SUMMARY
Although tuberculosis (TB) is one of the most common causes of morbidity and mortality in humans worldwide and diagnostic methods have been in place for more than 100 years, diagnosis remains a challenge. The main problems with diagnosis relate to the time needed to obtain a definitive result, difficulty in obtaining sputum, the primary clinical material used, and the ability of the causative agent, Mycobacterium tuberculosis, to cause disease in nearly any tissue within the body. In order to decrease incidence of TB, discovery of a novel interventions will be required, since current technologies have only been able to control numbers of infections, not reduce them. Diagnostic innovation is particularly needed because there are no effective pediatric or extrapulmonary TB diagnostic methods and multiple-drug resistance is only identified in less than 25% of those patients that are thought to have it. The most common diagnostic method worldwide remains acid-fast stain on sputum, with a threshold of ~10,000 bacteria/ml that is only reached ~5–6 months after development of symptoms. In order to obtain definitive diagnostic results earlier during the disease process, we have developed a diagnostic method designated reporter enzyme fluorescence (REF) that utilizes BlaC produced by M. tuberculosis and custom substrates to produce a specific fluorescent signal with as few as 10 bacteria/ml in clinical samples. We believe that the unique biology of the REF technique will allow it to contribute new diagnostic information that is complementary to all existing diagnostic tests as well as those currently known to be in development.
Keywords: Lactamase, Fluorescence, Diagnosis, Tuberculosis, Transmission, Infection
Worldwide incidence of tuberculosis (TB) has risen and fallen with the frequency of HIV infection from 1990 until 2012, rather than improvements in management practices for tuberculosis itself [20]. This observation suggests that our management practices need to change in order for us to have an impact on tuberculosis incidence. In our own laboratory, we have targeted diagnostic strategies, since they have the potential to change the TB management landscape due to the fact that current diagnostic methods often cannot make a diagnosis prior to transmission [1,15]. Recent optical imaging technological developments have created more sensitive ways to visualize and quantify mycobacteria within cells and in vivo [2,3,6–9,12,21]. Despite these advances, reduction in signal due to tissue depth make application to diagnosis in humans extremely difficult. Since invasive methods to diagnose TB are not desirable due to risk to the patient and the need sterile surgical facilities, diagnosis of TB is primarily limited to sputum. Sputum is an effective clinical material for TB diagnosis in the case of adults that have the pulmonary form of TB, but it is not sufficient for children where it is difficult to obtain sputum [11] and adults that have the extrapulmonary form of TB that occurs at a high frequency in HIV positive patients [4,10,14,17,18]. We chose to initially develop a diagnostic test for sputum to avoid interference in the current diagnostic path and because optical technologies based on reporter enzyme fluorescence (REF) should be complementary to existing diagnostic technologies.
REF is very sensitive because it uses the robust catalytic beta-lactamase enzyme produced by M. tuberculosis called BlaC to cleave custom fluorogenic substrates designed to be specific for this unique enzyme [3,21]. REF technology should produce complementary results as compared to existing diagnostic technologies because it requires viability of the bacteria due to the need for ATP in twin-arginine translocation (Tat) secretion of BlaC, but does not require complete bacterial replication as would be required for culture-based diagnosis. Acid-fast stain-based diagnosis requires the integrity of the mycobacterial cell wall, but does not require bacterial viability; whereas, REF requires only bacterial viability as measured by enzyme activity, which may not always involve a cell wall that stains acid-fast, since non-acid-fast staining Mtb have been observed during infection. PCR-based diagnosis requires DNA or RNA to be present, not viability, but REF requires viability and would not require the integrity of the DNA or RNA. Thus, the biology of REF diagnostic techniques is very different from that responsible for other TB diagnostic strategies, making it likely that even with great variability in diagnostic material and patient severity, REF would provide positive results in a different class of samples than the other technologies. We propose that because of its unique biology, REF-based diagnosis is likely to fill an important niche in TB diagnosis.
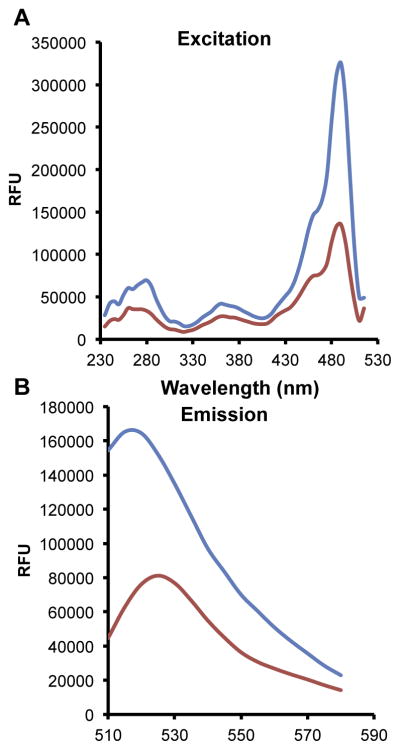
One of the first issues with use of a fluorogenic probe to detect tuberculosis is the optimal wavelength for reading the samples that will give maximal sensitivity. In order to determine the best wavelengths for our probe, designated CDG-3 [3], we scanned the excitation and emission of the substrate before and after exhaustive cleavage by BlaC (Figure 1) in 2-(N-morpholino)ethanesulfonic acid (MES) buffer as described previously [3]. Using the emission wavelength of 532 nm, we found that the best excitation wavelength was around 490 nm, giving a two- to three-fold difference in signal between the fully cleaved and un-cleaved substrate. We used the excitation wavelength of 492 nm and found that the peak difference in signal between cleaved and un-cleaved substrate is obtained at 510 nm. However, we chose 532 nm as the emission wavelength because it provides similar differences in signal between the cleaved ad un-cleaved substrate, but is further from the excitation wavelength, reducing the possibility of bleed-through from excitation filters in simple fluorescence reader systems. Despite this fact, there is a reasonably wide emission spectrum that would allow different filters to be used in readers that range from 510 nm to 540 nm with reasonably good sensitivity. Based on this information, we expect to choose filter systems that are cost-effective, yet still provide the highest sensitivity possible with clinical material that may itself absorb in this wavelength range due to contaminating hemoglobin or other biological material. Detailed analysis of multiple clinical samples to gain insight into the variability observed in absorbance should be the focus of future work to optimize reader design. In general, we expect the reader to be very low cost, with our first reader constructed from readily available materials and easily costing <$500 [21].
Figure 1.
We evaluated the capability of CDG-3 to detect BlaC efficiently using the difference in fluorescence signal between un-cleaved (red lines) and cleaved probe (blue lines). A concentration of 100 nM purified BlaC in MES buffer (pH 6.0; Fischer Scientific, Waltham, MA) was dispensed into 96 well plates. CDG-3 was added to the well at a final concentration of 2 μM. The reaction was allowed to proceed in the dark for 1 h. The wells were read using EnVision®(PerkinElmer, Waltham, MA). Excitation (A) at 230–505 nm and emission (B) spectra 510–580 nm were collected using 535 nm and 492 nm as the emission and excitation wavelengths, respectively, and data presented as relative fluorescence units (RFU). Data shown are from a representative experiment repeated with similar results three times. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
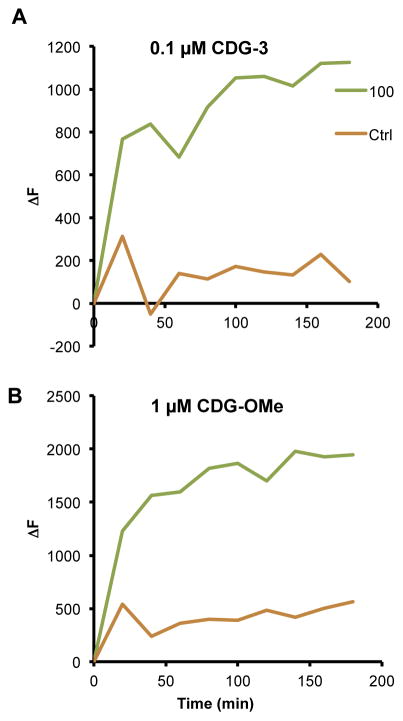
Once we had determined optimal excitation and emission conditions for CDG-3, we compared the sensitivity of this new substrate [3] to our first generation substrate CDG-OMe [21]. M. tuberculosis var. bovis strain bacillus Calmette-Guérin (BCG) was used in REF assays [3]. BCG was cultured in 7H9 broth (Difco, Detroit, MI) supplemented with 0.5% glycerol, 10% oleic acid albumin dextrose complex (OADC) and 0.05% Tween 80. The bacterial culture was incubated at 37 °C, 5% CO2 until an optical density at 600 nm of 0.5 was achieved. Bacterial dilutions were made in MES buffer and 100 CFU were added to TB-negative sputum in a final volume of 0.5 ml. Briefly, sputum with BCG was mixed with equal volumes (total volume of 1 ml) of Transport Stabilization Solution (TSS) and incubated at room temperature for 1 h. REF solution containing the desired probe concentration was then added and the sample was assayed at Ex/Em of 498/535 using an EnVision® (PerkinElmer, Waltham, MA). CDG-OMe concentration of 1 μM and a 10-fold lower concentration of CDG-3 (0.1 μM) were used. The samples were read every 20 min and ΔF was calculated by subtracting the fluorescence signal obtained at time zero. We found that CDG-3 displays greater sensitivity than CDG-OMe, even when used at 10-fold lower concentrations than CDG-OMe (Figure 2). Both substrates display good sensitivity for 100 CFU/ml of BCG in sputum after 20 min of incubation, but CDG-3 displays a three-fold difference between sputum alone and bacteria; whereas, CDG-OMe only displays a two-fold difference. The difference in fluorescence observed with BCG increases to over five-fold at most time points out to 3 h, but CDG-OMe only displays a three-fold difference at most time points. One of the reasons that we suspect this difference is observed is due to the greater aqueous stability of CDG-3 as compared to CDG-OMe. In general, we observe a steady increase in background signal with CDG-OMe over time in the absence of BCG, but CDG-3 displays a similar level of background for the full 3 h of incubation at room temperature. These data suggest that CDG-3 is a more stable substrate in biological materials and will future substrates based upon its backbone may further improve the desirable characteristics of high sensitivity and stability.
Figure 2.
CDG-3 (A) demonstrates improved signal-to-noise as compared to the previous generation probe CDG-OMe (B) in REF assays. BCG was diluted to 100 colony forming units (CFU)/ml in tuberculosis-negative human sputum and REF assays carried out with and without each substrate under the same conditions. Results are reported as change in fluorescence (ΔF) at each time point as compared to time zero. At each time point after 10 min, 10-fold lower concentrations of CDG-3 as compared to CDG-OMe were sufficient to allow detection of 100 CFU/ml of BCG (100) over sputum alone (Ctrl). Data shown are from a representative experiment repeated with similar results three times.
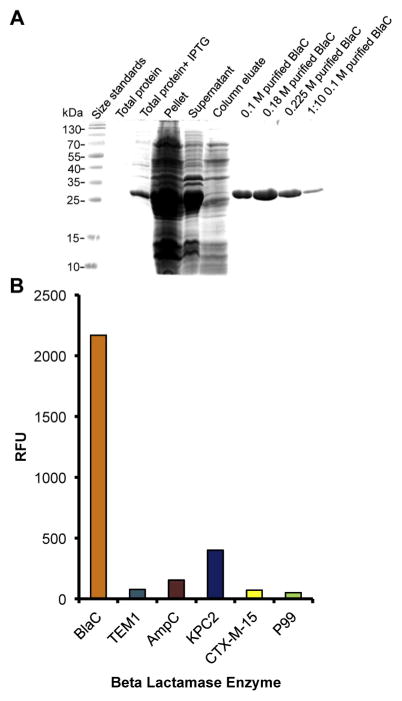
Because clinical materials are often contaminated with other bacterial species that can also express beta-lactamases [21] and other beta-lactamases could interfere with the ability of REF to detect TB in clinical samples, we were interested in the level of specificity observed with CDG-3. Recombinant BlaC beta-lactamase was expressed in E. coli BL21 (DE3) as described previously [19]. Briefly, cells were grown in 1 L of LB media containing 50 μg/mL of kanamycin to an OD600 of 0.6–0.8 at 37 °C before inducing it with 1 mM of IPTG. The culture was grown at 4 °C for 16 h with shaking. Bacterial pellet was obtained by centrifugation, re-suspended in 25 mM Tris-HCl (pH 8.0), 500 mM NaCl, and 2 mM beta-mercaptoethanol and lysed by sonication. After centrifugation, the supernatant containing the soluble proteins was loaded on a Ni2+ His-Trap chelating column, purified using an AKTA FPLC and eluted with an imidazole gradient (0–0.5 M). The purity of BlaC was determined by SDS-PAGE, and protein fractions were concentrated with 10-kDa cutoff protein filters. The protein concentration was determined using Bio-Rad Bradford protein assays. Beta-lactamase activity of BlaC was measured spectrophotometrically using nitrocefin assays [13]. Although our previous studies suggest that our fluorogenic substrate CDG-3 is highly specific [3], demonstrating approximately 10,000 fold specificity for BlaC over the common E. coli β-lactamase TEM-1, we examined additional beta-lactamases TEM-1, AmpC, KPC2, CTX-M-15 and P99 (Figure 3). Purified beta-lactamases were kindly provided by AstraZeneca (Waltham, MA). The enzymes were diluted in MES buffer (pH 6). Standard nitrocefin assays carried out at room temperature [13] were used to calculate active enzyme units in each purified protein preparation. Briefly, 200 μM nitrocefin was mixed with each beta-lactamase enzyme at concentrations of 0.1 nM–5.5 nM. Absorbance at 485 nm was measured after 80 min for each enzyme concentration and the number of active enzyme units calculated as a function of the change in absorbance over time. CDG-3 assays were then carried out for each enzyme and the data were normalized to the number of active units present for the Mtb BlaC enzyme. The fluorescent signal was read under conditions for CDG-3 30 min after addition of the substrate at Ex/Em of 498/535 and expressed relative to background. Although some background activity is observed with other beta-lactamases, the activity of BlaC is at least five-fold higher under these conditions. These data confirm our earlier observations that CDG-3 is specific for Mtb BlaC and should display very low background, even in the presence of other bacterial species expressing different beta-lactamases.
Figure 3.
(A) Recombinant BlaC was produced to high purity for analysis of the specificity of CDG-3 by liquid chromatography. Protein was separated on a 12% SDS-PAGE gel and stained with Coomassie blue. BlaC was expressed from the lac promoter in E. coli strain BL21 by induction with 1 mM IPTG. We show total protein lysates with and without IPTG as well as the insoluble (pellet) and soluble (supernatant) fractions. The soluble fraction was purified on a Ni2+ column and eluted with different concentrations of imidazole. We show 0.1 M, 0.18 M and 0.225 M imidazole eluates from the column, as indicated by the molarity next to the purified BlaC labels. The 0.1 M imidazole eluate was also run as a 1:10 dilution in MES buffer. (B) CDG-3 is highly specific to the β-lactamase produced by Mycobacterium tuberculosis BlaC. Equivalent enzyme units of purified β-lactamase enzymes were incubated with CDG-3 for 30 min at room temperature in a 96-well plate. The plates were then read at an Ex/ Em of 492/535 and data presented as relative fluorescent units (RFU). TEM-1 is a common plasmid-encoded β-lactamase in Gram negatives (Class A), AmpC is a common chromosomally-encoded β-lactamase in Gram negatives (Class C), KPC-2 is a common plasmid-encoded carbapenemase from Klebsiella (Class A), CTX-M-15 is one of the most common cefotaxime resistance proteins from Escherichia coli (Class A) and P99 is a common β-lactamase from Enterobacter (Class C). All enzyme and substrate dilutions were made in MES buffer.
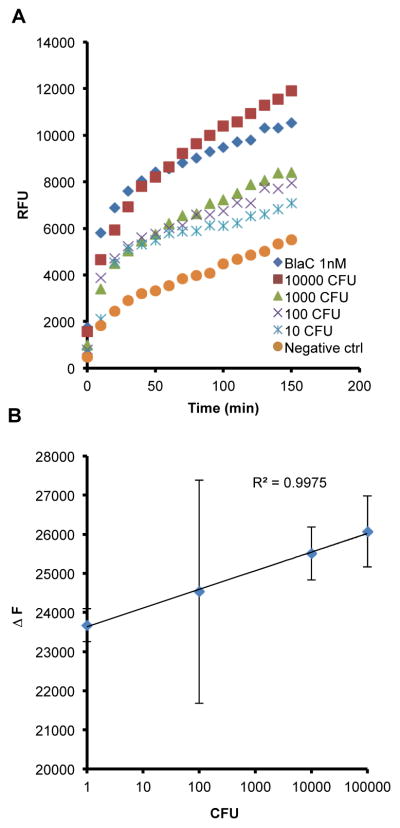
The good specificity of CDG-3 suggests that it could be useful for detection of Mtb in clinical samples that can be contaminated with other bacterial species, but our main concern was that the specificity had come at the price of sensitivity. However, we examined the ability of CDG-3 to detect Mtb in sputum and found that it had very good sensitivity and showed a good correlation with CFU from 10 to 100,000 CFU of BCG (Figure 4) as early as 20 min after addition of substrate. Bacterial dilutions were made in MES buffer and appropriate volume of the dilutions was added to TB negative sputum to obtain a final concentration, during the assay, of 10, 100, 1000 and 10,000 CFU/ml. BCG was incubated in TB negative sputum for 1 h and then mixed with equal volume of TSS, giving a final volume of 1 ml, and further incubated for an hour at room temperature. 1 nM of purified BlaC added to TB negative sputum was used as a positive control. The TSS incubation was followed by addition of REF solution containing the substrate. The samples were read continuously for 120 min using EnVision® (PerkinElmer, Waltham, MA) at an Ex/Em of 492/535 nm. These data demonstrate that CDG-3 can be used to detect very low numbers of BCG and suggest that detection of as low numbers of Mtb as 10 CFU should be possible, even in sputum. This detection level is 1000-fold better than acid fast stain and approximately equivalent to culture. These levels of sensitivity are such that, should a diagnostic system be produced with CDG-3, it would greatly facilitate detection of TB at thresholds that have previously been impossible without waiting the six weeks required for culture.
Figure 4.
CDG-3 is capable of detecting as few as 10 colony forming units (CFU) of BCG in tuberculosis (TB)-negative human sputum. (A) BCG (10–10,000 CFU) or 1 nM purified BlaC enzyme was incubated in TB-negative sputum in a final volume of 1 ml for 1 h. REF assays were carried out by adding 0.1 μM CDG-3. The samples were read continuously for 120 min using an EnVision®(PerkinElmer, Waltham, MA) plate reader at an Ex/Em of 492/535 nm. Data are compared to TB-negative sputum without BCG added (Negative ctrl). (B) The correlation between the change in fluorescence (ΔF) and CFU was determined by calculating the linear relationship between these two at 20 min post-addition of CDG-3. All experiments were carried out in duplicate and data points represent the mean and error bars the standard deviation.
Although autofluorescence has been utilized with some success to diagnose tuberculosis [5,16], the thresholds of detection are thought to be much higher than the 10 CFU needed for REF, requiring more than 106 bacteria/ml sputum [16]. Although it seems possible that at the wavelength ranges we are using (Ex/Em of 492/535 nm) some autofluorescence (Ex/Em of 405/475 nm) would be observed, no autofluorescence is observed when mycobacteria are excited at 488 nm [16]. This difference suggests that autofluorescence does not contribute significantly to the data obtained with REF, which is supported by our observation that Mycobacterium avium does not produce signal in REF assays. Although M. avium is autofluorescent at similar wavelengths to other mycobacteria, it does not produce BlaC and, thereby, does not produce signal with REF [16].
We envision the future test to arise out of the REF system to serve as a true point-of-care test that can be administered by any minimally trained person in any location throughout the world. Since the reader will only require an LED for illumination, it could easily be handheld and battery operated. We have created prototypes of this type of system and they perform similar to the plate readers that we used to generate the current data, though they only have a single cutoff and emission filter. A standard phone camera can be used to capture the images, as we describe previously [21], or more sophisticated plate readers can be used. We prefer the less costly option to allow use in economically disadvantaged areas where TB is common.
1. Conclusions
We have identified a fluorogenic substrate for REF-based assays that has desirable characteristics for use in a diagnostic system for TB. The substrate, CDG-3, has an optimal excitation 493 nm and emission at 510 nm. However, use of 493 nm excitation with 535 nm gives excellent specificity and sensitivity. CDG-3 is specific for the Mtb BlaC beta-lactamase over most other beta-lactamases and displays sensitive detection of 10 CFU in human sputum with a good correlation for change in fluorescence and CFU over 4-logs. Overall, these characteristics suggest that CDG-3 used in a REF system has promise as a diagnostic that could be applied at the point-of-care and provide sensitivity comparable to culture. This test could serve as either a solid stand-alone test or, potentially optimally, as a triage test upstream of additional diagnostic testing. Use of a CDG-3 REF diagnostic assay in this manner could have a profoundly positive impact on TB diagnosis.
Acknowledgments
This work was supported by grants 48523 from the Bill and Melinda Gates Foundation and AI104960 from the National Institutes of Health. We thank Dr. Bob Fader in the Department of Microbiology/Virology in Scott & White Memorial Hospital in Temple, TX for providing us sputum samples from TB-negative patients.
Funding
NIH grant AI104960. Bill and Melinda Gates Foundation grant 48523.
Footnotes
Competing interests
None declared.
Ethical approval
Not required.
References
- 1.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, Small PM. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353(9151):444–9. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 2.Chang M, Anttonen KP, Cirillo SL, Francis KP, Cirillo JD. Real-time bioluminescence imaging of mixed mycobacterial infections. PLoS One. 2014;9(9):e108341. doi: 10.1371/journal.pone.0108341. http://dx.doi.org/10.1371/journal.pone.0108341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y, Xie H, Sule P, Hassounah H, Graviss EA, Kong Y, Cirillo JD, Rao J. Fluorogenic probes with substitutions at the 2 and 7 positions of cephalosporin are highly BlaC-specific for rapid Mycobacterium tuberculosis detection. Angew Chem Int Ed Engl. 2014;53(35):9360–4. doi: 10.1002/anie.201405243. http://dx.doi.org/10.1002/anie.201405243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol. 1974;99(2):131–8. doi: 10.1093/oxfordjournals.aje.a121593. [DOI] [PubMed] [Google Scholar]
- 5.Joshi P, Singh M, Bhargava A, Singh M, Mehrotra R. Autofluorescence–an important ancillary technique for the detection of Mycobacterium tuberculosis: revisited. Diagn Cytopathol. 2013;41(4):330–4. doi: 10.1002/dc.21860. http://dx.doi.org/10.1002/dc.21860. [DOI] [PubMed] [Google Scholar]
- 6.Kong Y, Akin AR, Francis KP, Zhang N, Troy TL, Xie H, Rao J, Cirillo SL, Cirillo JD. Whole-body imaging of infection using fluorescence. Curr Protoc Microbiol. 2011;Chapter 2(Unit 2C):3. doi: 10.1002/9780471729259.mc02c03s21. http://dx.doi.org/10.1002/9780471729259.mc02c03s21. [DOI] [PubMed] [Google Scholar]
- 7.Kong Y, Cirillo JD. Reporter enzyme fluorescence (REF) imaging and quantification of tuberculosis in live animals. Virulence. 2010;1(6):558–62. doi: 10.4161/viru.1.6.13901. 13901 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong Y, Yang D, Cirillo SL, Li S, Akin A, Francis KP, Maloney T, Cirillo JD. Application of fluorescent protein expressing strains to evaluation of anti-tuberculosis therapeutic efficacy in vitro and in vivo. PLoS One. 2016;11(3):e0149972. doi: 10.1371/journal.pone.0149972. http://dx.doi.org/10.1371/journal.pone.0149972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kong Y, Yao H, Ren H, Subbian S, Cirillo SL, Sacchettini JC, Rao J, Cirillo JD. Imaging tuberculosis with endogenous beta-lactamase reporter enzyme fluorescence in live mice. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Proc Natl Acad Sci U S A. 2010;107(27):12239–44. doi: 10.1073/pnas.1000643107. http://dx.doi.org/10.1073/pnas.1000643107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewinsohn DA, Gennaro ML, Scholvinck L, Lewinsohn DM. Tuberculosis immunology in children: diagnostic and therapeutic challenges and opportunities. Int J Tuberc Lung Dis. 2004;8(5):658–74. [PubMed] [Google Scholar]
- 11.Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis [Review] Int J Tuberc Lung Dis. 2004;8(5):636–47. [PubMed] [Google Scholar]
- 12.Nooshabadi F, Yang HJ, Bixler JN, Kong Y, Cirillo JD, Maitland KC. Intravital fluorescence excitation in whole-animal optical imaging. PLoS One. 2016;11(2):e0149932. doi: 10.1371/journal.pone.0149932. http://dx.doi.org/10.1371/journal.pone.0149932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Callaghan CH, Morris A, Kirby SM, Shingler AH. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972;1(4):283–8. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai M, Lewinsohn DM. Interferon-gamma assays for tuberculosis: is anergy the Achilles’ heel? Am J Respir Crit Care Med. 2005;172(5):519–21. doi: 10.1164/rccm.2506003. [DOI] [PubMed] [Google Scholar]
- 15.Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, Spector S, Roscigno G, Nkengasong J. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24(2):314–50. doi: 10.1128/CMR.00059-10. http://dx.doi.org/10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patino S, Alamo L, Cimino M, Casart Y, Bartoli F, Garcia MJ, Salazar L. Autofluorescence of mycobacteria as a tool for detection of Mycobacterium tuberculosis. J Clin Microbiol. 2008;46(10):3296–302. doi: 10.1128/JCM.02183-07. http://dx.doi.org/10.1128/JCM.02183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pesanti EL. The negative tuberculin test. Tuberculin, HIV, and anergy panels. Am J Respir Crit Care Med. 1994;149(6):1699–709. doi: 10.1164/ajrccm.149.6.7710481. [DOI] [PubMed] [Google Scholar]
- 18.Starke JR, Jacobs RF, Jereb J. Resurgence of tuberculosis in children. J Pediatr. 1992;120(6):839–55. doi: 10.1016/s0022-3476(05)81949-3. [DOI] [PubMed] [Google Scholar]
- 19.Wang F, Cassidy C, Sacchettini JC. Crystal structure and activity studies of the Mycobacterium tuberculosis beta-lactamase reveal its critical role in resistance to beta-lactam antibiotics. Antimicrob Agents Chemother. 2006;50(8):2762–71. doi: 10.1128/AAC.00320-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Global tuberculosis report 2013. Geneva, Switzerland: World Health Organization; 2013. p. 289. [Google Scholar]
- 21.Xie H, Mire J, Kong Y, Chang M, Hassounah HA, Thornton CN, Sacchettini JC, Cirillo JD, Rao J. Rapid point-of-care detection of the tuberculosis pathogen using a BlaC-specific fluorogenic probe. Nat Chem. 2012;4(10):802–9. doi: 10.1038/nchem.1435. http://dx.doi.org/10.1038/nchem.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]