Abstract
Objective
To characterize administration of sedatives, analgesics, and paralytics in a large cohort of mechanically ventilated, premature infants.
Study design
Retrospective cohort study including all infants <1500 g birth weight and <32 weeks gestational age mechanically ventilated at 348 Pediatrix Medical Group neonatal intensive care units (NICU) from 1997–2012. The primary outcome is the proportion of mechanically ventilated days in which infants were administered drugs of interest. Multivariable logistic regression was used to evaluate the predictors of administration of drugs of interest.
Results
We identified 85,911 mechanically ventilated infants. Infants received a drug of interest (opioids, benzodiazepines, other sedatives, and paralytics) on 433,587/1,305,413 (33%) of mechanically ventilated infant-days. The administration of opioids increased during the study period from 5% of infant-days in 1997 to 32% in 2012. The administration of benzodiazepines increased during the study period from 5% of infant-days in 1997 to 24% in 2012. Use of paralytics and other drugs remained ≤1% throughout the study period. Predictors of drug administration included younger gestational age, small for gestational age status, male sex, major congenital anomaly, older postnatal age at intubation, exposure to high frequency ventilation, exposure to inotropes, more recent year of discharge, and NICU site.
Conclusion
Administration of opioids and benzodiazepines in mechanically ventilated premature infants increased over time. Because infant characteristics were unchanged, site-specific differences in practice likely explain our observations. Increased administration over time is concerning given limited evidence of benefit and potential for harm.
Keywords: comfort drugs, ventilated infants
Premature infants admitted to neonatal intensive care units (NICUs) frequently receive mechanical ventilation through an endotracheal tube.(1) Mechanical ventilation has been associated with pain, distress, and feelings of breathlessness in older children and adults.(2, 3) To alleviate these discomforts and facilitate gas exchange during mechanical ventilation, children and adults routinely receive sedatives and analgesics.(4, 5) However, routine administration of analgesics in premature infants receiving mechanical ventilation may be associated with harm.(6–11)
Randomized controlled trials of routine morphine administration demonstrate an increased incidence of hypotension, drug dependence, air leaks, prolonged duration of mechanical ventilation, and prolonged time to full enteral feeds.(6–11) Opioids also are associated with apoptosis in the developing brains of animals, and although it is unknown how extensively this occurs in humans, concerns about adverse neurocognitive outcomes associated with opioid use during infancy exist.(12–17) Based on available evidence, guidelines in 2006 recommended against routine use of sedatives and analgesics in premature infants receiving mechanical ventilation.(18, 19) Guidelines regarding use of paralytics in preterm infants do not currently exist. Even though some studies suggested possible benefits in infants with ventilator dyssynchrony, routine use of paralytics in preterm infants may be associated with sustained paralysis, joint contractures, and hearing loss.(20–23)
The frequency and determinants of sedative and analgesic use in premature infants receiving mechanical ventilation are not well described. Information regarding determinants of sedative and analgesic use in this population will help identify infants at risk for associated adverse neurocognitive outcomes, and will allow more efficient design of future trials investigating the risks and benefits of sedative and analgesic administration. Here, we seek to characterize the use of sedatives, analgesics, and paralytics over time in a large cohort of mechanically-ventilated premature infants, and to identify factors associated with use.
METHODS
We obtained data from the Pediatrix Medical Group Data Warehouse, which prospectively captures information from an electronic medical record of daily progress notes and other documentation prepared by clinicians involved in the care of infants. Information is collected regarding maternal history and demographics, drugs, laboratory results, culture results, mechanical ventilation and respiratory support, and diagnoses. Details of administered drug dose were not recorded during this study period.
We identified all infants <1500 g birth weight and <32 weeks gestational age (GA) who received ≥1 day of mechanical ventilation (high frequency or conventional) in the first 120 days of life during their initial admission to one of 348 NICUs from 1997 to 2012. We followed infants during mechanically-ventilated days, excluding days of a major surgical intervention and 6 days following the surgical intervention, from NICU admission until one of the following events occurred: death, discharge, or day 120 of admission. We evaluated use of sedatives, analgesics, and paralytics measured in infant-days of administration of drug during the study period.
We included the following drugs of interest: opioids (fentanyl, morphine, meperidine, methadone), benzodiazepines (midazolam, diazepam, clonazepam, lorazepam), paralytics (atracurium, mivacurium, pancuronium, rocuronium, vecuronium), and “other” drugs (chloral hydrate, dexmedetomidine, ketamine, clonidine). We measured drug use as the number of mechanically ventilated infant-days of administration for each drug of interest.
We defined the duration of mechanical ventilation as the total number of days of mechanical ventilation during the hospitalization of each infant, excluding the day of major surgery and 6 days thereafter. We defined surgical intervention as any surgical or catheter-based intervention (ie, cardiac catheterization) documented in the chart for which general anesthesia was likely. An infant was considered to have a major congenital anomaly if an anomaly presenting at birth had one or more of the following characteristics: (1) lethal; (2) life-shortening; (3) life threatening; (4) requiring major surgery; or (5) significantly affecting the infant’s quality of life. We grouped sites into quartiles of average annual site volume of mechanically-ventilated infants during the study period.
Statistical analyses
The primary unit of observation for this study was an infant-day of mechanical ventilation. We used standard summary statistics, including counts, percentages, medians, and 25th and 75th percentiles to describe the study variables. We compared the distribution of infants with any drug use across infant characteristics using Wilcoxon rank-sum tests. We evaluated trends over time using the Cochran-Armitage test for trend. To evaluate the independent associations between infant characteristics and daily exposure to sedatives, analgesics, or paralytics of interest, we used multivariable logistic regression with a robust variance estimator to account for the clustered nature of the data by infant. The final multivariable model included the following predictors: GA (categorical), small for GA status (SGA, binary), postnatal age at the time of intubation (categorical), race (categorical), sex (binary), exposure to inotropic support (binary), exposure to high frequency ventilation (binary), discharge year (categorical), and a binary indicator variable for each site. To investigate the potential effect on our results of outliers in mechanical ventilation duration we conducted a sensitivity analysis limited to the first 7 days of mechanical ventilation for each infant.
To characterize differences in drug administration by site, we used the final model without the indicator for site to calculate predicted probability of daily drug administration for each site.
We used STATA 13.1 (College Station, TX) to perform all statistical analyses. A two-sided p <0.05 was considered statistically significant for all tests. The study was approved by the Duke University Institutional Review Board without the need for written informed consent because the data were collected without identifiers.
RESULTS
We identified 85,911 infants who received mechanical ventilation for a total of 1,305,413 days at 329 sites. The median GA and postnatal age at initial intubation were 27 weeks (interquartile range; 25, 29), and 0 days (0, 0), respectively. Infants were ventilated for a median of 6 days (2, 22). 31,078 (36%) infants received high frequency ventilation on 297,751 (23%) days (Table I).
Table 1.
Demographics of mechanically ventilated premature infants by exposure to any sedatives, analgesics or paralytics from 1997 to 2012.
| Any exposure N=32,058 (%) |
No exposure N= 53,853 (%) |
|
|---|---|---|
| Male | 17,706 (55) | 28,224 (52) |
| SGA | 5550 (17) | 7526 (14) |
| GA (weeks) | ||
| ≤25 | 12,895 (40) | 12,345 (23) |
| 26–28 | 13,150 (41) | 23,758 (44) |
| 29–32 | 6013 (19) | 17,750 (33) |
| PNA (days) | ||
| ≤ 1 | 28,749 (90) | 49,516 (92) |
| 2–7 | 1283 (4) | 1917 (4) |
| 8–28 | 1253 (4) | 1718 (3) |
| >28 | 773 (2) | 702 (1) |
| Race/Ethnicity | ||
| White | 14,898 (48) | 23,967 (46) |
| Black | 8184 (26) | 14,377 (28) |
| Hispanic | 6476 (21) | 10,893 (21) |
| Other | 1562 (5) | 2660 (5) |
| Major congenital anomaly | 3355 (10) | 3275 (6) |
| High frequency ventilation | 18,281 (57) | 12,797 (24) |
| Inotropic support | 18,222 (57) | 12,514 (23) |
Over time, the median birth weight among infants in our cohort decreased, the median GA remained stable, and the proportion of infants born with a major congenital anomaly increased (957 g in 1997 and 890 g in 2012, p=0.001; 27 weeks in 1997 and 2012; and 6% in 1997 and 8% of infants in 2012, p=0.027). After 1998, the proportion of infants who received high frequency ventilation declined (38% in 1999 and 36% in 2012, p=0.003), as did the proportion receiving inotropes during ventilation (37% in 1999 and 33% in 2012, p=0.008).
Among infants in our cohort, 32,058 (37%) received ≥1 drug of interest during 433,587 (33%) ventilated days. Fentanyl, midazolam, and morphine were the most frequently administered drugs of interest: 215,663 (17%), 180,791 (14%) and 112,612 (9%) ventilated days. Chloral hydrate was the most frequently administered “other” drug, on 7410 (<1%) ventilated days, and vecuronium was the most frequently administered paralytic, on 9312 (<1%) days. Administration of 2 or 3 drugs of interest occurred on 175,437 (13%) and 16,121 (1%) days, and 4, 5, or 6 drugs of interest were administered on 1693, 126, and 22 (all <1%) days, respectively. Infants who received a drug of interest were less mature and of lower birth weight compared with those who never received any drug [median of 26 weeks (25, 28) and 27 weeks (26, 29), p<0.001; 830 g (665, 1063) and 990 g (771, 1213), p<0.001].
Providers typically administered opioids and benzodiazepines shortly after initiation of mechanical ventilation [median 1 day (0, 6) and 0 days (0, 7)], but administered paralytics and “other” sedatives later in the ventilation course [median 8 days (1, 20) and 38 days (24, 65)]. Infants exposed to a drug of interest during the course of mechanical ventilation were exposed for a median of 6 days (2, 19). Median duration of exposure to opioids, benzodiazepines, and paralytics were 7 (2, 20), 9 (3, 25), and 12 (4, 30) days, respectively. Although exposure to “other” sedatives was relatively rare (<1% of days), median duration of exposure to such sedatives was the longest at 28 days (12, 45). Overall, 8% of infants were exposed to drugs of interest for up to 25% of their ventilated days, and 17% were exposed for >75% of their ventilated-days.
On multivariable analysis, the following factors were associated with higher prevalence of sedative, analgesic, or paralytic administration on an infant-day: younger GA, SGA status, male sex, major congenital anomaly, older postnatal age at intubation, exposure to high frequency ventilation, and exposure to inotropes (Table II). When we limited our analysis to include only the first 7 days of mechanical ventilation for each infant in our cohort, GA, SGA status, and male sex were no longer significant predictors of administration of drugs of interest.
Table 2.
Adjusted odds ratios (95% confidence intervals)* of infant characteristics for any sedative, analgesic, or paralytic administration to premature infants receiving mechanical ventilation from 1997 to 2012
| Adjusted OR (95% CI) | |
|---|---|
| Male | 1.06 (1.02, 1.10) |
| SGA | 1.11 (1.05, 1.17) |
| GA (weeks) | |
| ≤ 25 | REF |
| 26–28 | 0.85 (0.81, 0.89) |
| 29–≤32 | 0.82 (0.77, 0.88) |
| PNA (days) | |
| ≤ 2 | REF |
| 3–7 | 1.07 (0.96, 1.19) |
| 8–28 | 1.07 (0.96, 1.19) |
| > 28 | 1.76 (1.47, 2.09) |
| Race/Ethnicity | |
| White | REF |
| Black | 0.99 (0.94, 1.04) |
| Hispanic | 0.96 (0.90, 1.02) |
| Other | 0.88 (0.81, 0.97) |
| Major congenital anomaly | 1.31 (1.22, 1.40) |
| High frequency ventilation | 2.58 (2.49, 2.67) |
| Inotropic support | 1.74 (1.68, 1.81) |
also adjusted for site and year of discharge
Administration of drugs of interest during mechanical ventilation varied substantially across sites [median proportion of days: 14% (2, 34) (Figure 1). Compared with lower volume centers, higher volume centers more frequently administered drugs of interest [median proportion of days: 18% in the first quartile of centers (≤4 infants per year) and 35% in the fourth quartile of centers (≥33 infants), p<0.001]. Among the 100 sites with the highest percent infant-days of administration of any of the drugs of interest during mechanically-ventilated infant days (minimum 0.5% of mechanically-ventilated infant days), administration of any drug of interest, benzodiazepines, or opioids occurred on 0.7–89%, 0.7–82%, and 0.7–78% of days, respectively. In Figure 1, such variability in drug administration among these 100 sites is demonstrated for any drug of interest, benzodiazepines, and opioids. As also shown in Figure 1 by the overlay of predicted probabilities of drug administration for each site, significant variability remained even among sites with similar predicted probabilities of drug administration after adjusting for infant characteristics at each site.
Figure 1.
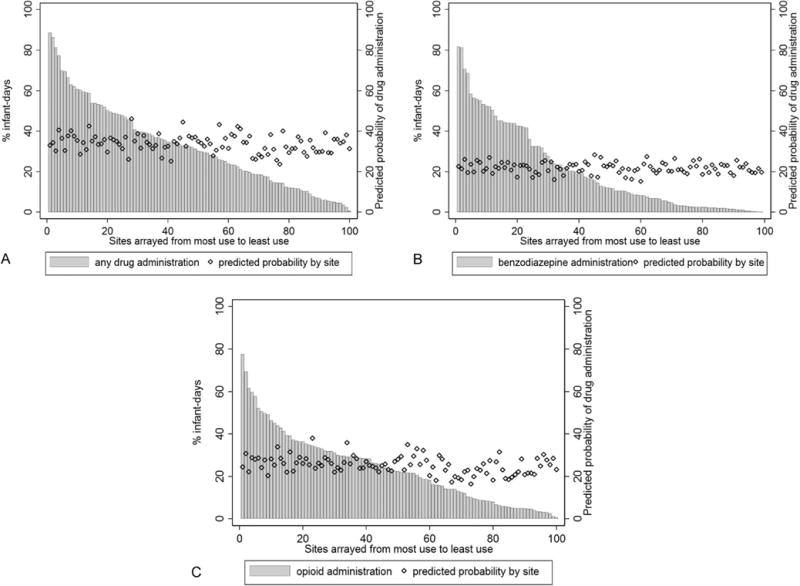
Predicted probability of drug administration by site, and administration of (a) any sedative, analgesic, or paralytic, (b) benzodiazepines, and (c) opioids among the 100 sites with most frequent drug administration [Footnote: dBased on characteristics of infants cared for at each site].
Administration of opioids during mechanical ventilation increased during the study period (678/14,800 days (5%) in 1997 to 29,426/91,418 (32%) in 2012, p <0.001) (Figure 2). Administration of benzodiazepines also initially increased (696/14,800 (5%) in 1997 to 28,891/102,583 (28%) in 2009 (p < 0.001)), but has since slightly decreased to 22,397/91,418 (24%) in 2012 (p< 0.001). Paralytic and “other” sedative administration remained stable throughout the study period with exposure to drugs in both categories remaining ≤1% of ventilator days.
Figure 2.
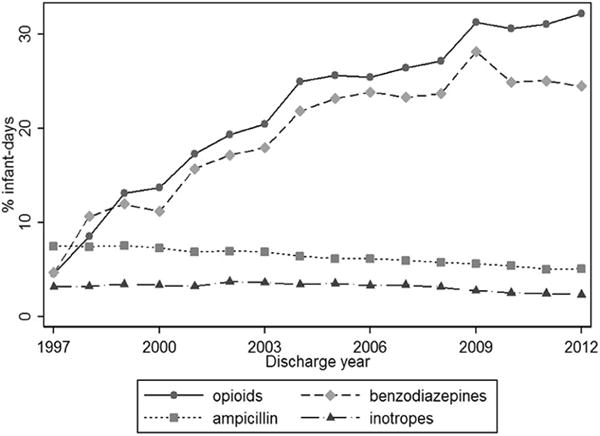
Opioid and benzodiazepine administration compared with ampicillin and inotrope administration over time.
DISCUSSION
We conducted a large retrospective cohort study to date evaluating the administration of sedatives, analgesics, and paralytics to premature infants receiving mechanical ventilation. We found that administration of opioids and benzodiazepines increased over time and varied significantly across sites. Our adjusted analysis indicated that patient characteristics, including surrogates of illness severity (i.e., the presence of a major congenital anomaly, and inotropic or high frequency ventilator support), GA, and postnatal age at intubation were independently associated with receipt of drugs of interest. Most importantly however, we found that site of hospitalization was an independent predictor of drug administration even when the probability of daily drug administration was adjusted for characteristics of infants at the site (C-statistic for model with infant characteristics alone and infant characteristics with site indicator variable, 0.62 and 0.80). This finding suggests that site specific factors, rather than infant risk factors alone, are associated with sedative and analgesic administration in this population, and presents an opportunity for quality improvement initiatives aimed at reducing exposure to potentially harmful drugs in premature infants.
The association between infant characteristics and sedative and analgesic administration has been previously demonstrated, and may have physiologic basis. (24–26) Administration of opioids and benzodiazepines may reduce infant-ventilator dyssynchrony and improve gas exchange in critically ill infants, particularly those supported with high frequency ventilation.(27–30) Further, compared with younger infants, older infants have increased body mass that may allow increased ability to move and display distress during mechanical ventilation. (24, 31)
Opioids and benzodiazepines may also decrease a patient’s stress response and metabolic demand.(5, 32, 33) In critically ill infants at high risk of mortality, administration of opioids and benzodiazepines may decrease oxygen consumption, avoid oxygen debt and associated acidosis, and decrease mortality.(5, 34, 35) Our study findings support this hypothesis by demonstrating that presence of a major congenital anomaly, high-frequency ventilation use, and exposure to inotropes were all associated with increased odds of drug exposure in an adjusted analysis. Unlike infant characteristics such as GA, SGA, and gender, which were not statistically significant in our analysis limited to the first 7 days, markers of illness severity are important contributors to the decisions to initiate and continue administration of drugs of interest in a particular patient.
If severity of illness was responsible for the increase in opioid and benzodiazepine administration over time, as suggested by minimal increase in the C-statistic from 0.62 to 0.65 when discharge year was added to infant characteristics in our model, we might expect increases in administration of other drugs given to infants with suspected or confirmed severe illness. In fact, the observed increases in opioid and benzodiazepine administration occurred in a cohort of infants in which administration of the example antibiotic, ampicillin, and inotropes decreased slightly over the study period (8% in 1997 and 5% in 2012, p<0.001; 3% in 1997 and 2% in 2012, p=0.046) (Figure 2).(36) This finding supports the hypothesis that severity of illness alone does not account for the increasing administration of opioids and benzodiazepines to mechanically-ventilated premature infants over the study period.
Site-level factors including training, institutional policies on assessment and management of pain and agitation, or individual patient encounters often influence provider decision making and practice, and likely explain the increasing administration of opioids and benzodiazepines to mechanically-ventilated, premature infants.(37, 38) In 2001, the Joint Commission established standards regarding the assessment and management of pain in patients, and a consensus statement recommended routine treatment of pain, including use of continuous opioid infusion, in mechanically-ventilated infants of any gestational age.(39–41) Such emphasis on pain management in combination with the increased availability of validated pain assessment scales in neonates (>25 available scales) likely influenced the increased administration of sedatives and analgesics over time, but sites appear to have adopted recommendations to varying degrees.(42–44)
In our study, administration of opioids and benzodiazepines varied widely across the 100 sites with the highest percent infant-days of administration, and we identified this variation even among sites with similar predicted administration of drugs of interest based on infant characteristics. Although not applicable to the majority of sites with limited drug administration, site-specific quality improvement initiatives may help decrease practice variability across NICUs with higher drug utilization.(45) This approach has been successfully employed by the Pediatrix Medical Group to limit exposure to histamine-2 receptor blockers and broad spectrum antibiotics.(46–48) Our study findings strongly support the implementation of a similar initiative for opioids and benzodiazepines, which may lead to improved short- and long-term outcomes of premature infants requiring mechanical ventilation.
The strengths of our study include the use of a multicenter, electronic health record-derived database to conduct the largest pharmacoepidemiologic study of sedative, analgesic, and paralytics in mechanically-ventilated, premature infants to date. Our access to detailed hospital records allowed us to identify several infant characteristics associated with drug exposure, and the longevity of the database provided us with an opportunity to demonstrate clear trends over time. Finally, the multi-institutional nature of the database, comprised of community and academic center-based NICUs that represent almost 25% of all NICU admissions in the United States over the study period, was essential to describe site variability and identify potential targets for quality improvement initiatives.(47)
Despite the strengths of our data source, some limitations exist. Although an electronic health record-derived database is representative of daily clinical practice, it has not undergone the scrutiny of a clinical trial database. A more detailed description of infant severity of illness, including mechanical ventilator measurements and markers of adequacy of gas exchange was not available. Instead, we reported surrogates of severity of illness previously used in Pediatrix data analyses.(24) Even though we were able to identify site variability in drug exposure, we lacked details about the characteristics of individual sites (including academic vs. private setting, number of providers, and site level) to completely describe sites with high and low drug use. Finally, we lacked information about drug dosing, exposure, or any other measures of drug tolerance to evaluate their associations with harm.
In summary, we found an increasing administration of potentially harmful sedatives and analgesics to mechanically ventilated premature infants, and this increase was not fully explained by infant severity of illness. Instead, large variability across NICUs suggests that site-specific quality improvement initiatives may successfully limit administration of these potentially harmful drugs, improving outcomes in this fragile population.
Acknowledgments
K.Z.is funded by the Duke Clinical and Translational Science Award (KL2TR001115–03). P.S. receives research support from Cempra Pharmaceuticals (HHS0100201300009C); serves as a paid consultant for AbbVie, Astellas Pharma US, Glaxo SmithKline, and Mission Pharma; receives salary support for research from the National Institutes of Health (NIH; 1R21HD080606-01ª1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD; HHSN275201000003I), and the Food and Drug Administration (1R18-FD005292-01). M.L. is supported by the US government for his work in pediatric and neonatal clinical pharmacology (HHSN267200700051C [PI: D.B.), the NICHD (K23 HD068497), and the National Heart, Lung, and Blood Institute (R34 HL124038). C.T. receives salary support for research from the Gerber Foundation. T.S. receives investigator-initiated research funding and support from the National Institute on Aging (R01/R56 AG023178) and NIH (R01 CA174453, R01 HL118255, R21-HD080214); receives salary support as Director of the Comparative Effectiveness Research Strategic Initiative, NC TraCS Institute, UNC Clinical and Translational Science Award (UL1TR001111) and as Director of the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck); the Department of Epidemiology, University of North Carolina at Chapel Hill receives research support from Amgen and AstraZeneca; and owns stock in Novartis, Roche, BASF, AstraZeneca, Johnsen & Johnsen, and Novo Nordisk. C.H. receives salary support for research from the National Center for Advancing Translational Sciences of the NIH (UL1TR001117). Sponsors had no involvement in the study design; the collection, analysis, and interpretation of data; the writing of the report; nor the decision to submit the manuscript for publication.
Abbreviations
- GA
Gestational age
- NICU
Neonatal intensive care unit
- SGA
Small for gestational age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
D.B. and R.C. declare no conflicts of interest.
References
- 1.Network SSGotEKSNNR. Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010 May 27;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelinas C, Fortier M, Viens C, Fillion L, Puntillo K. Pain assessment and management in critically ill intubated patients: a retrospective study. Am J Crit Care. 2004 Mar;13:126–35. [PubMed] [Google Scholar]
- 3.Horbar JD, Soll RF, Edwards WH. The Vermont Oxford Network: a community of practice. Clin Perinatol. 2010 Mar;37:29–47. doi: 10.1016/j.clp.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Alander M, Peltoniemi O, Saarela T, Anttila E, Pokka T, Kontiokari T. Current trends in paediatric and neonatal ventilatory care–a nationwide survey. Acta Paediatr. 2013 Feb;102:123–8. doi: 10.1111/j.1651-2227.2012.02830.x. [DOI] [PubMed] [Google Scholar]
- 5.Aranda JV, Carlo W, Hummel P, Thomas R, Lehr VT, Anand KJ. Analgesia and sedation during mechanical ventilation in neonates. Clin Ther. 2005 Jun;27:877–99. doi: 10.1016/j.clinthera.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Arnold JH, Truog RD, Orav EJ, Scavone JM, Hershenson MB. Tolerance and dependence in neonates sedated with fentanyl during extracorporeal membrane oxygenation. Anesthesiology. 1990 Dec;73:1136–40. doi: 10.1097/00000542-199012000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hall RW, Boyle E, Young T. Do ventilated neonates require pain management? Seminars in Perinatology. 2007 Oct;31:289–97. doi: 10.1053/j.semperi.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004 May 22;363:1673–82. doi: 10.1016/S0140-6736(04)16251-X. [DOI] [PubMed] [Google Scholar]
- 9.Simons SH, van Dijk M, van Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA. 2003 Nov 12;290:2419–27. doi: 10.1001/jama.290.18.2419. [DOI] [PubMed] [Google Scholar]
- 10.Roze JC, Denizot S, Carbajal R, Ancel PY, Kaminski M, Arnaud C, et al. Prolonged sedation and/or analgesia and 5-year neurodevelopment outcome in very preterm infants: results from the EPIPAGE cohort. Arch Pediatr Adolesc Med. 2008 Aug;162:728–33. doi: 10.1001/archpedi.162.8.728. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari V, Bergqvist LL, Kronsberg SS, Barton BA, Anand KJ. Morphine administration and short-term pulmonary outcomes among ventilated preterm infants. Pediatrics. 2005 Aug;116:352–9. doi: 10.1542/peds.2004-2123. [DOI] [PubMed] [Google Scholar]
- 12.Hammer RP, Ricalde AA, Seatriz JV. Effects of Opiates on Brain-Development. Neurotoxicology. 1989 Fal;10:475–83. [PubMed] [Google Scholar]
- 13.Seatriz JV, Hammer RP. Effects of Opiates on Neuronal Development in the Rat Cerebral-Cortex. Brain Research Bulletin. 1993;30:523–7. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- 14.Ricalde AA, Hammer RP. Perinatal Opiate Treatment Delays Growth of Cortical Dendrites. Neuroscience Letters. 1990 Jul 31;115:137–43. doi: 10.1016/0304-3940(90)90444-e. [DOI] [PubMed] [Google Scholar]
- 15.Kocek M, Wilcox R, Crank C, Patra K. Evaluation of the relationship between opioid exposure in extremely low birth weight infants in the neonatal intensive care unit and neurodevelopmental outcome at 2 years. Early Hum Dev. 2016 Jan;92:29–32. doi: 10.1016/j.earlhumdev.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 16.McGlone L, Mactier H. Infants of opioid-dependent mothers: neurodevelopment at six months. Early Hum Dev. 2015 Jan;91:19–21. doi: 10.1016/j.earlhumdev.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Lammers EM, Johnson PN, Ernst KD, Hagemann TM, Lawrence SM, Williams PK, et al. Association of fentanyl with neurodevelopmental outcomes in very-low-birth-weight infants. Ann Pharmacother. 2014 Mar;48:335–42. doi: 10.1177/1060028013514026. [DOI] [PubMed] [Google Scholar]
- 18.Batton DG, Barrington KJ, Wallman C. Prevention and management of pain in the neonate: an update. Pediatrics. 2006 Nov;118:2231–41. doi: 10.1542/peds.2006-2277. [DOI] [PubMed] [Google Scholar]
- 19.Bellu R, de Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD004212.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cools F, Offringa M. Neuromuscular paralysis for newborn infants receiving mechanical ventilation. Cochrane Database Syst Rev. 2005:CD002773. doi: 10.1002/14651858.CD002773.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Runkle B, Bancalari E. Acute cardiopulmonary effects of pancuronium bromide in mechanically ventilated newborn infants. J Pediatr. 1984 Apr;104:614–7. doi: 10.1016/s0022-3476(84)80563-6. [DOI] [PubMed] [Google Scholar]
- 22.Fanconi S, Ensner S, Knecht B. Effects of Paralysis with Pancuronium-Bromide on Joint Mobility in Premature-Infants. J Pediatr-Us. 1995 Jul;127:134–6. doi: 10.1016/s0022-3476(95)70274-1. [DOI] [PubMed] [Google Scholar]
- 23.Cheung PY, Tyebkhan J, Peliowski A, Ainsworth W, Chinnery H, Young B, et al. Prolonged use of pancuronium bromide and sensorineural hearing loss in childhood survivors of congenital diaphragmatic hernia. Pediatr Res. 1999 Apr;45:189a-a. doi: 10.1016/s0022-3476(99)70027-2. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman KO, Hornik CP, Ku L, Watt K, Laughon MM, Bidegain M, et al. Sedatives and Analgesics Given to Infants in Neonatal Intensive Care Units at the End of Life. J Pediatr. 2015 May 23; doi: 10.1016/j.jpeds.2015.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cruz MD, Fernandes AM, Oliveira CR. Epidemiology of painful procedures performed in neonates: A systematic review of observational studies. Eur J Pain. 2015 Jul 29; doi: 10.1002/ejp.757. [DOI] [PubMed] [Google Scholar]
- 26.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. JAMA. 2008 Jul 2;300:60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 27.Derdak S, Mehta S, Stewart TE, Smith T, Rogers M, Buchman TG, et al. High-frequency oscillatory ventilation for acute respiratory distress syndrome in adults: a randomized, controlled trial. Am J Respir Crit Care Med. 2002 Sep 15;166:801–8. doi: 10.1164/rccm.2108052. [DOI] [PubMed] [Google Scholar]
- 28.Dick CR, Sassoon CS. Patient-ventilator interactions. Clin Chest Med. 1996;17:423–38. doi: 10.1016/s0272-5231(05)70325-7. [DOI] [PubMed] [Google Scholar]
- 29.Sassoon CS, Foster GT. Patient-ventilator asynchrony. Curr Opin Crit Care. 2001;7:28–33. doi: 10.1097/00075198-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Tufano R. Analgesia and sedation in intensive care: a progress report. Minerva Anestesiol. 2003;69:735–6. 6–7. [PubMed] [Google Scholar]
- 31.Matthews AL, O’Conner-Von S. Administration of comfort medication at end of life in neonates: effects of weight. Neonatal Netw. 2008 Jul-Aug;27:223–7. doi: 10.1891/0730-0832.27.4.223. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard AR. Sedation and analgesia in intensive care. Medications attenuate stress response in critical illness. Postgrad Med. 2002;111:59–60. 3–4, 7–70. doi: 10.3810/pgm.2002.02.1107. passim. [DOI] [PubMed] [Google Scholar]
- 33.Kumba C, VdL P. Effects of sedative agents on the metabolic demand. French Annals of Anesthesia and Intensive Care. 2008;27:574–80. doi: 10.1016/j.annfar.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010 Sep;126:443–56. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Services DoHaH. User Guide to the 2013 period linked Birth/Infant seath public use file. [cited 2016 February 5]; Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/periodlinked/LinkPE13Guide.pdf.
- 36.Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK, Jr, Smith PB, et al. Medication use in the neonatal intensive care unit. American journal of perinatology. 2014 Oct;31:811–21. doi: 10.1055/s-0033-1361933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinlay JB, Potter DA, Feldman HA. Non-medical influences on medical decision-making. Social science & medicine. 1996 Mar;42:769–76. doi: 10.1016/0277-9536(95)00342-8. [DOI] [PubMed] [Google Scholar]
- 38.Hajjaj FM, Salek MS, Basra MK, Finlay AY. Non-clinical influences on clinical decision-making: a major challenge to evidence-based practice. Journal of the Royal Society of Medicine. 2010 May;103:178–87. doi: 10.1258/jrsm.2010.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001 Feb;155:173–80. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 40.Prevention and management of pain and stress in the neonate. American Academy of Pediatrics. Committee on Fetus and Newborn. Committee on Drugs. Section on Anesthesiology. Section on Surgery. Canadian Paediatric Society. Fetus and Newborn Committee. Pediatrics. 2000 Feb;105:454–61. [PubMed] [Google Scholar]
- 41.Lanser P, Gesell S. Pain management: the fifth vital sign. Healthc Benchmarks. 2001 Jun;8:68–70. 62. [PubMed] [Google Scholar]
- 42.Lago P, Guadagni A, Merazzi D, Ancora G, Bellieni CV, Cavazza A. Pain management in the neonatal intensive care unit: a national survey in Italy. Paediatr Anaesth. 2005 Nov;15:925–31. doi: 10.1111/j.1460-9592.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- 43.Eriksson M, Gradin M. Pain management in Swedish neonatal units–a national survey. Acta Paediatr. 2008 Jul;97:870–4. doi: 10.1111/j.1651-2227.2008.00826.x. [DOI] [PubMed] [Google Scholar]
- 44.de Melo GM, Lelis AL, de Moura AF, Cardoso MV, da Silva VM. Pain assessment scales in newborns: integrative review. Rev Paul Pediatr. 2014 Dec;32:395–402. doi: 10.1016/j.rpped.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell H, Duke T, Weber M, English M, Carai S, Tamburlini G, et al. Global initiatives for improving hospital care for children: state of the art and future prospects. Pediatrics. 2008 Apr;121:e984–92. doi: 10.1542/peds.2007-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clark RH, Spitzer AR. Patience is a virtue in the management of gastroesophageal reflux. J Pediatr. 2009 Oct;155:464–5. doi: 10.1016/j.jpeds.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system–tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010 Mar;37:49–70. doi: 10.1016/j.clp.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006 Jan;117:67–74. doi: 10.1542/peds.2005-0179. [DOI] [PubMed] [Google Scholar]


