Abstract
Background and Purpose
We sought to compare the effect of chronic treatment with commonly tolerated doses of Fasudil, a specific RhoA kinase (ROCK) inhibitor, and simvastatin (with pleiotropic effects including ROCK inhibition) on cerebral cavernous malformation (CCM) genesis and maturation in two models that recapitulate the human disease.
Methods
Two heterozygous murine models, Ccm1+/-Msh2-/- and Ccm2+/-Trp53-/-, were treated from weaning to 4-5 months of age with Fasudil (100 mg/kg/day), simvastatin (40 mg/kg/day) or with placebo. Mouse brains were blindly assessed for CCM lesion burden, non-heme iron deposition (as a quantitative measure of chronic lesional hemorrhage) and ROCK activity.
Results
Fasudil, but not simvastatin, significantly decreased mature CCM lesion burden in Ccm1+/-Msh2-/- mice, and in meta-analysis of both models combined, when compared to mice receiving placebo. Fasudil and simvastatin both significantly decreased the integrated iron density per mature lesion area in Ccm1+/-Msh2-/- mice, and in both models combined, compared to mice given placebo. ROCK activity in mature lesions of Ccm1+/-Msh2-/- mice was similar with both treatments. Fasudil, but not simvastatin, improved survival in Ccm1+/-Msh2-/- mice. Fasudil and simvastatin treatment did not affect survival or lesion development significantly in Ccm2+/-Trp53-/- mice alone, and Fasudil benefit appeared limited to males.
Conclusion
ROCK inhibitor Fasudil was more efficacious than simvastatin in improving survival and blunting the development of mature CCM lesions. Both drugs significantly decreased chronic hemorrhage in CCM lesions. These findings justify the development of ROCK inhibitors, and the clinical testing of commonly used statin agents in CCM.
Keywords: Cerebral cavernous malformation, cavernous angioma, ROCK, Fasudil, simvastatin, therapy
Introduction
Several therapies1-5 have already been proposed to treat cerebral cavernous malformations (CCMs), a disease which often leads to hemorrhagic stroke and seizures. These have largely arisen from the study of impact of downregulation of CCM genes on cultured endothelial cells. A recent study6 demonstrated that treatments targeting Rho or the ADAMTS proteases may be more efficacious in treating CCM disease than other therapies. In a previous report,2 using a limited number of animals, we showed that the Rho kinase (ROCK) inhibitor, Fasudil, decreased CCM lesion burden in a heterozygous murine model where lesions develop stochastically during life, with all phenotypic signatures recapitulating the human disease.7
We now aimed to replicate our previous results with Fasudil, using a larger cohort of animals with two heterozygous Ccm genotypes. We also treated the murine models with simvastatin, commonly used in humans, with weak ROCK inhibitor properties.8-11 Unlike specific ROCK inhibitors, statins have broader pleiotropic effects,8 and concerns have been raised whether they might increase brain hemorrhage.12-14 Hence, besides assessing the effect of treatment on the number, stage and size of CCM lesions in each animal brain, we also investigated quantitative non-heme iron deposition within the mature CCM lesions and carefully analyzed animal attrition in order to address safety and hemorrhagic propensity. A portion of the results from this work was presented in poster form at the 2015 International Stroke Conference held in Nashville, Tennessee.15
Materials and Methods
Ccm Murine Models
Animal procedures were approved by the Duke University Institutional Animal Care and Use Committee. The Ccm1+/-Msh2-/- and Ccm2+/-Trp53-/- murine models for CCM disease were developed as previously reported.7, 16 Based on previously demonstrated Knudsonian 2-hit mechanism (heterozygosity in the germ line and biallelic somatic mutations in lesions) these models create Ccm heterozygous mice in p53 or Msh2 deficient backgrounds, predisposing to somatic mutations and enhancing CCM lesion genesis.7, 16, 17 Immediately after weaning (P21), mice were randomly assigned to the respective treatment groups. One hundred forty two Ccm1+/-Msh2-/- mice (71 male, 71 female) and 72 Ccm2+/-Trp53-/-mice (61 male, 11 female) were included in the experiments. The breeding program for creating mice with the appropriate genotype has been described previously.7, 16, 17 This is detailed along with a treatment assignment scheme in the on-line Supplemental Methods and Figure I (please see http://stroke.ahajournals.org).
Randomized Assignment and Treatment Groups
For all groups, the National Institute of Neurological Disorders and Stroke (NINDS) guidelines for objectivity in preclinical research were followed including randomization, blinding of outcome assessment, appropriate sample-size estimation based on the primary outcome, and prespecified data analyses.18 Ccm1+/-Msh2-/- mice were randomized at weaning to receive Fasudil (100 mg/kg/day in the drinking water) or “placebo” with drug-free drinking water until 4-5 months of age. In a separate study, mice were randomized to receive simvastatin (40 mg/kg/day in the chow) or “placebo” controls with the same drug free-diet from weaning until 4-5 months of age. Ccm2+/-Trp53-/- mice similarly assigned to receive the same regimen of Fasudil or placebo, and simvastatin or placebo. Late, short term studies were completed for Ccm1+/-Msh2-/- treated for a 1-month duration, from 3 to 4 months of age, with Fasudil (100 mg/kg/day in the drinking water) or placebo. A concurrent randomly assigned cohort (15 mice) of the Ccm1+/-Msh2-/- models were used as placebos for the long term studies with simvastatin and for the late, short term studies with Fasudil. Notably, the body weights of the CCM models were not affected by Fasudil or simvastatin treatment (Table I, please see http://stroke.ahajournals.org).
CCM Lesion Analysis
After treatment brains were removed and assessed for lesion burden on serial histologic sections by a procedure previously described.2, 7 Briefly, formalin fixed brains were cut into 1-mm thick coronal slices, placed into cassettes and processed. After embedding the slices in paraffin, the slices were cut into 5-μm thick sections with a microtome. After staining with hematoxylin and eosin (H&E), the sections were assessed for the number of stage 1 pre-lesions (single ballooned capillaries > 100 micrometer in diameter) and stage 2 lesions (mature, multicavernous) by two observers (RS and either CS, CA or TM), with adjudication by a third observer (IAA), as was done previously.2, 7, 19-21 All assessors were blinded to the treatment assignment. The lesional area was determined by using the polygon area function of a microscope digital camera DP21 (Olympus) as described previously.19
H&E-stained sections from brains of mice that either died or euthanized due to a debilitating illness were examined for hemorrhage or tumors (Table II, please see http://stroke.ahajournals.org). Tumors on sections were confirmed by University of Chicago neuropathologist PP.
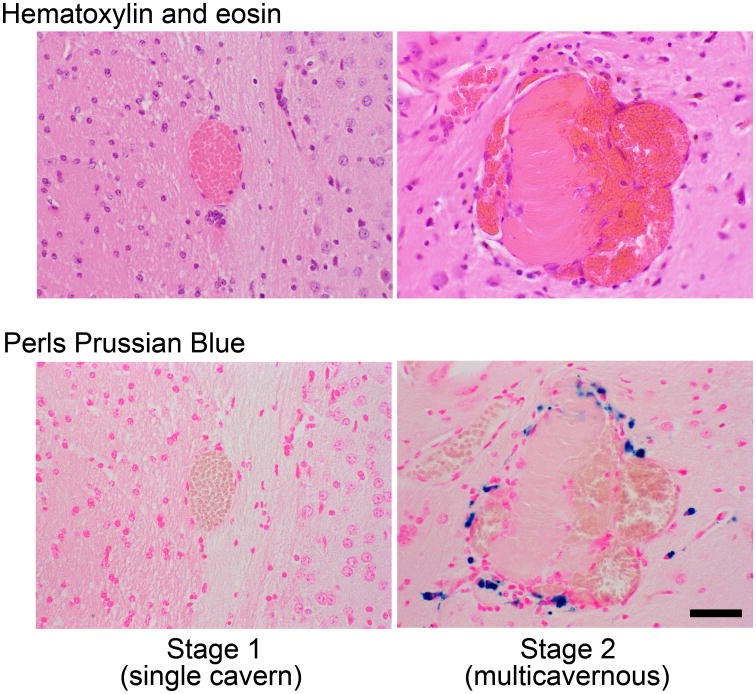
Blank sections from brains with stage 2 CCM lesions were processed quantitative assessment of non-heme iron deposition by Perls Prussian blue and for ROCK activity by intensity of phosphorylated myosin light chain (pMLC) through methods previously described.2, 7, 19 We had previously shown that stage 1 pre-lesions do not manifest iron deposition,7 and this was also true in the present study. Figure 1 illustrates typical stage 1 and 2 lesions, and associated iron depiction in the latter.
Figure 1.
Illustrative photomicrographs of stage 1 and 2 lesions, and typical non-heme iron deposition in stage 2, but not stage 1, lesions. Bar = 50μm.
Primary and secondary outcomes are defined and the statistical methods are presented in the Supplemental Methods (please see http://stroke.ahajournals.org).
Results
Fasudil, but Not Simvastatin, Decreases Lesion Burden in CCM Models
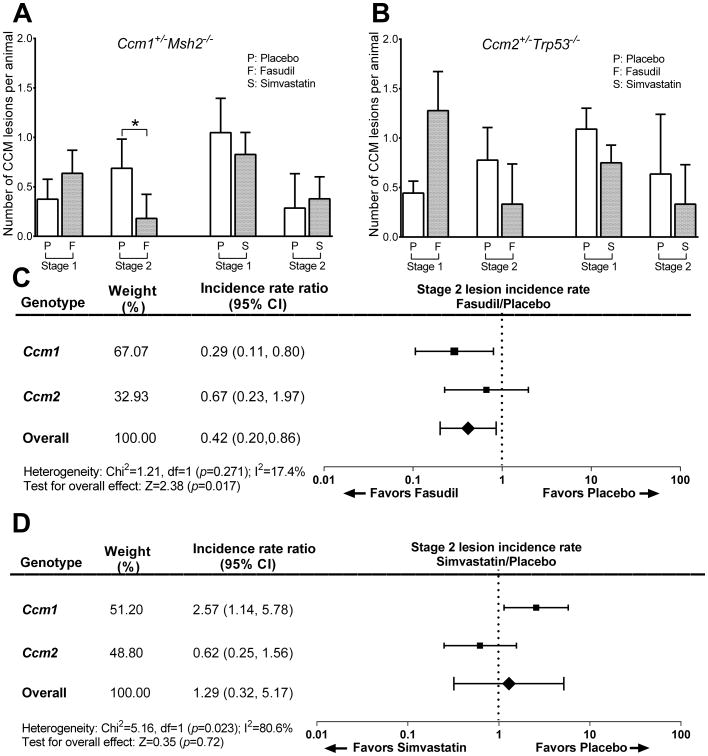
The number of mature multicavernous stage 2 lesions per mouse decreased significantly (P=0.02) by 74% from 0.68 ± 0.30 in placebos to 0.18 ± 0.24 in Fasudil-treated Ccm1+/-Msh2-/- animals (Figure 2A). Stage 2 lesion burden decreased, but not significantly (P=0.25), by 58% from 0.78 ± 0.33 in placebos to 0.33 ± 0.41 in Fasudil-treated Ccm2+/-Trp53-/- animals (Figure 2B). There were significant increases in stage 1 lesion counts, and no impact on total lesion counts, as the proportion of stage 2 lesions among total lesions decreased from 65% in placebos to 22% in the Fasudil-treated Ccm1+/-Msh2-/-mice (P=0.02) and decreased from 64% in placebos to 21% in the Fasudil-treated Ccm2+/-Trp53-/-murine model (P=0.002) indicating an effect of treatment on lesion maturation.
Figure 2.
Lesion burden in Ccm models with Fasudil and simvastatin treatment. A. Fasudil (n=16 placebo, n=22 Fasudil-treated mice), but not simvastatin (n=20 placebo, n=28 simvastatin-treated mice), decreased the multicavernous stage 2 lesion burden in the Ccm1+/-Msh2-/- murine model. B. Neither Fasudil (n=18 placebo, n=18 Fasudil-treated mice), nor simvastatin (n=11 placebo, n=12 simvastatin-treated mice), significantly affected lesion burden in Ccm2+/-Trp53-/- mice. Meta-analysis of combined effect in the two models showed that incidence of stage 2 lesions is decreased with Fasudil (C), but not with simvastatin (D). The DerSimonian and Laird method22 used for the meta-analyses considers the relative contribution of effect by the two models (weight %) and their different penetrance (incidence rate ratio).
In contrast, the number of stage 2 lesions per mouse and other lesion counts were not decreased by simvastatin in either of the genotypes compared to placebo. A meta-analysis of combined effect in the two genotypes showed a significantly decreased incidence of stage 2 lesions favoring Fasudil (P=0.017) (Figure 2C). A similar meta-analysis showed no significant effect of simvastatin on the incidence of stage 2 lesions in the two genotypes (Figure 2D). Neither Fasudil nor simvastatin significantly affected lesional cross sectional area in either genotype. But there was a trend of a smaller stage 2 lesional area in Fasudil treated mice, approaching statistical significance in meta-analysis of effect in combined genotypes (Figure II, please see http://stroke.ahajournals.org).
A few significant observations arose in the prespecified subgroup analysis of results per the animal's sex (Table III, please see http://stroke.ahajournals.org). In Ccm2+/-Trp53-/- male brains, there were significantly less stage 2 lesions in Fasudil-treated mice compared to placebos (P=0.04), an effect not present in the cohort as a whole. Among Ccm2+/-Trp53-/- females, there were significantly fewer total (stage 1 and 2) lesions/animal in simvastatin-treated mice than in placebos (P=0.02), an effect not present in the cohort as a whole. But we did note an imbalance in treatment assignment between sexes.
Fasudil given later in life for one month (at age 3-4 months) to Ccm1+/-Msh2-/- mice did not affect lesion burden when compared to placebo in the overall treated cohort (Figure III, please see http://stroke.ahajournals.org). However, in male mice, the total number of lesions per mouse was significantly lower, 2.89 ± 0.93 in 9 placebos versus 1.29 ± 0.68 in 7 Fasudil-treated animals (P=0.036). An opposite effect (greater lesion burden) was observed in female mice, but did not reach statistical significance. There was an imbalance in the placebo group, with females harboring fewer lesions.
Fasudil and Simvastatin Decrease Non-heme Iron in Mature CCM Lesions
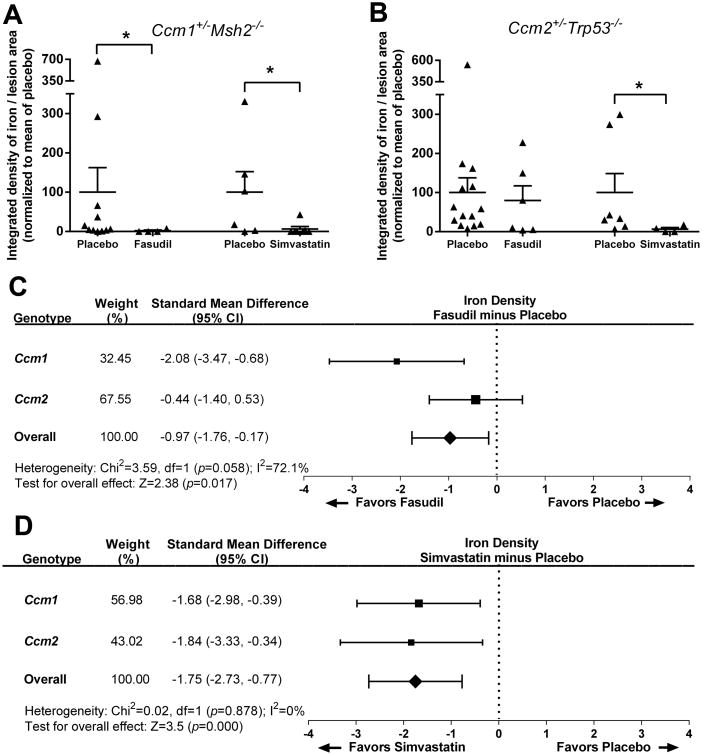
Both Fasudil (P<0.01) and simvastatin (P<0.05) both significantly decreased non-heme iron in the multicavernous stage 2 lesions of the Ccm1+/-Msh2-/- murine model, when compared to placebo treated animals (Figure 3). Simvastatin (P<0.05), but not Fasudil, decreased non-heme iron in the multicavernous lesions of the Ccm2+/-Trp53-/- murine model, when compared to placebo treated animals. Meta-analyses of effect on non-heme iron density per stage 2 lesional area in the two genotypes favored both Fasudil and simvastatin over placebo (P=0.017 and P<0.001 respectively). There was no differential sex effect of either treatment on iron deposit in lesions (Table IV, please see http://stroke.ahajournals.org).
Figure 3.
Non-heme iron deposition in multicavernous lesions with Fasudil and simvastatin treatment. A, Both Fasudil (n=11 and n=4 stage 2 lesions in placebo and Fasudil-treated mice, respectively) and simvastatin (n=6 and n=7 stage 2 lesions in placebo and simvastatin-treated mice, respectively) decreased non-heme iron deposition per lesion area in the multicavernous Stage 2 lesions in the Ccm1+/-Msh2-/- murine model. B, Simvastatin (n= 7 and n=4 stage 2 lesions in placebo and simvastatin-treated mice, respectively), but not Fasudil (n= 14 and n=6 stage 2 lesions in placebo and Fasudil-treated mice, respectively), significantly decreased non-heme iron deposition per lesion area in stage 2 lesions in the less penetrant Ccm2+/-Trp53-/- murine model. Meta-analysis of combined effect in the two models showed that non-heme iron deposition per lesion area in Stage 2 lesions is decreased with Fasudil (C) and with simvastatin (D). The DerSimonian and Laird method22 used for the meta-analyses considers the relative contribution of effect by the two models (weight %) and their difference in iron deposition per lesion.
Fasudil and Simvastatin Decrease ROCK Activity in Mature CCM Lesions
Stage 2 lesional endothelial cell ROCK activity in Ccm1+/-Msh2-/- murine brains (measured by the percentage of cells that were pMLC positive) was significantly decreased from 68.7% in placebos to 51.9% in Fasudil-treated mice (P=0.01 vs. placebo) and 57.0% in simvastatin-treated mice (P=0.007 vs. placebo). There was no significant difference of percent endothelial cell immunopositivity among individual lesions or mice in the placebo, Fasudil or simvastatin cohorts (Figure IV, please see http://stroke.ahajournals.org).
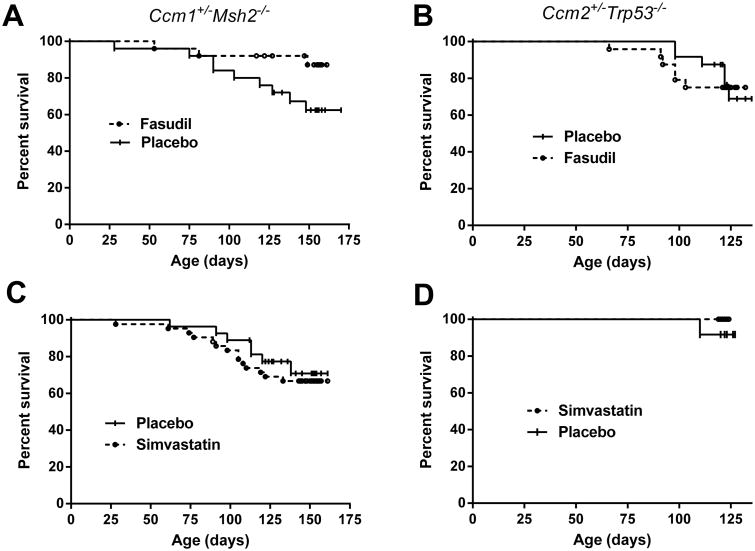
Fasudil Increases Survival in Ccm1 Models
The proportion of Ccm1+/-Msh2-/- mice that survived to complete the long term treatment was higher (P=0.05) with Fasudil compared to placebo (Figure 4). No significant difference on survival was observed between females and male animals. There was no effect on survival in this model with simvastatin treatment compared to placebos. For the Ccm2+/-Trp53-/- murine model no effect on survival was observed with either Fasudil or simvastatin treatment compared to placebos. In the Ccm1+/-Msh2-/- murine model treated from 3 to 4 months, there was a non-significant trend for increased survival with Fasudil treatment (17/18) compared to placebos (16/20). No increase in brain hemorrhage in association with animal attrition was observed with either Fasudil or simvastatin treatment compared to placebos in either genotype (Supplemental Table I, please see http://stroke.ahajournals.org).
Figure 4.
Kaplan–Meier plots of survival in CCM models with Fasudil and simvastatin treatment. Fasudil treatment increased (P=0.05) the survival of the Ccm1+/-Msh2-/- murine models (A; n=25 per group), while simvastatin did not affect survival of these animals (B; n=27 placebo, n=42 simvastatin). Both Fasudil treatment (C; n=24 per group) or simvastatin treatment (D; n=12 per group) did not affect the survival of Ccm2+/-Trp53-/- murine models.
Discussion
Two classes of murine models of CCM disease are available to test potential experimental therapies. Acute models with postnatal induced homozygous Ccm loss have been used to investigate mechanisms of CCM pathogenesis, including endothelial mesenchymal transition,3 sulindac therapy,4 delta-notch signaling,5 and MEKK3-KLF2/4 signaling.6 These models result in high lesion burden in the developing hindbrain and retina, and animal attrition before the first month of age. While allowing examination of signaling mechanisms involved in lesion genesis, these models neither assess CCM lesion maturation during life, nor the hemorrhage burden from lesions, cardinal features of the human disease. In contrast, the chronic heterozygous models we used herein7, 16, 17, 20 develop CCM lesions during the animal's lifespan, resembling the human disease more closely than the shorter lived acute models.
Our results provide evidence that long term Fasudil treatment, but not simvastatin, prevents the development of mature, multicavernous CCM lesions. We also noted that both drugs are more efficacious in the Ccm1+/-Msh2-/- compared to the Ccm2+/-Trp53-/- murine model. Meta-analysis of results from both genotypes confirmed a beneficial effect on the development of mature CCM lesions, favoring Fasudil treatment, but not simvastatin, over placebo. While both drugs at the chosen doses appeared to affect endothelial ROCK activity similarly in mature murine lesions, there is evidence that statins result in weaker systemic ROCK inhibition when compared to specific ROCK inhibitor drugs,9 and this could explain the differential benefit favoring Fasudil.
Fasudil and simvastatin both significantly decreased non-heme iron deposition in mature CCM lesions in two murine models. This effect was present even when adjusting for lesion area, and occurred with simvastatin in the absence of reduction in lesion burden. Simvastatin effect on lesional iron deposition was even greater than Fasudil in the Ccm2+/-Trp53-/- mice. Previous studies20, 23, 24 indicated that ROCK inhibition blocks endothelial hyperpermeability and actin stress fibers resulting from the heterozygous loss of CCM genes, both in cultured endothelial cells in vitro and in murine models in vivo, and simvastatin reverses dermal hyperpermeability in one of these models.25 Our group had previously demonstrated a correlation of quantitative vascular permeability with iron content in human CCM lesions evaluated by advanced magnetic resonance imaging (MRI) techniques.26 Blood leak from CCM lesions produces the most debilitating features of CCM disease, including hemorrhagic stroke and seizures. A favorable effect of either drug on lesional iron deposit is hence relevant clinically.
Long term (≥ 4 months) experiments in rodents treated with Fasudil in the drinking water or by gavage have used doses ranging from 10 to 228 mg/kg/day without any reported attrition or complications.27-32 In our proposed experiments, we used the midrange dose of 100 mg/kg/day, already shown to reduce CCM lesion burden in pilot studies.2 A lower dose of 10 mg/kg/day has been reported to be effective in suppressing cardiac allograft vasculopathy in mice.27 An effect at a lower dose was not investigated herein, but would be particularly encouraging for translating this therapy to humans, where oral doses as high as 240 mg/day have been well tolerated in trials on angina,33 and an intravenous dose of 90 mg/day is approved and has long been used in Japan for the prevention of cerebral vasospasm after subarachnoid hemorrhage.34 While treatment of transgenic mice indicates a potential therapeutic efficacy in CCM, Fasudil is a Rock1 and Rock2 inhibitor that was first approved in Japan in 1995 by Asahi Kasei Pharma Corporation for the treatment of cerebral vasospasm, and it has never been approved in the USA. Reported toxicity with Fasudil includes subcutaneous hemorrhage, subarachnoid hemorrhage, nausea, pyrexia, kidney failure and hypotension. Long term safety of Fasudil has not been assessed, in particular with concerns about hypotension and systemic side effects. Rock1 has widespread tissue distribution, but there is relatively little Rock1 in brain and skeletal muscle. Rock2 is mainly expressed in the central nervous system and is relatively low in liver, stomach, spleen, kidney and testis (BioAxone US patent 7,572,913). Inhibitors that target Rock2 are currently being developed, and would be expected to have much less systemic toxicity than inhibitors that target both kinases. For treatment of CCM, Rock2 inhibition promises to target cerebral microvessels and CCM vessels more selectively, and could increase targeted effectiveness and minimize systemic side effect.
We have chosen simvastatin as our statin test agent since this drug was shown to reverse dermal hyperpermeability in Ccm heterozygous mice.25 Simvastatin has been administered orally in the animals' chow, drinking water or by gavage in doses ranging from 2 to 100 mg/kg/day in chronic (≥ 4 months) studies without adverse effects.35-47 The target dose of simvastatin of 40 mg/kg/day, is at the mid-range used by other investigators, and is thought to be equivalent to adult human doses of 20-40 mg/day widely used for cardiovascular and cholesterol lowering effects.48 We should nevertheless be cautious about statin dose-effect, as statins have been reported to exhibit biphasic dose effects on inflammation-induced angiogenesis49 and higher doses are typically needed to achieve greater ROCK inhibition pleotropic effect.9
Because simvastatin at 40 mg/kg/day in the chow was less effective in reducing lesion burden than Fasudil, we are currently treating the Ccm1+/-Msh2-/- murine model with atorvastatin at 80 mg/kg/day in the chow (equivalent to simvastatin at 160 mg/kg/day in mice, and thought to be equivalent to the high dose atorvastatin 80 mg/day approved for clinical use in humans). A putative benefit on lesion burden and/or blunting CCM hemorrhage with higher dose statin will have implications regarding dose escalation in clinical trials that may be planned in humans. We are also in the process of treating Ccm3+/- murine models with Fasudil, simvastatin and atorvastatin, to determine if ROCK inhibitors can reduce lesion burden in another model with a more penetrant genotype.20
The benefit of Fasudil on lesion burden in the current study primarily impacted the development of mature multicavernous stage 2 lesions. Only stage 2 lesions have been associated with bleeding and inflammatory cell infiltrate in previous studies, 7 and we also noted no hemorrhage in stage 1 lesions in our current experiments. There was no effect on total lesion counts, and in fact we noted increases in stage 1 pre-lesion counts, and a greater prevalence of stage 1 lesions among total lesions. These results are all consistent with a therapeutic effect blunting lesion maturation into clinically relevant multicavernous phenotype, rather than lesion genesis. A trend was also noted of Fasudil on cross-sectional area of mature stage 2 lesions, as assessed by histologic morphometry. The combined effect of therapy on lesional count and cross sectional area promises to be more easily and accurately assessed in ongoing experiments using novel micro-computed tomography technique recently introduced and validated by our team, with volumetric quantification of CCM lesion burden in murine brains.50
We cannot comment on the relative benefits of ROCK inhibition versus B-cell depletion therapy, also demonstrated to be effective in chronic heterozygous Ccm murine models.19 Either therapy may have a role in the clinical setting, and it is possible that ROCK inhibition effect is in part mediated by modulation of the immune response in lesions. Indeed, we had previously shown that ROCK inhibition decreases B-cell infiltration in murine CCM lesions,2 and B-cell depletion decreases ROCK activity in lesional endothelium.19 Other compelling therapeutic targets have been identified in CCM, based on aberrant signaling in conjunction with acute postnatally induced Ccm homozygocity.3, 4, 6, 51 These therapeutic strategies have not been examined in chronic heterozygous models, and a recently identified primary CCM signaling effector was shown to act via downstream RhoA/ROCK activation.6
Because of the small numbers of animals, imbalance of treatment assignment and different phenotype severity by gender, definitive conclusions cannot be determined from subgroup analyses by sex. Nevertheless, we documented a greater benefit of Fasudil on lesion burden in males in both short term and long term treatments, a potential negative effect of short term Fasudil treatment, and a weaker phenotype in female mice receiving placebo. These may reflect mechanistic biologic effects, and will need to be confirmed in future studies. A weaker effect of Fasudil on lesion development in Ccm2+/-Trp53-/- mice, and a greater benefit of simvastatin than Fasudil on lesional iron in this model, may also represent differential biologic mechanisms. If these observations are confirmed in future studies, they may indicate a complex biology of ROCK inhibition requiring careful calibration.
Our experiments do not exclude pleiotropic effects of either drug, other than ROCK inhibition, as the mechanism of therapeutic effect. Animal weights were not affected by treatment in any cohort. And while we did not document blood pressure measurements, similar dose and duration of Fasudil treatment in mice did not significantly impact blood pressure in other studies.30, 52
Duration of therapy in mice cannot be easily translated into a prescribed course of treatment in humans. Beyond dose-effect questions, it is unclear when or how long to administer the treatment in order to blunt lesion development and maturation. Clinical trials of ROCK inhibition with specific drugs and or statins may target lesion formation and progression to larger clinically more relevant lesions. CCM hemorrhage is a most compelling outcome parameter, associated with neurologic sequelae,53 and this report identifies for the first time an effect of a drug on bleeding in CCM lesions. Yet it is unclear at what stage, and for how long a human lesion should be treated in order to prevent bleeding or rebleeding. It may be compelling to consider drug therapy for a lesion that has recently bled, and is hence most likely to rebleed in the next 2-3 years,54, 55 so ROCK inhibition might be conceived as lesion stabilization therapy. Dose escalation in clinical trials may be calibrated using dynamic contrast enhanced quantitative perfusion on MRI, thought to reflect ROCK induced hyperpermeability in vivo.26, 56, 57 Another MRI biomarker, quantitative susceptibility mapping, may be useful in clinical trials, to assess CCM lesional hemorrhage as a therapeutic outcome in humans,26, 57-59 as it was herein shown in mice.
Summary
ROCK-inhibitor Fasudil, but not simvastatin, blunted CCM lesion development into mature clinically significant forms. Both drugs significantly decreased lesional bleeding, the first such effect ever reported in CCM. These results justify the development of ROCK inhibitors as therapeutic agents for CCM, and the testing of statins, with cautious dose escalation, in humans.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported, in part, by a grant from the NIH, National Institute of Neurological Disorders and Stroke R01 NS077957 to Drs. Awad and Marchuk and the University of Chicago Medicine Comprehensive Cancer Center Support Grant (#P30 CA14599) and the services provided by the Immunohistochemistry Core Facility. The funding sources had no input on data analysis or interpretation of results.
Footnotes
Disclosures: JKL has consultancy for Asahi-Kasei Pharmaceuticals, Inc., Celgene, Pliant and Amgen.
References
- 1.Shi C, Shenkar R, Kinloch A, Henderson SG, Shaaya M, Chong AS, et al. Immune complex formation and in situ b-cell clonal expansion in human cerebral cavernous malformations. J Neuroimmunol. 2014;272:67–75. doi: 10.1016/j.jneuroim.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 2.McDonald DA, Shi C, Shenkar R, Stockton RA, Liu F, Ginsberg MH, et al. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke. 2012;43:571–574. doi: 10.1161/STROKEAHA.111.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, et al. Endmt contributes to the onset and progression of cerebral cavernous malformations. Nature. 2013;498:492–496. doi: 10.1038/nature12207. [DOI] [PubMed] [Google Scholar]
- 4.Bravi L, Rudini N, Cuttano R, Giampietro C, Maddaluno L, Ferrarini L, et al. Sulindac metabolites decrease cerebrovascular malformations in ccm3-knockout mice. Proc Natl Acad Sci U S A. 2015;112:8421–8426. doi: 10.1073/pnas.1501352112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wustehube J, Bartol A, Liebler SS, Brutsch R, Zhu Y, Felbor U, et al. Cerebral cavernous malformation protein ccm1 inhibits sprouting angiogenesis by activating delta-notch signaling. Proc Natl Acad Sci U S A. 2010;107:12640–12645. doi: 10.1073/pnas.1000132107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Z, Tang AT, Wong WY, Bamezai S, Goddard LM, Shenkar R, et al. Cerebral cavernous malformations arise from endothelial gain of mekk3-klf2/4 signalling. Nature. 2016;532:122–126. doi: 10.1038/nature17178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald DA, Shenkar R, Shi C, Stockton RA, Akers AL, Kucherlapati MH, et al. A novel mouse model of cerebral cavernous malformations based on the two-hit mutation hypothesis recapitulates the human disease. Hum Mol Genet. 2011;20:211–222. doi: 10.1093/hmg/ddq433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Q, Liao JK. Pleiotropic effects of statins. - basic research and clinical perspectives. Circ J. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: Differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119:131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rikitake Y, Liao JK. Rho gtpases, statins, and nitric oxide. Circ Res. 2005;97:1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CB, Li XX, Chen YG, Gao HQ, Bao MC, Zhang J, et al. Simvastatin exerts cardioprotective effects and inhibits the activity of rho-associated protein kinase in rats with metabolic syndrome. Clin Exp Pharmacol Physiol. 2012;39:759–764. doi: 10.1111/j.1440-1681.2012.05730.x. [DOI] [PubMed] [Google Scholar]
- 12.Ricard G, Garant MP, Carrier N, Leblanc N, Boulanger JM. Statins may increase intracerebral hemorrhage volume. Can J Neurol Sci. 2010;37:791–796. doi: 10.1017/s0317167100051453. [DOI] [PubMed] [Google Scholar]
- 13.Eisa-Beygi S, Wen XY, Macdonald RL. A call for rigorous study of statins in resolution of cerebral cavernous malformation pathology. Stroke. 2014;45:1859–1861. doi: 10.1161/STROKEAHA.114.005132. [DOI] [PubMed] [Google Scholar]
- 14.Amarenco P, Labreuche J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. doi: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- 15.Shenkar R, Shi C, McDonald D, Austin C, Rorrer A, Gallione C, et al. A comparison between fasudil and simvastatin treatment in two murine models with cerebral cavernous malformation. Stroke. 2015;46 AWP415 Abstract. [Google Scholar]
- 16.Plummer NW, Squire TL, Srinivasan S, Huang E, Zawistowski JS, Matsunami H, et al. Neuronal expression of the ccm2 gene in a new mouse model of cerebral cavernous malformations. Mamm Genome. 2006;17:119–128. doi: 10.1007/s00335-005-0098-8. [DOI] [PubMed] [Google Scholar]
- 17.Plummer NW, Gallione CJ, Srinivasan S, Zawistowski JS, Louis DN, Marchuk DA. Loss of p53 sensitizes mice with a mutation in ccm1 (krit1) to development of cerebral vascular malformations. Am J Pathol. 2004;165:1509–1518. doi: 10.1016/S0002-9440(10)63409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi C, Shenkar R, Zeineddine HA, Girard R, Fam MD, Austin C, et al. B-cell depletion reduces the maturation of cerebral cavernous malformations in murine models. J Neuroimmune Pharmacol. 2016;11:369–377. doi: 10.1007/s11481-016-9670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenkar R, Shi C, Rebeiz T, Stockton RA, McDonald DA, Mikati AG, et al. Exceptional aggressiveness of cerebral cavernous malformation disease associated with pdcd10 mutations. Genet Med. 2015;17:188–196. doi: 10.1038/gim.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shenkar R, Venkatasubramanian PN, Wyrwicz AM, Zhao JC, Shi C, Akers A, et al. Advanced magnetic resonance imaging of cerebral cavernous malformations: Part ii. Imaging of lesions in murine models. Neurosurgery. 2008;63:790–797. doi: 10.1227/01.NEU.0000315862.24920.49. discussion 797-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Stockton RA, Shenkar R, Awad IA, Ginsberg MH. Cerebral cavernous malformations proteins inhibit rho kinase to stabilize vascular integrity. J Exp Med. 2010;207:881–896. doi: 10.1084/jem.20091258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borikova AL, Dibble CF, Sciaky N, Welch CM, Abell AN, Bencharit S, et al. Rho kinase inhibition rescues the endothelial cell cerebral cavernous malformation phenotype. J Biol Chem. 2010;285:11760–11764. doi: 10.1074/jbc.C109.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via rho gtpases. Nat Med. 2009;15:177–184. doi: 10.1038/nm.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikati AG, Tan H, Shenkar R, Li L, Zhang L, Guo X, et al. Dynamic permeability and quantitative susceptibility: Related imaging biomarkers in cerebral cavernous malformations. Stroke. 2014;45:598–601. doi: 10.1161/STROKEAHA.113.003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Kaibuchi K, et al. Long-term treatment with a specific rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res. 2004;94:46–52. doi: 10.1161/01.RES.0000107196.21335.2B. [DOI] [PubMed] [Google Scholar]
- 28.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, et al. Long-term inhibition of rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 29.Ying H, Biroc SL, Li WW, Alicke B, Xuan JA, Pagila R, et al. The rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol Cancer Ther. 2006;5:2158–2164. doi: 10.1158/1535-7163.MCT-05-0440. [DOI] [PubMed] [Google Scholar]
- 30.Wu DJ, Xu JZ, Wu YJ, Jean-Charles L, Xiao B, Gao PJ, et al. Effects of fasudil on early atherosclerotic plaque formation and established lesion progression in apolipoprotein e-knockout mice. Atherosclerosis. 2009;207:68–73. doi: 10.1016/j.atherosclerosis.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 31.Kagiyama S, Matsumura K, Goto K, Otsubo T, Iida M. Role of rho kinase and oxidative stress in cardiac fibrosis induced by aldosterone and salt in angiotensin type 1a receptor knockout mice. Regul Pept. 2010;160:133–139. doi: 10.1016/j.regpep.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Steioff K, Rutten H, Busch AE, Plettenburg O, Ivashchenko Y, Lohn M. Long term rho-kinase inhibition ameliorates endothelial dysfunction in ldl-receptor deficient mice. Eur J Pharmacol. 2005;512:247–249. doi: 10.1016/j.ejphar.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, et al. Efficacy and safety of fasudil in patients with stable angina: A double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 34.Liao JK, Seto M, Noma K. Rho kinase (rock) inhibitors. J Cardiovasc Pharmacol. 2007;50:17–24. doi: 10.1097/FJC.0b013e318070d1bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachtigal P, Pospisilova N, Pospechova K, Jamborova G, Kopecky M, Jaynes R, et al. Mdoc and atorvastatin have potential antiinflammatory effects in vascular endothelium of apoe-/- mouse model of atherosclerosis. Life Sci. 2006;78:1983–1989. doi: 10.1016/j.lfs.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 36.Kempster S, Bate C, Williams A. Simvastatin treatment prolongs the survival of scrapie-infected mice. Neuroreport. 2007;18:479–482. doi: 10.1097/WNR.0b013e328058678d. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan NB, Gatto R. Synergistic benefits of erythropoietin and simvastatin after traumatic brain injury. Brain Res. 2010;1360:177–192. doi: 10.1016/j.brainres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Yudoh K, Karasawa R. Statin prevents chondrocyte aging and degeneration of articular cartilage in osteoarthritis (oa) Aging (Albany NY) 2010;2:990–998. doi: 10.18632/aging.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sparrow CP, Burton CA, Hernandez M, Mundt S, Hassing H, Patel S, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21:115–121. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 40.Ivanovski O, Szumilak D, Nguyen-Khoa T, Nikolov IG, Joki N, Mothu N, et al. Effect of simvastatin in apolipoprotein e deficient mice with surgically induced chronic renal failure. J Urol. 2008;179:1631–1636. doi: 10.1016/j.juro.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 41.Stone LM, Tan SS, Tam PP, Finger TE. Analysis of cell lineage relationships in taste buds. J Neurosci. 2002;22:4522–4529. doi: 10.1523/JNEUROSCI.22-11-04522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mao MJ, Hu JP, Chen FR, Zhang YY, Liu P. Effects of Chinese herbal medicine guanxinkang on lipid metabolism and serum c-reactive protein, amyloid a protein and fibrinogen in apolipoprotein e-knockout mice with atherosclerosis [in Chinese] Zhong Xi Yi Jie He Xue Bao. 2011;9:306–312. doi: 10.3736/jcim20110312. [DOI] [PubMed] [Google Scholar]
- 43.Qin W, Lu Y, Zhan C, Shen T, Dou L, Man Y, et al. Simvastatin suppresses apoptosis in vulnerable atherosclerotic plaques through regulating the expression of p(53), bcl-2 and bcl-xl. Cardiovasc Drugs Ther. 2012;26:23–30. doi: 10.1007/s10557-011-6347-z. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Mao S, Luo G, Wei J, Berggren-Soderlund M, Nilsson-Ehle P, et al. Effects of simvastatin on apolipoprotein m in vivo and in vitro. Lipids Health Dis. 2011;10:112. doi: 10.1186/1476-511X-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goncharova EA, Goncharov DA, Li H, Pimtong W, Lu S, Khavin I, et al. Mtorc2 is required for proliferation and survival of tsc2-null cells. Mol Cell Biol. 2011;31:2484–2498. doi: 10.1128/MCB.01061-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Zhang W, Zhu D, Zhu X, Pang X, Qu W. Hypolipidaemic and hypoglycaemic effects of total flavonoids from seed residues of hippophae rhamnoides l. In mice fed a high-fat diet. J Sci Food Agric. 2011;91:1446–1451. doi: 10.1002/jsfa.4331. [DOI] [PubMed] [Google Scholar]
- 47.Lei YF, Chen JL, Wei H, Xiong CM, Zhang YH, Ruan JL. Hypolipidemic and anti-inflammatory properties of abacopterin a from abacopteris penangiana in high-fat diet-induced hyperlipidemia mice. Food Chem Toxicol. 2011;49:3206–3210. doi: 10.1016/j.fct.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 48.Robson J. Lipid modification: Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. Heart. 2008;94:1331–1332. doi: 10.1136/hrt.2008.150979. [DOI] [PubMed] [Google Scholar]
- 49.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 50.Girard R, Zeineddine HA, Orsbon C, Tan H, Moore T, Hobson N, et al. Micro-computed tomography in murine models of cerebral cavernous malformations as a paradigm for brain disease. J Neurosci Methods. 2016;271:14–24. doi: 10.1016/j.jneumeth.2016.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gibson CC, Zhu W, Davis CT, Bowman-Kirigin JA, Chan AC, Ling J, et al. Strategy for identifying repurposed drugs for the treatment of cerebral cavernous malformation. Circulation. 2015;131:289–299. doi: 10.1161/CIRCULATIONAHA.114.010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YX, Martin-McNulty B, da Cunha V, Vincelette J, Lu X, Feng Q, et al. Fasudil, a rho-kinase inhibitor, attenuates angiotensin ii-induced abdominal aortic aneurysm in apolipoprotein e-deficient mice by inhibiting apoptosis and proteolysis. Circulation. 2005;111:2219–2226. doi: 10.1161/01.CIR.0000163544.17221.BE. [DOI] [PubMed] [Google Scholar]
- 53.Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA Angioma Alliance Scientific Advisory B. Hemorrhage from cavernous malformations of the brain: Definition and reporting standards. Angioma alliance scientific advisory board. Stroke. 2008;39:3222–3230. doi: 10.1161/STROKEAHA.108.515544. [DOI] [PubMed] [Google Scholar]
- 54.Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, et al. Untreated clinical course of cerebral cavernous malformations: A prospective, population-based cohort study. Lancet Neurol. 2012;11:217–224. doi: 10.1016/S1474-4422(12)70004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Horne MA, Flemming KD, Su IC, Stapf C, Jeon JP, Li D, et al. Clinical course of untreated cerebral cavernous malformations: A meta-analysis of individual patient data. [Accessed October 11, 2016];Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)00303-8. published online ahead of print December 1, 2015. http://www.sciencedirect.com/science/article/pii/S1474442215003038. [DOI] [PMC free article] [PubMed]
- 56.Mikati AG, Khanna O, Zhang L, Girard R, Shenkar R, Guo X, et al. Vascular permeability in cerebral cavernous malformations. J Cereb Blood Flow Metab. 2015;35:1632–1639. doi: 10.1038/jcbfm.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girard R, Fam MD, Zeineddine HA, Tan H, Mikati AG, Shi C, et al. Vascular permeability and iron deposition biomarkers in longitudinal follow-up of cerebral cavernous malformations. J Neurosurg. 2016:1–9. doi: 10.3171/2016.5.JNS16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan H, Liu T, Wu Y, Thacker J, Shenkar R, Mikati AG, et al. Evaluation of iron content in human cerebral cavernous malformation using quantitative susceptibility mapping. Invest Radiol. 2014;49:498–504. doi: 10.1097/RLI.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan H, Zhang L, Mikati AG, Girard R, Khanna O, Fam MD, et al. Quantitative susceptibility mapping in cerebral cavernous malformations: Clinical correlations. AJNR Am J Neuroradiol. 2016;37:1209–1215. doi: 10.3174/ajnr.A4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.