maternal ARV use among mothers with late-stage HIV was associated with lower rates of mortality, HIV-EU infants still had substantially higher rates of mortality than HIV-unexposed infants regardless of maternal HIV-stage and ARV use. These findings indicate that even though the provision of ARVs for mothers during pregnancy and lactation may partially mitigate the effects of HIV exposure on child health, it is unlikely to achieve the same health outcomes for HIV-EU infants as in HIV-unexposed infants.
Our findings on growth outcomes were surprising. Specifically, we found that HIV-EU infants had lower HAZ, WAZ and WHZ at 6 weeks of age, which is consistent with the few other studies that have assessed birth size in HIV-EU and HIV-unexposed infants in low-resource settings (5). Surprisingly, however, we found that the HIV-EU infants in our study experienced slower declines in HAZ, WAZ and WHZ than their unexposed peers over follow-up, and that ultimately, HIV-EU infants had lower rates of stunting from 6 weeks to 18 months, and higher mean HAZ scores by 18 months of age. There are several potential explanations for this counterintuitive result. First, the rate of mortality was higher in the HIV-EU population, and thus there is likely survival bias in that the infants who were most likely to become stunted in the HIV-EU sample were also at higher risk of death during follow-up (43). There were also two important systematic differences between our two trial populations that may explain these results. Because a previous trial in urban Dar es Salaam found that multivitamin supplementation (vitamins B-complex, C and E) for HIV-infected mothers during pregnancy and lactation slowed disease progression and delayed maternal mortality (44), all HIV-infected mothers in our first trial received multivitamin supplementation during pregnancy and lactation, but mothers in our second trial did not. Previous work from our group has shown that the provision of these supplements to HIV-infected women during pregnancy and lactation improves post-natal growth in their offspring (45). In addition, in accordance with the Tanzanian Ministry of Health and Social Welfare guidelines at the time of the trial, all HIV-EU infants all received prophylactic cotrimoxazole as a strategy to potentially reduce maternal-to-child transmission of HIV. A recent meta-analysis of 10 randomized controlled trials (46) has indicated that antibiotic use, and particularly cotrimoxazole as given as a prophylactic for HIV (47), has the potential to improve linear and ponderal growth in young children.
There are several limitations of our study. Given our study’s observational design, and particularly that our data come from two separate trials, we cannot rule out residual confounding. In our analyses, we adjusted for several socioeconomic and demographic characteristics that are strong predictors of child health outcomes, however we cannot eliminate the possibility that an unmeasured confounder is driving the observed relationships. In particular, although our two trials overlapped in duration, the first trial initiated randomization three years before the second trial, thus secular trends in Dar es Salaam could explain some, but likely not all of our findings – the national under 5 mortality rate in Tanzania fell only 7.1% from 2000 until 2012 (48). In addition, the majority of our participants enrolled and completed study follow-up in the pre-ARV era, so we have limited capacity to extrapolate our findings to populations where there is more access to ARVs. Due to the change in national guidelines on ARV use during our study, we were able to conduct sub-analyses accounting for maternal HIV-progression and ARV use; however, we had limited power for these analyses. It is worth noting that even after stratifying by maternal disease progression and ARV use, our findings on worse health outcomes for HIV-EU and HIV-infected infants did not change substantially.
Our study also has several strengths. The prospective, longitudinal study design and the collection of comprehensive data on socio-demographic characteristics, infant feeding practices, and maternal HIV disease progression and ARV usage allowed us to conduct rigorous analyses accounting for important confounders and effect modifiers. In addition, the frequent follow-up visits enabled us to include monthly morbidity and growth outcomes.
Our findings demonstrate that infants born to HIV-infected mothers have substantially poorer health outcomes than infants born to HIV-uninfected mothers, regardless of infant HIV infection. Although HIV-infected infants had dramatically inferior health and growth outcomes, HIV-EU infants in our study also experienced a nearly three-fold risk of mortality and higher rates of numerous morbidities compared with their unexposed peers. These poor health outcomes remained even after accounting for sociodemographic characteristics, infant feeding practices and maternal disease progression and ARV use. Interestingly, HIV-EU infants had improved growth outcomes during the 18 months of follow-up. Our findings highlight the importance of targeting not only HIV-infected, but also HIV-EU infants, in HIV care and treatment and child health programs in low-resource settings, if continued progress in improving child health is to be made.
Acknowledgments
We thank the mothers, children, and field teams, including physicians, nurses, midwives, supervisors, laboratory staff, and the administrative staff, who made this study possible. We also thank Ellen Hertzmark for her expert advice.
Supported by the National Institutes of Health (R01 HD043688-01, R01 HD048969-01, K24HD058795, and K24 DK104676). C.D serves on the Editorial Board of The Journal of Pediatrics.
ABBREVIATIONS
- ART
Antiretroviral therapy
- HIV-EU
HIV-Exposed-Uninfected
- PMTCT
Prevention of mother-to-child transmission
- HAZ
Height-for-age z-score
- WAZ
Weight-for-age z-score
- WHZ
Weight-for-height z-scores
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The other authors declare no conflicts of interest.
Trial registration Clinicaltrials.gov: NCT00197730 and NCT00421668
References
- 1.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic. 2013 [Google Scholar]
- 2.UNAIDS, editor. 2015 Progress Report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. Geneva, Switzerland: 2015. [Google Scholar]
- 3.Filteau S. The HIV‐exposed, uninfected african child. Tropical Medicine & International Health. 2009;14(3):276–87. doi: 10.1111/j.1365-3156.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 4.Newell M-L, Brahmbhatt H, Ghys PD. Child mortality and HIV infection in Africa: a review. AIDS. 2004;18:S27–S34. doi: 10.1097/00002030-200406002-00004. [DOI] [PubMed] [Google Scholar]
- 5.Isanaka S, Duggan C, Fawzi WW. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutrition reviews. 2009;67(6):343–59. doi: 10.1111/j.1753-4887.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newell M-L, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. The Lancet. 2004;364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 7.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. The Pediatric infectious disease journal. 2007;26(6):519–26. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 8.Heidari S, Mofenson L, Cotton MF, Marlink R, Cahn P, Katabira E. Antiretroviral drugs for preventing mother-to-child transmission of HIV: a review of potential effects on HIV-exposed but uninfected children. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;57(4):290–6. doi: 10.1097/QAI.0b013e318221c56a. [DOI] [PubMed] [Google Scholar]
- 9.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clinical & Experimental Immunology. 2014;176(1):11–22. doi: 10.1111/cei.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. The Lancet infectious diseases. 2012;12(4):330–40. doi: 10.1016/S1473-3099(11)70341-3. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen SD, Jeppesen DL, Kolte L, Clark DR, Sørensen TU, Dreves A-M, et al. Impaired progenitor cell function in HIV-negative infants of HIV-positive mothers results in decreased thymic output and low CD4 counts. Blood. 2001;98(2):398–404. doi: 10.1182/blood.v98.2.398. [DOI] [PubMed] [Google Scholar]
- 12.Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96(12):3866–71. [PubMed] [Google Scholar]
- 13.Cotton M, Schaaf H, Lottering G, Weber H, Coetzee J, Nachman S. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting [Short Communication] The international journal of tuberculosis and lung disease. 2008;12(2):225–7. [PubMed] [Google Scholar]
- 14.Thea DM, St Louis ME, Atido U, Kanjinga K, Kembo B, Matondo M, et al. A prospective study of diarrhea and HIV-1 infection among 429 Zairian infants. New England Journal of Medicine. 1993;329(23):1696–702. doi: 10.1056/NEJM199312023292304. [DOI] [PubMed] [Google Scholar]
- 15.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. The Lancet. 2007;369(9571):1440–51. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 16.Bailey RC, Kamenga MC, Nsuami MJ, Nieburg P, St Louis ME. Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. International journal of epidemiology. 1999;28(3):532–40. doi: 10.1093/ije/28.3.532. [DOI] [PubMed] [Google Scholar]
- 17.Ndirangu J, Newell M-L, Bland RM, Thorne C. Maternal HIV infection associated with small-for-gestational age infants but not preterm births: evidence from rural South Africa. Human reproduction. 2012:des090. doi: 10.1093/humrep/des090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halsey NA, Boulos R, Holt E, Ruff A, Brutus J-R, Kissinger P, et al. Transmission of HIV-1 infections from mothers to infants in Haiti: impact on childhood mortality and malnutrition. Jama. 1990;264(16):2088–92. [PubMed] [Google Scholar]
- 19.Patel D, Bland R, Coovadia H, Rollins N, Coutsoudis A, Newell M-L. Breastfeeding, HIV status and weights in South African children: a comparison of HIV-exposed and unexposed children. AIDS. 2010;24(3):437–45. doi: 10.1097/QAD.0b013e3283345f91. [DOI] [PubMed] [Google Scholar]
- 20.Chougrani Imene LD, Matheron Sophie, Mandelbrot Laurent, Azria Elie. Safety of Protease Inhibitors in HIV-infected Pregnant Women. HIV/AIDS - Research and Palliative Care. 2013;5:253–62. doi: 10.2147/HIV.S33058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powis KM, Smeaton Laura, Ogwu Anthony, Lockman Shahin, Dryden-Peterson Scott, van Widenfelt Erik, Leidner Jean, Makhema Joseph, Essex Max, Shapiro Roger L. Effects of in Utero Antiretroviral Exposure on Longitudinal Growth of HIV-Exposed Uninfected Infants in Botswana. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2011;56(2):131–8. doi: 10.1097/QAI.0b013e3181ffa4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powis KM, Smeaton L, Hughes MD, Tumbare EA, Souda S, Jao J, et al. In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. AIDS. 2016;30(2):211–20. doi: 10.1097/QAD.0000000000000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson DL, Patel K, Siberry GK, Van Dyke RB, DiMeglio LA, Geffner ME, et al. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: outcomes from the Pediatric HIV/AIDS Cohort Study. The American journal of clinical nutrition. 2011;94(6):1485–95. doi: 10.3945/ajcn.111.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duggan C, Manji KP, Kupka R, Bosch RJ, Aboud S, Kisenge R, et al. Multiple micronutrient supplementation in Tanzanian infants born to HIV-infected mothers: a randomized, double-blind, placebo-controlled clinical trial. The American Journal of Clinical Nutrition. 2012;96(6):1437–46. doi: 10.3945/ajcn.112.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald CM, Manji KP, Kisenge R, Aboud S, Spiegelman D, Fawzi WW, et al. Daily Zinc but Not Multivitamin Supplementation Reduces Diarrhea and Upper Respiratory Infections in Tanzanian Infants: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. The Journal of Nutrition. 2015 doi: 10.3945/jn.115.212308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupka R, Manji KP, Bosch RJ, Aboud S, Kisenge R, Okuma J, et al. Multivitamin Supplements Have No Effect on Growth of Tanzanian Children Born to HIV-Infected Mothers. The Journal of Nutrition. 2013;143(5):722–7. doi: 10.3945/jn.112.170498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Locks LM, Manji KP, McDonald CM, Kupka R, Kisenge R, Aboud S, et al. Effect of zinc and multivitamin supplementation on the growth of Tanzanian children aged 6–84 wk: a randomized, placebo-controlled, double-blind trial. The American Journal of Clinical Nutrition. 2016;103(3):910–8. doi: 10.3945/ajcn.115.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Standardization WECoB, Organization WH. Physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee: World Health Organization. 1995 [PubMed] [Google Scholar]
- 29.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weightfor-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. p. 156. [Google Scholar]
- 30.Graham SM. HIV and respiratory infections in children. Current opinion in pulmonary medicine. 2003;9(3):215–20. doi: 10.1097/00063198-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Duggan C, Fawzi W. Micronutrients and child health: studies in international nutrition and HIV infection. Nutrition reviews. 2001;59(11):358–69. doi: 10.1111/j.1753-4887.2001.tb06963.x. [DOI] [PubMed] [Google Scholar]
- 32.Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity Among Human Immunodeficiency Virus-exposed But Uninfected, Human Immunodeficiency Virus-infected, and Human Immunodeficiency Virus-unexposed Infants in Zimbabwe Before Availability of Highly Active Antiretroviral Therapy. The Pediatric Infectious Disease Journal. 2011;30(1):45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 33.Slogrove A, Reikie B, Naidoo S, De Beer C, Ho K, Cotton M, et al. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. Journal of tropical pediatrics. 2012;58(6):505–8. doi: 10.1093/tropej/fms019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;41(4):504–8. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 35.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child Mortality According to Maternal and Infant HIV Status in Zimbabwe. The Pediatric Infectious Disease Journal. 2007;26(6):519–26. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, et al. Does Severity of HIV Disease in HIV-Infected Mothers Affect Mortality and Morbidity among Their Uninfected Infants? Clinical Infectious Diseases. 2005;41(11):1654–61. doi: 10.1086/498029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venkatesh KK, de Bruyn G, Marinda E, Otwombe K, van Niekerk R, Urban M, et al. Morbidity and Mortality among Infants Born to HIV-Infected Women in South Africa: Implications for Child Health in Resource-Limited Settings. Journal of Tropical Pediatrics. 2010 doi: 10.1093/tropej/fmq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Organization WH. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: recommendations for a public health approach-2010 version. World Health Organization; 2010. [PubMed] [Google Scholar]
- 39.Ndirangu J, Newell M-L, Thorne C, Bland R. Treating HIV-infected mothers reduces under 5 years of age mortality rates to levels seen in children of HIV-uninfected mothers in rural South Africa. Antiviral therapy. 2011;17(1):81–90. doi: 10.3851/IMP1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mermin J, Were W, Ekwaru JP, Moore D, Downing R, Behumbiize P, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. The Lancet. 2008;371(9614):752–9. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- 41.Marazzi MC, Nielsen-Saines K, Buonomo E, Scarcella P, Germano P, Majid NA, et al. Increased Infant Human Immunodeficiency Virus-Type One Free Survival at One Year of Age in Sub-Saharan Africa With Maternal Use of Highly Active Antiretroviral Therapy During Breast-Feeding. The Pediatric Infectious Disease Journal. 2009;28(6):483–7. doi: 10.1097/INF.0b013e3181950c56. [DOI] [PubMed] [Google Scholar]
- 42.Sztam KA, Liu Enju, Manji Karim P, Kupka Roland, Kisenge Rodrick, Aboud Said, Fawzi Wafaie W, Bosch Ronald J, Duggan Christopher P. Maternal Antiretroviral Therapy is Associated with Lower Risk of Diarrhea in Early Childhood. Journal of Pediatrics. 2016 doi: 10.1016/j.jpeds.2016.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, Fawzi WW, et al. Associations of Suboptimal Growth with All-Cause and Cause-Specific Mortality in Children under Five Years: A Pooled Analysis of Ten Prospective Studies. Plos One. 2013;8(5) doi: 10.1371/journal.pone.0064636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fawzi WW, Msamanga GI, Spiegelman D, Wei R, Kapiga S, Villamor E, et al. A Randomized Trial of Multivitamin Supplements and HIV Disease Progression and Mortality. New England Journal of Medicine. 2004;351(1):23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 45.Villamor E, Saathoff E, Bosch RJ, Hertzmark E, Baylin A, Manji K, et al. Vitamin supplementation of HIV-infected women improves postnatal child growth. The American Journal of Clinical Nutrition. 2005;81(4):880–8. doi: 10.1093/ajcn/81.4.880. [DOI] [PubMed] [Google Scholar]
- 46.Gough EK, Moodie EEM, Prendergast AJ, Johnson SMA, Humphrey JH, Stoltzfus RJ, et al. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348 doi: 10.1136/bmj.g2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prendergast A, Walker AS, Mulenga V, Chintu C, Gibb DM. Improved growth and anemia in HIV-infected African children taking cotrimoxazole prophylaxis. Clinical infectious diseases. 2011;52(7):953–6. doi: 10.1093/cid/cir029. [DOI] [PubMed] [Google Scholar]
- 48.Afnan-Holmes H, Magoma M, John T, Levira F, Msemo G, Armstrong CE, et al. Tanzania’s Countdown to 2015: an analysis of two decades of progress and gaps for reproductive, maternal, newborn, and child health, to inform priorities for post-2015. The Lancet Global Health. 3(7):e396–e409. doi: 10.1016/S2214-109X(15)00059-5. [DOI] [PubMed] [Google Scholar]


