Abstract
Objective
To characterize regional differences in brain water distribution and content during diabetic ketoacidosis (DKA) in children and determine whether these differences correlate with regional vascular supply
Study design
We compared changes in brain water distribution and water content in different brain regions during DKA by analyzing magnetic resonance diffusion weighted imaging data collected during DKA and after recovery in 45 children (<18 years). We measured the apparent diffusion coefficient of water (ADC) in the frontal and occipital cortex, basal ganglia, thalamus, hippocampus and medulla. Brain water content was also measured in a subset of patients.
Results
ADC values were elevated (suggesting vasogenic cerebral edema) in the frontal cortex, basal ganglia, thalamus and hippocampus during DKA. In contrast, ADC values in the medulla and the occipital cortex were not increased during DKA, and ADC changes in the medulla tended to be negatively correlated with other regions. Regions supplied by the anterior / middle cerebral artery circulation had greater elevations in both ADC and brain water content during DKA compared with regions supplied by the posterior cerebral artery circulation.
Conclusions
ADC changes during DKA in the brainstem contrast with those of other brain regions, and changes in both ADC and brain water content during DKA vary according to regional vascular supply. These data suggest that brainstem blood flow might possibly be reduced during DKA concurrent with hyperemia in other brain regions.
Keywords: diabetes, diabetic ketoacidosis, brain injury, cerebral edema, magnetic resonance imaging
Clinically-apparent cerebral injury occurs in 0.7–0.9% of DKA episodes in children.(1, 2) Signs and symptoms of this include a decline in mental status, often with other signs of neurological dysfunction.(3) Notably, case reports of children with DKA-related brain injuries describe sudden onset of signs of brainstem dysfunction, such as respiratory depression, bradycardia and hypotension.(4–6) This may suggest the occurrence of cerebral herniation. Imaging findings consistent with herniation, however, are infrequent and imaging studies may be normal in spite of these ominous clinical signs.(3, 5) For example, among 25 children with severe DKA-related cerebral injury (resulting in death or permanent disability), 96% were unresponsive when DKA-related cerebral injury was diagnosed or suspected, 40% had abnormal respirations or suffered respiratory arrests, 56% had absent or abnormal pupillary reflexes, 64% had bradycardia without hypertension, and 12% had hypotension that could not be attributed to causes other than cerebral injury. Only three of these patients had imaging findings suggesting herniation or impending herniation.3 An alternative explanation for the presence of clinical signs of brainstem dysfunction in these children must therefore be considered.
Sub-clinical cerebral edema occurs commonly in children with diabetic ketoacidosis. The cerebral ventricles are narrowed during DKA treatment(7, 8) and subtle cerebral dysfunction may be apparent.(7) Magnetic resonance imaging (MRI) studies demonstrate findings consistent with vasogenic cerebral edema, even in apparently asymptomatic children.(9–13) One previous study, however, showed that findings in the occipital cortex differ from those of other regions, suggesting that the brain’s response to DKA may not be uniform and that regional differences in the brain should be investigated further. (9)
We hypothesized that clinical signs of brainstem dysfunction during severe DKA-related brain injury might result from alterations in brainstem perfusion. To investigate this hypothesis, we analyzed MRI data from children with DKA to compare regional alterations in brain water distribution (Apparent Diffusion Coefficients [ADC]) and brain water content. During vasogenic cerebral edema and hyperemia, ADC values are typically elevated and brain water content is increased. In contrast, reductions in cerebral blood flow are associated with decreased ADC values. In the current study, we determined whether regional changes in ADC values and brain water content vary according to differences in regional vascular supply and whether ADC changes in the brainstem suggest either hyperemia (elevated ADC) or hypoperfusion (decreased ADC).
Methods
We evaluated data from children with DKA (without overt signs of brainstem dysfunction) who underwent MRI as part of previous research protocols. The ADC data analyzed in this study were collected during two prospective studies (n=27 and n=18).(11, 14) For the purposes of the current study, additional data were collected from the stored MR images obtained in one previous study, including measurements of ADC values in brain regions not previously investigated and measurements of brain water content. Identical protocols were used to collect ADC data in both studies.
Database 1 (n=27)11 included: (1) ADC measurements from children with uncomplicated DKA, 2–12 hours after beginning therapy and comparison ADC measurements after recovery, >72 hours after beginning therapy; (2) images obtained in frontal and occipital cortex, basal ganglia and thalamus; (3) three clinical centers conducted imaging studies (78% at UC Davis Medical Center); and (4) no stored MRIs available for additional measurements
Database 2 (n=18)14 included: (1) ADC measurements from children with uncomplicated DKA 3–6 hours and/or 9–12 hours after beginning therapy with comparison measurements after recovery, >72 hours after beginning therapy; (2) images obtained in frontal and occipital cortex, hippocampus, basal ganglia and thalamus; (3) all images obtained at a single center (UC Davis Medical Center); (4) brain water measurements recorded in an exploratory subset of patients (n=10); and (5) additional ADC data collected from stored images of the medulla (n=16)
Both prospective studies enrolled patients who were younger than 18 years old, diagnosed with type 1 diabetes mellitus, and had DKA (serum glucose concentration > 300 mg/dl, venous pH < 7.25 or serum bicarbonate < 15 mEq/l, and a positive test for urine ketones or serum ketones > 3 mmol/L).
DKA treatment protocols
Patients enrolled in the prospective MRI studies were treated with DKA protocols in compliance with guidelines from the International Society for Pediatric and Adolescent Diabetes.(15) The only variation from these guidelines was that each patient received an initial intravenous fluid bolus of either 10 or 20 mL per kg of 0.9% saline, rather than administering fluid boluses at the discretion of the provider. Intravenous fluid infusions then replaced an estimated deficit of 70–100 cc per kg over 48 hours using 0.45–0.9% saline. Insulin was administered intravenously (0.1 units per kg per hour) after completion of intravenous fluid bolus(es). Intravenous fluid treatment was continued until acidosis resolved (serum bicarbonate > 18 mmol/L). Neurological status was assessed hourly using Glasgow Coma Scale (GCS) scores(16) for all patients, and every 30 minutes or more frequently for patients with altered mental status (GCS scores below 14).
Diffusion Weighted Imaging (DWI)
MR DWI data were collected 3 to 12 hours after beginning DKA treatment. Patients in the database underwent DWI at either one or two time points during the 3–12 hour window. DWI measures the ease of diffusion of water molecules in cerebral tissues, quantified as the ADC. Areas with cell swelling (cytotoxic edema) or tissue dehydration (reduced extracellular water content) are characterized by low ADC. Areas with increased extracellular water content (vasogenic edema) are characterized by elevated ADC. Comparison studies were obtained after recovery from DKA (>72 hours after beginning treatment). Methods for DWI were described previously.(11, 14) ADC values were measured in the basal ganglia, thalamus, hippocampus, medulla, frontal cortex (gray and white matter) and occipital cortex (gray and white matter). ADC measurements were collected by one of two radiologists who were blinded to the patients’ clinical data and to the study hypothesis. The means of ADC measurements on the right and left sides of the brain were used to determine regional values.
Brain Water Content Measurement
In a sub-set of patients (n=10), brain water content was assessed using the following protocol. Brain water was measured on a 3.0T MRI system (Excite HDx, Ver.12x, GE Healthcare, Waukesha, WI) with a 8-channel RF head coil (In-vivo, Inc. Gainesville, FL) using 3D fast spoiled gradient echo (FSPGR) scans (8.76 ms TR, 2.80 ms TE, 24.0 cm FOV, 256×256 matrix, 2.0 mm thickness, 32 slices) with five different flip angles (3, 6, 9, 12, 15, 29°), followed by non-linear curve fitting for generation of proton density (M0) and T1 maps, and finally by calculation of regional brain water by division of M0 map values by the reference average signal values in four 100% water reference vials attached to the RF coil. An FSPGR scan with 6° flip angle using the body coil for signal reception was also obtained, and post-processing algorithms were developed to estimate the RF coil sensitivity and the B1 field (flip angle) spatial distributions to refine the curve fitting for M0 and T1 maps. Brain water content was recorded in seven regions (basal ganglia, thalamus, hippocampus, occipital gray and white matter and frontal gray and white matter) by one of two radiologists who were blinded to the patients’ clinical data as well as to the study hypothesis. In both the frontal and occipital cortices, gray and white matter values were averaged to calculate brain water content. The brain water content protocol did not allow for measurements in the brainstem or any region caudal to the pontomidbrain junction.
Statistical analyses
We performed statistical analyses using version 9.4 of SAS. MRI measures were log-transformed for analysis. Imaging data were occasionally missing as a result of patient motion, distortion due to dental hardware, inability to tolerate MRI, or safety concerns related to prolonged time away from the critical care unit. No patients were missing MRI data in database 1 (n=27). In database 2 (n=18), four patients were missing ADC data from the first time point during DKA treatment in all measured regions, and one patient was missing ADC data in all regions from the second time point during DKA treatment. One patient was missing ADC data from the hippocampus only at the second time point during DKA treatment and two patients were missing ADC data from the basal ganglia and frontal cortex at all three time points. In the subset of patients who underwent brain water assessment (n=10), two patients were missing brain water measurements at the first time point during DKA treatment and one additional patient was missing data at all three time points in the hippocampus only. To address intermittently missing imaging data, we used multiple imputation. The imputation model was based on multivariate analyses of correlations among the longitudinal measurements in each region and, at most, one other highly correlated region. Overall, 140 of 636 analyzed ADC measurements (22%) and 18 of 210 analyzed brain water measures (9%) were imputed in each of 100 multiple imputation datasets. Following imputation, early and late treatment measures were averaged and the recovery measurement was subtracted to determine the treatment to recovery difference. Because measures were log-transformed, the resulting difference is equivalent to a log-transformed ratio of treatment to recovery values on the untransformed scale. Pooled point and 95% Confidence Interval (CI) estimates of mean values, between-region differences in means, and Fisher Z-transformed correlation coefficients were generated using standard multiple imputation analysis procedures. Point and interval estimates involving means were back-transformed. Hence, mean treatment to recovery differences are expressed as geometric mean ratios (GMR) and between-region differences in treatment to recovery differences are expressed as relative geometric mean ratios (RGMR). Finally, we conducted sensitivity analysis to address the possibility that patients missing some imaging data may have had more abnormal values in comparison with patients who were able to tolerate all imaging procedures. To do that, we replaced imputed values with values that were 10% higher and assessed whether conclusions were substantively affected.
Results
MRI data from 45 children with DKA were analyzed (Table I). All children recovered fully, without any evident neurological deficits. Five (11%) of these children had declines in mental status during DKA treatment (GCS score <14), but none had visible evidence of cerebral edema or cerebral injury on imaging. Follow-up MRI scans were done before hospital discharge (72 to 99 hours after DKA) in 55% of patients, and in 40% after hospital discharge, 5 days to 3 months after the DKA episode. Data on timing of the follow-up MRI were not available for two patients.
Table 1.
Clinical Data at Presentation in Children with DKA Evaluated with MRI, mean (SD)
| all patients (n= 45) | subset evaluated for brain water content (n=10) | |
|---|---|---|
| age (years) | 12.5 (3.2) | 13.0 (3.0) |
| serum glucose (mg/dL) | 612 (231) | 609 (291) |
| serum sodium (mEq/L) | 132 (5) | 134 (7) |
| serum potassium (mEq/L) | 4.8 (0.9) | 4.8 (0.8) |
| serum bicarbonate (mEq/L) | 8.8 (3.2) | 10.4 (2.9) |
| serum urea nitrogen (mg/dL) | 22 (8) | 22 (9) |
| pH (mmHg) | 7.11 (0.10) | 7.13 (0.11) |
| pCO2 (mmHg) | 24 (9) | 26 (7) |
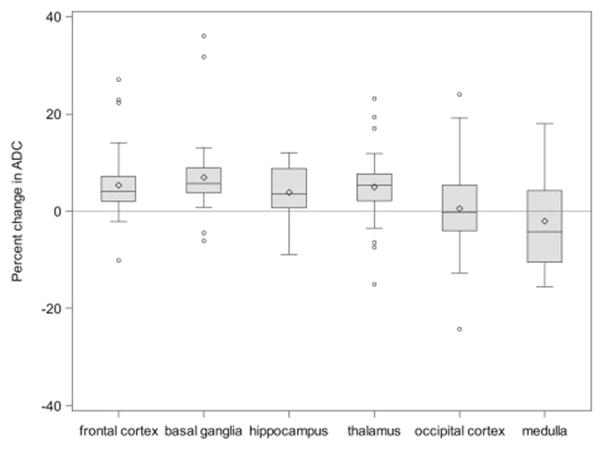
Patterns of ADC change differed among brain regions. In most regions (frontal cortex, basal ganglia, thalamus and hippocampus), ADC values were significantly elevated during DKA treatment, compared with values measured after recovery (Figure 1) suggesting increased water volume in the extracellular space (vasogenic edema). In contrast, this pattern was not observed in the medulla or occipital cortex. These findings suggest that extracellular fluid volumes in these areas are not increased and may be reduced in some patients during DKA treatment. Statistical comparisons of regional ADC changes confirmed that changes in the medulla and occipital cortex differed significantly from those in most other regions (Figure 1). In addition, ADC changes in brain regions supplied by the anterior / middle cerebral artery circulation (mean of frontal cortex and basal ganglia) were significantly greater than those in regions supplied by the posterior cerebral artery circulation (mean of occipital cortex and thalamus; RGMR = 1.04, 95% CI: 1.01, 1.06).
Figure 1.
Differences in ADC values during DKA compared with post-recovery (percent changes). The line in the middle of each box indicates the median (50% percentile), the top and bottom indicate the 75th / 25th percentiles, and the diamond indicates the mean. The whiskers extend toward the maximum and minimum values to a maximum length of 1.5 interquartile ranges. Individual values beyond the whiskers are possible outliers. For imputed values, the depicted value is based on the geometric mean treatment to recovery ratio across the 100 multiple imputation datasets (n=45 for occipital cortex and thalamus, n=43 for frontal cortex and basal ganglia, n=18 for hippocampus, n=16 for medulla).
| GMR (95% CI) | RGMR (95% CI), Relative to Occipital Cortex | RGMR (95% CI), Relative to Medulla | |
|---|---|---|---|
| Frontal cortex | 1.05 (1.03, 1.07) | 1.05 (1.01, 1.09) | 1.08 (1.02, 1.15) |
| Basal ganglia | 1.07 (1.04, 1.09) | 1.07 (1.03, 1.11) | 1.09 (1.01, 1.17) |
| Hippocampus | 1.04 (1.01, 1.07) | 0.99 (0.95, 1.03) | 1.06 (1.001, 1.13) |
| Thalamus | 1.05 (1.02, 1.07) | 1.05 (1.01, 1.08) | 1.06 (0.98, 1.14) |
| Occipital cortex | 1.00 (0.97, 1.03) | Reference | 1.07 (1.01, 1.15) |
| Medulla | 0.98 (0.93, 1.03) | 0.93 (0.87, 0.99) | Reference |
Pooled estimates from 100 multiple imputation datasets. Boldface indicates estimate is statistically significantly different from 1 (p<0.05).
When we examined the correlations of ADC changes among different regions (Table II), we found positive correlations among areas supplied by the anterior / middle cerebral artery circulation (frontal cortex and basal ganglia) and among areas supplied by the posterior cerebral artery circulation (thalamus and occipital cortex), suggesting that ADC changes are not independent but instead may stem from differences in the vascular supply. Notably, evaluations of ADC changes in the medulla suggested a tendency toward inverse correlations with ADC changes in other regions. Although the number of ADC measurements in the medulla was small, limiting the power to detect significant correlations, the negative correlation between ADC changes in the medulla and those of the thalamus (r = −0.48, 95% CI: −0.78, 0.01) was nearly significant (p=0.053).
Table 2.
Correlation Coefficients for Regional ADC changes during DKA
| basal ganglia | thalamus | occipital cortex | hippocampus | medulla | |
|---|---|---|---|---|---|
| frontal cortex | 0.44* (0.08, 0.70) (n=43, p=0.02) | −0.08 (−0.40, 0.26) (n=43, p=0.66) | 0.22 (−0.12, 0.52) (n=43, p=0.20) | −0.01 (−0.52, 0.50) (n=16, p=0.96) | −0.34 (−0.74, 0.24) (n=16, p=0.25) |
| basal ganglia | 0.34 (−0.01, 0.62) (n=43, p=0.06) | 0.27 (−0.10, 0.58) (n=43, p=0.14) | 0.27 (0.27, 0.68) (n=16, p=0.32) | −0.33 (−0.72, 0.22) (n=16, p=0.23) | |
| thalamus | 0.49* (0.18, 0.71) (n=45, p=0.003) | 0.17 (−0.33, 0.59) (n=18, p=0.52) | −0.48 (−0.78, 0.01) (n=16, p=0.053) | ||
| occipital cortex | 0.38 (−0.13, 0.73) (n=18, p=0.14) | −0.03 (−0.50, 0.46) (n=16, p=0.92) | |||
| hippocampus | −0.08 (−0.55, 0.42) (n=16, p=0.76) |
p<0.05
Note: Correlation coefficients and 95% confidence interval estimates computed by back-transforming pooled estimates of Fisher Z-transformed Pearson correlation coefficients in 100 multiple imputation dataset.
Regional differences in brain water content during DKA treatment
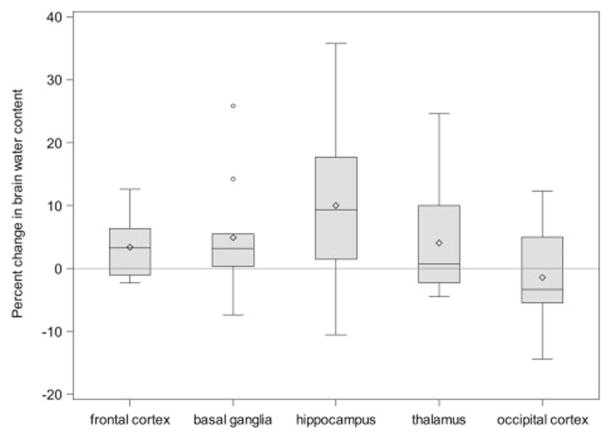
In an exploratory subset (n=10, Table I, Figure 2), we measured brain water content during DKA treatment and after recovery. Brain regions supplied by the anterior / middle cerebral artery circulation (frontal cortex and basal ganglia) had significantly greater elevations in water content during DKA compared with those supplied by the posterior cerebral artery circulation (occipital cortex and thalamus; RGMR = 1.04, 95% CI: 1.005, 1.07). Point estimates for mean brain water content in most regions exhibiting elevated ADC values during DKA treatment (frontal cortex, basal ganglia, hippocampus), were higher than recovery values but these differences were not statistically significant in this small exploratory subset.
Figure 2.
Differences in brain water content measurements during DKA compared with post-recovery (n=10), expressed as percent changes. The line in the middle of each box indicates the median (50% percentile), the top and bottom indicate the 75th / 25th percentiles, the diamond indicates the mean. The whiskers extend toward the maximum and minimum values to a maximum length of 1.5 interquartile ranges. Individual values beyond the whiskers are possible outliers. For imputed values, the depicted value is based on the geometric mean treatment to recovery ratio across the 100 multiple imputation datasets.
| GMR (95% CI) | RGMR (95% CI), Relative to Occipital Cortex | |
|---|---|---|
| Frontal cortex | 1.03 (0.999, 1.07) | 1.05 (0.99, 1.12) |
| Basal ganglia | 1.05 (0.99, 1.11) | 1.06 (0.99, 1.14) |
| Hippocampus | 1.09 (0.90, 1.32) | 1.11 (0.92, 1.34) |
| Thalamus | 1.04 (0.98, 1.10) | 1.06 (0.98, 1.14) |
| Occipital cortex | 0.98 (0.92, 1.05) | Reference |
Pooled estimates from 100 multiple imputation datasets.
Sensitivity analyses for the missing data imputation model provided substantively similar conclusions for both ADC and brain water content comparisons. In addition, comparisons of patients who had post-recovery MRI scans done prior to hospital discharge (72–99 hours after DKA) with those who had scans done after discharge (5 days-3 months) did not show any significant differences in mean ADC changes in any region (data not shown).
Discussion
Signs of brainstem dysfunction occur frequently in children with severe DKA-related cerebral injuries, although, imaging findings of herniation are uncommon. These data raise the possibility that oxygen delivery to the brainstem may be altered ny mechanisms other than herniation. In this study, we demonstrate that changes in brain water distribution during DKA treatment vary among different regions. Whereas ADC values in most brain regions are increased by approximately 5% during DKA, suggesting vasogenic cerebral edema, these trends are not evident in the occipital cortex and medulla. ADC changes among regions within the same arterial territories are positively correlated and both ADC changes and brain water content measurements in regions supplied by the anterior / middle cerebral artery circulation are significantly greater than those in areas supplied by the posterior cerebral artery circulation. Comparisons of regional ADC changes suggest the possibility that ADC changes in the medulla may be negatively correlated with those of cortical regions.
Our observations suggest that regional ADC changes may not be independent and that alterations in cerebral blood flow (CBF) in some vascular territories may influence blood flow changes in others. Although these findings are based on a relatively small dataset and are therefore preliminary, the data raise the possibility that the medulla may suffer reduced CBF while other regions experience elevated CBF and vasogenic edema during DKA treatment. In this scenario, signs of brainstem dysfunction in children with severe DKA-related cerebral injuries might reflect diversion of blood flow away from the brainstem.
Previous studies have mainly demonstrated increased CBF and increased ADC values during DKA, consistent with vasogenic cerebral edema.(9, 12) Children with DKA frequently have decreased size of the lateral cerebral ventricles suggesting edema in adjacent regions.(7, 8, 17) Similarly, studies using near infrared spectroscopy suggest cerebral hyperemia in the frontal cortex during DKA treatment.(12, 13)
Elevated regional oxygen saturation values in these studies suggest blood flow in excess of metabolic demand. Studies using transcranial Doppler ultrasound in children with DKA similarly show elevated CBF velocity and impairment of cerebral autoregulation.(13, 18)
There are few data regarding regional differences in the brain’s response to DKA. One previous study showed that ADC values were variable and often decreased in the occipital cortex during DKA treatment, contrasting with trends in other brain regions.(9) Perfusion weighted imaging studies showed increased cerebral perfusion in most regions, but measures reflecting perfusion in the occipital cortex were not significantly elevated. Another study showed that changes in white matter volume and water diffusivity (reflecting vasogenic edema) during DKA were more prominent in the frontal cortex compared with other brain regions.(10)
Under normal circumstances, CBF is tightly regulated to ensure consistent oxygen delivery to the brain and is highly responsive to changes in pCO2. In the setting of prolonged hypocapnia, however, a return to normal pCO2 levels causes hyperemia, likely as a result of reduced extracellular fluid buffer capacity.(19, 20) Responsiveness of CBF to changes in pCO2 differs among regions with different vascular supplies, and there is disparate blood flow regulation in the brainstem versus the cerebral cortex, and the anterior versus the posterior cerebral circulation.(21, 22) The capacity for autoregulation of CBF also may differ among brain regions.(21, 22) Studies of global cerebral vasodilation demonstrate that vascular beds with greater vasodilatory reserve may “steal” blood flow from regions with lesser reserve, causing ischemia.(23, 24)
We and others have hypothesized that abnormal vasodilation during DKA treatment may result from cerebral hypoperfusion during untreated DKA followed by reperfusion, or from the effects of rising pCO2 levels after prolonged hypocapnia, or a combination of the two.(12, 25, 26) It is possible that brainstem hypoperfusion may occur during DKA treatment when blood flow is diverted to regions with greater hyperemic responses. Support for this hypothesis also comes from a recent study using trans-cranial Doppler to evaluate CBF in children with severe DKA.(27) That study found impaired cerebral autoregulation in nearly all of these children and significantly reduced basilar artery flow velocity in children with clinical or radiographic findings consistent with cerebral edema.
The current study has several limitations. The sample size for ADC measurements in the medulla was small, limiting our statistical power to detect differences. Although correlation coefficient estimates describing the relationship between the ADC changes in the medulla and those of the rest of the brain were uniformly negative, these were not statistically significant in most areas and of borderline significance in the thalamus. The findings of the present study therefore are not definitive, but suggest hypotheses to be further investigated. In addition, some patients did not have complete datasets because they were unable to tolerate all of the imaging studies or were felt to be too unstable to leave the critical care unit at one of the study time points. Missing values were imputed, however, and sensitivity analyses demonstrated consistent results, confirming that the study conclusions were not influenced by data imputation. In addition, brain water content studies were obtained in a small subset because many patients were unable to remain immobile for sufficient periods to accomplish MRI studies or were too seriously ill to remain outside of the critical care unit for these additional studies. Although we had limited statistical power to detect brain water content differences, some differences were nonetheless identified. Technical limitations of the protocol also prevented brain water content measurements in the medulla. Finally, the timing of follow-up imaging varied among patients. Approximately 50% of patients had follow-up images obtained within three to four days of DKA. It is possible that this time period was insufficient to allow complete resolution of alterations caused by DKA, thereby diminishing the apparent magnitude of changes and limiting our ability to detect significant differences.
Acknowledgments
Funded by American Diabetes Association (7-01-CR-14 [to N.G]) and by National Institute of Neurological Disorders and Stroke (R01 NS048610 [to NG)].
We thank Michael Buonocore, MD, for his implementation of MRI programs for noninvasive measurement of physiological processes, including development of the protocol for measurement of brain water content that was used in this study.
Abbreviations
- DKA
diabetic ketoacidosis
- MRI
magnetic resonance imaging
- DWI
diffusion weighted imaging
- CBF
cerebral blood flow
- ADC
apparent diffusion coefficient
- GMR
geometric mean ratios
- RGMR
relative geometric mean ratios
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edge J, Hawkins M, Winter D, Dunger D. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001;85:16–22. doi: 10.1136/adc.85.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, et al. Risk Factors for Cerebral Edema in Children with Diabetic Ketoacidosis. N Engl J Med. 2001;344:264–9. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 3.Muir A, Rosenbloom A, Williams L, Yang M, Quisling R. Cerebral edema in childhood diabetic ketoacidosis: Natural history, radiographic findings and early identification. Diab Care. 2004;27:1541–6. doi: 10.2337/diacare.27.7.1541. [DOI] [PubMed] [Google Scholar]
- 4.Lufkin E, Reagan T, Doan D, Yanagihara T. Acute cerebral dysfunction in diabetic ketoacidosis: Survival followed by panhypopituitarism. Metabolism. 1977;26:363–9. doi: 10.1016/0026-0495(77)90103-2. [DOI] [PubMed] [Google Scholar]
- 5.Roberts M, Slover R, Chase H. Diabetic ketoacidosis with intracerebral complications. Pediatr Diabetes. 2001;2:103–14. doi: 10.1034/j.1399-5448.2001.002003109.x. [DOI] [PubMed] [Google Scholar]
- 6.Taubin H, Matz R. Cerebral edema, diabetes insipidus and sudden death during the treatment of diabetic ketoacidosis. Diabetes. 1968;17:108–9. doi: 10.2337/diab.17.2.108. [DOI] [PubMed] [Google Scholar]
- 7.Glaser N, Wooton-Gorges S, Buonocore M, Marcin J, Rewers A, Strain J, et al. Frequency of SubClinical Cerebral Edema in Children with Diabetic Ketoacidosis. Pediatr Diab. 2006;7:75–80. doi: 10.1111/j.1399-543X.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 8.Krane E, Rockoff M, Wallman J, Wolfsdorf J. Subclinical brain swelling in children during treatment of diabetic ketoacidosis. N Engl J Med. 1985;312:1147–51. doi: 10.1056/NEJM198505023121803. [DOI] [PubMed] [Google Scholar]
- 9.Glaser N, Gorges S, Marcin J, Buonocore M, DiCarlo J, Neely E, et al. Mechanism of Cerebral Edema in Children with Diabetic Ketoacidosis. J Pediatr. 2004;145:164–71. doi: 10.1016/j.jpeds.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 10.Cameron F, Scratch S, Nadebaum C, Northam E, Koves I, Jennings J, et al. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. 2014;37:1554–62. doi: 10.2337/dc13-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser N, Marcin J, Wooton-Gorges S, Buonocore M, Rewers A, Strain J, et al. Correlation of Clinical and Biochemical Findings with DKA-related Cerebral Edema in Children using Magnetic Resonance Diffusion Weighted Imaging. J Pediatr. 2008;153:541–6. doi: 10.1016/j.jpeds.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 12.Glaser N, Tancredi D, Marcin J, Caltagirone R, Lee Y, Murphy C, et al. Cerebral hyperemia measured with near infrared spectroscopy during treatment of diabetic ketoacidosis in children. J Pediatr. 2013;163:1111–6. doi: 10.1016/j.jpeds.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts J, Vavilala M, Schenkman K, Shaw D, Martin L, Lam A. Cerebral hyperemia and impaired cerebral autoregulation associated with diabetic ketoacidosis in critically ill children. Crit Care Med. 2006;34:2217–23. doi: 10.1097/01.CCM.0000227182.51591.21. [DOI] [PubMed] [Google Scholar]
- 14.Glaser N, Wooton-Gorges S, Buonocore M, Tancredi D, Marcin J, Caltagirone R, et al. Subclinical cerebral edema in children with diabetic ketoacidosis randomized to 2 different rehydration protocols. Pediatrics. 2013;131:e73–80. doi: 10.1542/peds.2012-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfsdorf J, Craig M, Daneman D, Dunger D, Edge J, Lee W, et al. ISPAD Clinical Practice Consensus Guidleines 2009 Compendium: Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diab. 2009;10:118–33. doi: 10.1111/j.1399-5448.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 16.Reilly P, Simpson D, Sprod R, Thomas L. Assessing the conscious level in infants and young children: a paediatric version of the Glasgow Coma Scale. Child’s Nerv Syst. 1988;4:30–3. doi: 10.1007/BF00274080. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman W, Steinhart C, El Gammal T, Steele S, Cuadrado A, Morse P. Cranial CT in children and adolescents with diabetic ketoacidosis. AJNR. 1988;9:733–39. [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman W, Pluta R, Fisher A, Wagner M, Yanovski J. Transcranial doppler ultrasound assessment of intracranial hemodynamics in children with diabetic ketoacidosis. J Clin Ultrasound. 1995;23:517–23. doi: 10.1002/jcu.1870230903. [DOI] [PubMed] [Google Scholar]
- 19.Muizelaar J, Marmarou A, Ward J, Kontos H, Choi S, Becker D, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: a randomized clinical trial. J Neurosurg. 1991;75:731–39. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- 20.Muizelaar J, van der Poel H, Li Z, Kontos H, Levasseur J. Pial arteriolar vessel diameter and CO2 reactivity during prolonged hyperventilation in the rabbit. J Neurosurg. 1988;69:923–7. doi: 10.3171/jns.1988.69.6.0923. [DOI] [PubMed] [Google Scholar]
- 21.Skow R, Mackay C, Tymko M, Willie C, Smith K, Ainslie P, et al. Differential cerebrovascular CO reactivity in anterior and posterior cerebral circulations. Respir Physiol Neurobiol. 2013;189:76–86. doi: 10.1016/j.resp.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Willie C, Macleod D, Shaw A, Smith K, Tzeng Y, Eves N, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol. 2012;15:3261–75. doi: 10.1113/jphysiol.2012.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willie C, Yu-Chieh T, Fisher J, Ainslie P. Integrative regulation of human brain blood flow. J Physiol. 2014;592(5):841–59. doi: 10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobczyk O, Battisti-Charbonney A, Fierstra J, Mandell D, Poublanc J, Crawley A, et al. A conceptual model for CO-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. Neuroimage. 2014;92:56–68. doi: 10.1016/j.neuroimage.2014.01.051. [DOI] [PubMed] [Google Scholar]
- 25.Glaser N. Cerebral injury and cerebral edema in children with diabetic ketoacidosis: could cerebral ischemia and reperfusion injury be involved? Pediatr Diab. 2009;10:534–41. doi: 10.1111/j.1399-5448.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney C, Vlcek B, Del Aguila M. Risk factors for developing brain herniation during diabetic ketoacidosis. Pediatr Neurol. 1999;21:721–27. doi: 10.1016/s0887-8994(99)00079-x. [DOI] [PubMed] [Google Scholar]
- 27.Chung M, Maa T, O’Brien N. Prolonged abnormal cerebral autoregulation in children with moderate to severe diabetic ketoacidosis. Pediatr Crit Care Med. 2013;(supplement 12) abstract #986. [Google Scholar]