Abstract
Background
There is an urgent need for methods that can rapidly and accurately assess therapeutic responses in patients with active tuberculosis (TB) in order to predict treatment outcomes. Exposure to bacterial pathogens can rapidly activate the plasma contact system, triggering the release of bradykinin (BK) and its metabolite desArg9-bradykinin (DABK) to induce inflammation and innate immune responses. We hypothesized that serum BK and DABK levels might act as sensitive immune response signatures for changes in Mycobacterium tuberculosis (Mtb) burden, and therefore examined how serum levels of these markers corresponded with anti-TB therapy in a small cohort of active TB cases.
Methods
Nanotrap Mass-Spectrometry (MS) was used to analyze serial blood specimens from 13 HIV-negative adults with microbiologically confirmed active TB who were treated with first-line anti-TB chemotherapy. MS signal for BK (m/z 1060.5) and DABK (m/z 904.5) serum peptides were evaluated at multiple time-points (before, during, and after treatment) to evaluate how BK and DABK levels corresponded with disease status.
Results
Serum BK levels declined from pretreatment baseline levels during the early stage anti-TB therapy (induction phase) and tended to remain below baseline levels during extended treatment (consolidation phase) and after therapy completion. BK levels were consistent with induction phase sputum culture conversions indicative of decreased Mtb burden reflecting good treatment responses. Serum DABK levels tended to increase during the induction phase and decrease at consolidation and post-therapy time points, which may indicate a shift from active disease to chronic inflammation to a disease free state. Elevated BK and DABK levels after treatment completion in one patient may be related to the subsequent recurrent TB disease.
Conclusions
Our pilot data suggests that changes in the circulating BK and DABK levels in adult TB patients can be used as potential surrogate markers of the host response both early and late in anti-TB treatment for both pulmonary and extrapulmonary TB patients. We will further exploit these host-response signatures in the future as biomarkers in combination with other clinical and microbiologic tools which may improve treatment efficacy and facilitate the development of host-directed therapy.
Keywords: Tuberculosis, Bradykinin, Antibiotics, Treatment response, Recurrence, Outcome
1. Introduction
Effective treatment of active tuberculosis (TB) requires a multi-antibiotic drug regimen that is able to eliminate Mycobacterium tuberculosis (Mtb) bacilli and persistent subpopulations of intermittently metabolizing bacilli [1]. Although current chemotherapeutic regimens are known to achieve relapse-free outcomes in a majority of patients with drug-susceptible Mtb, about 5% of TB patients will have recurrent TB within 2 years of treatment completion [2,3]. Multidrug-resistant (MDR) TB is estimated to account for 3.3% of incident cases and 20% of all cases [2], indicating that MDR TB represents a growing public health threat. Faithful adherence to a treatment regimen with an effective combination of drugs, dosages and treatment duration is one of the most efficient ways to prevent MDR TB development [4]. However, recent bacteriologic and pathology evidence highlights the existence of highly heterogeneous pulmonary Mtb lesions (PTB), where lesion growth, structure, and microenvironment may differentially affect antibiotic penetration and access [5]. This phenomenon may partially explain why no single antibiotic is able to eliminate all drug-susceptible mycobacterial subpopulations and why some patients develop recurrent TB diseases after long-term treatment. Multiple high-dose antibiotics are commonly used to ensure patient cures, which can lead to frequent side-effects that contribute to poor treatment adherence and thereby promote treatment failure and/or MDR TB development.
Sputum culture conversion (SCC) after 2 months of treatment is used to analyze initial treatment efficacy in culture positive PTB patients during the induction phase, but sputum culture is not quantitative and cannot assess the extent of Mtb bacilli remaining during the consolidation stage when sputum cultures are negative [6]. Most other TB diagnostic assays are also not appropriate for treatment evaluation or require optimization and additional validation before they can be used for this purpose. Xpert MTB/RIF does not differentiate between live and dead bacilli, yielding high false positive results, and thus is not recommended to replace standard smear microscopy and culture methods for monitoring treatment response [7]. Persistently positive interferon-gamma release assays results may indicate a failure of therapy, but this approach has not proven useful for monitoring treatment response in large and longitudinal studies, which found assay reversion results in individuals with or without clinical response to treatment [8,9]. There is thus an urgent need for new serum-based methods that can rapidly predict therapeutic responses and the risk of disease recurrence to improve treatment efficacy and reduce the development of new MDR TB cases.
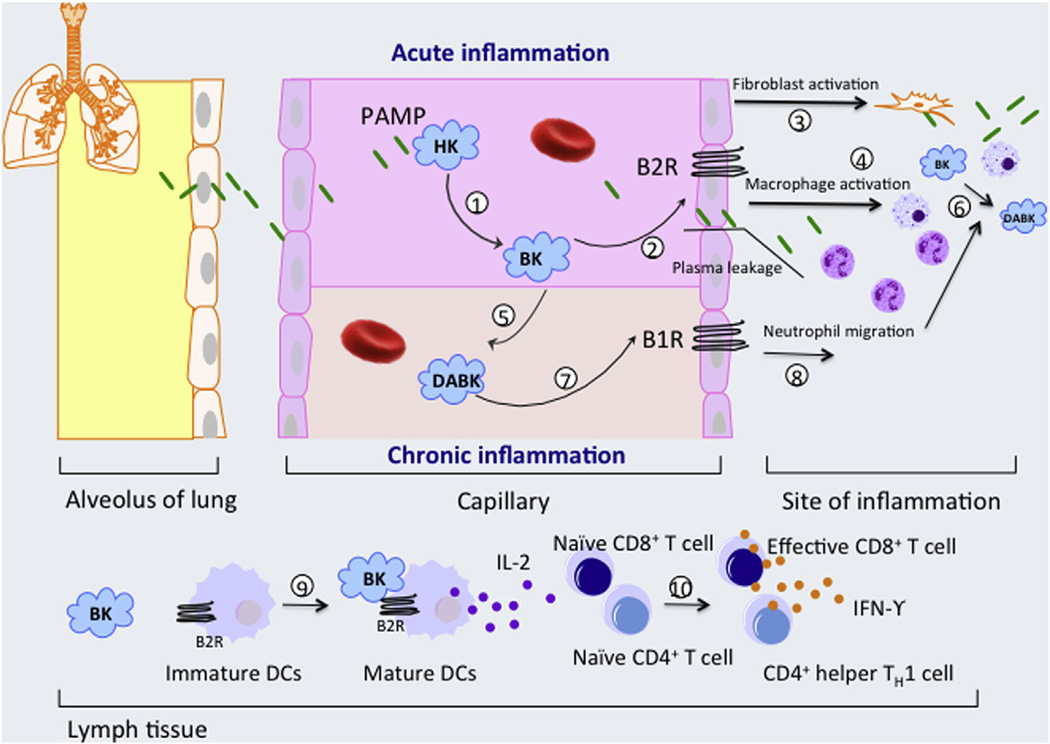
Most Mtb assays detect either Mtb bacilli (e.g. AFB smear, mycobacterial culture, Xpert MTB/RIF assays) or ex vivo host responses to Mtb antigens (e.g. interferon gamma (IFN-γ) release assays) as a means for TB diagnosis; however, blood levels of host response biomarkers may also yield useful diagnostic information. The plasma contact system, also known as the kallikrein/kinin system, plays a role in the initiation of acute inflammation and maintenance of chronic inflammation during certain infectious diseases [10–12]. Interaction of circulating Factor XII with specific microbial pathogens leads to contact activation and causes a cascade of proteolytic activation steps that ultimately results in the cleavage of high-molecular weight kininogen (HK) to liberate the pro-inflammatory peptide bradykinin (BK) and its metabolite desArg9-BK (DABK), which play important roles in inflammation responses and represent important surveillance and protective functions in defense against bacterial infections (Fig. 1) [13–15]. However, many bacterial proteinases can also trigger BK production, either by activating kallikrein to stimulate HK cleavage or directly cleaving HK to increase BK release [16,17]. Regardless of its origin, BK-mediated signaling through the kinin B2 receptor (B2R), can activate alveolar macrophages, induce neutrophils and monocyte chemotaxis [18,19], stimulate the migration of immature human monocyte-derived dendritic cells (DC) to inflammation sites [20], induce DC maturation and trigger pro-inflammatory CD4+ Th1 responses [21], including the release of IFNγ and IL-2, which have positive effects on DC maturation and cytotoxic T lymphocytes (CTL) function [22]. Interaction of BK with B2R increases vasodilation and vascular permeability [23], a scenario that might be expected to favor Mtb invasion and colonization. BK is rapidly inactivated by circulating hydrolases, particularly angiotensin converting enzyme; however, removal of the C-terminal BK arginine residue, by carboxypeptidase N (CPN) in circulation or carboxypeptidase M (CPM) at sites of inflammation, produces DABK, which also has proinflammatory activity but exhibits a much longer half-life (~20-fold) in serum [24,25]. Emerging evidence indicates that BK-mediated signaling through the B2R dominates the acute phase of bacterial infection while DABK signaling through the kinin B1 receptor (B1R) is involved in chronic inflammation [23,26]. B1R expression can be induced by inflammation [27] or chronic bacterial infection [15], which may enhance DABK signaling to promoting an increase in the production of inflammatory mediators, and is upregulated during persistent inflammation in animal models [25,28]. CPM upregulation accounted for an increase in DABK and B1R agonists in bacterial lipopolysaccharide induced vascular inflammation [29], and soluble CPM can be released from plasma membranes by bacterial phosphatidylinositol-specific phospholipase C activity, which is known to contribute to Mtb virulence [30]. DABK-induced B1R signaling can induce vascular smooth muscle cells growth [31], leukocyte accumulation [32] and modulate the life span of neutrophils at sites of inflammation [33]. We therefore hypothesized that pro-inflammatory BK and DABK (Fig. 1) levels might differentially correspond with changes in bacterial load during active TB disease and responses to anti-TB therapy.
Fig. 1.
Role of pathogen–associated molecular pattern (PAMP) contact activation in inflammation. (1) In circulation, PAMPs interact with high molecular weight kininogen (HK) to activate the contact system and liberate bradykinin (BK) from HK [15]. (2) BK interacts with bradykinin B2 receptors (B2Rs) on vascular endothelial cells to augment plasma extravasation during acute inflammation [23], while also activating lung fibroblasts (3) and macrophages to promote (4) pulmonary neutrophil and monocyte accumulation [18,19,50]. BK is also converted to DABK by CPN in circulation (5) and CPM at sites of inflammation (6), which can act through B1R to regulate permeability and chemokine production properties of endothelial cells as well as enhance neutrophil migration, accumulation and modulate the life span of neutrophils at sites of inflammation (8). BK also induces the migration and maturation of dendritic cell (DC) via the B2R signaling pathways (9). Mature DC producing IL-12 and presenting peptide-MHC complexes, and therefore promote priming and differentiation of naive CD4+ T cells and CD8+ T lymphocytes into CD4+ Th1 and CD8+ cytotoxic effector T cells (10). B1 receptor (B1R) can be induced by BK and other pro-inflammatory cytokines. Also, monocyte stimulation after bacterial toxin release can secrete cytokines which in turn trigger the increase of B1R [51]. The function of DABK via B1R is not completely understood but its up-regulation was found in persistent chronic inflammation [30].
To address this hypothesis we used an approach that combined nanotrap-based peptide enrichment and matrix-assisted laser desorption/ionization time-of flight-mass spectrometry (MALDITOF-MS) analysis [34,35] to rapidly and accurately detect BK and DAPK peptides in small (5 µl) serum samples of TB patients receiving first-line anti-TB chemotherapy. We systematically evaluated patient BK and DABK levels, clinical characteristics, treatment regimens and therapy outcomes. Our results suggest that changes in BK and DABK serum levels in these patients are related to their anti-TB treatment response.
2. Materials and methods
2.1. Patients
Medical records from the Houston Tuberculosis Initiative (HTI) database [36,37] were retrospectively reviewed, and archived serum samples from 13 adult patients with microbiologically-confirmed tuberculosis and without history of an immunosuppressive condition were obtained for analysis of BK and DABK levels. All study subjects were notified of the potential risks of participation and had given written informed consent, were recruited, enrolled, had specimens obtained and analyzed using Institutional Review Board (IRB)-approved questionnaires, consent forms and procedures. This study was approved by the IRB of the Houston Methodist Hospital, USA (Pro00000546, Pro00005327) and Baylor College of Medicine and its Affiliates, Houston, Texas, USA (H-8182). Diagnosis of active TB was defined by the American Thoracic Society/Centers for Disease Control and Prevention (CDC) guidelines [38] based on clinical and bacteriologic evidence. Serum samples were collected from blood draws performed before, during and after anti-TB therapy completion when available. All subjects had pre-treatment samples and at least one on-treatment sample, but several subjects did not have post-treatment blood samples. All patients underwent HIV testing and were reported HIV negative. Baseline characteristics of the study population are summarized in Table 1. All patients were alive at last contact.
Table 1.
Patient demographic and clinical data.
| ID | Age (y) | Ethnicity | Diagnosis | TB history |
TST | Jail | Ever homeless |
Current smoker | Alcohol abuse |
Drug use | TB exposure | Comorbidities | Symptoms | Thoracic radiographics | SCC (mo) | DOT (mo) | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | White | PTB | N | N | Y | N | Y | C | Y | N | Type 2 diabetes | Y | Cavities | 1.7 | 7.0 | Cured, completed |
| 2 | 48 | Black | PTB | N | NA | N | N | Y | C | Y | N | Hypertension | Y | Cavities, local infiltrates | 3.0 | 6.4 | Cured, completed |
| 3 | 41 | White | PTB | N | P | N | Y | Y | C | Y | N | Liver disease | Y | Local infiltrates | 1.6 | 9.0 | Cured, completed |
| 6 | 63 | White | PTB | N | NA | N | Y | Y | C | N | N | Asthma | Y | Local infiltrates | 5.8 | 7.0 | Cured, completed |
| 7 | 42 | Hispanic | PTB | N | P | N | N | Y | N | N | N | N | Y | N | 0.9 | 11.0 | Cured, completed |
| 8 | 49 | Hispanic | PTB | N | P | N | Y | Y | C | N | Y | Liver disease | Y | Cavities, local infiltrates | 2.6 | 7.5 | Cured, completed |
| 9 | 50 | White | PTB | N | P | N | Y | Y | C | Y | N | N | Y | Local infiltrates | 1.5 | 10.0 | Cured, completed |
| 13 | 43 | Black | PTB | N | P | Y | N | Y | N | Y | Y | N | N | Local infiltrates, hilar lymphadenopathy |
2.5 | 3.0 | Lost to follow-up |
| 12 | 42 | Black | PTB, EPTB | Y | P | Y | N | N | F | Y | N | Hypertension, liver disease |
Y | Cavities, local infiltrates, pleural effusion |
7.0 | 9.0 | Cured, completed |
| 4 | 52 | Asian | EPTB | N | P | N | N | Y | N | N | N | N | N | N | NA | 11.0 | Cured, completed |
| 5 | 50 | Asian | EPTB | N | P | N | N | N | N | N | N | N | N | N | N | 6.0 | Cured, completed |
| 10 | 37 | Black | EPTB | N | P | N | N | N | C | N | N | N | Y | Pleural effusion | N | 6.5 | Cured, completed |
| 11 | 47 | Black | EPTB | Y | P | N | N | N | F | N | N | Hypertension | N | N | N | 10.0 | Cured, completed |
Abbreviations: N, no; Y, yes; NA, not available; PTB, pulmonary tuberculosis; EPTB, extra-pulmonary tuberculosis; TST, previous tuberculin skin-test (P, positive; N, negative); Jail, incarceration history; Alcohol abuse (C, current; F, former; N, no history); Drug use, illicit drug use history; SCC, time to initial negative sputum culture conversion after starting treatment (months); DOT, directly observed treatment duration (months). Neg.: patient No. 5, 10 and 11 had positive culture from biopsy specimen but negative sputum culture status at the treatment start date.
2.2. Treatment and standard care
All patients were started on standard TB regimens at time of diagnosis, but drug regimens for specific individuals were adjusted as indicated to account for drug resistance determined by in vitro drug susceptibility tests initiated at diagnosis and during treatment. Most (12 of 13) patients were subjected to a standard TB treatment regimen, consisting of 2-month induction phase with isoniazid (INH), rifampin (RMP), ethambutol (EMB) and pyrazinamide (PZA), followed by a 4-month consolidation phase with at least INH and RMP. Patient 3 started treatment with para-aminosalicylic acid, ciprofloxacin, cycloserine, EMB and streptomycin due to advanced liver disease, alcoholic cirrhosis in the setting of hepatitis C infection; however, after normalization of liver function, this patient was switched to RMP, Ciprofloxacin, EMB and Streptomycin until the treatment completion. Patients were seen daily by clinicians during hospitalization (2 weeks) and required directly observed treatment (DOT) while receiving outpatient therapy. Standard laboratory tests (sputum smear and culture, drug-susceptibility testing) were performed during DOT. Treatment duration ranged from 6 to 12 months.
2.3. Nanotrap-based assay and MALDI-TOF-MS analysis
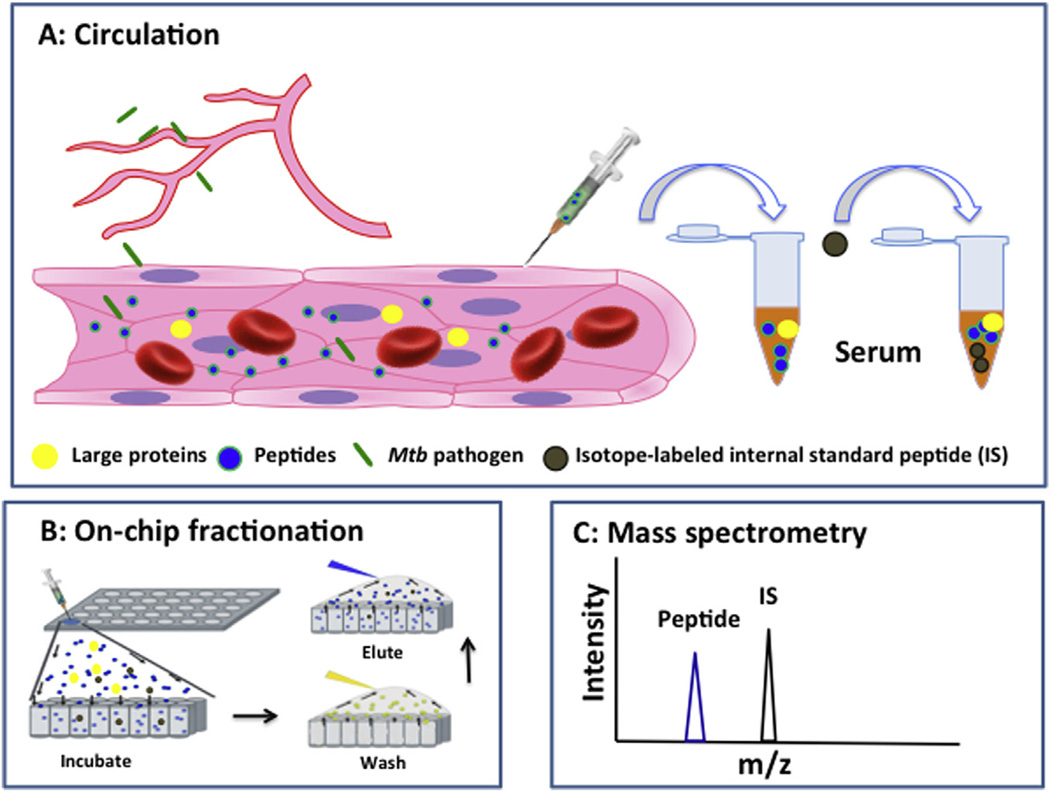
On-chip nanotrap-based peptide fractionation followed by a MALDI-TOF MS analysis [35]was used to detect BK (m/z 1060.5) and DABK (m/z 904.5) peptide signals. Nanoporous silica (NPS) thin films were used to capture and enrich low-molecular-weight peptides from serum samples, which were analyzed by MS after a wash step to remove excluded high-molecular-weight peptides and proteins (Fig. 2). Briefly, each chip well was filled with 5 µl serum, incubated for 30 min at room temperature in a humidified chamber, and then aspirated and washed four times with 10 µl deionized water to remove unbound material. Wells were then incubated for 90 s with 5 µl of a 50% acetonitrile (ACN) and 0.1% trifluoroacetic acid (TFA) solution, and eluted material was spotted onto a target plate, allowed to completely dry (≈10 min), after which 0.5 µl of matrix solution (5 g/l of a-cyano-4-hydroxycinnamic acid (CHCA) in 50% ACN and 0.1% TFA) which was spotted on the target plate and allowed to completely dry. Serum peptide m/z peaks were acquired in positive reflection mode in the 800–3500 Da range by MALDITOF MS analysis (Applied Biosystems 4700 MALDI TOF/TOF Analyzer, Applied Biosystems) [34]. Serum samples were spiked with 5 nM concentrations of stable, isotope-labeled internal standard (IS) peptides (m/z 1070.5 for BK and m/z 914.5 for DABK; synthesized by GenScript USA Inc.) which were used to normalize the intensity ratios of serum BK and DABK MS peptide signals (Fig. 2).
Fig. 2.
Schematic approach for serum BK and DABK analyses. Serum of patients with active TB were collected (a) and processed by on-chip nanotrap fractionation to enrich low-molecular-weight peptides (b). BK and DABK were detected by mass spectrometry (MS) (c). BK and DABK molar amounts were measured by normalization of their target peptide MS intensity ratio to that of a stable isotope-labeled internal standard (IS) peptide (d).
2.4. Statistical analyses
All statistical analyses were conducted using the SPSS 16.0-software (SPSS, Inc., Chicago, Illinois, USA). To analyze the fold change in peptides among subjects at baseline, SCC and treatment completion, t-test or the nonparametric Wilcoxon signed rank test or Kruskal Wallis test was used. A p-value of 0.05 was considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics
Median patient age was 46.9 years, with a range of 37–63 years (Table 1). Patients were diagnosed with pulmonary tuberculosis (PTB; 8 of 13 patients), extra-pulmonary tuberculosis (EPTB; 4 of 13), or PTB and EPTB with plural effusion (patient 12). All 9 PTB and PTB/EPTB cases were sputum culture positive and all 4 EPTB patients had culture positive biopsy specimens. Radiographic evidence of TB disease was observed in 7 of the 8 PTB cases as well as the PTB/EPTB case, most of whom showed signs of cavitation and/or local infiltrates, although one subject (patient 13) exhibited hilar lymphadenopathy and focal infiltrates. Most patients (11 of 13) were reported as incident TB cases. Patients 11 and 12 were identified as recurrent TB cases, but Mtb strain information was not available for all episodes in these patients. Drug susceptibility assays found that all patients were initially susceptible to first-line anti-TB drugs, but patient 2 developed isoniazid and streptomycin resistance 4 months post-treatment and was switched to RMP, EMB and PZA therapy until achieving a cure.
3.2. Initial sputum culture conversion time
Sputum culture conversion (SCC), which remains the standard measure of effective early treatment response in culture confirmed cases [6], was achieved by all the PTB and PTB/EPTB patients who completed their treatment regimens (8 of 9 patients), while the remaining patient was lost to follow-up (patient 13). Median initial SCC time was 2.9 months, ranging from 0.9 to 7 months. Patient 7 achieved SCC at 0.9 months treatment, was culture-positive at 4 months treatment, and then achieved a second SCC at 7 months treatment. All EPTB patients had an initial positive biopsy culture but their culture status during treatment was not available for review.
3.3. Serum BK levels tend to decrease and DABK levels tend to increase in the induction phase
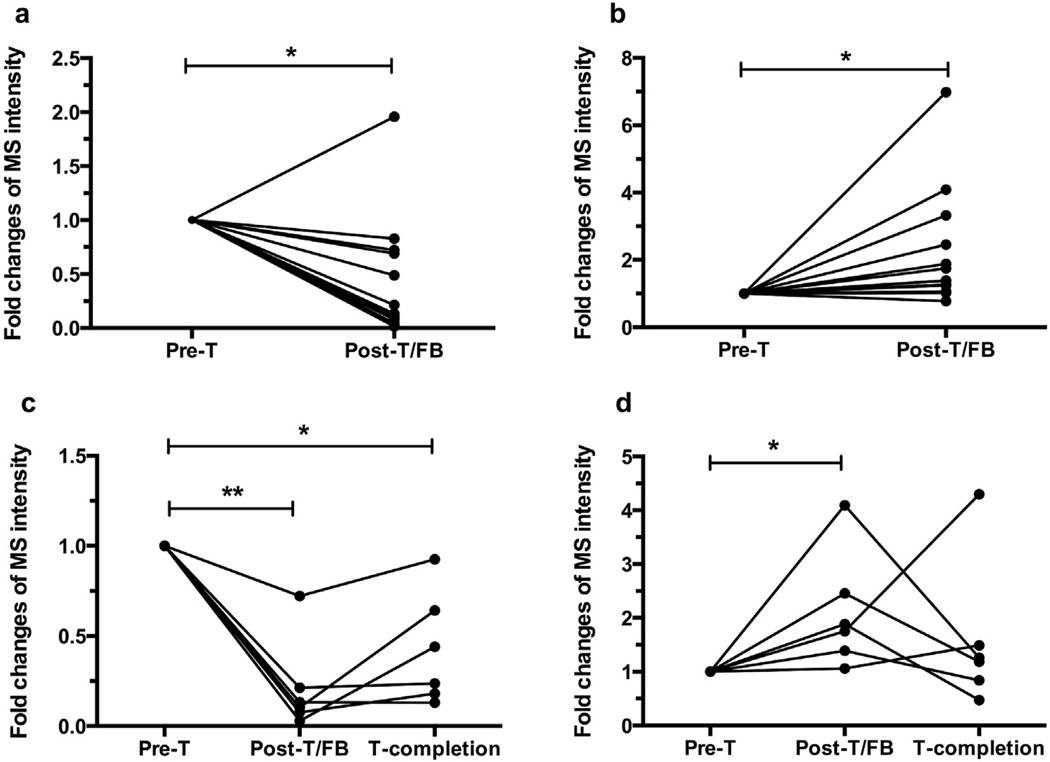
Circulating BK levels decreased (Fig. 3a) and DABK levels increased (Fig. 3b) in 12 of 13 patients post-treatment initiation (Figs. 4 and 5). BK declines and DABK increases corresponded with initial SCC times in 8 of 9 culture-positive patients. BK levels modestly increased post-treatment initiation in patient 8, prior to their third Mtb positive culture, but then declined after SCC was achieved at 2.5 months post-treatment initiation. Patient 13 revealed a progressive BK increase and DABK decrease after starting therapy, prior to initial SCC at 2.5 months post-treatment initiation, but was lost to follow-up before post-SCC blood samples could be obtained to measure BK and DABK values.
Fig. 3.
Serum BK and DABK changes in response to anti-TB treatment. (a) BK declined and (b) DABK increased at earlier stage in 13 patients (data were analyzed based on the first blood draw after treatment. It can be time points either at sputum culture conversion or after induction phase; patient 9 was included as they had only 2 sampling times). Six patients (No. 5–7 and 10–12) had serum samples through treatment completion. These 6 patients revealed that (c) BK declined and tended to remain low after treatment initiation while (d) also showing a significant increase in DABK after induction phase and a tendency for a DABK decline at treatment completion, which did not significantly differ between induction phase and treatment completion. *p < 0.05, **p < 0.001. Pre-T: pre-treatment; Post-T/FB: post-treatment (first blood draw); T-completion: treatment completion.
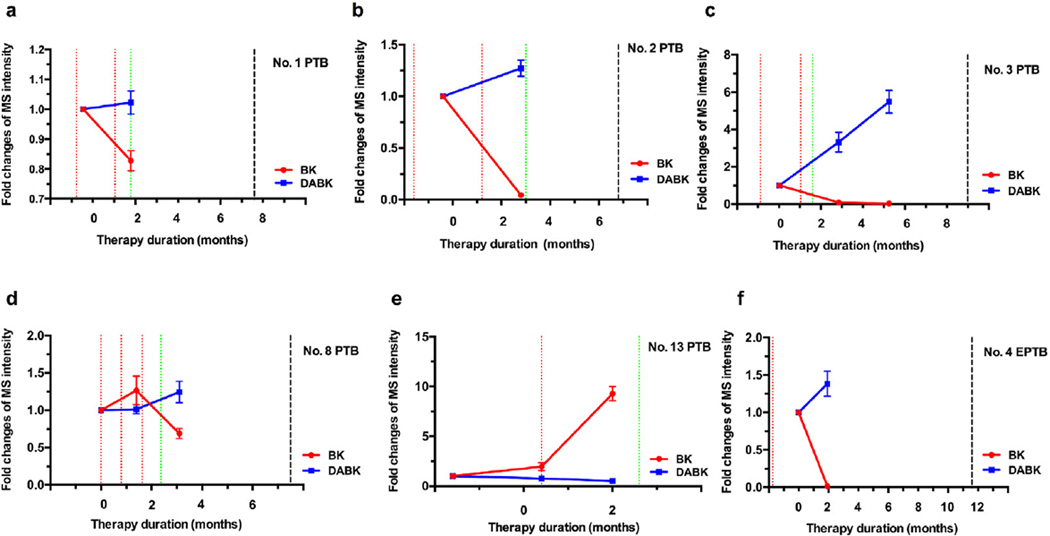
Fig. 4.
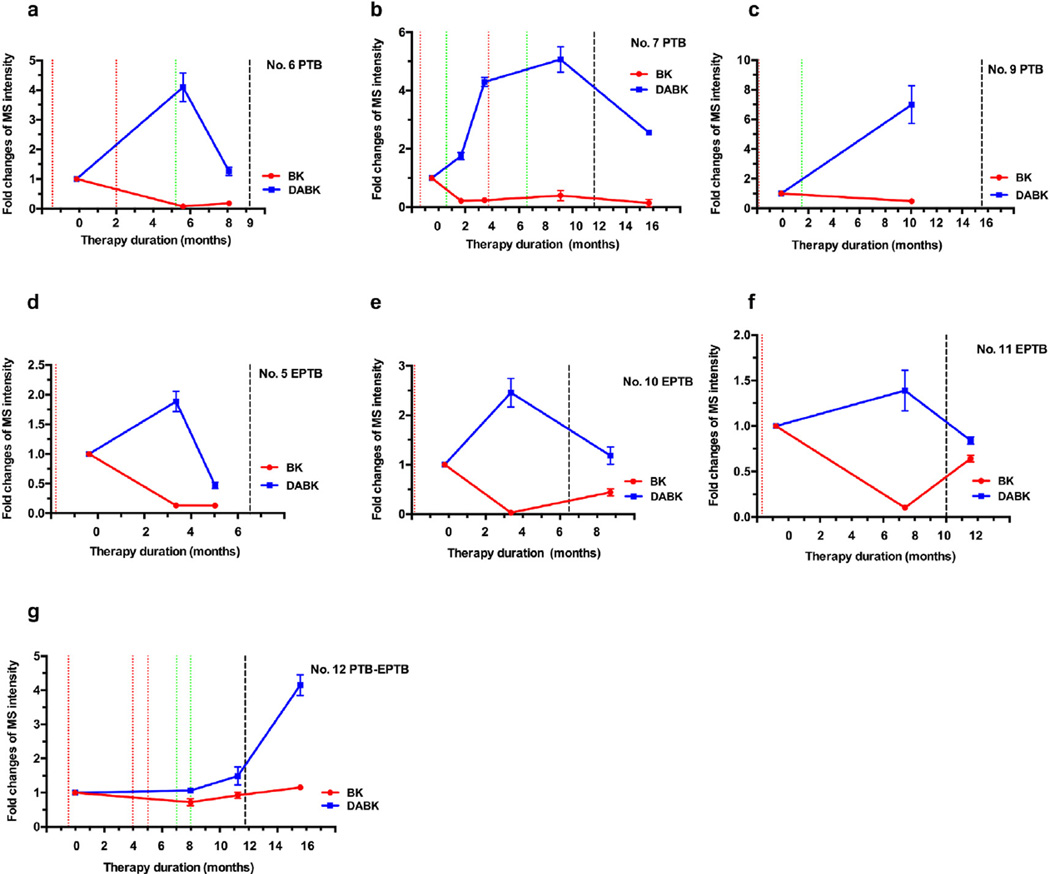
Changes in serum BK and DABK during the induction phase of TB therapy. BK decreases and DABK increases corresponded with the SCC of TB patient (a–d). One patient showed elevated bradykinin with modest change of DABK before SCC and was lost to follow up (e). The EPTB patient (patient 4) exhibited reduced BK and increased DABK at 2-month post treatment (f). Red line: positive sputum culture; Green line: negative sputum culture; Black line: end of treatment; The EPTB patient had an initial positive Mtb culture from a biopsy specimen. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Changes in serum BK and DABK during the consolidation phase of TB therapy. DABK decreases and modest BK changes were observed versus induction phase values in most patients (a, b, d, e), although two patients lacked induction phase data, preventing this comparison (c, f) and one patient had reduced DABK and rising BK levels after their discharge date (e). One patient with second episode of TB disease showed both elevated DABK and BK after their treatment discharge date, suggesting treatment failure, corresponding to a suspected third episode of recurrent TB disease (g). Red line: positive sputum culture; Green line: negative sputum culture; Black line: end of treatment; All EPTB patients had an initial positive Mtb culture from a biopsy specimen. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. DABK decrease during treatment consolidation near therapy completion
Most patients (12 of 13) completed their treatment regimens and achieved clinical cures, although only 7 subjects (patients 5–7 and 9–12) had blood samples drawn late in their consolidation phase and close to treatment completion. Reduced BK and elevated DABK levels in the induction phase may indicate a chronic inflammatory state or condition in response to the existence of persistent bacilli or shed antigens. We hypothesize that declining DABK levels during the consolidation phase (Fig. 3d) indicate a strong therapeutic response reflecting a shift from chronic inflammation to a quiescent state (Fig. 3c). Near the date of treatment completion, 5 patients [5–7,10,11] showed declining DABK levels compared to the induction phase (Fig. 5). Patients 7 and 12 showed fluctuating peptide levels and are discussed below.
Patient 7 showed BK and DABK serum concentration changes that were consistent with sputum culture status at their initial SCC. However, this patient also exhibited increases in both DABK and BK shortly prior to a positive culture results and following a second SCC, both of which decreased at a blood draw several months after the last treatment dose (Fig. 5b).
Patient 12, who was hospitalized after losing 15 pounds in one month, was diagnosed with PTB 4 years previously and had received first-line anti-TB treatment for 13 months. The patient was diagnosed as a recurrent TB case for the second episode and received a first-line anti-tuberculosis regimen. This patient had three positive sputum cultures: at 12 days pre-treatment and at 4 and 5 months post-treatment initiation (Fig. 5g). Chest radiographs showed a pulmonary interval increase until 5 months post-treatment initiation, with initial SCC achieved at 7 months, but no follow-up chest radiographs were taken until after the end of therapy. Following initial SCC, modest BK decreases and DABK increases were consistent with a shift to an attenuated chronic infection. The patient remained culture negative 3 months later, despite being off-treatment for 2 months between the two blood draws, but revealed BK and DABK increases. Three months after treatment completion the patient still showed elevated BK and DABK levels compared to baseline measurements. Eight months after the treatment completion, the patient was suspected of a third episode of TB recurrence. The chest radiograph showed scarring in the bilateral upper lobes with pleural thickening in the bilateral apices and new cavitary lesions in the left upper lobe compared to old radiographs. It was not possible to determine if the TB case was a relapse or re-infection due to lack of culture and genotype information. Review of the first case found that SCC was achieved at almost one year post-treatment initiation, and that initial SCC was achieved at 7 months post-treatment in the current study. Based on the long delay to initial SCC, elevated BK and DABK serum levels after treatment completion, and suspected third TB episode within one year of treatment completion, it appears likely this patient's disease was not adequately treated during their second TB disease episode.
BK reduction and DABK increases were also achieved after treatment initiation in EPTB patients with negative baseline sputum cultures but positive biopsy cultures. Patient 11 presented with a complaint of pain in their left elbow and was diagnosed with a second episode of left elbow tuberculosis arthritis. Given the clinical signs of improvement and the declining BK level and rising DABK serum levels at 7.7 months after treatment start (Fig. 5f), this combination of lab and clinical findings may indicate a good response to anti-TB treatment. However, the last blood-draw, taken 1.8 months after treatment completion, showed a rising BK level and a declining DABK level. There was no follow-up data available to further explain this phenomenon. We assume TB disease activity may have been limited to the left elbow as no clear determination for dissemination can be deduced from the clinical data, but BK and DABK changes representing the host anti-TB response were detectable in serum. For patient No. 10, there was also no data available to explain why an elevated BK level and declining DABK level occurred 2.6 months post-treatment completion (Fig. 5e). Interestingly bacteriologic results showed a positive culture only from the lung biopsy and all subsequent sputum cultures were negative. Patient No.4 received 11 months of treatment and was cured, but only one blood was drawn and collected at 2 months after treatment start. However, the change of BK and DABK levels were similar to those of PTB patients at the time SCC was achieved (Fig. 4f).
Additional risk factors such as alcohol consumption, smoking status, comorbidities (such as hypertension and liver disease) should be taken into account for patients, but did not show significant differences or impact peptide levels for these patients.
4. Discussion
This case series depicts dynamic changes in serum BK and DABK levels in active TB patients during anti-TB therapy. Clinical cure of TB disease can be characterized by negative Mtb culture, resolution or improvement of symptoms and changes in chest radiography. In this study, we identified the host response signatures, BK and DABK, appearing to be valuable in predicting treatment efficacy in adult TB patients and could aid in the decision making with clinical markers such as sputum culture and chest radiography. Based on our findings, we suggest that the changes in serum BK and DABK level in the induction phase and DABK level in consolidation phase may be valuable biomarkers of treatment efficacy.
BK produced by a host immune response to Mtb exposure functions as a proinflammatory factor which can regulate both innate and adaptive immune response. We hypothesized that eradication of drug-susceptible Mtb during the induction phase of anti-TB therapy should decrease circulating BK concentrations, and found that BK declining tended to correspond with initial SCC during the induction phase. BK levels tended to remain below baseline levels during the consolidation phase and after therapy completion, consistent with negative sputum culture or radiographic findings. Serum DABK levels, however, tended to increase during the induction phase and tended to decline in consolidation phase. The mechanism responsible for DABK increases during treatment is not clear, but it has been previously shown that increased DABK via unregulated B1R may play a role in persistent inflammation [23,26,39]. We propose that changes in activated macrophage responses and granulomatous inflammation during the induction and consolidation phase may regulate DABK production and its circulating level.
Long-term host-pathogen interactions within the granuloma can produce a wide spectrum of granuloma structures due to adaptive changes in bacilli and immune cell numbers and phenotypes, and the levels of their regulatory factors. CPM activity, which plays an important role in BK production, is expressed on the plasma membrane of a broad variety of cells and tissues, including blood vessels, pneumocytes, macrophages and granulomas [24], preferentially induced in epithelioid cell (EPC) clusters of all granuloma types [40] and can be released from plasma membranes by bacterial phosphatidylinositol-specific phospholipase C activity [30]. Moreover, upregulation of CPM correlates to increasing endogenous levels of DABK and B1R agonists in bacterial lipopolysaccharide-induced inflammatory responses [29], while the DABK receptor B1R can be induced on cellular components of granulomas, including epithelial cells, macrophages, fibroblasts and lymphocytes [15,41,42]. Increased B1R but not B2R expression was found on macrophages at the center of granulomas in Crohn's disease [43], suggesting that DABK but not BK may play an important role in granuloma function. DABK production by CPM is thus likely to regulate the local actions of DABK in pulmonary granulomas and may also have systemic effects.
Reductions in Mtb-infected macrophages, resolution of TB granulomas and/or phenotype changes in granuloma during therapy consolidation may result in a decrease in CPM activity and a corresponding decrease in serum DABK levels. Based on this hypothesis, declining Mtb burden during the initiation phase would be expected to decrease systemic BK production, while clearance of Mtb bacilli or persistence of Mtb-infected macrophages as sites of infection might increase CPM activity to increase systemic DABK levels, which would be expected to resolve with the final clearance of these sites of infection. These hypotheses need to be explored in future studies.
Persistent Mtb after initial successful treatment completion may contribute to recurrent TB disease, as individual-specific interactions between host and pathogen determine whether relapse to active TB disease will occur. Our data from one patient suggest that BK and DABK serum levels may relate to recurrent TB disease after treatment completion. Patient 12 was diagnosed with an episode of recurrent TB disease and received first-line chemotherapy for 12 months. The serum DABK level of this patient increased after SCC and markedly increased by 3.5 months after treatment completion, with a modest accompanying increase in BK. This patient was suspected of another recurrent TB episode at 7 months post-treatment, presenting with new radiologic evidence of scarring and cavities. Baseline cavitary lesions and a positive sputum culture at 2 months after treatment initiation are risk factors for recurrent TB according to U.S. guidelines, which recommend extension of consolidation therapy in such cases [44]. This PTB-EPTB patient had baseline cavities and achieved SCC at 7 month post-treatment initiation, but was considered cured at 12 months of treatment, after two negative sputum culture results and resolution of their symptoms; however, the elevated rather than decreased DABK and BK levels observed at 3.5 months after treatment completion appear to indicate a persistent disease state. Thus, we suggest that analysis of circulating DABK and BK levels in combination with sputum culture and chest radiography results may more accurately evaluate treatment efficacy.
For patients with EPTB, a definitive TB diagnosis can only be made by culturing Mtb bacilli from a biopsy specimen and it is therefore difficult to define the criteria for ending treatment, as it is difficult to obtain follow-up biopsies to determine if a patient has achieved culture conversion and is thus considered disease free. Therapy responses are thus often judged by clinical evaluations on the basis of clinical and radiographic findings. We found that BK levels decreased while DABK levels tended to increase during treatment induction and decline during the consolidation phase of patients who achieved cures based on clinical evaluation, suggesting that circulating BK and DABK levels might be a useful additional means of evaluating therapy responses and clinical cures.
Host responses to invading pathogens are important indicators of both primary infections and the persistence of long-term infections, and are heterogeneous in nature [6]. A balance of pro- and anti-inflammatory immune responses are essential for controlling bacterial proliferation and the resolution of immune lesions, such as granulomas, over time. Recent studies have identified blood transcriptional signatures that correlate with disease severity and reversion in response to treatment [45] or patients who relapse rather than achieve lasting cures [46]. Other studies [47–49] have evaluated the serum host biomarkers reflecting treatment responses at earlier time points or in relation to sputum culture conversion. One important advantage of our findings, is that circulating BK and DABK levels represent the host's response to pathogen–associated molecular patterns and may offer a rational route forward in the prediction of real-time treatment outcomes at both early and late treatment stages and allow individualization of treatment regimens, dosing, and treatment duration. The MS-based nanotrap fractionation approach used in this study is a rapid, highly DABK- and BK-specific high-throughput assay that requires only a few microliters of serum. This approach employs equipment already in use in most major clinical laboratories and can be used in patients where sputum samples are difficult to obtain or sometimes non-informative (e.g. EPTB patients at all stage of treatment or PTB patients after symptomatic improvement). The rapid sample-to-result time of this assay also offers significant advantages for monitoring patient responses to treatment, unlike culture assays that require extended development time. Xpert MTB/RIF assays, offer similarly rapid response times but require sputum or biopsy material and do not provide quantitative information. We believe our approach has the potential to significantly improve current therapy management. The ability to rapidly monitor responses to TB treatment and identify treatment endpoints predicting long-term outcomes should also allow increased opportunity for therapy personalization to improve treatment outcomes. Large scale prospective studies which employ frequent sampling at defined time points in conjunction with patient symptom and treatment response data are thus important to establish and validate the sensitivity and specificity of these biomarkers.
Limitations of this study include its retrospective nature, limited cohort size, and structural inability to correlate BK or DABK with sputum culture conversion or radiographic findings. This study also could not assess the ability of BK or DABK to predict recurrent disease, as only one patient developed a suspected case of TB recurrence. The small size, cohort structure, and variable collection times of the current study do not allow us to address the sensitivity and specificity of circulating DABK and BK changes for culture-conversion and clinical cures. Nor can we directly determine the precise correspondence of the BK and DABK switch with culture conversion. However, our observations provide a foundation for future prospective studies with the cohort structure and frequent, defined blood draw time points needed to evaluate these parameters. We are currently planning a prospective study recruiting patients with PTB and EPTB in Shandong Providence Chest Hospital, P.R. China. The blood samples will be collected at defined time-points pre- and post-treatment initiation to determine assay sensitivity for Mtb clearance and time points and thresholds predictive of long-term TB cures.
Acknowledgments
This research was supported in part by US National Institute of Allergy and Infectious Diseases grant R01Al113725-01A1 and R01AI122932-01A1, US Department of Defense TATRC grant W81XWH-11-02-0168, and John S. Dunn Foundation award.
Footnotes
Potential conflicts of interest
All authors have no reported conflicts of interest.
References
- 1.Mitchison D, Davies G. The chemotherapy of tuberculosis: past, present and future. Int J Tuberc lung Dis Off J Int Union Against Tuberc Lung Dis. 2012;16(6):724–732. doi: 10.5588/ijtld.12.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report. 2015 Available online, http://www.who.int/tb/data [Internet] [Google Scholar]
- 3.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet. 2004;364(9441):1244–1251. doi: 10.1016/S0140-6736(04)17141-9. [DOI] [PubMed] [Google Scholar]
- 4.van der Werf MJ, Langendam MW, Huitric E, Manissero D. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39(6):1511–1519. doi: 10.1183/09031936.00125711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenaerts A, Barry CE, 3rd, Dartois V. Heterogeneity in tuberculosis pathology, microenvironments and therapeutic responses. Immunol Rev. 2015;264(1):288–307. doi: 10.1111/imr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallis RS, Doherty TM, Onyebujoh P, Vahedi M, Laang H, Olesen O, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis. 2009;9(3):162–172. doi: 10.1016/S1473-3099(09)70042-8. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich SO, Rachow A, Saathoff E, Singh K, Mangu CD, Dawson R, et al. Pan African consortium for the evaluation of anti-tuberculosis A. Assessment of the sensitivity and specificity of Xpert MTB/RIF assay as an early sputum biomarker of response to tuberculosis treatment. Lancet Respir Med. 2013;1(6):462–470. doi: 10.1016/S2213-2600(13)70119-X. [DOI] [PubMed] [Google Scholar]
- 8.Chee CB, KhinMar KW, Gan SH, Barkham TM, Koh CK, Shen L, et al. Tuberculosis treatment effect on T-cell interferon-gamma responses to Mycobacterium tuberculosis-specific antigens. Eur Respir J. 2010;36(2):355–361. doi: 10.1183/09031936.00151309. [DOI] [PubMed] [Google Scholar]
- 9.Hill PC, Brookes RH, Fox A, Jackson-Sillah D, Jeffries DJ, Lugos MD, et al. Longitudinal assessment of an ELISPOT test for mycobacterium tuberculosis infection. PLoS Med. 2007;4(6):e192. doi: 10.1371/journal.pmed.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oschatz C, Maas C, Lecher B, Jansen T, Bjorkqvist J, Tradler T, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34(2):258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Oehmcke S, Herwald H. Contact system activation in severe infectious diseases. J Mol Med. 2010;88(2):121–126. doi: 10.1007/s00109-009-0564-y. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan AP, Joseph K, Shibayama Y, Nakazawa Y, Ghebrehiwet B, Reddigari S, et al. Bradykinin formation. Plasma and tissue pathways and cellular interactions. Clin Rev Allergy Immunol. 1998;16(4):403–429. doi: 10.1007/BF02737659. [DOI] [PubMed] [Google Scholar]
- 13.Frick IM, Akesson P, Herwald H, Morgelin M, Malmsten M, Nagler DK, et al. The contact system–a novel branch of innate immunity generating antibacterial peptides. EMBO J. 2006;25(23):5569–5578. doi: 10.1038/sj.emboj.7601422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frick IM, Bjorck L, Herwald H. The dual role of the contact system in bacterial infectious disease. Thromb Haemost. 2007;98(3):497–502. [PubMed] [Google Scholar]
- 15.Phagoo SB, Reddi K, Silvallana BJ, Leeb-Lundberg LM, Warburton D. Infection-induced kinin B1 receptors in human pulmonary fibroblasts: role of intact pathogens and p38 mitogen-activated protein kinase-dependent signaling. J Pharmacol Exp Ther. 2005;313(3):1231–1238. doi: 10.1124/jpet.104.083030. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Yamamoto T, Kamata R, Maeda H. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J Biochem. 1984;96(3):739–749. doi: 10.1093/oxfordjournals.jbchem.a134892. [DOI] [PubMed] [Google Scholar]
- 17.Potempa J, Pike RN. Corruption of innate immunity by bacterial proteases. J Innate Immun. 2009;1(2):70–87. doi: 10.1159/000181144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koyama S, Sato E, Numanami H, Kubo K, Nagai S, Izumi T. Bradykinin stimulates lung fibroblasts to release neutrophil and monocyte chemotactic activity. Am J Respir Cell Mol Biol. 2000;22(1):75–84. doi: 10.1165/ajrcmb.22.1.3752. [DOI] [PubMed] [Google Scholar]
- 19.Sato E, Koyama S, Nomura H, Kubo K, Sekiguchi M. Bradykinin stimulates alveolar macrophages to release neutrophil, monocyte, and eosinophil chemotactic activity. J Immunol. 1996;157(7):3122–3129. [PubMed] [Google Scholar]
- 20.Bertram CM, Baltic S, Misso NL, Bhoola KD, Foster PS, Thompson PJ, et al. Expression of kinin B1 and B2 receptors in immature, monocyte-derived dendritic cells and bradykinin-mediated increase in intracellular Ca2+ and cell migration. J Leukoc Biol. 2007;81(6):1445–1454. doi: 10.1189/jlb.0106055. [DOI] [PubMed] [Google Scholar]
- 21.Aliberti J, Viola JP, Vieira-de-Abreu A, Bozza PT, Sher A, Scharfstein J. Cutting edge: bradykinin induces IL-12 production by dendritic cells: a danger signal that drives Th1 polarization. J Immunol. 2003;170(11):5349–5353. doi: 10.4049/jimmunol.170.11.5349. [DOI] [PubMed] [Google Scholar]
- 22.Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol. 2010;22(1):109–117. doi: 10.1016/j.coi.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57(1):27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 24.Erdös EGSR. Metabolism of bradykinin by peptidases in health and disease, the kinin system. London: Academic Press; 1997. [Google Scholar]
- 25.Couture R, Harrisson M, Vianna RM, Cloutier F. Kinin receptors in pain and inflammation. Eur J Pharmacol. 2001;429(1–3):161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- 26.Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16(3):99–104. doi: 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- 27.Christiansen SC, Eddleston J, Woessner KM, Chambers SS, Ye R, Pan ZK, et al. Up-regulation of functional kinin B1 receptors in allergic airway inflammation. J Immunol. 2002;169(4):2054–2060. doi: 10.4049/jimmunol.169.4.2054. [DOI] [PubMed] [Google Scholar]
- 28.Marceau F, Hess JF, Bachvarov DR. The B1 receptors for kinins. Pharmacol Rev. 1998;50(3):357–386. [PubMed] [Google Scholar]
- 29.Schremmer-Danninger E, Offner A, Siebeck M, Roscher AA. B1 bradykinin receptors and carboxypeptidase M are both upregulated in the aorta of pigs after LPS infusion. Biochem Biophys Res Commun. 1998;243(1):246–252. doi: 10.1006/bbrc.1997.7999. [DOI] [PubMed] [Google Scholar]
- 30.Raynaud C, Guilhot C, Rauzier J, Bordat Y, Pelicic V, Manganelli R, et al. Phospholipases C are involved in the virulence of Mycobacterium tuberculosis. Mol Microbiol. 2002;45(1):203–217. doi: 10.1046/j.1365-2958.2002.03009.x. [DOI] [PubMed] [Google Scholar]
- 31.Christopher J, Velarde V, Jaffa AA. Induction of B(1)-kinin receptors in vascular smooth muscle cells: cellular mechanisms of map kinase activation. Hypertension. 2001;38(3 Pt 2):602–605. doi: 10.1161/01.hyp.38.3.602. [DOI] [PubMed] [Google Scholar]
- 32.Ahluwalia A, Perretti M. Involvement of bradykinin B1 receptors in the polymorphonuclear leukocyte accumulation induced by IL-1 beta in vivo in the mouse. J Immunol. 1996;156(1):269–274. [PubMed] [Google Scholar]
- 33.Araujo RC, Kettritz R, Fichtner I, Paiva AC, Pesquero JB, Bader M. Altered neutrophil homeostasis in kinin B1 receptor-deficient mice. Biol Chem. 2001;382(1):91–95. doi: 10.1515/BC.2001.014. [DOI] [PubMed] [Google Scholar]
- 34.Wu HJ, Li Y, Fan J, Deng Z, Hu Z, Liu X, et al. Antibody-free detection of Mycobacterium tuberculosis antigen using customized nanotraps. Anal Chem. 2014;86(4):1988–1996. doi: 10.1021/ac4027669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Y, Peng Y, Lin K, Shen H, Brousseau LC, 3rd, Sakamoto J, et al. Surface engineering on mesoporous silica chips for enriching low molecular weight phosphorylated proteins. Nanoscale. 2011;3(2):421–428. doi: 10.1039/c0nr00720j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Sahly HM, Wright JA, Soini H, Bui TT, Williams-Bouyer N, Escalante P, et al. Recurrent tuberculosis in Houston, Texas: a population-based study. Int J Tuberc Lung Dis Off J Int Union Against Tuberc Lung Dis. 2004;8(3):333–340. [PubMed] [Google Scholar]
- 37.Qiu L, Teeter LD, Liu Z, Ma X, Musser JM, Graviss EA. Diagnostic associations between pleural and pulmonary tuberculosis. J Infect. 2006;53(6):377–386. doi: 10.1016/j.jinf.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the american thoracic society and the centers for disease Control and prevention was adopted by the ATS board of directors, july 1999. This statement was endorsed by the council of the infectious disease society of America, september 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 39.Phagoo SB, Poole S, Leeb-Lundberg LM. Autoregulation of bradykinin receptors: agonists in the presence of interleukin-1beta shift the repertoire of receptor subtypes from B2 to B1 in human lung fibroblasts. Mol Pharmacol. 1999;56(2):325–333. doi: 10.1124/mol.56.2.325. [DOI] [PubMed] [Google Scholar]
- 40.Tsakiris I, Torocsik D, Gyongyosi A, Dozsa A, Szatmari I, Szanto A, et al. Carboxypeptidase-M is regulated by lipids and CSFs in macrophages and dendritic cells and expressed selectively in tissue granulomas and foam cells. Lab Investig J Tech Methods Pathol. 2012;92(3):345–361. doi: 10.1038/labinvest.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prat A, Weinrib L, Becher B, Poirier J, Duquette P, Couture R, et al. Bradykinin B1 receptor expression and function on T lymphocytes in active multiple sclerosis. Neurology. 1999;53(9):2087–2092. doi: 10.1212/wnl.53.9.2087. [DOI] [PubMed] [Google Scholar]
- 42.Prat A, Biernacki K, Pouly S, Nalbantoglu J, Couture R, Antel JP. Kinin B1 receptor expression and function on human brain endothelial cells. J Neuropathol Exp Neurol. 2000;59(10):896–906. doi: 10.1093/jnen/59.10.896. [DOI] [PubMed] [Google Scholar]
- 43.Stadnicki A, Pastucha E, Nowaczyk G, Mazurek U, Plewka D, Machnik G, et al. Immunolocalization and expression of kinin B1R and B2R receptors in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2005;289(2):G361–G366. doi: 10.1152/ajpgi.00369.2004. [DOI] [PubMed] [Google Scholar]
- 44.American thoracic S, cdc, infectious diseases society of a. Treatment of tuberculosis. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Reports/Centers Dis Control. 2003;52(RR-11):1–77. [PubMed] [Google Scholar]
- 45.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cliff JM, Cho JE, Lee JS, Ronacher K, King EC, van Helden P, et al. Excessive cytolytic responses predict tuberculosis relapse after apparently successful treatment. J Infect Dis. 2016;213(3):485–495. doi: 10.1093/infdis/jiv447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayakumar A, Vittinghoff E, Segal MR, MacKenzie WR, Johnson JL, Gitta P, et al. Serum biomarkers of treatment response within a randomized clinical trial for pulmonary tuberculosis. Tuberculosis. 2015;95(4):415–420. doi: 10.1016/j.tube.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Groote MA, Nahid P, Jarlsberg L, Johnson JL, Weiner M, Muzanyi G, et al. Elucidating novel serum biomarkers associated with pulmonary tuberculosis treatment. PloS One. 2013;8(4):e61002. doi: 10.1371/journal.pone.0061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Almeida ML, Barbieri MA, Gurgel RQ, Abdurrahman ST, Baba UA, Hart CA, et al. alpha1-acid glycoprotein and alpha1-antitrypsin as early markers of treatment response in patients receiving the intensive phase of tuberculosis therapy. Trans R Soc Trop Med Hyg. 2009;103(6):575–580. doi: 10.1016/j.trstmh.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 50.Koyama S, Rennard SI, Robbins RA. Bradykinin stimulates bronchial epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Physiol. 1995;269(1 Pt 1):L38–L44. doi: 10.1152/ajplung.1995.269.1.L38. [DOI] [PubMed] [Google Scholar]
- 51.Bengtson SH, Sanden C, Morgelin M, Marx PF, Olin AI, Leeb-Lundberg LM, et al. Activation of TAFI on the surface of Streptococcus pyogenes evokes inflammatory reactions by modulating the kallikrein/kinin system. J Innate Immun. 2009;1(1):18–28. doi: 10.1159/000145543. [DOI] [PMC free article] [PubMed] [Google Scholar]