Abstract
Previous studies have shown the functional neural circuitry differences before and after an explicitly learned motor sequence task, but have not assessed these changes during the process of motor skill learning. Functional magnetic resonance imaging activity was measured while participants (n=13) were asked to tap their fingers to visually presented sequences in blocks that were either the same sequence repeated (learning block) or random sequences (control block). Motor learning was associated with a decrease in brain activity during learning compared to control. Lower brain activation was noted in the posterior parietal association area and bilateral thalamus during the later periods of learning (not during the control). Compared to the control condition, we found the task-related motor learning was associated with decreased connectivity between the putamen and left inferior frontal gyrus and left middle cingulate brain regions. Motor learning was associated with changes in network activity, spatial extent, and connectivity.
Keywords: motor learning, motor skill, functional connectivity, gPPI
1. INTRODUCTION
Motor learning is a process by which new movements are acquired. This motor learning, commonly termed motor skill learning, signifies increased spatial and temporal accuracy of the acquired movement patterns for a performance goal. The increased spatial and temporal accuracy can be achieved through practice alone, or by training (in cases where motor performance involves greater complexity of movement sequences across multiple joints, or inter-limb coordination). Motor skill learning also includes the establishment of new associations between environmental signals or targets and the motor actions. As such, the motor skill learning may involve development of the link between previously acquired movement patterns or environmental signals experienced in the context of the [new] performance goal.[1, 2] Motor skill learning is associated with changes in the brain circuitry underlying the motor performance.[3–6] During motor skill learning, functional connectivity among a broad array of regions including frontal, parietal, and limbic association areas involved in performance transition to a pattern of more efficient circuitry between premotor and dorsolateral prefrontal cortical regions and the dorsal striatum.[7, 8] The performance of well-learned motor skills, such as walking is thought to be efficient and nearly automatic. Age-related brain changes can disrupt this neural circuitry. In response to this age-related disruption, the brain pattern of activation for performance of the once well-learned motor skill may regress to a pre-automatization phase;[9] a phase in which the brain activation associated with performance is inefficient (i.e. a broad spatial pattern of brain regions activated during performance).[10] This pattern of activation in brain circuitry for performance may have a secondary impact on the efficiency of brain networks for performance in other motor and cognitive behaviors.[5, 11]
To better understand age-related changes in the neural circuitry of well-learned motor skill performance and potential intervention strategies to facilitate adaptive or restorative change in performance, it is important to have a greater understanding of motor skill learning-induced changes. Especially changes in neural circuitry during the process of acquiring or adapting the movement pattern.[9] Most investigations of changes in brain activation patterns with motor skill learning have involved performance of motor actions that can be done lying on the back, and require a small range of motion, usually of a single limb to avoid motion artifacts to the signal, such as a finger-tapping movement task.[12–16]
Brain activation patterns for phases of motor skill learning in finger-tapping tasks have previously been described.[4, 17, 18] Much of the previous work has examined changes in the brain activation pattern but not network functional connectivity changes.[4, 18, 19] In addition, the functional neural circuitry changes have been defined before and after a period of practice of an explicitly learned finger-tapping motor sequence.[3–5, 17–19] The explicitly learned task is more representative of conscious learning of the defined sequence of the movements of the performance. Explicitly learned movement task are, however, less reflective of the movement-related procedural learning characteristic of motor skill learning, or ‘learning while doing’ the finger-tapping task. Newer methods of functional connectivity (FC) analysis of fMRI signal processing,[20] like psychophysiological interaction (PPI),[21–24] have made it possible to define the connectivity (task-related functional communications) among brain regions or circuits underlying motor performance.
In this study, we used FC methods, specifically generalized PPI (gPPI),[23] to define motor skill learning induced changes in the neural circuitry associated with repeated action of a finger-tapping sequenced task (e.g. practice). [20, 24, 25] Generalized PPI investigates context-dependent differences in functional connectivity from a specified seed region of the brain. Based on our understanding of motor skill learning,[1, 2] and previous findings of brain activation patterns related to motor skill learning [3–7, 26] we expected that spatial activation and functional connectivity would shift from an initial, inefficient pattern of high activity across a broad cortical network of prefrontal and premotor association areas, posterior parietal association areas, cingulate motor, and anterior cingulate areas to a more efficient pattern of neural activations. We expect, after repeated action of the simple finger-tapping task, the motor skill learning would induce the functional connectivity to be more restricted to a cortical-striatal network of premotor and dorsolateral prefrontal cortex with the putamen (motor component of the striatum). Changes in functional neural circuitry during motor skill learning may help explain changes in motor performance efficiency and in the identification of effective rehabilitation approaches to facilitate adaptive or restorative changes in motor skills.
2. METHODS
2.1. Study Design and Subjects
Functional MR imaging was performed on 13 (self-reported) right-handed healthy adult volunteers (mean age 23.8 ± 3.1 years); 6 males and 7 females. Participants had no history of a neurological disorder, were not currently taking medications known to alter brain function, and were eligible to undergo MR imaging. The University of Pittsburgh Institutional Review Board approved the study, and all subjects provided written informed consent for participation.
2.2. MRI Data Collection
All scanning was conducted using a 3T Siemens Trio TIM scanner located at the Magnetic Resonance Research Center at the University of Pittsburgh using a 12-channel coil. A high-resolution T1-weighted 3D sequence was collected (TR=19ms/TE=4.92/FA=25) with a field of view 176×256 x192 and voxel dimensions of 1.17×1.17×1.0mm3. T2*-weighted BOLD acquisition using gradient-echo echo-planar imaging (EPI) was collected during functional tasks (TR=2000ms/TE=30ms/FA=90) with a field of view 64×64×34 and voxel dimensions of 3.12×3.12×3.00 mm3. The head was immobilized using cushions to minimize motion artifacts.
2.3. Motor Learning Task
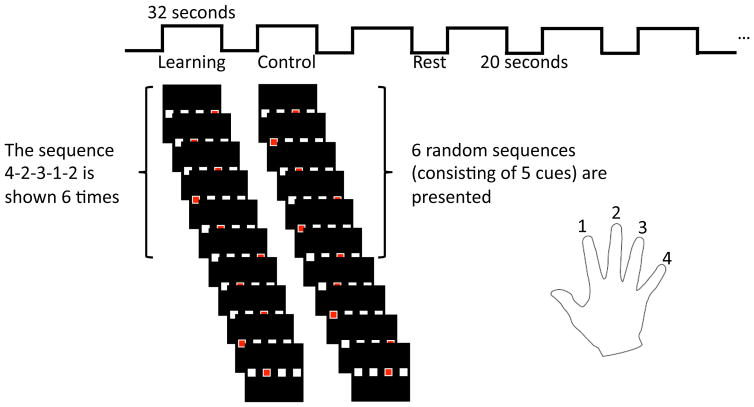
Subjects performed motor skill learning and a comparison control motor task. The sequencing of movements, which involves individual movements sequenced in a spatial pattern or in time, is a common component of motor skill learning for performance of a specific task. The motor skill learning studied involved performance of a finger-tapping motor sequence-learning task. Subjects used their dominant hand (right in all subjects). Specifics of the finger-tapping task are outlined in Figure 1. The finger-tapping motor skill-learning task involved blocks of repeated learning sequences alternated with blocks of control sequences, with an equivalent number of learning and control sequences performed. Each functional scan (11min) had six 32s learning blocks and six 32s control blocks with 20s rest periods in between. In each task block, subjects were presented with five visual cues for the finger-tapping movement sequence. In learning blocks, the motor learning sequence was a pre-determined, 5-movement sequence (e.g. 4-2-3-1-2 with the index finger designated 1, and the little finger as 4). The sequence was repeated a total of five times within each 32s block. The same pre-determined learning sequence was repeated across all six learning blocks within a functional scan. To control for improvement in the finger motor performance alone, in the control blocks participants were presented with random sequences of 5 finger movements; each tap was 1 second. During the interweaved 20s rest blocks, subjects were instructed to stare at the white cross hair and stay awake.
Figure 1.
Illustration of the task used in this study. Six learning and control blocks are presented with rest in between. Subjects are presented with the sequence 4-2-3-1-2 during the learning block a total of 6 times. During the control blocks 6 random sequences are shown to the subject.
Participants were positioned supine in the scanner and viewed the visual cues provided through a mirror fixed above the head coil. Worn on the right hand, an instrumented glove was used to record participant responses to the visual cues for the finger-tapping sequences viewed. A red box in one of four white boxes placed, side to side, visually-cued a finger tap of the sequence. Each white box corresponded to one finger of the right hand, starting with the second digit (index finger) to the fifth digit (little finger). When a red box appeared in the box corresponding to one of the four fingers, subjects had been instructed to press the corresponding button as fast and as accurately as possible. Participants were not told to expect repeated (learning) sequences interspersed with random (control) finger-tapping motor sequences. Stimuli were presented with E-prime software (E-prime Version 2.0 Psychology Software Tools; Pittsburgh PA).
2.4. Reaction time behavioral changes
To determine changes in reaction time and differences between the finger-tapping task conditions, a 2-way repeated measures ANOVA was performed with the following factors: condition (control versus learning) and time (blocks 1–6). The change in mean reaction time was determined across each task block.
2.5. MRI Preprocessing
Statistical Parametric Mapping (SPM12) toolbox was utilized to preprocess the fMRI data. The functional scans were first motion corrected using a linear coregistration procedure to correct for motion artifacts (using the mean image as the reference, mutual information similarity metric, and a 4th degree B-spline interpolation). For all the subjects, the motion was less than 2mm and no subjects were discarded. These aligned images were then co-registered (via affine transformation, normalized mutual information similarity metric, and a 4th degree B-spline interpolation) to the same-session structural image (a skull stripped image was used to improve co-registration between functional and structural data), which only stored a transformation (to avoid interpolation in a high resolution space). The images were normalized and warped into the standard MNI space using SPM12 via the structural image (via unified segmentation/ normalization procedure). A deformation field is generated from this process and this was then applied to the aligned functional scans. After registration and normalization (to MNI space which has a 2mm isotropic resolution), the functional images were smoothed with an 8mm Gaussian kernel.
2.6. General Linear Model
A general linear model (GLM) analysis was applied to the functional data where we modeled the learning and control tasks (convolved with a hemodynamic response function) as well as the mean of the signal and motion parameters from the realignment. A high-pass filter (1/128Hz) was used to account for slow drift in the fMRI signal and serial correlations in the fMRI time series were accounted for using an autoregressive AR(1) model during parameter estimation. We then computed the learning compared to control contrast (a difference in parameter estimates) and used these maps in a subsequent group level analysis using a one-sample t-test. This tested whether there were significant differences between learning and control hemodynamic evoked responses across the participants in the sample.
We further wanted to test whether there were differences between the first half of the finger-tapping task and the second half. We performed another GLM where we modeled the first three learning/control blocks independently of the second three learning/control blocks. We then computed contrasts for each block (learning/control first and second half of the paradigm). We used these contrasts in a subsequent group level analysis using one-sample t-tests, to test for differences in activation between the first and second halves of the blocks.
2.7. Connectivity of the Putamen using gPPI
We used gPPI, a region of interest (ROI) method, to investigate the effects of a specific seed ROI on the neural circuitry.[23] We used an a priori ROI seed from the putamen to perform the gPPI, because the putamen is both involved in cortical to striatal circuitry related to the sequencing of movements for a well-learned motor task, and the putamen is involved in the selection and timing of movements of acquired motor skills.[5, 17, 27, 28] The automatic anatomical labeling (AAL)[29] template was used to identify the putamen bilaterally.
This analysis models the association between the time-series in the chosen ROI with task-based activation in every other region of the brain. In brief, the method first uses the ROI to generate a principal time series using principal components analysis. This time series is then deconvolved with a standard hemodynamic response function which estimates the neural activity from the ROI.[30] An interaction term is generated between each task regressor (i.e., learning, control) and the estimated neural activity. The interaction term is then convolved with the standard hemodynamic response function. Subsequently, another general linear model was performed to model the two tasks (learning and control), the ROI principal time series, and the two interaction terms (between the ROI and learning, and ROI and control). After this model estimation, contrasts were generated for each subject to compare the learning and control interaction terms. The contrasts were entered into a subsequent group level analysis. This analysis was used to test whether there were differences in the following interaction terms: (a) learning related brain activity and activity in the putamen compared to (b) non-learning (control) related brain activity and the activity in the putamen.
2.8. Multiple Comparisons Correction
A permutation method for peak-cluster level error correction was applied for this whole-brain analysis, as implemented in 3dClustSim[31] by taking into account the significance of the peak voxel (threshold, p-value <0.005, uncorrected), controlling for multiple comparisons. The smoothness of the residuals was estimated using 3dFWHMx.[31] Essentially, using 3dFWHMx the smoothness of the residuals is estimated, and then 3dClustSim is used to estimate the minimum cluster size required to control for multiple comparisons. It does this by performing alpha probability simulations where it computes the probability of a random field of noise (with a certain smoothness and a given cluster threshold, in this case p<0.005) to produce a cluster of a given size. A minimum of 115 voxels was needed to attain a ClustSim corrected level of p < 0.05. All statistical testing was performed only in the gray matter (generated using the MNI space SPM template). This approach was used to limit the number of voxels tested and increase sensitivity.
3. RESULTS
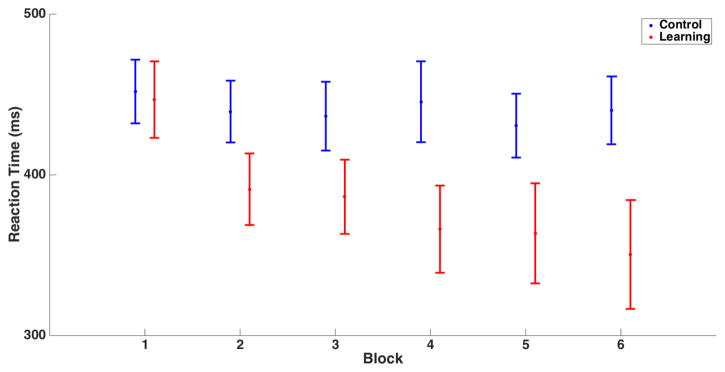
As shown in figure 2, the group-averaged changes in reaction times illustrate that motor learning occurred as expected for the task conditions. The mean reaction times for the learning and control blocks were 384±100ms and 441±74ms, respectively. In the learning blocks, reaction time decreased sharply over the early blocks, with little or no change in reaction time over the control blocks (Figure 2). Using a 2-way ANOVA interaction between condition and time, we found that the difference in trajectory of reaction times across trials was different between conditions, F(5,60) = 6.9, p<0.005.
Figure 2.
Mean and standard error of reaction time for the six control (blue) and learning (red) blocks. While the control block reaction times show little change, reaction time decreased over the learning blocks.
3.1. General Linear Model: Learning vs. Control
Regions of statistically significant increased or decreased signals relative to rest during the learning and control task as well as regions where there was a significant difference between the tasks are shown in table 1. The following regions had increased signal during the learning task: left primary motor and sensory cortex, left lateral premotor, left middle frontal gyrus, and left posterior parietal cortex, left putamen, bilateral medial (supplementary motor and pre-supplementary motor) and lateral premotor areas, right primary motor and middle frontal gyrus, and right posterior parietal cortex. Decreased signal was noted in the right insula and superior temporal cortex in the learning condition. The same regions showed increased signal in the control as in the learning condition, but with a greater extent of the left and right posterior parietal cortex regions activated, and additional increased signal of the left thalamus, left insula, left and right visual association areas, and bilateral cerebellum.
Table 1.
Regions activated during the learning and control tasks as well as regions that showed a significant difference (note negative parameter estimate differences mean control is greater than learning).
| Task | Region | x | y | z | T-max | Cluster Size |
|---|---|---|---|---|---|---|
| Learning | Left Precentral / Postcentral Gyrus / Lateral Premotor Area / Middle Frontal Gyrus / Superior/Inferior Parietal Lobule | −34 | 0 | 48 | 7.6 | 3804 |
| Left Putamen | −24 | −2 | 6 | 4 | 135 | |
| Bilateral Supplementary Motor Area / Lateral Premotor Area | −10 | 4 | 50 | 5.5 | 678 | |
| Right Precentral / Middle Frontal Gyrus | 58 | 8 | 32 | 8.3 | 829 | |
| Right Superior Parietal Lobule / Angular Gyrus | 28 | −54 | 44 | 6.1 | 297 | |
| Right Insula / Superior Temporal Gyrus | 62 | −24 | 6 | −5.1 | 319 | |
|
| ||||||
| Control | Left Precentral / Postcentral Gyrus / Lateral Premotor Area / Middle Frontal Gyrus / Supplementary Motor Area / Superior /Inferior Parietal Lobule / Precuneus | −56 | −26 | 42 | 13.3 | 7794 |
| Left Middle Occipital Gyrus | −44 | −72 | 10 | 96.4 | 179 | |
| Left Putamen | −26 | −2 | 4 | 5.3 | 322 | |
| Left Thalamus | −14 | −18 | 6 | 5 | 165 | |
| Left Insula | −50 | −24 | 18 | 4.7 | 146 | |
| Right Supramarginal / Postcentral Gyrus | 56 | −38 | 24 | 4.6 | 276 | |
| Right Middle Occipital Gyrus | 30 | −88 | 2 | 5.7 | 117 | |
| Right Putamen | 20 | 8 | 4 | 4.1 | 133 | |
| Right Superior / Inferior Parietal Lobule / Precuneus | 26 | −64 | 46 | 6.9 | 779 | |
| Bilateral Cerebellum Vermis/Culmen | 4 | −58 | −10 | 4.3 | 124 | |
|
| ||||||
| Learning-Control | Left Superior Parietal Lobule / Precuneus | −20 | −64 | 58 | −5.5 | 146 |
| Bilateral Thalamus | −4 | −20 | 12 | −4.5 | 488 | |
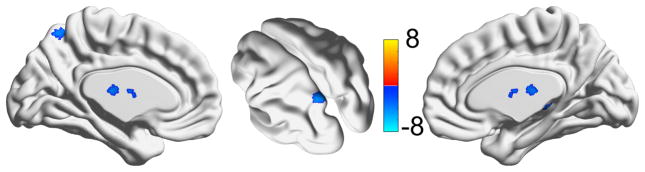
Lower activation during the learning compared to the control condition was found in bilateral thalamus and the left lateral and medial posterior parietal cortex. No brain regions were found where activation was greater in the learning than in the control condition. These are shown in figure 3. We further investigated and found that the difference between learning and control condition was in fact a result of increased signal in both conditions (where control just had a greater increase in signal than learning in those areas).
Figure 3.
Regions that show significantly greater hemodynamic evoked responses in the control compared to the learning task (blue). No regions showed the opposite effect (red). The color bar indicates the t-statistic from a one-sample t-test (testing whether the contrast learning compared to control was significantly greater than zero).
3.2. General Linear Model: Differentiating Early and Late Learning
To further understand whether there was a different pattern of activation during the initial or early and the later period of motor learning, we performed one-sample t-tests to compare the first and second halves of the paradigm (for both learning and control). The results from these tests are shown in table 2 and in figure 4.
Table 2.
Regions activated during the learning/control during the first and second halves (learning/control 1 and 2, respectively).
| Task | Region | x | y | z | T-max | Cluster Size |
|---|---|---|---|---|---|---|
| Learning 1 | Left Precentral/ Postcentral / Superior / Middle Frontal Gyrus Supplementary Motor Area / Medial Frontal Gyrus / Anterior Cingulate Cortex / Precuneus / Superior/ Inferior Parietal Lobule / Middle Occipital Gyrus |
−38 | −28 | 46 | 9 | 5522 |
| Left Hippocampus / Parahippocampus | −28 | −2 | −28 | −5.3 | 170 | |
| Left Superior Frontal Gyrus | −20 | 36 | 48 | −4 | 141 | |
| Right Middle / Inferior Frontal / Precentral Gyrus | 40 | −4 | 56 | 5.7 | 1138 | |
| Right Middle Frontal Gyrus | 36 | 34 | 26 | 7.6 | 149 | |
| Right Superior / Middle Occipital Gyrus / Precuneus / Cuneus / Superior Parietal Lobule / Right Angular Gyrus | 24 | −86 | 2 | 7 | 1250 | |
|
| ||||||
| Learning 2 | Left Precentral /Postcentral / Middle Frontal Gyrus | −34 | −18 | 50 | 5.4 | 2048 |
| Left Supplementary Motor Area / Medial Frontal Gyrus | −8 | −4 | 52 | 4 | 147 | |
| Right Insula / Superior Temporal Gyrus | 60 | −6 | 6 | −6.4 | 602 | |
| Right Cuneus / Precuneus / Posterior Cingulate Cortex | −16 | −64 | 18 | −7.8 | 1551 | |
| Right Precuneus / Middle Cingulate Cortex | 2 | −46 | 56 | −5.6 | 543 | |
|
| ||||||
| Control 1 | Left Precentral / Postcentral / Middle / Inferior Frontal Gyrus Supplementary Motor Area / Medial Frontal Gyrus / Precuneus / Superior / Inferior Parietal Lobule / Middle Occipital Gyrus |
−34 | −40 | 50 | 10.6 | 8391 |
| Left Putamen / Thalamus | −22 | −8 | 2 | 4.1 | 171 | |
| Right Inferior Frontal Gyrus | 42 | 10 | 26 | 4.8 | 310 | |
| Right Middle Occipital Gyrus | 28 | −86 | 2 | 6.5 | 312 | |
| Right Superior Temporal Gyrus | 52 | −46 | 16 | 4.7 | 148 | |
| Right Precuneus / Superior Parietal / Occipital Gyrus | 28 | −74 | 28 | 5.8 | 779 | |
| Right Anterior Cerebellum Vermis/Culmen | 4 | −58 | −10 | 4.4 | 118 | |
| Right Supplementary Motor Area | 4 | −20 | 52 | −3.9 | 118 | |
|
| ||||||
| Control 2 | Left Precentral/Postcentral / Superior / Middle/Inferior Frontal Gyrus / Supplementary Motor Area / Medial Frontal / Cingulate Gyrus / Precuneus / Superior / Inferior Parietal Lobule / Supramarginal Gyrus | −56 | 2 | 34 | 8 | 7968 |
| Left Putamen / Thalamus | −26 | −2 | 2 | 5.7 | 534 | |
| Right Postcentral Gyrus | 52 | −24 | 44 | 5.3 | 213 | |
| Right Supplementary Motor Aresa | 4 | −20 | 52 | −3.9 | 118 | |
| Right Precuneus / Superior Parietal Lobule | 28 | −52 | 44 | 6.4 | 368 | |
| Right Middle Occipital Gyrus | 26 | −84 | 0 | 6.6 | 188 | |
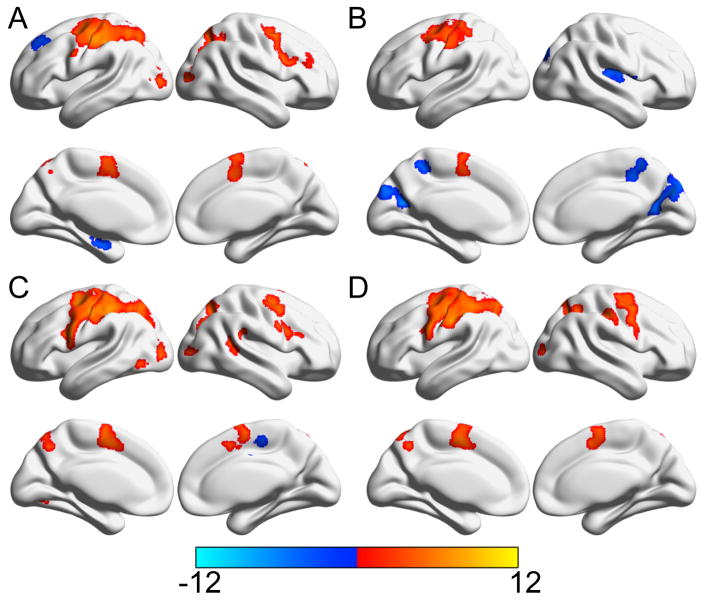
Figure 4.
Areas significantly activated during the first (A) and second (B) halves of the learning blocks as well as the first (C) and second (D) halves of the control blocks. Color bar indicates the t-statistic from one-sample t-tests (tested whether the control and learning blocks from the first/second halves were significantly greater than zero). Areas significantly positively associated with the task regressor are in red while those negatively associated are shown in blue.
We found that the first half of the learning block (figure 4A) showed increased signal in the left primary motor and sensory cortex, left lateral premotor, left prefrontal cortex, left medial premotor areas, left anterior cingulate cortex, left posterior parietal cortex, and left occipital association cortical areas, right primary motor, right lateral premotor, and right prefrontal cortex, right middle frontal gyrus, and right posterior parietal and occipital association cortical areas. Decreased signals were observed in the left hippocampus and medial temporal cortex, and left superior frontal gyrus in this early period of the learning condition. However, in the second half of learning block (figure 4B) we found increased signals in only the left primary motor and sensory cortex finger region, adjacent left premotor cortex (possibly frontal eye fields, Brodmann’s Area 8) and left medial premotor areas. Decreased signals were noted in the right insula and superior temporal gyrus, right posterior parietal and posterior cingulate cortex, and right medial posterior parietal and middle cingulate cortex.
In the first half of the control block (figure 4C) increased signal was found in the left primary motor and sensory cortex, left lateral premotor, left prefrontal cortex, left medial premotor areas, left posterior parietal and occipital association cortical areas, left putamen and thalamus, right inferior frontal, right posterior parietal and occipital association cortical areas, right superior temporal gyrus, and right cerebellum. The right premotor area showed decreased signal. We found that the second half of control block (figure 4D) showed a very similar pattern of increased signal with variations in a few regions. Increased signal observed in the left primary motor and sensory cortex, left lateral premotor, left prefrontal, left medial premotor areas, left posterior parietal and cingulate cortex, left putamen and thalamus, right primary sensory, right medial premotor and right posterior parietal and occipital association cortex. There were decreased signals in the later period of the control condition.
3.3. Connectivity of the Putamen using gPPI
Regions of significant connectivity (interaction term of the gPPI) for learning and control as well as the difference between the interaction terms of the gPPI are given in table 3. The following regions showed significant positive interactions (or task-based putamen connectivity) with the putamen during the learning task: left primary motor and premotor, left lateral and medial premotor and left cingulate motor area, left inferior frontal and insula, right dorsal prefrontal, right ventral prefrontal, right insula and amygdala, right middle temporal gyrus, right parahippocampus and lingual gyrus, and bilateral thalamus.
Table 3.
Regions that showed significant interaction with the putamen during learning and control as well as regions that showed a significant difference (note negative parameter estimate differences mean control is greater than learning).
| Task | Region | x | y | z | T-max | Cluster Size |
|---|---|---|---|---|---|---|
| gPPI: Learning | Left Precentral / Middle Frontal Gyrus | −30 | 0 | 60 | 4.1 | 328 |
| Left Superior / Medial Frontal Gyrus / Supplementary Motor Area / Middle Cingulate | −4 | 16 | 52 | 4.6 | 974 | |
| Left Insula / Inferior Frontal Gyrus | −30 | −10 | −6 | 5.4 | 1258 | |
| Left Lingual Gyrus | −20 | −60 | −6 | 4.2 | 145 | |
| Right Superior / Middle Frontal Gyrus | 30 | 4 | 56 | 4.6 | 467 | |
| Right Inferior Frontal Gyrus / Insula /Amygdala | 26 | 2 | −2 | 7.1 | 2172 | |
| Right Middle Temporal Gyrus | 54 | −36 | 0 | 3.9 | 198 | |
| Right Parahippocampus / Lingual Gyrus | 22 | −40 | −10 | 5 | 450 | |
| Bilateral Thalamus | −2 | −12 | 4 | 3.8 | 252 | |
|
| ||||||
| gPPI: Control | Left Anterior Cingulate Cortex | −2 | 48 | −2 | 4 | 123 |
| Left Parahippocampus | −20 | 10 | −4 | 5.4 | 996 | |
| Left Parahippocampus / Brainstem | −20 | −36 | −6 | 4.4 | 595 | |
| Left Middle Temporal Gyrus | −56 | −42 | 0 | 4.1 | 250 | |
| Right Superior / Middle Frontal Gyrus | 26 | 2 | 56 | 3.8 | 220 | |
| Right Insula / Superior Temporal Gyrus | 50 | −22 | 10 | 4 | 252 | |
| Right Cuneus / Calcarine / Lingual Gyrus | 0 | −72 | 0 | 4.1 | 630 | |
| Right Amygdala / Parahippocampus | 26 | 4 | 0 | 4.9 | 991 | |
| Bilateral Middle /Anterior / Posterior Cingulate / Cingulate Motor Area / Supplemental Motor Area / Lateral Premotor / Superior Frontal Gyrus | 6 | −36 | 50 | 6.5 | 4117 | |
|
| ||||||
| gPPI: Learning-Control | Left Inferior Frontal Gyrus | −40 | 46 | 14 | −7.6 | 133 |
| Left Middle Cingulate Cortex | −6 | −38 | 46 | −6.7 | 118 | |
The following regions showed significant positive interaction (or task-based putamen connectivity) with the putamen during the control task: left anterior cingulate cortex, left parahippocampus, left parahippocampus and brainstem, left middle temporal gyrus, right prefrontal cortex, right posterior parietal and occipital association cortex, right insula and superior temporal gyrus, right medial temporal lobe, and bilateral lateral and medial premotor, bilateral cingulate motor, bilateral posterior cingulate and dorsal prefrontal areas.
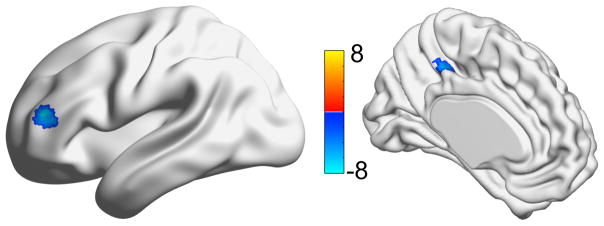
Using a group gPPI analysis on the learning compared to control interaction terms (using the putamen as an ROI), the left inferior frontal gyrus and left middle cingulate were identified as regions where the control interaction terms were greater than the learning interaction terms. This means that the task-based putamen connectivity of these regions was greater during the control compared to the learning condition. There were no significant areas where the learning interaction terms were greater than control interaction terms. These are shown in Figure 5.
Figure 5.
Regions where the differences between the gPPI interaction terms are significantly different. Color bar indicates the t-statistic from a one-sample t-test (testing whether the learning compared to control gPPI interaction terms was significantly greater than zero). Blue shows regions where the interaction term was greater in the control compared to the learning, while red shows the opposite (none).
4. DISCUSSION
To study motor skill learning in a finger-tapping paradigm, we applied the functional connectivity method, gPPI, to define condition-related change in a motor task-related neural network. Compared to previously reported brain activity of a finger-tapping motor sequence learning task,[23] our fMRI study of the task is unique in two ways, we 1) used a time series approach to examine functional changes in the functional connectivity between brain ROIs in the task-related neural network, and 2) examined these changes during the process of motor skill learning the finger-tapping motor sequence, not after a period of practice and task mastery. We interpret the findings of both lower (both by spatial extent and intensity) functional brain activity, and lower functional connectivity of the putamen with fewer brain regions during the learning compared to the control condition, to be an indication of the motor skill learning induced increase in neural efficiency in performance.
The motor skill learning facilitated changes in performance were evident in the basic GLM analyses. In the learning compared to the control condition, brain activity was lower and there were fewer brain regions activated. By differentiating early and late motor learning, this greater ‘efficiency’ was further demonstrated. In the early period of learning and control (learning 1, control 1, Table 2) conditions, and the later period of the control (control 2, Table 2) condition, the task-related brain activation was similar. Associated with these conditions was a pattern of brain activity consistent with the cortical-subcortical network of task-related motor skill learning,[32] in addition to some brain region correlates of the cognitive control[33] or attention network.[34, 35] The additional cognitive control network regions were activated particularly in the control conditions. Only in the later period of the learning (learning 2, Table 2) condition is a marked reduction (in terms of spatial extent and intensity) in brain activations observed. In the learning condition the specific decreased activations in the cognitive control network regions, the frontal eye fields (middle frontal gyrus) and the area bordering on the intraparietal sulcus may be an indication of little or no need to attend to the visually-cued sequence in the later learning condition trials. Motor skill learning is associated with quick recognition of the environmental conditions for performance and enhanced selection of the appropriate motor plan for the task[7, 26, 28, 36, 37] The need for movement guidance information for the task, such as position, direction and trajectory, and expected performance goal, is minimized.[5]
During the learning relative to the control condition, the lower functional connectivity (i.e. gPPI analyses) between the putamen and the inferior frontal and cingulate regions illustrates aspects of the task-related motor learning. Part of the inferior frontal gyrus can be included in the ventral lateral prefrontal cortex (VLPFC) known to be involved in the ‘what’ pathway, or the selection and maintenance of stimulus information needed to guide actions or behavior.[38, 39] The VLPFC has been considered the cognitive control of the ventral pathway from the inferior parietal and/or temporal association areas to the ventral premotor and prefrontal cortical areas. These ventral areas of the frontal and parietal association areas are involved in recognition of object characteristics and calculations used to adjust motor behaviors to be appropriate for the task. In the learning condition, the finger-tapping motor learning of the repeated tapping sequences led to a reduced need for stimulus information to guide the motor task performance. This was not true for the random finger-tapping sequences in the control condition for which motor learning was not expected. Thus in the control condition, the task-related communication between the putamen and the VLPFC was maintained for stimulus information, and translation of the sequence into accurate performance of the motor task.
The reduction in functional connectivity of the putamen with the middle cingulate region in the learning compared to control condition likely illustrates the lower reliance on internal planning and programming movement sequences appropriate for the motor task expectations. The cingulate motor area (i.e. mid-dorsal cingulate gyrus, posterior aspect of anterior cingulate) in conjunction with other medial premotor regions, pre-supplementary motor area and supplementary motor area, has a major role in programming internally-generated motor sequenced actions to meet a goal. The cingulate motor area also works with the prefrontal cortex to define the temporal order of goal-oriented movements. Motor skill learning enhances the chance for movement success; motor experts for a motor task demonstrate less activation in the limbic brain regions than novices in performance of the same task.[26] In the learning compared to the control condition the reduced functional connectivity of the putamen with VLPFC and cingulate motor area is consistent with the lower need for ‘recognition’ of the motor sequence task and selection and maintenance of the finger-tapping sequence (i.e. stimulus) with learning. Thus as the finger-tapping motor sequence task was acquired, there was a reduction in the decision-making necessary to both time and program the motor actions. Under the challenge of a motor sequence that repeatedly changed in the control condition, the cingulate’s role in weighting the decision to move would be unchanged as the chance of success was unlikely to be different across the trials. In fact, for some individuals in the control condition, the behavioral consequences and limbic connectivity may have even increased as concern about performance accuracy or inaccuracy on previous movement sequences pervaded thinking about the next sequence. Given the accuracy achieved in both learning and control conditions, the participants clearly valued correct performance throughout the tasks.
The findings illustrate the enhanced neural efficiency in brain connectivity with motor skill learning. In the early period of visually cued motor skill learning, task-related brain functional connectivity was lower in the motor learning condition compared to the random motor sequence [control] condition. Similarly, using a gPPI analysis Wu et al [5] reported a substantial reduction of brain network connectivity in the automatic stage compared to the novel [pre-training] stage of motor sequence task performance. Even in the early minutes of motor skill learning examined in this study, the brain was adapting in response to the motor skill learning. This pattern of reduced connectivity with the inferior frontal and cingulate brain regions has similarities to the changes observed in later stages of learning described by others, including the automatic stage of sequential movement task learning.[5, 6, 25, 40] In this early learning of a simple motor sequence task, the motor skill learning was associated with lower involvement of the cognitive control/cortical attention, guidance network.[5, 24, 32, 41, 42]
While we studied a simpler 5 step-movement compared to the 12-step [musical note cued] movement sequence examined by Basset et al,[20] our findings have some similarities to their brain network reconfiguration with motor skill learning. The increase in the BOLD signal during this early learning (minutes scale) for both the motor skill learning and control [random sequence] task conditions, but less of an increase in the learning condition, may be a reflection of an increase and subsequent decrease in neural network flexibility as the brain reconfigures the local properties of the networks to adapt to learning. In this study, the inferior frontal and cingulate brain regions that appear to highlight the lower connectivity in motor skill learning overlap the brain regions in the previous study in which connectivity changes during an initial task predicted learning in subsequent motor skill learning task sessions.[20]
The current study has several limitations. We have a relatively small sample size and future studies are needed to assess the reliability of these results. This study was performed on a relatively young and healthy sample, thus these results may not generalize to older populations or non-healthy populations. The finger-tapping task may have been too simple as the participants very quickly reduced their reaction time, which may have not allowed for observation of the changes that occur as an intermediary step. We used a study design that allowed us to study changes that occurred during the task and not permanent changes in the functional activation/connectivity, as such we cannot speculate on whether any permanent changes occurred.
In the very early stages of the process of motor skill learning of a simple finger-tapping motor sequence task, motor skill learning induced greater efficiency in the underlying motor task-related neural circuitry. The evidence derived from this application of a context-dependent gPPI method to functional connectivity changes during motor skill learning of simple motor sequence task provides evidence of the rapid motor skill learning induced reorganization of brain network connectivity in the process. Even in this simple finger-tapping motor sequence task, the motor skill learning was associated with a dramatic reduction in the task-related neural network size and activity. The findings lend support to consideration of motor skill learning-based exercise approaches to acquire or recover motor skill in performance as a means to both reduce brainwork during rehabilitation, and conserve brain functional capacity in the presence of restricted brain resources.
Highlights.
Brain activity was measured during motor sequence learning task.
Posterior parietal and thalamus activated less during learning (vs. control).
Putamen to frontal/mid. cingulate connectivity lower during learning (vs. control).
Learning was associated with changes in brain activity/spatial extent/connectivity.
Acknowledgments
Funding was provided by the University of Pittsburgh Older Americans Independence Center Grant NIH P30 AG024827.
Footnotes
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: TJH, KIE, PJS, ES, JMV; Methodology: TJH, KIE, PJS, ES, JMV; Formal Analysis: HTK; Investigation: HTK, MEW, TJH, PJS, JMV; Resources: TJH, KIE, PJS; Writing – Original Draft: HTK, JMV; Writing – Review and Editing: HTK, TJH, KIE, MEW, PJS, ES, JMV; Visualization: HTK; Supervision: TJH, JMV; Project Administration: HTK, MEW, TJH, JMV; Funding Acquisition: TJH, KIE, PJS, ES, JMV.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanes JN. Neocortical mechanisms in motor learning. Current opinion in neurobiology. 2003;13(2):225–231. doi: 10.1016/s0959-4388(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 2.Willingham DB. A neuropsychological theory of motor skill learning. Psychological review. 1998;105(3):558. doi: 10.1037/0033-295x.105.3.558. [DOI] [PubMed] [Google Scholar]
- 3.Coynel D, Marrele G, Perlbarg V, Pelegrini-Issac M, Van de Moortele P, Ugurbil K, Doyon J, Benali H, Lehericy S. Dynamics of motor-related functional integration during motor sequence learning. Neuroimage. 2010;49:759–766. doi: 10.1016/j.neuroimage.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orban P, Peigneux P, Lungu O, Debas K, Barakat M, Bellec P, Benali H, Maquet P, Doyon J. Functional neuroanatomy associated with the expression of distinct movement kinematics in motor sequence learning. Neuroscience. 2011;179:94–103. doi: 10.1016/j.neuroscience.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Wu T, Chan P, Hallett M. Modifications of the interactions in the motor networks when a movement becomes automatic. J Physiol. 2008;586:4295–4304. doi: 10.1113/jphysiol.2008.153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu T, Hallett M. The influence of normal human ageing on automatic movements. J Physiol. 2005;562(2):605–615. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milton J, Small S, Solodkin A. On the road to automatic: dynamic aspects in the development of expertise. J Clin Neurophys. 2004;21:134–143. doi: 10.1097/00004691-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Wulf G, Shea C, Lewthwaite R. Motor skill learning and performance: a review of influential factors. Med Educ. 2010;44:75–84. doi: 10.1111/j.1365-2923.2009.03421.x. [DOI] [PubMed] [Google Scholar]
- 9.VanSwearingen JM, Studenski SA. Aging, motor skill, and the energy cost of walking: implications for the prevention and treatment of mobility decline in older persons. Journals of Gerontology Series A: Biological Sciences & Medical Sciences. 2014;69(11) doi: 10.1093/gerona/glu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graydon F, Friston K, Thomas C, Brooks V, Menon R. Learning-related fMRI activation associated with a rotational visuo-motor transformation. Cogn Brain Res. 2005;22:373–383. doi: 10.1016/j.cogbrainres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 11.VanSwearingen J, Studenski S. Aging, motor skill and the energy cost of walking: Implications for the prevention and treatment of mobility decline in older persons. J Gerontol Biol Med Sci. 2014;16:1429–1436. doi: 10.1093/gerona/glu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allali G, Van der Meulen M, Beauchet O, Rieger S, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. J Gerontol Med Sci. 2014;69:1389–1398. doi: 10.1093/gerona/glt207. [DOI] [PubMed] [Google Scholar]
- 13.Bakker M, deLange F, Helmich R, et al. Cerebral correlates of motor imagery of normal and precision gait. Neuroimage. 2008;41:998–1010. doi: 10.1016/j.neuroimage.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Godde B, Voelchker-Rehage C. More automation and less cognitive control of imagined walking movement in high- verusu low-fit older adults. Frontiers in Aging Neurosci. 2010;2(139):1–12. doi: 10.3389/fnagi.2010.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malouin F, Richards C, Jackson P, et al. Brain activations dufing motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapping. 2003;19:47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging. 2012;33:1073–1084. doi: 10.1016/j.neurobiolaging.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H. Contributions of the basal ganglia and functionally related brain studtures to motor learning. Beh Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Karni A, et al. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 19.Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Tahar A, Bellec P, Karni A, Underleider L, Benali H, Doyon J. Brain plasticity related to the consolidation of motor sequence learning and motor adaption. Proc Natl Acad Sci USA. 2010;107:17839–17844. doi: 10.1073/pnas.1013176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett D, Wymbs N, Porter M, Mucha P, Carlson J, Grafton S. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci USA. 2011;108:7641–7646. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cisler J, Bush K, Steele J. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–1062. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 23.McLaren D, Ries M, Xu G, Johnson S. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movement in Parkinson’s disease. Neuroimage. 2010;49:2581–2587. doi: 10.1016/j.neuroimage.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wymbs N, Bassett D, Mucha P, Porter M, Grafton S. Differential recruitment of the sensorimotor putamen and frontoparietal cortex during motor chunking in humans. Neuron. 2012;74:936–946. doi: 10.1016/j.neuron.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milton J, Solodkin A, Hlustik P, Small S. The mind of expert motor performance is cool and focused. Neuroimage. 2007;35:804–813. doi: 10.1016/j.neuroimage.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Nenadic I, Gaser C, Volz H, Rammsayer T, Hager F, Sauer H. Processing of temporal information and the basal ganglia: new evidence from fMRI. Exp Brain Res. 2003;148:238–246. doi: 10.1007/s00221-002-1188-4. [DOI] [PubMed] [Google Scholar]
- 28.Toni I, Rowe J, Stephan K, Passingham R. Change of cortico-striatal effective connectivity during visuomotor learning. Cerebral Cortex. 2002;12:1040–1097. doi: 10.1093/cercor/12.10.1040. [DOI] [PubMed] [Google Scholar]
- 29.Rolls E, Joliot M, Tzourio-Mazoyer N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage. 2015;122:1–5. doi: 10.1016/j.neuroimage.2015.07.075. [DOI] [PubMed] [Google Scholar]
- 30.Gitelman D, Penny W, Ashburner J, Friston K. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 31.Cox R, Hyde J. Software tools for analysis and visualization of FMRI data. NMR in Biomedicine. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. in press. [DOI] [PubMed] [Google Scholar]
- 32.Hardwick R, Rottschy C, Miall R, Eickoff S. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2013;67:283–297. doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cole M, Pathak S, Schneider W. Identifying the brain’s most globally connected regions. Neuroimage. 2010;49:3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Fox M, Snyder A, Vincent J, Corbetta M, Van EDC, Raichle M. The human brain is intrisically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teasdale J, Dritschel B, Taylor M, Proctor L, Lloyd C, Nimmo-Smith I, Baddeley A. Stimulu-independent thought depends on central executive resources. Memory Cognit. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- 36.Szameitat A, Shen S, Sterr A. Motor imagery of complex everyday movements, and fMRI study. Neuroimage. 2007;34:702–713. doi: 10.1016/j.neuroimage.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Brooks V. The Neural Basis of Motor Control. Oxford University Press; New York: 1986. [Google Scholar]
- 38.O’Reilly R. The What and How of prefrontal cortical organization. Trend Neurosci. 2008;33:355–361. doi: 10.1016/j.tins.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodale M, Milner A. Separate visual pathways for perception and action. Trend Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 40.Pammi V, Miyapuram K, Ahmed A, Samejima K, Bapi R, Doya K. Changing the structure of complex viso-motor sequences selectively activates the fronto-parietal network. Neuroimage. 2012;50:1180–1189. doi: 10.1016/j.neuroimage.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Gobel E, Parrish T, Reber P. Neural correlates of skill acquisition: decreased cortical activity during a serial interception sequence learning task. Neuroimage. 2011;58:1150–1157. doi: 10.1016/j.neuroimage.2011.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poldrack R, Sabb F, Foerde K, Tom S, Asarnow R, Bookheimer S, Knowlton B. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–5364. doi: 10.1523/JNEUROSCI.3880-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]