Abstract
At age 6 years, patients with hypoplastic left heart syndrome had mean age-adjusted z-scores for weight (WAZ) and height (HAZ) below and body mass index (BMIZ) similar to the normative population. Higher birth weight infants had the greatest decrease in WAZ and HAZ. Males had the greatest increase in BMIZ.
Trial registration ClinicalTrials.gov: NCT00115934
Keywords: Hypoplastic left heart syndrome, pediatrics, growth, risk factors
Growth failure among infants with hypoplastic left heart syndrome (HLHS) is common.1,2 We previously used the database from the National Heart, Lung, and Blood Institute-sponsored Pediatric Heart Network Single Ventricle Reconstruction (SVR) trial to assess growth patterns in the first 3 years after the Norwood procedure.3 Because the factors contributing to impaired growth may change over time, particularly after the Fontan procedure, we sought to expand this analysis.
Methods
Neonates with HLHS or a related single morphologic right ventricular (RV) anomaly were randomized to either a modified Blalock-Taussig shunt or right ventricle-to-pulmonary artery shunt in the SVR trial and follow-up was subsequently extended to age 6 years.4 Sociodemographic and clinical characteristics, medical and surgical variables, unanticipated procedures, complications, outcome data, and core laboratory analysis of echocardiographic images were collected.3, 4
We extended our analysis of changes in z-scores-for-age for weight (WAZ), height (HAZ), and body mass index (BMIZ) using all available data (Table I; available at www.jpeds.com) on subjects from birth to age 6 years. To assess the effect of the Fontan procedure, we analyzed measurements obtained between the pre-Fontan visit and age 6 years. We included growth data up to death (N=153), transplant (n=21) or biventricular repair (N=3). The shunt type in place when the subject left the operating room following the Norwood procedure was used for this analysis.
Table 1.
Candidate Predictors Considered in Models Predicting Change in Weight-for-age Z-score, Height-for-age Z-score and Body Mass Index Z-score for the Designated Time Intervals
| A. Between Birth and age 6 years |
| General |
| Center |
| Socioeconomic status and Hollingshead scores at birth |
| Percentage of residents below federal poverty level |
| Gender |
| Gestational age |
| Race/ethnicity |
| Anatomy subtype |
| Obstructed pulmonary venous return |
| Birth weight |
| Birth weight <2,500 grams |
| Multiple birth |
| Maternal age |
| Paternal age |
| History of consanguinity |
| Exposures during pregnancy |
| Smoking |
| Prescription drugs or general anesthesia Recreational drugs |
| Number of complications during pregnancy |
| Number of infections during pregnancy |
| Number of miscarriages or stillbirths |
| Number of terminations for a cardiac defect |
| Number of full and half siblings deaths Shunt type |
| Type of Fontan (extracardiac vs. lateral tunnel) |
| Genetics |
| Diagnosed with a genetic syndrome or genetic abnormalities |
| APOE genotype |
| Interventional catheterizations |
| Cumulative number |
| Concomitant surgeries |
| Cumulative number |
| Total number of non-cardiac surgeries |
| Total number of adverse events |
| Age at procedure |
| Age at Norwood |
| Age at stage II |
| Urgency of stage II (elective/nonelective) |
| Age at Fontan |
| Feeding method |
| Any enteral feedings pre-Norwood |
| Oral feeding at all times |
| Oral feeding with tube feeding supplementation at any time |
| Tube feeding at all times |
| Tube feeding at any time |
| Feeding issues |
| Feeding issues at any time |
| Therapy for feeding issues |
| Therapy for feeding issues at any time |
| Diet |
| Increased caloric density at Norwood discharge |
| Solid foods 13–36 months |
| Caloric-supplemented diet at any time |
| Digoxin use at any time |
| Monitoring or early intervention services (speech/language/occupational therapy) at any time |
| NYHA Heart failure classification >Class 1 at age 5 or 6 years |
| Arrhythmias |
| Arrhythmias treated with medications or implantable cardioverter defibrillator(ICD) at any time |
| Arrhythmias treated with medications at any time |
| Arrhythmias treated with ICD at any time |
| Echocardiography |
| Right ventricular area change |
| Tricuspid regurgitation ≥mild at any time |
| Total number of complications |
| Intraoperative variables |
| Cumulative bypass support time |
| Cumulative cross-clamp time |
| Cumulative regional perfusion time |
| Cumulative circulatory arrest time |
| Extracorporeal membrane oxygenation at any hospitalization |
| Cardiopulmonary resuscitation at any hospitalization |
| Length of hospital stay |
| Cumulative length of stay for all admissions |
| Unplanned readmissions |
| Number of unplanned readmissions |
| B. Between Fontan and age 6 years |
| General |
| Center |
| Socioeconomic status and Hollingshead scores at birth |
| Percentage of residents below federal poverty level |
| Gender |
| Race/ethnicity |
| Anatomy subtype |
| Obstructed pulmonary venous return |
| Birth weight |
| Birth weight <2,500 grams |
| Diagnosed with a genetic syndrome or genetic abnormalities |
| APOE genotype |
| Pre-Norwood |
| Enteral feedings before Norwood |
| Norwood hospitalization |
| Age at Norwood |
| Shunt type in place when leaving the OR |
| Length of hospital stay |
| At Norwood discharge |
| Feeding method (categorized as oral, oral with tube supplementation, tube) |
| Home monitoring (weight or oxygen) program (yes/no) |
| Post Norwood echocardiogram |
| Right ventricular area change |
| Tricuspid valve regurgitation ≥moderate (yes/no) |
| Pre-stage II procedure |
| Feeding method (categorized as oral, oral with tube supplementation, tube) |
| End diastolic ventricular pressure |
| Right ventricular area change |
| Tricuspid valve regurgitation ≥ moderate (yes/no) |
| Pre-Fontan catheterization |
| Pulmonary artery abnormalities |
| Systemic ventricular end-diastolic pressure |
| Pre-Norwood to 6 years |
| Cumulative cardiac surgeries |
| Cumulative non-cardiac surgeries |
| Cumulative all surgeries |
| Cumulative serious adverse events |
| Cumulative complications |
| Cardiopulmonary resuscitation at any hospitalization |
| Extracorporeal membrane oxygenation at any hospitalization |
| Cumulative interventional catheterizations |
| Cumulative length of stay all hospitalizations |
| Unplanned readmissions |
| Number of unplanned hospital readmissions |
| Digoxin use at any time |
| Tricuspid regurgitation ≥ moderate at any time |
| Speech/language/occupational therapy at any time |
| Feeding issues at any time |
| Caloric-supplemented diet at any time point |
| Therapy for feeding issues at any time |
| Stage II hospitalization |
| Urgency (elective/nonelective) |
| Age |
| Length of stay |
| 12 or 14 months to 3 years |
| Number of medications |
| Feeding method 13–24 months (oral, oral with tube supplementation) |
| Feeding method 25–36 months |
| Solid foods at 2 years |
| Solid foods at 3 years |
| Pre-Fontan echocardiogram |
| Right ventricular area change |
| Tricuspid regurgitation ≥ moderate |
| Pre-Norwood to 6 years (continued): |
| Arrhythmia treated with medication, pacemaker or |
| ICD at any time |
| Arrhythmia treated with medication at any time |
| Early intervention at any time |
| Oral feeding supplemented with tube at any time |
| Receiving only tube feedings at any data collection point between Norwood discharge and 6 years (yes/no) |
| NYHA Heart failure classification >Class 1 at age 5 or 6 years (yes/no) |
| Fontan hospitalization |
| Age at Fontan |
| Type of Fontan |
| Use of cardiopulmonary bypass |
| Use of regional cerebral perfusion |
| Number of additional cardiac surgeries |
| Cardiopulmonary resuscitation |
| Extracorporeal membrane oxygenation |
| Number of interventional catheterization procedures |
| Number of other surgical procedures |
| ICD |
| Number of complications |
| Length of stay |
| Readmission within 30 days post-Fontan |
| Readmission length of stay |
The primary outcomes were the change in WAZ, HAZ, and BMIZ between birth and age 6 years. Anthropometric measurements were obtained at study visits as previously described.3 Reliable height data were available only at birth, pre-Fontan, and annually from ages 3–6 years.
Analyses were performed between birth and age 6 years and between pre-Fontan and age 6 years with subgroup analyses for those subjects with WAZ <−2 at Norwood discharge. A one-sample t-test was used to determine if the mean z-score for a growth of the study population differed from a z-score of 0 for the normative population. Simple linear regression was used to obtain initial estimates of association of each candidate predictor with change in WAZ, HAZ, and BMIZ. Nonlinear relationships with continuous outcomes were assessed by categorizing continuous variables into groups based on quartiles; in addition, relationships between the natural logarithm of predictors with skewed distributions and the outcomes were explored. Variables with unadjusted p<0.20 were used as candidate predictors for multivariable modeling. For variables with >5% missing data, mean imputation was performed prior to conducting multivariable modeling. Stepwise linear regression was employed to develop multivariable models, in conjunction with bootstrapping (1000 samples) to obtain reliability estimates. The criteria to enter and remain in the model were p<0.15 and p<0.05, respectively. All terms in the final multivariable models have a reliability >50% and p<0.05. Separate models were constructed adjusting, and not adjusting, for center.
Results
Birth to age 6 years
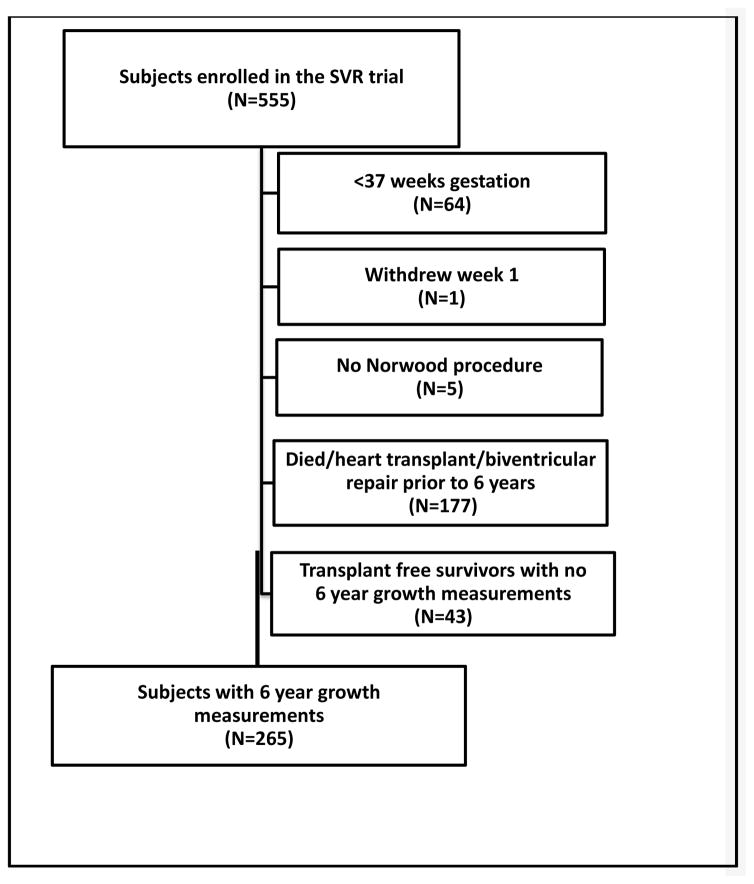
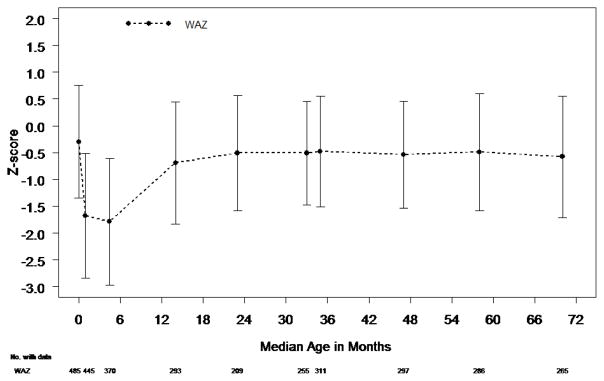
The analysis of anthropometric measurements at age 6 years included 265 subjects enrolled between May 2005 and July 2008 (Figure 1; available at www.jpeds.com). At age 6 years, 19 SVR transplant-free survivors had not undergone the Fontan procedure. Mean WAZ (−0.58±1.13) and mean HAZ (−1.03±1.18) were below the mean of the normative population (p<0.001 for both). WAZ was less impaired than HAZ (p<0.001). By 6 years of age, 37% of subjects had WAZ <−1 and 51% had HAZ <−1; WAZ was <−2 in 9% and HAZ was <−2 in 18%. Mean BMIZ (0.09±1.11) was similar to the normative population (p=0.17) with 13% of subjects having BMIZ <−1 and 3% with BMIZ <−2.
FIGURE 1.
on line: Flow diagram showing the study sample used for the growth analysis from birth to age 6 years (without intermediate time points).
Mean WAZ and mean HAZ decreased between birth and age 6 years (p<0.001 for both) (Table II; available at www.jpeds.com). WAZ decreased ≥0.5 SD in 49% of subjects and HAZ decreased by ≥0.5 SD in 61%. In contrast to WAZ and HAZ, mean BMIZ increased between birth and age 6 years. During this time, BMIZ decreased by ≥0.5 SD in 28% of subjects and increased by ≥0.5 SD in 52%. At 6 years of age, 9% of SVR survivors had BMIZ >1.5 and 4% had BMIZ >2.
Table 2.
Changes in Anthropometric Measurements for Each Time Period
| Time period | Change in Z-score | ||||
|---|---|---|---|---|---|
| Mean ± Standard Deviation (n) | |||||
| Time between measurements (months) | All subjects* | MBTS | RVPAS | P-value comparing shunts** | |
| Weight-for-age Z-score | |||||
| Birth to 6 years | 70.0±3.5 | −0.34±1.30 (265) | −0.38±1.37 (125) | −0.30±1.23 (140) | 0.62 |
| Pre-Fontan to 6 years | 36.0±10.3 | −0.07±0.82 (237) | −0.01±0.89 (114) | −0.11±0.76 (123) | 0.36 |
| Height-for-age Z-score | |||||
| Birth to 6 years | 70.0±3.5 | −1.04±1.65 (259) | −1.03±1.59 (120) | −1.05±1.71 (139) | 0.91 |
| Pre-Fontan to 6 years | 36.1±10.3 | −0.09±1.10 (231) | 0.00±1.18 (109) | −0.18±1.01 (122) | 0.20 |
| Body Mass Index-for-age Z-score | |||||
| Birth to 6 years | 70.0±3.6 | 0.54±1.72 (246) | 0.43±1.55 (115) | 0.64±1.85 (131) | 0.34 |
| Pre-Fontan to 6 years | 36.1±10.3 | 0.04±1.12 (231) | 0.07±1.16 (109) | 0.02±1.10 (122) | 0.76 |
MBTS, modified Blalock Taussig shunt; RVPAS, right ventricle to pulmonary artery shunt
Each mean was based on data available at the respective time point
The p-value used to compare shunts was derived from a two-sample t-test
The univariable results for candidate predictors that remained significant in any of the final multivariable models presented (both reliability >50% and p<0.05) are shown in Table III (available at www.jpeds.com). Higher birth weight (p<0.001), longer cumulative length of stay (LOS) for all admissions (p=0.001) and being white/non-Hispanic (p=0.02) were independently associated with a greater decrease in WAZ (Table IV). Higher birth weight was the only factor independently associated with a greater decrease in HAZ (p<0.001). Males had a greater increase in BMIZ over the 6-year study period. A Hollingshead score of >54.5 (highest socioeconomic status) was associated with the smallest increase in BMIZ, but the relationship was not linear. Tube feeding at any time point correlated with a greater decrease in BMIZ. Independent predictors were similar whether or not center was included in the model and explained only 11% of the variation in change in BMIZ. Shunt type was not associated with change in WAZ, HAZ, or BMIZ.
Table 3.
Univariable Regression Results for Change in Anthropometric Z-score1
| Change in Z-scores Between Birth and Age 6 Years | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight-for-age | Length-for-age | BMI-for-age | |||||||
| N | Mean ± SD | P-value | N | Mean ± SD | P-value | N | Mean ± SD | P-value | |
| Hollingshead category | 0.11 | 0.42 | 0.04 | ||||||
| 3.0–27.0 | 63 | −0.13±1.29 | 60 | −0.80±1.22 | 56 | 0.53±1.81 | |||
| >27.0–43.0 | 54 | −0.63±1.37 | 52 | −1.22±1.71 | 50 | 0.26±1.53 | |||
| >43.0–54.5 | 70 | −0.27±1.09 | 69 | −1.25±1.96 | 65 | 1.04±1.79 | |||
| >54.5–66.0 | 54 | −0.55±1.27 | 54 | −1.06±1.49 | 51 | 0.21±1.72 | |||
| Missing | 24 | 0.02±1.63 | 24 | −0.60±1.79 | 24 | 0.54±1.42 | |||
| Sex | 0.77 | 0.25 | 0.04 | ||||||
| Male | 165 | −0.32±1.32 | 163 | −1.13±1.72 | 156 | 0.71±1.76 | |||
| Female | 100 | −0.37±1.27 | 96 | −0.89±1.53 | 90 | 0.25±1.61 | |||
| Race/ethnicity | 0.02 | 0.02 | 0.76 | ||||||
| Hispanic | 48 | −0.16±1.28 | 45 | −0.86±1.33 | 43 | 0.51±1.77 | |||
| Non-Hispanic white | 181 | −0.50±1.30 | 178 | −1.24±1.61 | 170 | 0.50±1.75 | |||
| Non-Hispanic black | 26 | 0.26±1.16 | 26 | −0.37±1.62 | 25 | 0.69±1.48 | |||
| Non-Hispanic other | 6 | 0.21±0.99 | 6 | −0.25±1.27 | 5 | 1.28±1.64 | |||
| Missing | 4 | 0.01±1.57 | 4 | 0.25±4.52 | 3 | 0.85±1.57 | |||
| Birth weight | |||||||||
| Slope ± SE | 265 | −0.0015±0.0001 | <0.001 | 259 | −0.0015±0.0002 | <0.001 | 246 | −0.0007±0.0002 | 0.004 |
| Low birth weight (<2,500 grams) | <0.001 | <0.001 | 0.009 | ||||||
| Yes | 18 | 1.48±0.98 | 18 | 1.00±1.84 | 11 | 1.86±1.12 | |||
| No | 247 | −0.47±1.21 | 241 | −1.19±1.53 | 235 | 0.48±1.72 | |||
| Multiple birth | 0.009 | 0.03 | 0.38 | ||||||
| Yes | 4 | 1.33±0.76 | 4 | 0.70±1.38 | 2 | 1.61±0.23 | |||
| No | 261 | −0.36±1.29 | 255 | −1.07±1.64 | 244 | 0.54±1.72 | |||
| Cumulative length of stay for all admissions | 0.02 | 0.39 | 0.30 | ||||||
| 1–30 days | 31 | 0.34±1.12 | 31 | −0.67±1.92 | 31 | 1.02±2.12 | |||
| 31–44 days | 89 | −0.36±1.27 | 84 | −0.95±1.52 | 78 | 0.38±1.64 | |||
| 45–67 days | 75 | −0.43±1.12 | 75 | −1.24±1.26 | 73 | 0.63±1.61 | |||
| >67 days | 70 | −0.51±1.49 | 69 | −1.10±2.01 | 64 | 0.42±1.71 | |||
| Tube feeding at any time point | 0.004 | 0.54 | 0.008 | ||||||
| Yes | 97 | −0.52±1.44 | 95 | −1.13±1.81 | 86 | 0.44±1.77 | |||
| No | 168 | −0.23±1.20 | 164 | −0.99±1.56 | 160 | 0.60±1.69 | |||
| Caloric supplemented diet at any time pre-Norwood to age 6 years | 0.42 | 0.97 | 0.37 | ||||||
| Yes | 99 | −0.42±1.48 | 98 | −1.05±1.86 | 89 | 0.41±1.78 | |||
| No | 166 | −0.29±1.18 | 161 | −1.04±1.52 | 157 | 0.62±1.69 | |||
| Right ventricular area change at baseline | 0.04 | 0.10 | 0.46 | ||||||
| 9%–29% | 65 | −0.63±1.32 | 63 | −1.47±1.52 | 62 | 0.52±1.67 | |||
| >29%–35% | 59 | 0.04±1.27 | 57 | −0.76±1.39 | 55 | 0.78±1.56 | |||
| >35%–40% | 61 | −0.33±1.22 | 61 | −0.89±1.86 | 58 | 0.36±1.91 | |||
| >40% | 63 | −0.35±1.26 | 61 | −1.10±1.78 | 56 | 0.78±1.67 | |||
| Missing | 17 | −0.52±1.50 | 17 | −0.68±1.44 | 15 | −0.38±1.72 | |||
| Change in Z-scores Between Pre-Fontan and Age 6 Years | |||||||||
| Weight-for-age | Length-for-age | BMI-for-age | |||||||
| N | Mean ± SD | P-value | N | Mean ± SD | P-value | N | Mean ± SD | P-value | |
|
| |||||||||
| Length of hospital stay at Stage II procedure2 | 0.01 | 0.13 | 0.17 | ||||||
| ≤6 days | 66 | 0.10±0.76 | 64 | 0.17±0.93 | 64 | 0.03±1.09 | |||
| 7–8 days | 70 | −0.21±0.76 | 67 | −0.23±1.02 | 67 | −0.07±1.05 | |||
| 9–14 days | 55 | −0.25±0.77 | 54 | −0.23±1.14 | 54 | −0.08±1.00 | |||
| >14 days | 46 | 0.15±0.98 | 46 | −0.09±1.32 | 46 | 0.36±1.37 | |||
| Feeding method 13–24 months2 | <0.001 | 0.07 | 0.08 | ||||||
| Oral | 160 | 0.06±0.78 | 156 | −0.02±0.97 | 156 | 0.14±1.06 | |||
| Combination (Oral + Tube) | 16 | −0.66±0.63 | 16 | −0.41±0.84 | 16 | −0.51±0.82 | |||
| Tube | 16 | −0.41±1.04 | 15 | −0.52±1.15 | 15 | −0.03±1.59 | |||
| Missing | 45 | −0.18±0.82 | 44 | −0.10±1.50 | 44 | −0.10±1.20 | |||
Unless otherwise indicated, all means and standard deviations are based on the subjects with 6 year growth measurements and available data on the change from birth to age 6 years. Continuous variables were categorized into groups based on the quartiles of the distribution using all available data.
Means and standard deviations are based on the subjects with 6 year growth measurements and available data on the change from pre-Fontan to age 6 years.
Table 4.
Multivariable Models for Change in Anthropometric Z-scores from Birth to Age 6 Years.*
| Weight-for-age Z-score (n=261, adjusted R2=0.34) | ||||
|---|---|---|---|---|
| Variable | Estimate | Standard Error | P-value | Reliability (%) |
| Birth weight, kg | −0.001 | 0.0001 | <0.001 | 82 |
| Cumulative length of stay: all admissions (days) | 0.001 | 64 | ||
| ≤30 | 0.914 | 0.229 | ||
| 31–44 | 0.351 | 0.170 | ||
| 45–67 | 0.242 | 0.179 | ||
| >67 | Reference | |||
| Race/ethnicity | 0.02 | 56 | ||
| Hispanic | −0.229 | 0.459 | ||
| White non-Hispanic | −0.471 | 0.440 | ||
| Black non-Hispanic | 0.193 | 0.479 | ||
| Other non-Hispanic | Reference | |||
| Height-for-age Z-score (n=259, adjusted R2=0.20) | ||||
| Variable | Estimate | Standard Error | P-value | Reliability (%) |
|
| ||||
| Birth weight, kg | −0.002 | 0.0002 | <0.001 | 85 |
| Body Mass Index-for-age Z-score (n=246, adjusted R2=0.07) | ||||
| Variable | Estimate | Standard Error | P-value | Reliability (%) |
|
| ||||
| Hollingshead score | 0.014 | 69 | ||
| <27.0 | 0.296 | 0.323 | ||
| 27.0–43.0 | 0.158 | 0.302 | ||
| 43.0–54.5 | 0.921 | 0.312 | ||
| >54.5 | reference | |||
| Tube feeding--any time | 0.003 | 59 | ||
| Yes | −0.700 | 0.232 | ||
| No | Reference | |||
| Sex | 0.040 | 54 | ||
| Male | 0.457 | 0.221 | ||
| Female | Reference | |||
Results were similar with and without center included as a potential predictor in the model. Only those without center are presented.
Relation of the Fontan Procedure to Anthropometric Measurements at Age 6 Years
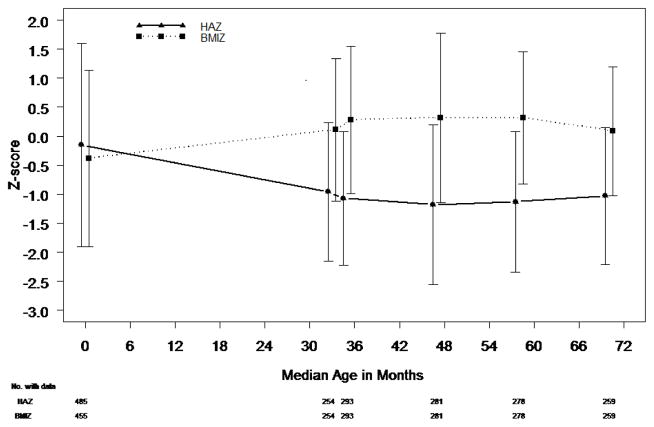
Among the 255 subjects who had a Fontan procedure (33.8±9.6 months of age), 1 subject underwent cardiac transplantation and 12 subjects died before age 6 years. At the pre-Fontan visit, mean WAZ (−0.51±0.97) and HAZ (−0.96±1.19) were significantly below the normative mean (p<0.001). WAZ was <−1 in 31% of subjects and <−2 in 5%. Mean WAZ decreased between the pre-Fontan visit and age 6 years (Table II), but compared with the Norwood and stage II procedures,3 the Fontan procedure had the least impact on WAZ (Figure 2). During this time interval, WAZ decreased by ≥0.5 SD in 24% of subjects.
Figure 2.
WAZ over all time periods (top). HAZ and BMIZ are demonstrated at birth, pre-Fontan and annually between ages 3–6 years (bottom). Each mean is based on all data available at the respective time point. Error bars represent one standard deviation. Age at surgery (months): Norwood 0.2±0.4; stage II 4.7±1.8; Fontan 33.8±9.6.
HAZ was <−1 in 50% and <−2 in 15% at the pre-Fontan assessment. Similar to WAZ, HAZ decreased from the pre-Fontan visit to age 6 years (Table II) with a decrease of ≥0.5 SD in 26% of subjects. In contrast to WAZ and HAZ, at the pre-Fontan visit, mean BMIZ was similar to the normative population; 16% were <−1 and 2% were <−2. Overall, mean BMIZ increased between Fontan and age 6 years. During this time, 29% of subjects had a decrease in BMIZ of ≥0.5 SD and 28% had an increase of ≥0.5 SD.
Center was independently associated with change in WAZ (p=0.03). With center removed from the model, being white/non-Hispanic was independently associated with a decrease in WAZ. Longer LOS at the stage II hospitalization but shorter cumulative LOS at all hospitalizations correlated with a greater increase in WAZ. Being fed a caloric-supplemented diet at any time point was the only variable associated with a greater decrease in HAZ. Center was associated with change in BMIZ from pre-Fontan to 6 years but explained only 14% of the variation. The mean change in BMIZ ranged from a decrease of 0.94 SD to an increase of 0.88 SD (p=0.002). The type of shunt in place after the Norwood procedure had no association with change in any anthropometric measurement.
Age 6 Years Anthropometric Measurements for Subjects with WAZ <−2 at Norwood Discharge
Of the 445 subjects discharged from the Norwood hospitalization, 169 (38%) had WAZ <−2. The prevalence of WAZ <−2 was similar between the group of subjects who died or were transplanted (35/97, 36%) and the group who survived (134/348, 39%, p=0.72). At 6 years of age, WAZ for these subjects was lower than the group discharged with WAZ ≥−2 (−1.08±1.01 vs. −0.24±1.08, p<0.001). RV area change at the pre-Norwood echocardiogram had a non-linear association with increased weight gain between Norwood discharge and age 6 years. Multiple births and low birth weight were also independently associated with better weight gain between Norwood and age 6 years in this subgroup. Between the Fontan and age 6 years, being black non-Hispanic and oral feeding at 13–24 months of age were associated with better weight gain. At age 6 years, HAZ was similar between those discharged with and those without WAZ <−2 (p=0.25). BMIZ was 0.26 SD below normal in those discharged with WAZ <−2 and 0.34 SD above normal in those with WAZ ≥−2 (p<0.001). Of those discharged with WAZ <−2, BMIZ was >1.5 in 5% and none had BMIZ >2.0 at age 6 years.
Discussion
This longitudinal assessment of growth from birth to age 6 years in a well characterized cohort of subjects with HLHS and related single RV anomalies enrolled in the SVR trial demonstrated that the Fontan procedure had little effect on growth at age 6 years. Similar to our findings at age 3 years3, both mean WAZ and HAZ for this study population remained below mean values for the normative population, with HAZ more impaired than WAZ at age 6 years. Despite the drop in both mean WAZ and HAZ, mean BMIZ increased >0.5 SD in over 50% of the SVR survivors. These data highlight both the trends and the variance in growth for 6 years old transplant-free survivors of the Norwood procedure.
It is possible that the increase in BMIZ may be from increasing adipose rather than increasing lean body mass. Because of the strong associations reported between poor weight gain in infancy and increased morbidity, including prolonged hospitalization, and impaired neurodevelopment,5,6 many centers have focused on improving weight gain by increasing caloric intake with varying degrees of success.1, 2, 5 Increasing weight by adding adipose tissue without a proportional increase in height, however, may have long-term detrimental effects.
In this cohort, factors associated with better weight gain were inconsistent, varied by time interval and, at best, explained only a small percentage of the variability in growth over the first 6 years of life. Shorter total LOS for all hospitalizations correlated with a greater increase in growth between Fontan and age 6 years likely reflecting better operative outcomes or less severe disease. Non-Hispanic black subjects had the greatest increase and higher birth weight resulted in a greater decline in growth between birth and age 6 years. These findings are consistent with previous reports and have been thought to reflect a regression toward the mean.3, 5 This explanation may be too simplistic, however, as HAZ remains more impaired than WAZ and no association was found between birth weight and BMIZ at age 6 years.
Feeding predictor variables were not associated with growth. Tube feeding at any time was associated with a greater decrease in BMIZ between birth and age 6 years. Being fed a caloric-supplemented diet at any time was associated with a greater decrease in HAZ between pre-Fontan and age 6 years and may indicate that increasing calories alone has little effect on height in this population. Because caloric intake was not collected in the SVR database, however, these associations are difficult to interpret beyond noting that feeding seems more associated with change in BMIZ rather than change in WAZ or HAZ alone.
It is noteworthy that infants with WAZ <−2 at Norwood discharge experienced increases in WAZ indicating that even the smallest subjects were capable of gaining weight if they survived. Mean HAZ at age 6 years was similar between the group with WAZ <−2 and the group with WAZ ≥2 at Norwood discharge.
Although previous reports documented short stature in older Fontan patients,3, 6 height has not historically been the focus of efforts to improve early growth. Height, not weight, has been associated with poorer socioeconomic and neurodevelopmental outcomes, however.5, 7, 8 Impairment of linear growth is not readily modifiable by increasing caloric intake.
De novo mutations likely play a role in impairment of linear growth.9 Genetic polymorphisms or pathologic copy number variants have been associated with short stature and worse neurodevelopmental outcomes in children with single ventricles but the role of genetic factors is still being explored.10 Disruption of the neuroendocrine growth axis,11 and the long-term effects of an unfavorable fetal environment8 are also being investigated.
This study has several limitations. Growth measurements were not standardized as outcome measurements in the SVR trial. These measurements were used for clinical care, however, and are typically carefully obtained in this vulnerable population. Because height is known to be unreliable in infants3, we included only birth length and height in older children. The anthropometric data are conditional on transplant-free survival to each respective time point, using all available data to maximize precision of our estimates. As a result, qualitative comparisons of the change in anthropometric measurements among the time intervals are based on diminishing cohort size and subject to survivor bias. Genotyping was not used to define genetic syndromes. Children with potentially pathogenic copy number variants or de novo mutations often have no obvious clinical findings9, 10 potentially accounting for the failure to find an association between genetic syndrome and poor linear growth or weight gain in this study. Blood samples were stored for future genetic studies that may be enlightening.
Thus, at 6 years of age, height remained more impaired than weight for SVR transplant-free survivors. Efforts to avoid excessive weight gain in these children warrant attention because they are particularly vulnerable to the morbidity associated with obesity. Future studies should consider the impact of other factors beyond caloric intake to improve height in this population.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute. The authors declare no conflicts of interest.
Abbreviations
- SVR
Single Ventricle Reconstruction trial
- RV
right ventricle
- WAZ
weight-for-age z-score
- HAZ
height-for-age z-score
- BMIZ
body mass index-for-age z-score
- HLHS
hypoplastic left heart syndrome
- LOS
length of stay
Appendix
Additional Pediatric Heart Network Investigators include (*no longer at the institution listed): National Heart, Lung, and Blood Institute--Gail Pearson, Victoria Pemberton, Kristin Burns, Jonathan Kaltman, Mario Stylianou, Frank Evans.
Protocol Chair: University of Texas Southwestern Medical Center, Lynn Mahony
Data Coordinating Center--New England Research Institutes: Lynn Sleeper*, Sharon Tennstedt*, Steven Colan, Minmin Lu*, Shan Chen, Julie Schonbeck*, Melissa Lamberti, David F. Teitel.
Core Clinical Site Investigators--Boston Children’s Hospital: Jane W. Newburger (PI), Peter Laussen*, Pedro del Nido, Roger Breitbart, Jami Levine, Carolyn Dunbar-Masterson, Bethany Trainor; Children’s Hospital of New York: Wyman Lai (PI)*, Ismee Williams*, Rosalind Korsin; Children’s Hospital of Philadelphia: J. William Gaynor (Study Co-Chair), Chitra Ravishankar, Tonia Morrison; Cincinnati Children’s Hospital Medical Center: James Cnota (PI), Catherine Dent Krawczeski*, Kathryn Hogan, Teresa Barnard, Kathleen Ash; North Carolina Consortium (Duke University, East Carolina University, Wake Forest University): Jennifer Li, Mingfen Xu; Medical University of South Carolina: Andrew Atz, J. Philip Saul (PI)*, Scott Bradley, Eric Graham, Teresa Atz, Carolyn Taylor, Girish Shirali*, Patricia Infinger; Primary Children’s Hospital and the University of Utah, Salt Lake City, Utah: L. LuAnn Minich (PI), Richard V. Williams (PI), Phillip T. Burch, Thomas A. Miller, Linda M. Lambert, Marian E. Shearrow, Michelle L. Robinson; Hospital for Sick Children, Toronto, Canada: Brian McCrindle (PI), Seema Mital, Elizabeth Radojewski*, Patricia Walter; University of Michigan: Caren S. Goldberg (PI), Richard G. Ohye (PI), Suzanne Welch; Children’s Hospital of Wisconsin and Medical College of Wisconsin: Nancy S. Ghanayem (PI), James S. Tweddell*, Kathleen A. Mussatto, Michele A. Frommelt, Peter C. Frommelt, Lisa Young-Borkowski*.
Auxiliary Sites--Children’s Hospital Los Angeles: Alan Lewis (PI), Hesham Mahmoud;
Johns Hopkins All Children’s Heart Institute: Jeffrey P. Jacobs (PI), Tracey Cox; Emory University: William Mahle (PI), Kirk Kanter, Jeryl Huckaby; Nemours Cardiac Center: Christian Pizarro (PI), Carol Prospero, Erica Sood, Gina M. Baffa, Wolfgang A. Radtke, Jane Vetter; University of Texas Southwestern Medical Center: Ilana Zeltzer (PI), Deborah McElroy*. Echocardiography Core Laboratories--Children’s Hospital of Wisconsin, Peter C. Frommelt. Protocol Review Committee--Michael Artman (Chair); Erle Austin; Timothy Feltes, Julie Johnson, Thomas Klitzner, Jeffrey Krischer, G. Paul Matherne.
Data and Safety Monitoring Board--John Kugler (Chair); Rae-Ellen Kavey, Executive Secretary; David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Holly Taylor, Catherine L. Webb.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Di Maria MV, Glatz AC, Ravishankar C, Quartermain MD, Rush CH, Nance M, et al. Supplemental tube feeding does not mitigate weight loss in infants with shunt-dependent single-ventricle physiology. Pediatr Cardiol. 2013;34:1350–1356. doi: 10.1007/s00246-013-0648-x. [DOI] [PubMed] [Google Scholar]
- 2.McCrary AW, Clabby ML, Mahle WT. Patient and practice factors affecting growth of infants with systemic-to-pulmonary shunt. Cardiol Young. 2013;23:499–506. doi: 10.1017/S1047951112001382. [DOI] [PubMed] [Google Scholar]
- 3.Burch PT, Gerstenberger E, Ravishankar C, Hehir DA, Davies RR, Colan SD, et al. Longitudinal assessment of growth in hypoplastic left heart syndrome: results from the Single Ventricle Reconstruction Trial. J Am Heart Assoc. 2014;3:e000079. doi: 10.1161/JAHA.114.000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams RV, Zak V, Ravishankar C, Altmann K, Anderson J, Atz AM, et al. Factors affecting growth in infants with single ventricle physiology: a report from the Pediatric Heart Network Infant Single Ventricle Trial. J Pediatr. 2011;159:1017–22. doi: 10.1016/j.jpeds.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MI, Bush DM, Ferry RJ, Spray TL, Moshang T, Wernovsky G, et al. Somatic growth failure after the Fontan operation. Cardiol Young. 2000;10:447–457. doi: 10.1017/s1047951100008118. [DOI] [PubMed] [Google Scholar]
- 7.Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. 2009;123:101–9. doi: 10.1542/peds.2008-1352. [DOI] [PubMed] [Google Scholar]
- 8.Miller TA, Zak V, Shrader P, Ravishankar C, Pemberton VL, Newburger JW, et al. Growth asymmetry, head circumference, and neurodevelopmental outcomes in infants with single ventricles. J Pediatr. 2016;168:220–225. doi: 10.1016/j.jpeds.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, et al. De novo mutations in histone modifying genes in congenital heart disease. Nature. 2013;498:220–223. doi: 10.1038/nature12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey A, Liang L, Edwards J, Brandt T, Mei H, Sharp A, et al. The impact of CNVs on outcomes for infants with single ventricle defects. Circ Cardiovasc Genet. 2013;6:444–451. doi: 10.1161/CIRCGENETICS.113.000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avitabile CM, Leonard MB, Brodsky JL, Whitehead KK, Ravishankar C, Cohen MS, et al. Usefulness of insulinlike growth factor 1 as a marker of heart failure in children and young adults after the Fontan palliation procedure. Am J Cardiol. 2015;115:816–20. doi: 10.1016/j.amjcard.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]