Abstract
Background and Purpose
Little is known about the clinical outcomes associated with post-hemorrhage anticoagulation resumption for atrial fibrillation. This study had two objectives: first, to evaluate anticoagulation use after a first major bleed on warfarin or dabigatran; and second, to compare effectiveness and safety outcomes between patients discontinuing anticoagulation after a major bleed and patients restarting warfarin or dabigatran.
Methods
Using 2010-2012 Medicare Part D data, we identified atrial fibrillation patients who experienced a major bleeding event while using warfarin (n=1135) or dabigatran (n=404) and categorized them by their post-hemorrhage use of anticoagulation. We followed them until an ischemic stroke, recurrent hemorrhage, or death through December 31, 2012. We constructed logistic regression models to evaluate factors impacting anticoagulation resumption, and Cox Proportional Hazard models to compare the combined risk of ischemic stroke and all-cause mortality, and the risk of recurrent bleeding between treatment groups.
Results
Resumption of anticoagulation with warfarin (hazard ratio (HR) 0.76; 95%CI, 0.59-0.97) or dabigatran (HR0.66; 95%CI 0.44-0.99) was associated with lower combined risk of ischemic stroke and all-cause mortality than anticoagulation discontinuation. The incidence of recurrent major bleeding was higher for patients prescribed warfarin after the event than for those prescribed dabigatran (HR2.31; 95%CI, 1.19-4.76) or whose anticoagulation ceased (HR1.56; 95%CI, 1.10-2.22), but did not differ between patients restarting dabigatran and those discontinuing anticoagulation (HR0.66; 95% CI, 0.32-1.33).
Conclusions
Dabigatran was associated with a superior benefit/risk ratio than warfarin and anticoagulation discontinuation in the treatment of atrial fibrillation patients who have survived a major bleed.
Keywords: Anticoagulants, Atrial Fibrillation, Intracranial Hemorrhage, Ischemic Stroke, Quality and Outcomes
INTRODUCTION
Anticoagulation therapy reduces the risk of stroke associated with atrial fibrillation (AF) by around 60%.1 Anticoagulation, however, is not free of risks, being an important determinant of bleeding. The optimal management of AF patients who have experienced a major bleeding complication is uncertain, since there are competing risks from both the resumption and the discontinuation of anticoagulation: while patients experiencing a major bleed are at increased risk of recurrent bleeding events,2 they are also at a high risk of thromboembolic events, if anticoagulation is not reinitiated.3-6 The uncertainty surrounding decisions about the post-hemorrhage use of anticoagulation is very relevant from the clinical perspective, particularly because patients who are at highest risk of bleeding are also at highest risk of stroke.2, 7
Previous studies that examined the clinical outcomes of patients who resumed versus those who discontinued anticoagulation after a major bleed found that resumption of anticoagulation was associated with lower risk of thromboembolic events, but higher risk of bleeding.3-6 Nevertheless, in comparing clinical outcomes between these 2 groups of patients, these studies did not account for the type of anticoagulation agent used, and used data that preceded the market entry of the non-vitamin K antagonist oral anticoagulants (NOACs).3-6 With no requirement for routine coagulation assay monitoring, and with a lower risk of intracranial bleeding, the therapeutic management and bleeding profile of the NOACs are considerably different from those of warfarin.8 Consequently, the clinical outcomes associated with the resumption of anticoagulation after a major bleeding event may differ between patients reinitiating warfarin therapy and those reinitiating NOACs. Therefore, it is important to separately evaluate the risks of stroke and recurrent bleeding among patients who resume anticoagulation with warfarin, those who reinitiate anticoagulation with the NOACs, and those who discontinue all anticoagulation.
Therefore, our present analysis had two objectives: first, to evaluate the patterns of oral anticoagulation use after a major bleeding event on dabigatran or warfarin and to identify predictors for post-hemorrhage resumption of oral anticoagulation; and second, to compare the combined risk of ischemic stroke and all-cause mortality and the risk of recurrent bleeding events between patients who resume anticoagulation with warfarin or dabigatran versus those whose anticoagulation is ceased.
METHODS
Data Source and Study Population
We obtained 2010-2012 data for a 5% random sample of Medicare beneficiaries from the Centers for Medicare and Medicaid Services (CMS). First, we identified all patients who had a diagnosis of AF9 and filled a prescription for dabigatran or warfarin between October 19, 2010 (date of dabigatran approval) and June 30, 2012 (Figure 1). To make sure that the warfarin group was representative of patients initiating warfarin and hence, comparable to the dabigatran group, we excluded all individuals who had filled a prescription for warfarin during the six months before October 19, 2010. We followed 10,059 dabigatran users and 79,714 warfarin users from the date of the first prescription of dabigatran or warfarin after October 19, 2010 through December 31, 2012 until the first of the following events: major bleeding, discontinuation of treatment, defined as a gap in treatment for over 60 days, 10 switch of anticoagulant, or death. Second, we selected those who experienced a major bleeding event that required hospitalization (index major hemorrhage) and identified those who were discharged alive. Third, we collected their prescriptions for oral anticoagulant agents filled after the date of the index major hemorrhage and categorized them according to the oral anticoagulation agent used. Patients who filled a prescription for dabigatran or warfarin after the bleeding event were followed from the date of the first anticoagulant prescription after index major hemorrhage (post-hemorrhage follow-up start date) through December 31, 2012 or until the occurrence of a stroke, a recurrent bleeding event, or death. To set the post-hemorrhage follow-up start date for patients who never filled a prescription for an oral anticoagulant agent after the index major hemorrhage, we performed frequency matching. Further details on frequency matching can be found in the Supplemental Methods at http://stroke.ahajournals.org. Patients who switched to rivaroxaban were not included in the study because of the small sample size of this treatment group (n=8 in the dabigatran cohort, and n=9 in the warfarin cohort). This study was approved by the Institutional Review Board at the University of Pittsburgh as exempt.
Figure 1. Selection of the Study Sample.

Notes:
Abbreviations: AF=Atrial Fibrillation.
Outcomes
Effectiveness outcomes included ischemic stroke, all-cause mortality, and the composite of ischemic stroke and all-cause mortality. Ischemic stroke was defined as having one inpatient, emergency room or outpatient claim with primary or secondary International Classification of Diseases, Ninth Revision (ICD-9) codes 433, 434 or 436.11, 12 Safety outcomes included recurrent major bleeding and any recurrent bleeding event (definitions in the Supplemental Methods at http://stroke.ahajournals.org.).
Covariates
We evaluated how different demographic factors, clinical characteristics, anatomical location and severity of the index major hemorrhage affected the post-hemorrhage use of oral anticoagulation. All covariates were measured at the time of the index major hemorrhage. Demographic characteristics included age, gender, race and eligibility for Medicaid coverage. Clinical covariates included CHA2DS2-VASc score,7 HAS-BLED score,2 and a number of other CMS priority comorbidities. Because Medicare claims data does not contain information on the international normalized ratio (INR), we calculated the HAS-BLED score as the sum of all previous factors except labile INR. We categorized the anatomical location of the index major hemorrhage into four groups: intracranial bleeding, gastrointestinal hemorrhage, genitourinary hemorrhage, and other bleeding events, which included hemoperitoneum, epistaxis, hemoptysis, hemarthrosis, conjunctival and vaginal hemorrhage and not-otherwise specified hemorrhage. Measures of the severity of the index major hemorrhage included length of inpatient stay, intensive care unit admission, blood transfusion therapy, and whether the patients underwent corrective procedures in the same anatomical area of the bleeding. The definitions of covariates can be found in the Supplemental Methods at http://stroke.ahajournals.org.
Statistical Analysis
We compared patient characteristics of three post-hemorrhage treatment groups in each cohort at the time of index major hemorrhage using chi-square tests, Fisher’s exact tests and ANOVA, as appropriate. To predict the probability of restarting the same anticoagulation agent used before the index bleeding event or switching to another agent as opposed to discontinuing oral anticoagulation, we constructed a multinomial logistic regression model with generalized logit link function, where the outcome variable was the post-hemorrhage treatment group, and covariates included all variables listed in the Covariates Section.
Kaplan-Meier time-to-event curves were constructed to compare the cumulative incidence rates of effectiveness and safety outcomes at 3 months, 6 months and 1 year post-hemorrhage follow-up among the post-hemorrhage treatment groups. To further control for potential confounders in comparing effectiveness and safety outcomes, we constructed Cox Proportional Hazard models. Cox models built to compare effectiveness outcomes controlled for age, CHA2DS2-Vasc score, HAS-BLED score and an indicator variable for the location of the index major hemorrhage (1 if intracranial, 0 otherwise). Cox models built to compare safety outcomes controlled for CHA2DS2-Vasc, HAS-BLED score, an indicator variable for the location of the index major hemorrhage (1 if intracranial, 0 otherwise), and the measures of the severity of the index bleeding event, as detailed above. For all time-to-event analyses, time 0 was the post-hemorrhage follow-up start date (defined in the Data Source and Study Population Section). The time at risk was censored at the end of the study period (December 31, 2012) or at the time of death, except for the Kaplan-Meier and Cox models whose outcome included mortality. In those analyses, the time at risk was only censored at the end of the study period. All of these analyses were performed separately for the dabigatran and the warfarin cohorts. In a secondary analysis, we grouped patients from the warfarin and dabigatran cohorts according to the treatment used after the index major hemorrhage, and compared effectiveness and safety outcomes using Cox models in a similar manner, as described above. All analyses were conducted with statistical software SAS 9.4 (Cary, NC).
Sensitivity Analysis
Post-hemorrhage clinical outcomes of patients who experienced an intracranial bleeding are likely to differ from those who bled on other anatomical locations. To examine how this may have affected our results for the comparative risk of post-hemorrhage clinical outcomes, we repeated our analysis after excluding patients who experienced an intracranial bleeding.
RESULTS
Post-Hemorrhage Anticoagulation Use and Patient Characteristics
The proportion of patients who reinitiated anticoagulation after the index major hemorrhage was similar between the warfarin and dabigatran cohorts (49% for dabigatran and 47% for warfarin, p-value=0.497). However, dabigatran users were more likely to switch to warfarin after the bleeding event than warfarin users were to switch to dabigatran (17% versus 2%, p -value<0.001). In addition, resumption of the same oral anticoagulation agent used before the index major hemorrhage was more common in the warfarin cohort than in the dabigatran cohort (41% vs. 28%, with p-value <0.001). In the dabigatran cohort, the mean time from index bleeding to anticoagulation resumption was 45 days for patients who resumed dabigatran, and 73 days for those who switched to warfarin (p-value=0.005). In the warfarin cohort, the average time from index bleeding to anticoagulation resumption was 60 days for patients who resumed warfarin, and 70 days for those who switched to dabigatran (p-value=0.501). The average follow-up time for each group and cohort can be found in Table II at http://stroke.ahajournals.org
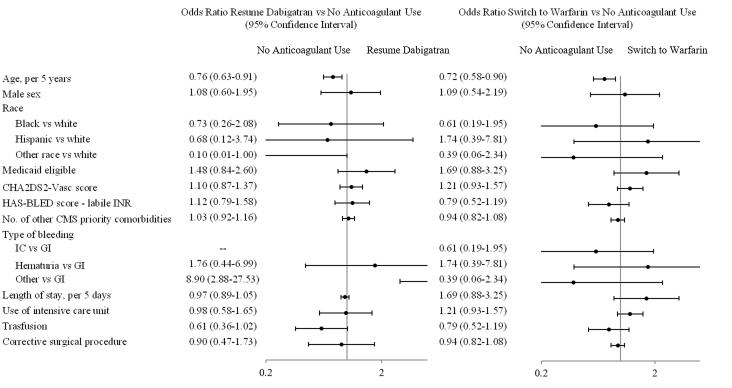
Table 1 shows how patient characteristics at baseline compare among post-hemorrhage treatment groups. Older patients were more likely to discontinue anticoagulation after the index hemorrhage in both cohorts. Specifically, the odds of resuming dabigatran or switching to warfarin compared to discontinuing anticoagulation decreased by 24% (95% CI, 9%-37%) and 28% (95% CI, 10%-42%) for every 5 years increase in age, respectively (Figure 2). In the warfarin cohort, patients who experienced an intracranial bleeding, were admitted to the intensive care unit, or received a blood transfusion, were more likely to cease anticoagulation (Figure 3).
Table 1.
Baseline Characteristics of the Cohorts, by Use of Anticoagulation after Index Major Hemorrhage.
| Dabigatran Cohort (N=404) |
Warfarin Cohort (N=1135) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Resumed Dabigatran (n=117) |
No Oral Anticoagulation (n=217) |
Switched to Warfarin (n=70) |
P- Value |
Resumed Warfarin (n=484) |
No Oral Anticoagulation (n=626) |
Switched to Dabigatran (n=25) |
P- Value |
| Age, mean (SD) | 79.64 (8.67) | 81.9 (7.63) | 78.73 (8.34) | 0.005 | 77.95 (9.40) | 80.20 (8.96) | 76.15 (6.79) | <0.001 |
| Male sex, N (%) | 41 (35.0) | 68 (31.3) | 23 (32.9) | 0.788 | 219 (45.3) | 247 (39.5) | 13 (52.0) | 0.093 |
| Race, N (%) | 0.745 | 0.657 | ||||||
| White | 106 (90.6) | 188 (86.6) | 60 (85.7) | 400 (82.6) | 521 (83.2) | 20 (80.0) | ||
| Black | 8 (6.8) | 15 (6.9) | 5 (7.1) | 54 (11.2) | 72 (11.5) | 2 (8.0) | ||
| Hispanic | 2 (1.7) | 6 (2.8) | 3 (4.3) | 23 (4.8) | 26 (4.2) | 3 (12.0) | ||
| Other | 1 (0.9) | 8 (3.7) | 2 (2.9) | 7 (1.5) | 7 (1.1) | 0 (0.0) | ||
| Medicaid eligibility, N (%) | 43 (36.8) | 60 (27.7) | 25 (35.7) | 0.170 | 166 (34.3) | 194 (29.4) | 6 (24.0) | 0.158 |
| CHA2DS2-VASc score, mean (SD) | 5.96 (1.77) | 5.89 (1.58) | 5.77 (1.99) | 0.773 | 4.92 (1.57) | 5.08 (1.60) | 4.28 (1.56) | 0.018 |
| HAS-BLED score, mean (SD) | 4.16 (0.96) | 4.12 (0.95) | 3.94 (1.08) | 0.306 | 4.06 (0.90) | 4.12 (0.95) | 4.00 (0.76) | 0.478 |
| Use of antiplatelet agents, N (%) | 16 (13.7) | 33 (15.2) | 5 (7.1) | 0.225 | 67 (13.8) | 110 (17.6) | 5 (20.0) | 0.210 |
| No. of other CMS priority comorbidities, mean (SD)* | 7.21 (2.53) | 7.06 (2.32) | 6.53 (2.80) | 0.178 | 6.79 (2.48) | 7.00 (2.40) | 5.92 (2.60) | 0.051 |
| Type of bleeding, N (%) | <0.001 | <0.001 | ||||||
| IC | 0 (0.0) | 23 (10.6) | 5 (7.1) | 20 (4.1) | 116 (18.5) | 3 (12.0) | ||
| GI Hemorrhage | 92 (78.6) | 184 (84.8) | 53 (74.7) | 337 (69.6) | 426 (68.1) | 19 (76.0) | ||
| Genitourinary hemorrhage | 5 (4.3) | 5 (2.3) | 2 (2.86) | 42 (8.7) | 18 (2.9) | 1 (4.0) | ||
| Other | 20 (17.1) | 5 (2.3) | 10 (14.3) | 85 (17.6) | 66 (10.5) | 2 (8.0) | ||
| Length of stay,median (IQR) | 4.0 (3.0-5.0) | 4.0 (3.0-7.0) | 4.0 (2.0-7.0) | 0.768 | 4.0 (3.0-6.0) | 5.0 (3.0-8.0) | 4.0 (2.0-7.0) | 0.253 |
| Use of intensive care unit, N (%) | 39 (33.3) | 88 (40.6) | 35 (50.0) | 0.078 | 418 (30.6) | 268 (42.8) | 14 (56.0) | <0.001 |
| Trasfusion, N (%) | 39 (33.3) | 105 (48.4) | 35 (50.0) | 0.018 | 210 (43.4) | 329 (52.6) | 13 (52.0) | 0.010 |
| Surgical procedures in area affected, N (%) | 18 (15.4) | 38 (17.5) | 16 (22.9) | 0.427 | 99 (20.5) | 115 (18.4) | 6 (24.0) | 0.575 |
NOTES:
Abbreviations: CMS=Centers for Medicaid Services; IC=Intracranial; GI=Gastrointestinal; NOS=Not Otherwise Specified.
The list of other CMS priority comorbidities can be found in the Supplemental Methods at http://stroke.ahajournals.org.
Figure 2. Odds Ratio of Post-Hemorrhage Anticoagulation Use for the Dabigatran Cohort.
Notes:
Abbreviations: CMS=Centers for Medicare and Medicaid Services; IC=Intracranial; GI=Gastrointestinal.
Results from a multinomial logistic regression model. The odds ratio for restarting dabigatran as opposed to not using any oral anticoagulation for patients experiencing an intracranial bleeding compared to those experiencing a gastrointestinal bleeding could not be estimated because none of the patients undergoing a gastrointestinal bleeding resumed dabigatran therapy after the index bleeding event.
Figure 3. Odds Ratio of Post-Hemorrhage Anticoagulation Use for the Warfarin Cohort.
Notes:
Abbreviations: CMS=Centers for Medicare and Medicaid Services; IC=Intracranial; GI=Gastrointestinal.
Results from a multinomial logistic regression model. The odds ratio for switching to dabigatran as opposed to interrupting anticoagulation for patients of other race compared to white patients could not be estimated because none of the patients belonging to other racial minorities switched to dabigatran after the index bleeding event.
Ischemic Stroke
Before adjustment, there was no difference in the risk of stroke among treatment groups in two cohorts: the cumulative incidence of ischemic stroke at 1 year was 0.20(95% CI, 0.12-0.29) for dabigatran users resuming dabigatran, 0.15(95% CI, 0.08-0.21) for dabigatran users who discontinued anticoagulation, 0.21(95% CI, 0.10-0.32) for dabigatran users switching to warfarin, 0.17(95% CI, 0.13-0.21) for warfarin users resuming warfarin, 0.14(95%CI, 0.11-0.18) for warfarin users who discontinued anticoagulation, and 0.25(95% CI, 0.06-0.44) for warfarin users switching to dabigatran (Table III at http://stroke.ahajournals.org). After adjustment for potential confounders, the risk of ischemic stroke did not differ between patients who resumed dabigatran (hazard ratio (HR) 1.29; 95% CI, 0.69-2.43) or switched to warfarin (HR 1.26; 95% CI, 0.63-2.65) and those who did not reinitiate anticoagulation. In the warfarin cohort, similarly, the risk of ischemic stroke did not differ among treatment groups (HR 0.75; 95% CI, 0.57-0.98 for resumption of warfarin vs. discontinuation of anticoagulation, and HR 0.37; 95% CI, 0.23-0.58 for switching to warfarin vs. discontinuation of anticoagulation). When the two cohorts were analyzed simultaneously based on the treatment received after the index hemorrhage, once again, there was no difference in the risk of ischemic stroke among post-hemorrhage treatment groups.
Ischemic Stroke and All-Cause Mortality
The cumulative incidence of all-cause mortality at 1 year was higher for patients who discontinued anticoagulation (0.13;95%CI, 0.08-0.18 for patients on the dabigatran cohort and 0.15;95% CI, 0.12-0.18 for patients on the warfarin cohort) than for those who restarted anticoagulation (0.02;95%CI, 0.00-0.04 for dabigatran users resuming dabigatran and 0.07;95% CI, 0.04-0.09 for warfarin users resuming warfarin) (Table III at http://stroke.ahajournals.org). After adjustment for potential confounders, the risk of all-cause mortality was lower for patients on the dabigatran cohort who resumed dabigatran (hazard ratio (HR) 0.13; 95% CI, 0.03-0.58) or switched to warfarin (HR 0.21; 95% CI, 0.05-0.91) than for those who did not reinitiate anticoagulation (Figure 4). In the warfarin cohort, resumption of warfarin was associated with lower composite risk of ischemic stroke and all-cause mortality (HR 0.75; 95% CI, 0.57-0.98) and lower risk of all-cause mortality (HR 0.37; 95% CI, 0.23-0.58) than discontinuation of anticoagulation.
Figure 4.
Adjusted Hazard Ratios of Post-Hemorrhage Clinical Outcomes.
Notes:
Bold denotes statistical significant results.
Hazard ratios were estimated with Cox Proportional Hazard Models.
When the two cohorts were analyzed simultaneously based on the treatment received after the index hemorrhage, we found that the composite risk of ischemic stroke and all-cause mortality was lower for patients who were prescribed warfarin (HR 0.76; 95% CI, 0.59-0.97) or dabigatran (HR 0.66; 95% CI, 0.44-0.99) than for those whose anticoagulation was discontinued after the major bleeding event. Furthermore, resumption of anticoagulation with warfarin (HR 0.35; 95% CI, 0.23-0.53) or with dabigatran (HR 0.13; 95% CI, 0.04-0.41) was associated with decreased mortality, compared to discontinuation of anticoagulation.
Recurrent Bleeding
There were no differences in the unadjusted risk of bleeding events among post-hemorrhage treatment groups in the dabigatran cohort (Table III at http://stroke.ahajournals.org): the cumulative incidence of major recurrent bleeding at 1 year was 0.07(95%CI, 0.02-0.11) for dabigatran users who resumed dabigatran, 0.09 (95%CI, 0.04-0.14) for those who discontinued anticoagulation, and 0.09(95%CI, 0.01-0.17) for those switching to warfarin. However, in the warfarin cohort, the unadjusted risk of recurrent major bleeding at 1 year was lower for patients who discontinued anticoagulation (0.10;95%CI, 0.07-0.13) than for those who restarted warfarin after the index hemorrhage (0.17;95%CI, 0.13-0.21). These unadjusted results were consistent with the findings of the adjusted analysis: The risks of major and any bleeding events were similar for 3 treatment groups in the dabigatran cohort (Figure 4). In the warfarin cohort, however, the risk of major bleeding was higher for patients resuming warfarin compared to those discontinuing all anticoagulation (HR 1.60; 95% CI, 1.09-2.36).
When the two cohorts were combined based on the treatment received after the index hemorrhage, we found that the risk of major hemorrhage was higher for patients who were prescribed warfarin than for those who were prescribed dabigatran or who discontinued anticoagulation therapy. Specifically, the hazard ratio of recurrent major bleeding was 0.42 (95% CI, 0.21-0.84) for dabigatran compared to warfarin, and 1.59 (95% CI, 1.10-2.22) for warfarin compared to anticoagulation discontinuation. The risk of bleeding did not differ between patients who were prescribed dabigatran after the index hemorrhage and those whose anticoagulation was discontinued (HR 0.66; 95% CI, 0.32-1.33).
Table IV at http://stroke.ahajournals.org shows the anatomical location of the recurrent bleeding events, stratified by the anatomical location of the index hemorrhage. The highest incidence of recurrent intracranial hemorrhage was for patients in the warfarin cohort who resumed warfarin (25%).
Sensitivity Analyses
Our results for the hazard ratios of post-hemorrhage clinical outcomes were robust to the exclusion of patients who experienced an intracranial bleeding event (Table V at http://stroke.ahajournals.org).
DISCUSSION
To the best of our knowledge, our study is the first real-world analysis comparing clinical outcomes after a major hemorrhage among patients who reinitiated anticoagulation therapy with dabigatran or warfarin, and those who never resumed anticoagulation. Our study has four main findings: First, we found that post-hemorrhage use of warfarin was more common than that of dabigatran in 2010-2012. Second, we observed that the CHA2DS2-VASc and HAS-BLED scores did not impact the likelihood of reinitiating anticoagulation after a major bleeding event. In contrast, age, anatomical location and severity of the index bleeding event were the most important determinants of resuming anticoagulation. Third, compared to discontinuation of all anticoagulation, resumption of anticoagulation therapy with either dabigatran or warfarin was associated with higher rates of survival and stroke-free survival. Fourth, the risk of recurrent major hemorrhage was higher for patients who were prescribed warfarin after a first major bleeding compared to those who were prescribed dabigatran or those whose anticoagulation was never reinitiated.
Our estimate for the hazard ratio of all-cause mortality for patients who reinitiated warfarin compared to those who discontinued anticoagulation (HR 0.35; 95% CI,0.23-0.54) is similar to the one reported by Staerk and collaborators (HR 0.39; 95% CI, 0.34-0.46).4 Regardless of the consistency of these findings, the association of anticoagulation resumption with increased survival may be subject to residual confounding, because patients who discontinued anticoagulation had higher burden of disease than those who resumed anticoagulation. In our analyses, we controlled for CHA2DS2-VASc and HAS-BLED scores; however, these prediction tools do not distinguish the severity of the risk factors included in their calculation. Furthermore, conditions other than the ones included in the calculation of CHA2DS2-VASc and HAS-BLED scores may have been unbalanced between patients who restarted anticoagulation and those who did not. Consequently, our results for the comparative risk of all-cause mortality between patients who reinitiated and those who discontinued anticoagulation should be interpreted with caution.
Study Implications
Our study contributes significantly to the existing literature because, as opposed to previous work, it stratified treatment groups into two cohorts according to the type of anticoagulation agent used after the index bleeding event. In doing so, we demonstrate the benefit of the use of anticoagulation therapy after a major bleeding event. More specifically, we found that the resumption of anticoagulation therapy after a major hemorrhage was associated with a lower incidence of stroke and all-cause mortality than anticoagulation discontinuation. In contrast, less than half of the patients who survived a major hemorrhage in 2010-2012 restarted anticoagulation; which likely represents prescribers’ aversion to the perceived high risk of recurrent hemorrhage. However, in our study, we found that the risk of recurrent major bleeding was lower than the risk of ischemic stroke for all treatment groups, and that the risk of recurrent major bleeding did not differ between treatment groups in the dabigatran cohort. These results should encourage clinicians to resume anticoagulation among patients who survived a major bleeding event. When comparing outcomes associated with the resumption of warfarin and dabigatran, we found that the benefit/risk ratio of post-hemorrhage dabigatran use is superior to that of warfarin because, with comparable effectiveness, dabigatran was associated with lower rates of recurrent bleeding. In contrast, we observed that the use of dabigatran was substantially less common than the use of warfarin among patients who survived a major bleeding event in 2010-2012. The lower tendency to prescribe dabigatran as compared to warfarin after a major hemorrhage in 2010-2012 may be explained by two reasons. First, whereas warfarin therapy requires routine INR monitoring, laboratory coagulation markers are not routinely monitored for patients on dabigatran. In this context, clinicians may be under the impression that they have more control over the coagulation status of patients on warfarin than those on dabigatran, particularly in the early aftermath of a major bleeding event. Second, clinicians may have been especially risk-averse to prescribe dabigatran during our study period because of the warnings on the risk of severe bleeding with dabigatran released by the main international regulatory agencies throughout 2011, as well as the lack of antidote to reverse the anticoagulation effects of dabigatran in the time period that this study captures. In this scenario, patients who were prescribed dabigatran after the index hemorrhage were likely to be those at lowest risk of recurrent bleeding. These risk-averse prescription patterns of dabigatran may have introduced residual confounding in our results for the comparative risk of bleeding events with warfarin and dabigatran. With the approval in October 2015 of idarucizumab, a dabigatran-binding monoclonal antibody fragment, prescribers may become more comfortable using dabigatran in patients who have already suffered a major bleeding event on anticoagulation.13 Therefore, it will be important to repeat analyses similar to ours as newer Medicare Part D data that represents the period after the approval of idarucizumab become available.
Study Limitations
In addition to the fact that our results reflect the early experience with dabigatran, our study is subject to three main limitations. First, claims data do not contain laboratory results and therefore, we did not have information about the INR levels of our study subjects, which may have affected the decision to restart anticoagulation therapy in patients who bled on warfarin. Second, we did not stratify our analyses by the anatomical location of the index bleeding event. The post-hemorrhage clinical outcomes of patients experiencing an intracranial bleeding, for example, are likely to be different from those who presented with a gastrointestinal bleeding. Third, we did not stratify by the dose of dabigatran used. Nevertheless, the use of dabigatran 75mg was relatively uncommon in the period that our study represents—less than 10% of Medicare beneficiaries with AF on dabigatran were prescribed dabigatran 75 mg in the first two years after dabigatran approval.14
Conclusions
In this observational study, the resumption of anticoagulation with either dabigatran or warfarin after a major bleeding event was associated with increased survival and stroke-free survival, compared to discontinuing anticoagulation. In addition, dabigatran was associated with lower risk of recurrent hemorrhage than warfarin. Our findings suggest that the benefit/risk ratio of dabigatran in the prevention of stroke among AF patients who have survived a major hemorrhage is superior to that of warfarin therapy or anticoagulation discontinuation, but will need to be validated in other patient cohorts and with more recent data.
Supplementary Material
Acknowledgements
We thank Andrew Lau for his help preparing the figures.
Sources of Funding
We acknowledge funding from Commonwealth Foundation and Agency for Healthcare Research and Quality (No. R01 HS018657), and from the National Institute of Mental Health (No. R21 MH100721). Hernandez has received a scholarship from “La Caixa” Foundation, Spain.
Footnotes
Disclosures
Dr. Saba reports having received research support from Boston Scientific, Medtronic Inc., and St. Jude Medical.
Contributor Information
Inmaculada Hernandez, Department of Pharmacy and Therapeutics, School of Pharmacy, University of Pittsburgh, PA USA.
Yuting Zhang, Department of Health Policy and Management, Graduate School of Public Health, University of Pittsburgh, PA, USA.
Maria M. Brooks, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, PA, USA.
Paul K.L. Chin, Department of Medicine, University of Otago, Christchurch, New Zealand.
Samir Saba, Heart and Valvular Institute, University of Pittsburgh Medical Centre PA, USA.
References
- 1.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 2.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (has-bled) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro heart survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi W, Mittal C, Patsias I, Garikapati K, Kuchipudi A, Cheema G, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol. 2014;113:662–668. doi: 10.1016/j.amjcard.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 4.Staerk L, Lip GY, Olesen JB, Fosbol EL, Pallisgaard JL, Bonde AN, et al. Stroke and recurrent haemorrhage associated with antithrombotic treatment after gastrointestinal bleeding in patients with atrial fibrillation: Nationwide cohort study. BMJ. 2015;16:h5876. doi: 10.1136/bmj.h5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengupta N, Feuerstein JD, Patwardhan VR, Tapper EB, Ketwaroo GA, Thaker AM, et al. The risks of thromboembolism vs. Recurrent gastrointestinal bleeding after interruption of systemic anticoagulation in hospitalized inpatients with gastrointestinal bleeding: A prospective study. Am J Gastroenterol. 2015;110:328–335. doi: 10.1038/ajg.2014.398. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen PB, Larsen TB, Skjoth F, Gorst-Rasmussen A, Rasmussen LH, Lip GY. Restarting anticoagulant treatment after intracranial haemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality and bleeding: A nationwide cohort study. Circulation. 2015;132:517–525. doi: 10.1161/CIRCULATIONAHA.115.015735. [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez I. Time to reconsider dabigatran 110 mg in the USA. Am J Cardiovasc Drugs. 2015;15:307–309. doi: 10.1007/s40256-015-0137-0. [DOI] [PubMed] [Google Scholar]
- 9.Center for Medicare and Medicaid Services Chronic Conditions Data Warehouse 27 chronic condition algorithm 2014. 2015 https://www.ccwdata.org/cs/groups/public/documents/document/ccw_condition_categories.pdf. Accessed February 22.
- 10.Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2015;175:18–24. doi: 10.1001/jamainternmed.2014.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thigpen JL, Dillon C, Forster KB, Henault L, Quinn EK, Tripodis Y, et al. Validity of international classification of disease codes to identify ischemic stroke and intracranial hemorrhage among individuals with associated diagnosis of atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2015;8:8–14. doi: 10.1161/CIRCOUTCOMES.113.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lichtman JH, Jones SB, Watanabe E, Allen NB, Wang Y, Howard VJ, et al. Elderly women have lower rates of stroke, cardiovascular events, and mortality after hospitalization for transient ischemic attack. Stroke. 2009;40:2116–2122. doi: 10.1161/STROKEAHA.108.543009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack CV, Jr., Reilly PA, Bernstein R, Dubiel R, Eikelboom J, Glund S, et al. Design and rationale for re-verse ad: A phase 3 study of idarucizumab, a specific reversal agent for dabigatran. Thromb Haemost. 2015;114:198–205. doi: 10.1160/TH15-03-0192. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez I, Zhang Y. Risk of bleeding with dabigatran in 2010-2011 medicare data. JAMA Intern Med. 2015;175:1245–1247. doi: 10.1001/jamainternmed.2015.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.