Abstract
Introduction
Prior studies on the cause and effect of surgical variation have been limited by utilization of administrative data. The Vascular Quality Initiative (VQI), a robust national clinical registry, provides anatomic and perioperative details allowing a more robust analysis of variation in surgical practice.
Methods
The VQI was used to identify all patients undergoing infrainguinal open bypass or endovascular intervention from 2009 to 2014. Asymptomatic patients were excluded. The 16 regional groups of the VQI were used to compare variation in patient selection, operative indication, technical approach, and process measures. χ2 analysis was used to assess for differences across regions where appropriate.
Results
A total of 52,373 interventions were included (31%). Of the 16145 bypasses, 5% were performed for asymptomatic disease, 26% for claudication, 56% for chronic limb threatening ischemia (CLI) (61% of these for tissue loss), and 13% for acute limb ischemia (ALI). Of the 35,338 endovascular procedures, 4% were for asymptomatic disease, 40% for claudication, 46% for CLI (73% tissue loss), and 12% for ALI. Potentially unwarranted variation included proportion of prosthetic conduit for infrapopliteal bypass in claudication (13–41%, median 29% P<.001), isolated tibial endovascular intervention for claudication (0.0–5.0%, median 3.0% P<.001), discharge on antiplatelet and statin (Bypass: 62–84%, P<.001; Endo: 63–89%, P<.001), and ultrasound guidance for percutaneous access (Claudication: range 7–60%, P<.001; CLI: 5–65%, P<.001). Notable areas needing further research with significant variation include proportion of CLI vs. claudication treated by bypass (38–71%, P < .001) and endovascular intervention (28- 63%, P < .001), and use of closure devices in percutaneous access (claudication 26–76%, P < .001; CLI 30–78%, P < .001).
Conclusion
Significant variation exists both in areas where evidence exists for best practice and therefore potentially unwarranted variation, and in areas of clinical ambiguity. Quality improvement efforts should be focused on reducing unwarranted variation. Further research should be directed at identifying best practice where no established guidelines and high variation exists.
Introduction
In the current healthcare climate there is an increasing emphasis on efficient resource utilization while simultaneously optimizing outcomes. Variation in surgical practice has been discussed in this context as both a reason for inefficient resource utilization and cause of suboptimal outcomes.[1] The Dartmouth Atlas project estimates that 20–30% of the nations healthcare spending adds no increased benefit to outcomes and is therefore unnecessary.[2] In an attempt to understand surgical variation Birkmeyer et al proposed five categories to explain variation in surgical care; patient demand/disease burden, physician beliefs on indication for a given procedure, patient preferences, regionalized practice patterns, and environmental factors (which include availability and advancements in technology, financial incentives, supply of surgeons, etc.).[1, 3] The Society for Vascular Surgery (SVS), in an attempt to reduce variation where strong evidence exists, has produced multiple consensus statements for the management of vascular disease.[4–7] The SVS created the Vascular Quality Initiative (VQI), with prospective collection of detailed clinical data on common vascular procedures with a goal of improving patient care. [8–10] One major opportunity for improving care is to initially determine and subsequently disseminate best practices for vascular surgical care and subsequent dissemination of information. The Trans-Atlantic Inter-Society Consensus (TASC) and the American Heart Association (AHA) have produced similar guidelines and consensus statements to reduce variation in care. [11, 12] The federal government, through the Affordable Care Act, established the Patient Centered Outcomes Research Institute (PCORI) to improve patient care through the identification of best practices, with respect to patient-centered outcomes, and has been given a budget of approximately $3.6 billion over 10 years.
The treatment of Peripheral Artery Disease (PAD) has undergone rapid expansion, in available procedures and interventions performed, with the incorporation of endovascular techniques.[13] The resulting variation in surgical care for PAD has been a topic of national concern, with a recent New York Times article citing the potential overuse of endovascular therapy for PAD in the Medicare population, and demonstrates the need to address variation in surgical practice for PAD.[14] The Society for Vascular Surgery has produced consensus statements for guidelines on appropriate treatment of PAD, but as prior work has demonstrated, this does not always result in adherence to such guidelines and the subsequent presumed reduction in variation of surgical practice.[1, 15]
The purpose of this study is to describe the regional variation that currently exists for the treatment of PAD with respect to patient selection, treatment, and process measures.
Methods
Dataset
We utilized the VQI database to identify all infrainguinal bypass procedures and endovascular interventions from participating hospitals across the United States, from 2009–2014. The VQI is a national clinical registry, set up as a collaboration between regional quality groups in an effort to improve patient care through the collection of clinical data, and includes 16 regions and close to 300 participating hospitals with 1300 physicians. More information about the VQI can be found at www.vascularqualityinitiative.org/. The Beth Israel Deaconess Medical Center Institutional Review Board approved this study and waived patient consent due to the de-identified nature of the data.
Cohorts and variables
All patients undergoing infrainguinal bypass or endovascular intervention below the infrainguinal ligament were selected from years 2009 to 2014. Patients were excluded if their symptom status was missing. Data were stratified by symptoms, intervention type, and region. Chronic limb threatening ischemia (CLI) was defined as rest pain or tissue loss and acute limb threatening ischemia (ALI) was considered separately. Medians reported are at the regional level to not be skewed by regions that contribute more cases.
Analysis and Figures
Forest Plots were used to demonstrate regional variability with each repeating symbol along a line representing an individual region and a perpendicular vertical line representing the VQI median. Bar charts were used to display the relationship of paired variables by region arranged from low to high for one of the paired variables. The data were stratified by symptom status, claudication versus chronic limb threatening ischemia, and type of intervention (bypass versus endovascular). All variables analyzed had < 5% missing data with the exception of skin prep (7% missing) and operative time (7%) for bypass and TASC class (12%) and fluoroscopy time (7%) for endovascular interventions. Variation between regions was assessed by χ2 analysis, with P-value < 0.05 considered statistically significant. All analyses were performed using SPSS Version 22.0 (IBM Inc., Chicago, IL).
Results
A total of 52,373 interventions were identified (31% open and 69% endovascular). For bypass, 2% of patients were excluded for missing documentation of symptom status. Of the remaining 16,145 bypasses, 5% were performed for asymptomatic PAD. Of these 54% were for aneurysmal disease, 17% for occlusive disease (of whom 31% had prior bypass), and 29% for unknown pathology (of whom 14% had prior bypass). Of the remaining patients, 26% for claudication, 56% for chronic limb threatening ischemia (61% of these for tissue loss), and 13% for acute limb ischemia. The range for regional procedure volume over the study period ranged widely from 124 to 5573 bypasses per region.
For endovascular procedures, 2% were excluded for having no recorded symptom status. Of the 35,338 remaining endovascular interventions, 4% were for no symptoms. Of these 19% were for aneurysmal disease, 58% for occlusive disease (of whom 35% had prior bypass), and 23% for unknown pathology (of whom 11% had prior bypass). Of the remaining patients, 40% for claudication, 44% for chronic CLI (73% of these for tissue loss), and 12% for acute limb ischemia. The range for regional procedure volume ranged from 412 to 7545.
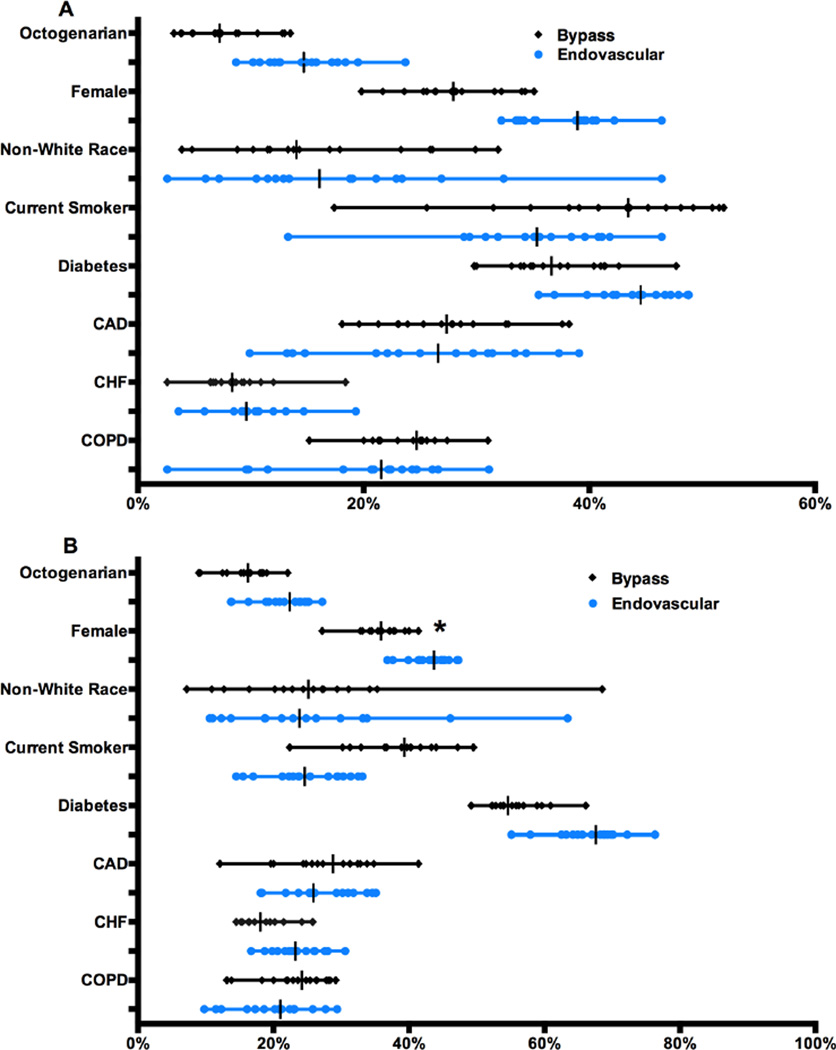
Figure 1A shows the baseline characteristics of patients with claudication who underwent bypass or endovascular revascularization and Figure 1B shows the same characteristics for those with CLI. For both bypass and endovascular interventions there was significant variation in the proportion of octogenarians being treated for claudication (Bypass: regional range 3.2–14%, P<.001; Endo: 8.7–24%, P<.001) and CLI (Bypass: 9.0–22%, P<.001; Endo: 14–27%, P<.001) and women being treated for claudication (Bypass: 20–35%, P<.001; Endo: 32–46%, P<.001) and for CLI in the endovascular group (38–47%, P<.001). There was variation in the proportion of females treated by bypass but this did not reach significance (Bypass: 27–41%, P = .23). There was also large variation in the proportion of non-white patients being treated regardless of symptom status across both bypass and endovascular interventions. Common co-morbidities in this population, such as diabetes mellitus, coronary artery disease, congestive heart failure, and chronic obstructive pulmonary disease also showed significant variation within each treatment type for patients with both claudication and CLI.
Figure 1. Patient characteristics for Claudication (A) and Critical Limb Ischemia (B) by intervention type.
Diabetes includes both insulin and non-insulin dependent, CAD=coronary artery disease, CHF=congestive heart failure, COPD=chronic obstructive pulmonary disease. All variation illustrated above was significant (to P < .001) except *. Each symbol on a line represents a region with a vertical line for the region-level median
There was significant variation in prior ipsilateral procedures in the bypass group for patients with claudication (inflow bypass 4–15%, P<.001; inflow percutaneous transluminal angioplasty (PTA)/stent 5–19%, P<.001; outflow bypass 4–20%, P<.001; outflow PTA/stent 17–33%, P<.001) and CLI (inflow bypass 2–10%, P<.001; inflow PTA/stent 10–20%, P<.001; outflow bypass 9–26%, P<.001; outflow PTA/stent 21–37%, P<.001).
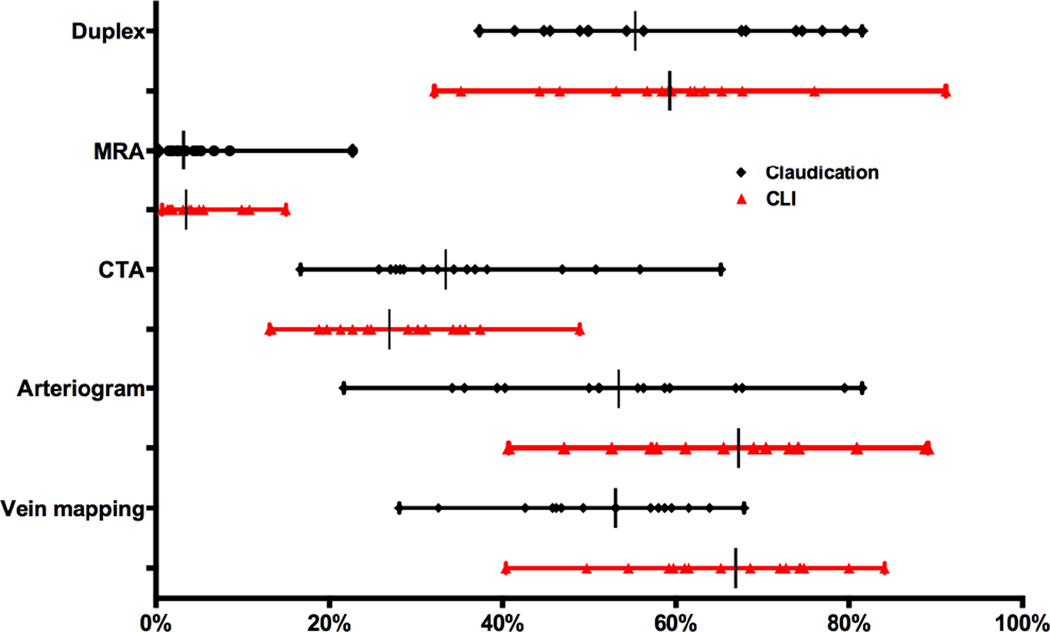
Figure 2 demonstrates significant variation across all preoperative imaging modalities and in particular for preoperative vein mapping (claudication 28–68%, P<.001; CLI 40–84%, P<.001).
Figure 2. Preoperative imaging in Bypass.
MRA = magnetic resonance angiogram, CTA = computed tomography angiography. All variation illustrated above was significant (P < .001). Emergent cases excluded from CLI.
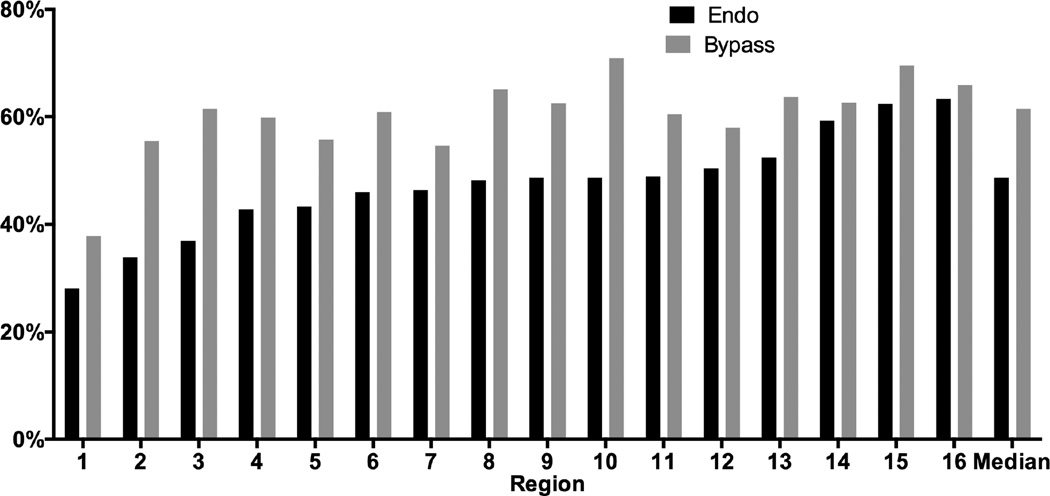
The proportion treated for CLI compared to claudication varied significantly across regions for both bypass (38–71%, P < .001) and endovascular interventions (28–63%, P < .001) (Figure 3). Among patients with CLI, tissue loss was more common than rest pain for both bypass and endovascular interventions (61% and 73% respectively), but was also significantly variable across regions (Bypass: 51–68%, P < .001; Endo: 65–84%, P < .001).
Figure 3. Proportion of CLI (vs. Claudication) for elective cases only by procedure type and region (de-identified).
There was also a wide range of patients undergoing treatment for acute limb ischemia (Bypass: 5–25%, P < .001; Endo: 3–23%, P < .001) and asymptomatic disease without lower extremity aneurysms (Bypass: 0.6–5%, P < .001; Endo: 2–10%, P<.001). There was wide variation in regional utilization of endovascular intervention (vs. bypass) to treat claudication, ranging from 68 to 90%, and CLI, ranging from 44 to 77%. There were also differences in proportion of non-elective cases presenting for CLI (Bypass: 6–52%, P < .001; Endo: 1–60%, P < .001).
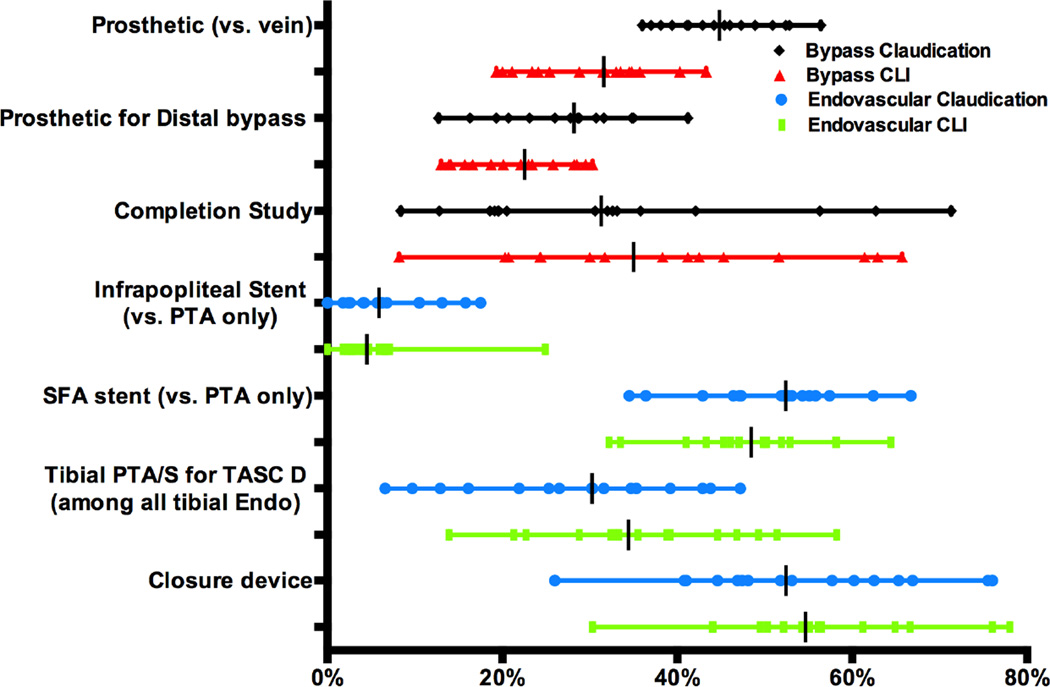
A large percentage of patients with claudication were treated with prosthetic conduits versus vein for bypass (36–56%, P<.001), with 14% of these patients undergoing re-do bypass. Similarly there was a high proportion treated with prosthetic conduit vs. vein for distal bypass, defined as below-knee popliteal or more distal target, in patients with claudication (13–41%, P<.001), 23% of whom were undergoing a redo bypass, and CLI (14–30%, P < .001) (Figure 4). During endovascular interventions infrapopliteal stents, versus angioplasty alone, were utilized at a significantly variable rate for patients with claudication (0–18%, P<.001) and CLI (0–25%, P<.001). There was also significant variation in proportion of TASC D lesions treated out of total tibial endovascular interventions (Claudication: 6.6–47%, P<.001; CLI 12–59%, P<.001) and utilization of closure devices after any percutaneous intervention (Claudication 26–76%, P < .001; CLI 30–78%, P < .001).
Figure 4. Treatment details.
Distal bypass defined as below-knee popliteal and more distal targets, Completion study defined as arteriogram or duplex, SFA = superficial femoral artery. All variation was significant (P < .001)
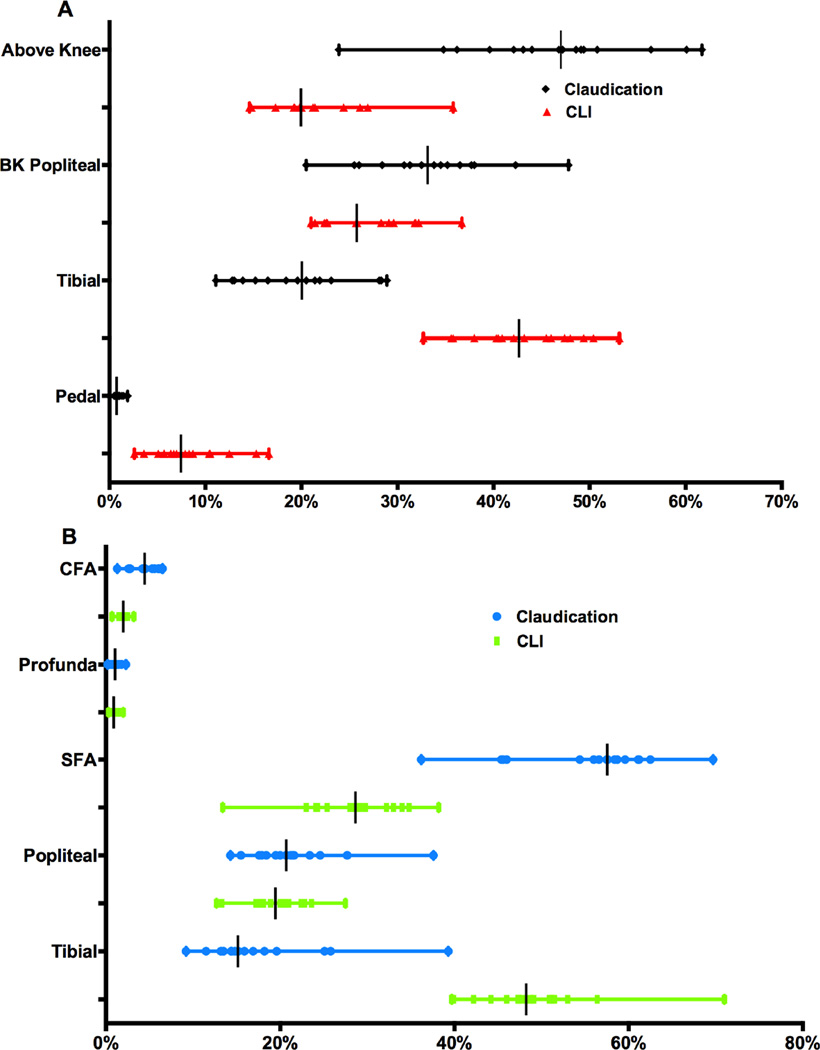
Figure 5A and 5B illustrate the distribution of distal targets for bypass and most distal vessel treated for endovascular interventions, respectively. There was significant variation in single-vessel (vs. multivessel) endovascular intervention across regions for patients with claudication (44–71%, P<.001) and CLI (33–57%, P<.001). The proportion of isolated tibial endovascular interventions, out of all endovascular interventions, for claudication was low but still significantly varied across regions (0–5%, P<.001)
Figure 5. Proportion of target vessels for bypass (A) and most distal vessel treated for Endovascular intervention (B).
Above knee includes CFA = common femoral artery, SFA = superficial femoral artery, Profunda, above-knee popliteal. BK = below-knee. Tibial defined as tibioperoneal trunk, anterior tibial, posterior tibial, and peroneal. Pedal = dorsalis pedal, posterior tibial at ankle level, and tarsal/plantar. Endovascular-popliteal intervention not stratified to above or below knee.
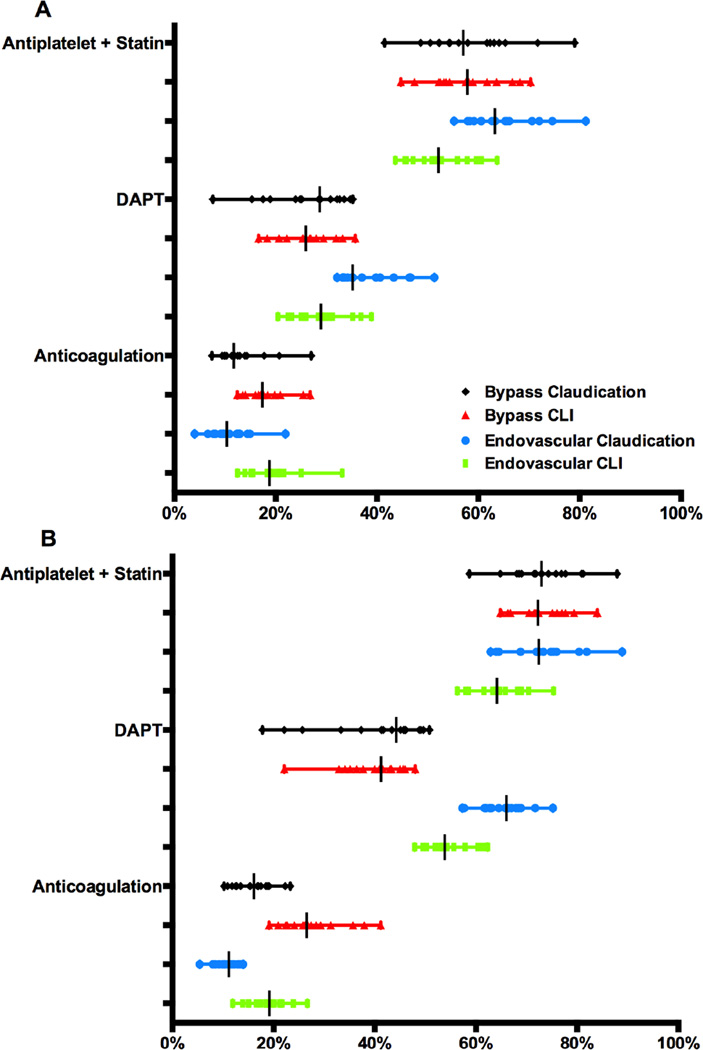
Figure 6A and 6B illustrate the proportions of patients on any antiplatelet agent and a statin, on dual antiplatelet therapy, and on anticoagulation, at admission and discharge. The discharge medication proportions excluded patients who were non-compliant or not able to take one of the medications for medical reasons. Although many regions met the Society for Vascular Surgery’s suggested goal of having 80% of postoperative vascular patients discharged on an antiplatelet and statin medication there was substantial variation and the median fell below 80%. Notably, no region met that standard in the endovascular-treated CLI cohort (56–75%, P<.001). There was significant variation amongst all stratified groups for patients discharged on dual antiplatelet therapy, which was more commonly prescribed after endovascular intervention.
Figure 6. Medications both pre-operatively (A) and at time of discharge (B) by intervention type and symptoms.
DAPT = dual antiplatelet theraby. Pre-operative anticoagulation only for years 2012–14 due to reporting of this variable. All variation was significant (P < .001).
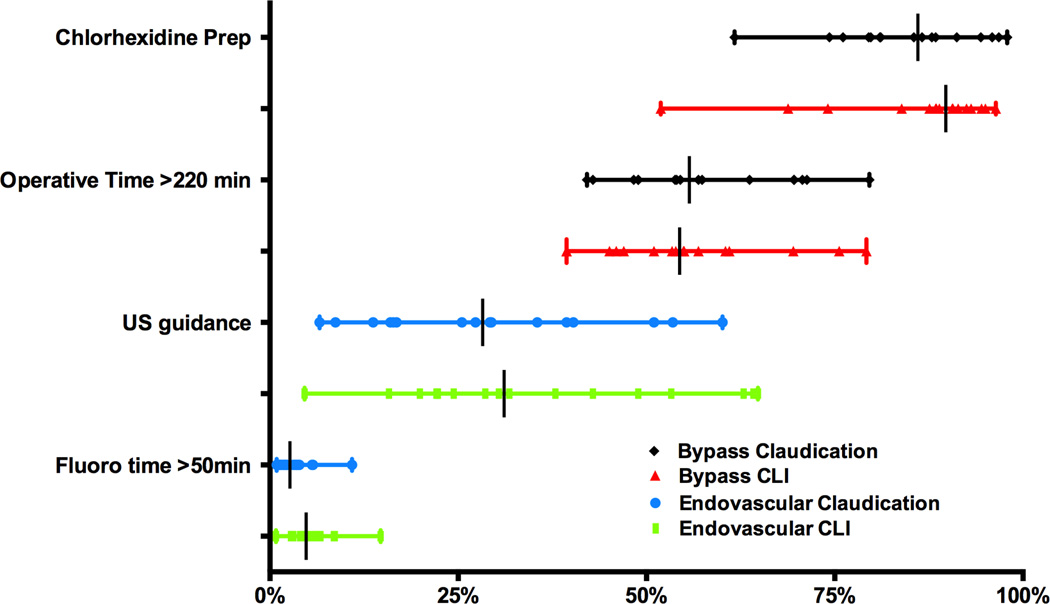
Several additional process measures with accumulating data warrant analysis (Figure 7). Chlorhexidine-based prep solutions were used in 86% of claudication (62–98%, P < .001) and 90% of CLI patients (52–96%, P < .001). Ultrasound guidance for percutaneous access showed considerable variation (Claudication: 7–60%, P<.001; CLI 5–64%, P<.001). Other potential quality metrics, with significant variation, include prolonged operative time (> 220 minutes) in bypasses for claudication and prolonged fluoroscopy time (> 50 minutes) during any endovascular intervention for PAD.
Figure 7. Process Measures for Bypass and Endovascular Intervention.
US guidance = Ultrasound guidance. All variation significant (P < .001).
Discussion
By illustrating the variation that exists in lower extremity vascular surgical care across the VQI, this descriptive study serves a two-fold purpose. First, it highlights the amount of variation in selection and treatment of this population, and second, it helps divide the data into potentially unwarranted variation and variation of unknown significance.
Currently, there are a number of clinical areas where clear guidelines exist, from both the Society for Vascular Surgery and the American Heart Association, and yet our study finds continued variation across regions (Table I).[7, 11, 12, 16] We suggest this variation is unwarranted and believe quality efforts should focus on education about and adherence to already established best practices. Practice patterns that have strong enough evidence to expect more uniform practice include antiplatelet and statin medications at discharge and use of chlorhexidine prep solutions for bypass.[17–19] Our analysis showed significant variation within these measures over the study period. It should be noted that much of the evidence supporting the above factors came after the initiation of our study period, and this could be leading to some of the variation we report. In addition, we do not know what percent of providers were aware of these guidelines. However, we know that historically there has been slow acceptance of guidelines and benchmarks set by professional societies and therefore we believe much of the variation within this group reflects the most current practice.[3, 20] Facilitators associated with improved uptake of guidelines have been identified in the process for creation of guideline content and how effectively and efficiently that content is communicated to its audience.[21] Creation of content begins with involvement of stakeholders, and also includes appropriate synthesis of evidence, clinical relevance, and implementation feasibility, including associated resources and costs.
Table I.
Classification of Endpoints
| Existing Recommendations | YearA |
|---|---|
| Antiplatelet and statin at time of Disharge for all Symptomatic PAD [7,11,12,17,18] | 2007 |
| Chlorhexidine Prep for all Bypass Operations [19] | 2014 |
| Limited to no angioplasty/stenting for isolated tibial disease in patients with claudication [7,12] | 2013 |
| Limited to no angioplasty/stenting of profunda femoris in patients with claudication [7,12] | 2013 |
| Avoidance of Bypass or Endovascular intervention for Asymptomatic PAD with no aneurysmal disease [7] | 2015 |
| Avoidance when possible of prosthetic conduit for below-knee bypass in patients with claudication [7,12] | 2013 |
| Mounting Evidence | |
| Routine use of ultrasound guidance for access in percuteneous interventions [21,22] | 2015 |
| High rate of vein mapping prior to first vascular intervention [7] | 2015 |
| Potential New Quality Measures | |
| Fluoroscopy time < 50 minutes (as surrogate for total radiation dose) | |
| Bypass operative time < 220 minutes for patients with claudication [32] | 2012 |
| Endpoints of Unknown Significance | |
| Optimal pre-operative imaging to evaluate severity of disease and intervention options | |
| Utilization of open vs. endovascular intervention for both claudication and CLI | |
| Optimal medical management (dual antiplatelet, anticoagulation, etc.) | |
Year represents earliest year of publication for a guideline or supporting evidence
Unwarranted variation also includes clinically relevant variation from zero for “almost never events” that should occur at a zero or exceedingly low rate. Examples of events that should be very low or never occur include, isolated tibial angioplasty or below knee stenting for claudication, and endovascular intervention of the profunda femoris artery for claudication.[7, 12, 16] Within this category it is also important to highlight the proportion of patients undergoing both bypass and endovascular intervention for asymptomatic PAD with no documented aneurysmal disease. The SVS guidelines have recommended against treatment of asymptomatic disease in general given its unclear benefit.[7, 12] From the data available we could not discern why exactly these asymptomatic patients underwent intervention and had no way of verifying the symptom status was accurate. The SVS guidelines have also recommended against intervention on isolated infrapopliteal artery lesions for claudication.[7]
Use of prosthetic conduit for below-knee bypass in patients with claudication should be minimized given its inferior long-term performance compared to vein, but our analysis showed significant variation with some regions reaching over 40% of such cases being performed.[7] Possible reasons for the persistence of such practice variation include reluctance to change practice pattern, other environmental influences on treatment strategy, or unique clinical presentations poorly captured in the registry database that do warrant such interventions.
We believe there is also an intermediate category of potentially unwarranted variation where data are mounting and regions may want to consider whether adequate data exist or if more should be collected and analyzed. For example, routine ultrasound use for percutaneous endovascular intervention has been shown to reduce access complications in both single-center and regional level studies and is now being analyzed at the national level.[22, 23]
It is difficult to identify unwarranted variation in areas of clinical ambiguity, where no consensus on best treatment exists; however wide variation suggests an opportunity for further evaluation and possible quality improvement. In terms of patient selection, there is evidence that disease burden varies by region but prior work focusing on regional variation has also found that regional differences in co-morbidities only account for a small proportion of variation in who gets surgery and who does not, and the decision to operate may be more related to where a patient lives rather than patient-level factors which makes the analysis of surgical practice patterns that much more imperative.[1, 24]
Related to a surgeons’ belief in appropriate threshold for intervention, pre-operative imaging behaviors can directly affect rates of intervention. This has been shown in the cardiac literature where rates of coronary intervention (both percutaneous coronary intervention and coronary artery bypass graft) were found to be directly proportional to rates of cardiac catheterization.[25] There have been no clear guidelines on the pre-operative imaging modality of choice, as discussed in the Society for Vascular Surgery and American Heart Association guidelines, and this lack of clarity was reflected in the variation seen in our analysis.[7, 12] We hypothesize that much of this variation is a result of hospital or provider preference. Furthermore, we cannot verify if all this imaging was done prior to making the decision to intervene, which may also affect choice of modality. However, identifying the best imaging modality (or sequence of imaging) for a particular disease process may have a large impact on patient care. Further, there is already general agreement that vein mapping should be performed for any patient being considered for bypass and the variation seen in our analysis suggests that this is not the case; however the VQI does not collect data on prior harvest or presence of AV fistula and may miss vein mapping performed by the surgeon outside of the vascular lab without official documentation of this testing.[7, 26]
In terms of procedure indication, it remains unclear what the appropriate proportion of claudication to chronic limb threatening ischemia patients should be for a given region, and whether this is even a value that can be established, however outlier regions in either direction from our study may be over or undertreating those with claudication. Reasons for the development of practice patterns that disproportionately treat claudication or CLI within the VQI are likely multifactorial and could include patient preference, provider belief in appropriate indication for an intervention, timing of referral, financial incentives, availability of vascular specialists, or teaching paradigm these specialists were trained under.[1, 3, 24] The VQI is incorporating the WiFI classification schema into the registry moving forward to help better define disease severity at time of intervention and may help better elucidate how much variation actually exists in terms of threshold for intervention.[27] Similarly it is unclear what is an appropriate proportion of octogenarians undergoing revascularization. Older age has been a strong risk factor for adverse events historically but more recent data, focusing on endovascular interventions, have pointed towards acceptable common endpoints in this population.[28–31]
Within the VQI there were a larger proportion of patients with CLI who underwent bypass compared to those with claudication, which was in line with the American Heart Association Practice Guidelines for peripheral artery disease that recommends surgical bypass in more extensive peripheral vascular disease.[12] The low rate of tibial/pedal bypass compared to endovascular interventions is concerning due to the questionable long-term durability of these endovascular interventions, although the ongoing BEST CLI randomized controlled trial is trying to conclusively answer this question of treatment choice.[11] Recent data from the Board of Vascular Surgery suggest that many graduating vascular fellows are not adequately trained in performance of tibial or pedal bypass, which could serve to exacerbate this pattern.[32]
In addition to the more established process measures discussed above, we propose additional quality metrics, including prolonged operative and fluoroscopy time (as a surrogate for total fluoroscopy dose) for bypass and endoscopic interventions respectively. Operative time greater than 220 minutes in bypass for claudication warrants consideration due to its demonstrated increase in surgical site infection rates and hospital length of stay.[33] Similarly, increasing radiation doses, which fluoroscopy time can be utilized as an indirect measure for, has been associated with higher risk of radiation-induced skin damage.[34] However, we recognize that measures of total radiation dose, and not fluoroscopy time, would be optimal and therefore we suggest that tracking of this metric should be incorporated into clinical registries.
Medical management of peripheral artery disease is another area of potential quality improvement. There has been recent evidence that dual antiplatelet therapy in patients undergoing lower extremity revascularization may improve short-term patency and have long-term benefits, when compared to monotherapy, and our data showed there was use of dual antiplatelet therapy, although additional cardiac indications for such a regimen also exist and were not controlled for in our analysis.[35]
The VQI, with its participating regional quality groups, has a structure to help identify best practice, though identifying high performers in established quality metrics, and an administrative body in place to disseminate information and promote adherence to guidelines. The VQI has adopted a reporting system comparing “your hospital” to peers in the region as well as nation, in addition to risk-adjusted outcomes to help hospitals compare themselves. We believe this internally driven, self-monitored approach has high buy-in from participating centers and early efforts have shown it to be effective in changing practice, as reported for bovine patch use and protamine administration in the Vascular Study Group of New England.[36, 37] Future analyses focused on adherence rates to new guidelines will need to factor in the confounding effect of the addition of new hospitals to the VQI.
This study has several limitations. Most notably, it is a retrospective analysis of a prospectively collected database and has potential issues of miscoding and misreporting. However, the VQI conducts annual audits to ensure consecutive procedure entry and to eliminate any inconsistencies between claims data and clinical data entered by each hospital. The centers and regions are also de-identified and therefore have no benefit from misreporting. As VQI is a growing registry some regions low volumes for particular procedures may be more a reflection of fewer participating hospitals and not reflect true volume in the region. Statistical testing was used to assess if the variation across all regions differed significantly, and does not actually identify those particular regions that lead to the significant difference. Additionally our data are unadjusted and furthermore do not account for the geographic reasons for variation based on disease burden. Also, in subset analyses we lose significance for variation in certain variables, despite a wide range, due to limited sample size, and thus there may still be room for quality improvement projects even in areas where variation was not significantly different. However, the purpose of this analysis is to be descriptive and not to make assumptions about quality of care in a particular region based on our data alone. Additionally, the lack of adjustment allows for comparison with SVS guidelines, which also do not adjust for patient demographics. Another important limitation is that patients managed non-operatively for PAD are not currently represented in the VQI, and additionally the VQI includes only a subset of hospitals providing vascular care and may not be fully representative of national practice patterns. Related to this, we cannot comment on the intensity of medical therapy and exercise regimens, especially for patients without symptoms or claudication, to fully capture the practice variation for PAD. Finally, hypotheses can be drawn about the reasons for variation but without feedback from the providers and patients themselves it is difficult to do more than speculate on the reasons for variation in practice. This analysis of variation is a step towards identifying opportunities for improving care, which will become increasingly important to address as we move toward basing reimbursement on performance. Further analyses are necessary before we can reliably comment on what is appropriate or not beyond what has already been stated through the respective societal guidelines. It will also be important to identify which of these areas of variation lead to worse risk-adjusted outcomes to determine priority targets for quality improvement. This is beyond the scope of our current analysis but will be addressed in subsequent reports.
Conclusion
Within this national clinical dataset there is wide variation in areas of known best practice, so called unwarranted variation, and even more variation in areas of clinical ambiguity, where further research is warranted, for the treatment of peripheral artery disease.
Acknowledgments
Supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Poster Presentation at the Society for Vascular Surgery Annual Meeting 2014, June 5–7th, Boston MA
References
- 1.Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121–1129. doi: 10.1016/S0140-6736(13)61215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dartmouth Atlas: overtreatment still common. Capitation Rates Data. 2008;13(8):8–10. 12. [PubMed] [Google Scholar]
- 3.McCulloch P, Nagendran M, Campbell WB, Price A, Jani A, Birkmeyer JD, et al. Strategies to reduce variation in the use of surgery. Lancet. 2013;382(9898):1130–1139. doi: 10.1016/S0140-6736(13)61216-7. [DOI] [PubMed] [Google Scholar]
- 4.Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(2 Suppl):3S–21S. doi: 10.1016/j.jvs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Hobson RW, 2nd, Mackey WC, Ascher E, Murad MH, Calligaro KD, Comerota AJ, et al. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48(2):480–486. doi: 10.1016/j.jvs.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 6.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50(4 Suppl):S2–S49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Society for Vascular Surgery Lower Extremity Guidelines Writing G. Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61(3 Suppl):2S–41S. doi: 10.1016/j.jvs.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Cronenwett JL, Kraiss LW, Cambria RP. The Society for Vascular Surgery Vascular Quality Initiative. J Vasc Surg. 2012;55(5):1529–1537. doi: 10.1016/j.jvs.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Woo K, Eldrup-Jorgensen J, Hallett JW, Davies MG, Beck A, Upchurch GR, Jr, et al. Regional quality groups in the Society for Vascular Surgery(R) Vascular Quality Initiative. J Vasc Surg. 2013;57(3):884–890. doi: 10.1016/j.jvs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Murad MH, Montori VM, Sidawy AN, Ascher E, Meissner MH, Chaikof EL, et al. Guideline methodology of the Society for Vascular Surgery including the experience with the GRADE framework. J Vasc Surg. 2011;53(5):1375–1380. doi: 10.1016/j.jvs.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(13):1425–1443. doi: 10.1161/CIR.0b013e31828b82aa. [DOI] [PubMed] [Google Scholar]
- 13.Schanzer A, Steppacher R, Eslami M, Arous E, Messina L, Belkin M. Vascular surgery training trends from 2001–2007: A substantial increase in total procedure volume is driven by escalating endovascular procedure volume and stable open procedure volume. J Vasc Surg. 2009;49(5):1339–1344. doi: 10.1016/j.jvs.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Abelson JCaR. Medicare Payments Surge for Stents to Unblock Blood Vessels in Limbs. New York Times. 2015 [Google Scholar]
- 15.Birkmeyer JD, Dimick JB. Understanding and reducing variation in surgical mortality. Annu Rev Med. 2009;60:405–415. doi: 10.1146/annurev.med.60.062107.101214. [DOI] [PubMed] [Google Scholar]
- 16.Conte MS, Pomposelli FB. Society for Vascular Surgery Practice guidelines for atherosclerotic occlusive disease of the lower extremities management of asymptomatic disease and claudication. Introduction. J Vasc Surg. 2015;61(3 Suppl):1S. doi: 10.1016/j.jvs.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 17.De Martino RR, Eldrup-Jorgensen J, Nolan BW, Stone DH, Adams J, Bertges DJ, et al. Perioperative management with antiplatelet and statin medication is associated with reduced mortality following vascular surgery. J Vasc Surg. 2014;59(6):1615–1621. 1621 e1. doi: 10.1016/j.jvs.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Martino RR, Hoel AW, Beck AW, Eldrup-Jorgensen J, Hallett JW, Upchurch GR, et al. Participation in the Vascular Quality Initiative is associated with improved perioperative medication use, which is associated with longer patient survival. J Vasc Surg. 2015;61(4):1010–1019. doi: 10.1016/j.jvs.2014.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalish JA, Farber A, Homa K, Trinidad M, Beck A, Davies MG, et al. Factors associated with surgical site infection after lower extremity bypass in the Society for Vascular Surgery (SVS) Vascular Quality Initiative (VQI) J Vasc Surg. 2014;60(5):1238–1246. doi: 10.1016/j.jvs.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Rudarakanchana N, Halliday AW, Kamugasha D, Grant R, Waton S, Horrocks M, et al. Current practice of carotid endarterectomy in the UK. Br J Surg. 2012;99(2):209–216. doi: 10.1002/bjs.7810. [DOI] [PubMed] [Google Scholar]
- 21.Kastner M, Bhattacharyya O, Hayden L, Makarski J, Estey E, Durocher L, et al. Guideline uptake is influenced by six implementability domains for creating and communicating guidelines: a realist review. J Clin Epidemiol. 2015;68(5):498–509. doi: 10.1016/j.jclinepi.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Lo RC, Fokkema MT, Curran T, Darling J, Hamdan AD, Wyers M, et al. Routine use of ultrasound-guided access reduces access site-related complications after lower extremity percutaneous revascularization. J Vasc Surg. 2015;61(2):405–412. doi: 10.1016/j.jvs.2014.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalish J, Eslami M, Gillespie D, Schermerhorn M, Rybin D, Doros G, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. 2015;61(5):1231–1238. doi: 10.1016/j.jvs.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Cronenwett JL, Birkmeyer JD. The Dartmouth Atlas of Vascular Health Care. Cardiovasc Surg. 2000;8(6):409–410. [PubMed] [Google Scholar]
- 25.Lucas FL, Siewers AE, Malenka DJ, Wennberg DE. Diagnostic-therapeutic cascade revisited: coronary angiography, coronary artery bypass graft surgery, and percutaneous coronary intervention in the modern era. Circulation. 2008;118(25):2797–2802. doi: 10.1161/CIRCULATIONAHA.108.789446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeger JM, Schmidt JH, Flynn TC. Preoperative saphenous and cephalic vein mapping as an adjunct to reconstructive arterial surgery. Ann Surg. 1987;205(6):733–739. doi: 10.1097/00000658-198706000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills JL, Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59(1):220–234. e1–e2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50(6):1462–1473. e1–e3. doi: 10.1016/j.jvs.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 29.Brosi P, Dick F, Do DD, Schmidli J, Baumgartner I, Diehm N. Revascularization for chronic critical lower limb ischemia in octogenarians is worthwhile. J Vasc Surg. 2007;46(6):1198–1207. doi: 10.1016/j.jvs.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 30.Dosluoglu HH, Lall P, Cherr GS, Harris LM, Dryjski ML. Superior limb salvage with endovascular therapy in octogenarians with critical limb ischemia. J Vasc Surg. 2009;50(2):305–315. 316 e1–362 e2. doi: 10.1016/j.jvs.2009.01.004. discussion 315-6. [DOI] [PubMed] [Google Scholar]
- 31.Jones DW, Siracuse JJ, Graham A, Connolly PH, Sedrakyan A, Schneider DB, et al. Safety and effectiveness of endovascular therapy for claudication in octogenarians. Ann Vasc Surg. 2015;29(1):34–41. doi: 10.1016/j.avsg.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Nandivada P, Lagisetty KH, Giles K, Pomposelli FB, Chaikof EL, Schermerhorn ML, et al. The impact of endovascular procedures on fellowship training in lower extremity revascularization. J Vasc Surg. 2012;55(6):1814–1820. doi: 10.1016/j.jvs.2012.01.082. [DOI] [PubMed] [Google Scholar]
- 33.Tan TW, Kalish JA, Hamburg NM, Rybin D, Doros G, Eberhardt RT, et al. Shorter duration of femoral-popliteal bypass is associated with decreased surgical site infection and shorter hospital length of stay. J Am Coll Surg. 2012;215(4):512–518. doi: 10.1016/j.jamcollsurg.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(1 Suppl):15S–21S. doi: 10.1016/j.jvs.2010.06.175. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong EJ, Anderson DR, Yeo KK, Singh GD, Bang H, Amsterdam EA, et al. Association of dual-antiplatelet therapy with reduced major adverse cardiovascular events in patients with symptomatic peripheral arterial disease. J Vasc Surg. 2015;62(1):157–165 e1. doi: 10.1016/j.jvs.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 36.Patel RB, Beaulieu P, Homa K, Goodney PP, Stanley AC, Cronenwett JL, et al. Shared quality data are associated with increased protamine use and reduced bleeding complications after carotid endarterectomy in the Vascular Study Group of New England. J Vasc Surg. 2013;58(6):1518–1524 e1. doi: 10.1016/j.jvs.2013.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodney PP, Nolan BW, Eldrup-Jorgensen J, Likosky DS, Cronenwett JL Vascular Study Group of Northern New E. Restenosis after carotid endarterectomy in a multicenter regional registry. J Vasc Surg. 2010;52(4):897–904. 905 e1–905 e2. doi: 10.1016/j.jvs.2010.05.005. discussion 904-5. [DOI] [PubMed] [Google Scholar]