Abstract
Previous studies show that mice with Ywhae deficiency show abnormalities in brain development including defects in neuronal migration of post-mitotic pyramidal neurons as well as neuronal differentiation and proliferation in neuronal progenitor cells. Also, our previous research indicated that the Ywhae knockout mice show moderate defects in working memory and anxiety-like behavior. This previous work was performed using heterozygous mutant mice. Here we performed behavioral analyses using homozygous Ywhae knockout mice and found that the homozygous Ywhae knockout mice have increased locomotor activity, decreased working memory, and increased sociability. Taken together with the results obtained from the previous pathophysiological analyses in the Ywhae knockout mice, the Ywhae knockout mouse is useful for pathophysiological analyses of neuropsychiatric disorders caused by defects during neurodevelopment.
Keywords: Neurobehavior, 14-3-3, locomotor activity, memory, sociability
The family of 14-3-3 proteins was first identified as abundant proteins in the brain (approximately 1% of total brain protein) [1].There are seven 14-3-3 isoforms in mammals with high homology among them [2]. The 14-3-3 proteins bind to phosphorylated serine or threonine motifs on their binding partners to function inside cells [3]. 14-3-3 proteins are multifunctional proteins involved in a wide variety of cellular events such as cell cycle regulation, cell proliferation, and membrane trafficking [4]. These observations have mainly been obtained from in vitro studies using primary cells and cell lines. To date, mice independently deficient for four out of the seven 14-3-3 isoforms have been generated, including mice deficient for Ywhas (14-3-3sigma), Ywhag (14-3-3gamma), Ywhaz (14-3-3zeta) or Ywhae (14-3-3epsilon). The Ywhas deficient mice were originally identified as mice with a genetic epidermal syndrome with repeated epilation [5]. However, the functions of 14-3-3sigma, also known as Stratifin (Sfn), in neurons and in the brain are poorly understood. The adult Ywhag deficient mice appear normal and show no significant defects in brain morphology [6]. However, we recently found that the knockdown of 14-3-3gamma protein in migrating post-mitotic neurons in the cerebral cortex resulted in neuronal migration delay and morphological abnormality in pyramidal neurons [7]. Also, there is evidence to suggest that the Ywhag gene is associated with neurobehavioral disorders [8, 9]. We previously found that the Ywhaz knockout mice show behavioral abnormalities related to schizophrenia [10]. Also, we found that the heterozygous Ywhae knockout mice (+/KO) show a variety of behavioral defects including increased motor activity and decreased working memory. These defects were moderate because of the use of the Ywhae heterozygous knockout mice (+/KO) [11]. Thus, 14-3-3 proteins are important for a wide range of cellular phenomena as well as neurobehavior. Since we previously used heterozygous Ywhae deficient mice (+/KO) to test behavioral abnormalities, here we collected homozygous Ywhae deficient mice (KO/KO) and test if they show more severe defects in working memory and locomotor activity as well as in other defects in neuropsychiatric behaviors.
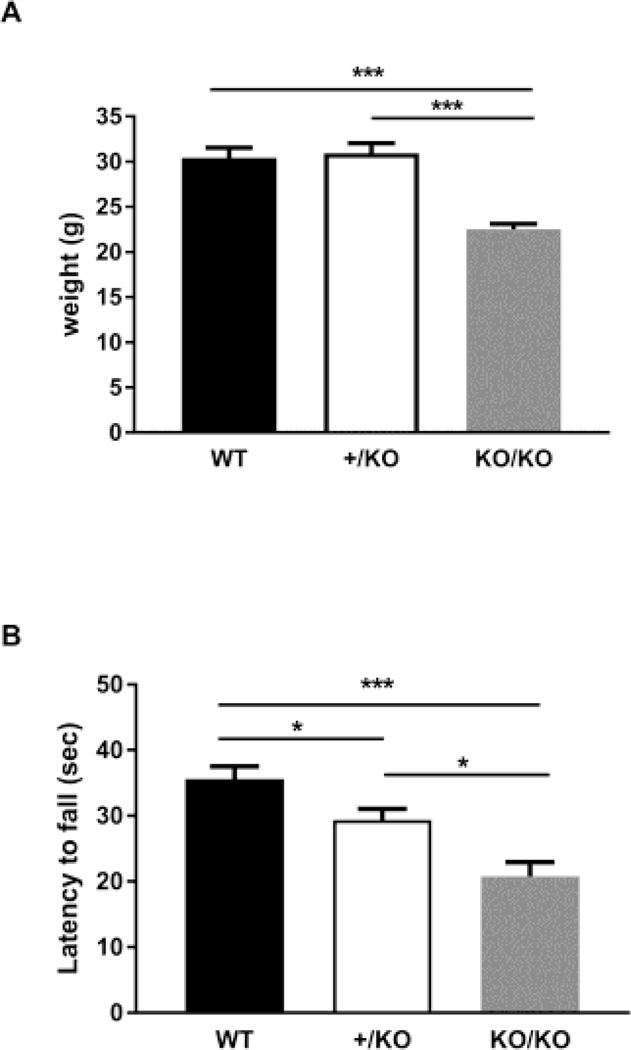
Genetic background can largely affect results in behavioral tests. Therefore, we backcrossed the Ywhae knockout mice with two genetic backgrounds, 129SVE and C57BL/6, which are commonly used for producing knockout/transgenic mice as well as for use in behavioral tests. However, we have not been able to obtain Ywhae homozygous deficient mice (KO/KO) with an inbred genetic background. Therefore, we decided to use the Ywhae homozygous deficient mice (KO/KO) with mixed genetic background. Even with the mixed genetic background, almost all of the Ywhae homozygous knockout mice (KO/KO) die soon after birth [12]. However, with difficulty, we obtained 13 Ywhae homozygous deficient adult male mice and 7 female mice (age 3–4 months) from 77 litters. We used only male mice for the experiments in this study. These mice displayed lower body weight than heterozygous and wild-type mice (WT, 30.40g ± 1.133, n=15: +/KO, 30.93g ± 1.106, n=15: KO/KO, 22.50g ± 0.6268, n=8, One-way ANOVA, F(2, 35)=13.52, p=4.5 × 10−5, WT vs +/KO: p>0.99, WT vs KO/KO: p=0.00062, +/KO vs KO/KO: p=0.00012, Figure 1A). As seen in heterozygotes in our previous study, the homozygotes (KO/KO) showed weaker motor activity than the WT mice (+/+) revealed by cage top hang test, and to the same extent less than the heterozygotes (+/KO) (WT, 35.60s ± 1.932, n=15: heterozygote, 29.33s ± 1.715, n=15: KO/KO, 20.75s ± 2.169, n=8, One-way ANOVA, F(2, 35)=12.21, p=9.5 × 10−5, WT vs +/KO: p=0.045, WT vs KO/KO: p=0.00023, +/KO vs KO/KO: p=0.022, Figure 1B).
Figure 1.
General assessment of the Ywhae knockout mice. (A) The homozygous Ywhae knockout mice (KO/KO) are smaller than the control wild-type mice (+/+). The weight of wild-type (+/+), heterozygotes (+/KO) and homozygotes (KO/KO) was measured at 3-months of age. (B) Cage top hang test revealed motor defects in the Ywhae deficient mice. Significance was analyzed by one-way ANOVA with Bonferroni’s post-hoc test. * p<0.05 and *** P<0.001.
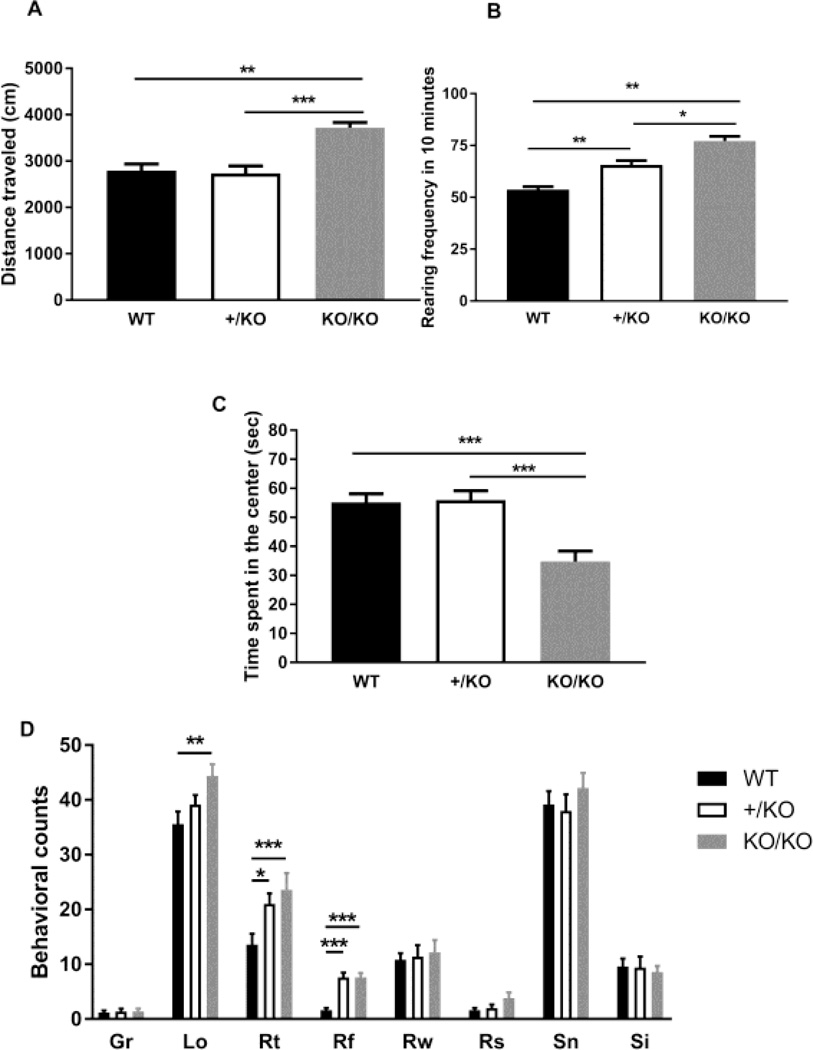
Our previous study using heterozygotes (+/KO) and unpublished observations on home cage activity indicated that deficiency of the 14-3-3epsilon protein results in hyperactivity [11]. Therefore, we performed an open field test using homozygotes (KO/KO) (Figure 2). The test mouse was placed in the corner of the open field arena (41×41×41cm) and allowed to freely move for 10 minutes. Mouse behavior was recorded by CCD camera and analyzed by ImageOF plugin for ImageJ (available from the Mouse Phenotype Database, http://www.mouse-phenotype.org/) [13]. Total travel distance was calculated. Total travel distance was significantly increased in homozygotes (KO/KO) compared to the heterozygotes (+/KO) and the control wild-type mice (+/+) (WT, 2,794cm ± 140.8, n=15: heterozygote, 2,735cm ± 157.2, n=15: KO/KO, 3,718cm ± 114.5, n=8, One-way ANOVA, F(2, 35)=9.990, p=0.00041, WT vs +/KO: p>0.99, WT vs KO/KO: p=0.0012, +/KO vs KO/KO: p=0.00056, Figure 2A). Also, vertical activity was calculated as the number of rearing events when the mice would stand on their hind feet. Vertical activity was increased both in heterozygotes (+/KO) and homozygotes (KO/KO) compared to the control mice (+/+) (WT, 53.67s ± 1.427, n=15: +/KO, 65.60s ± 2.095, n=15: KO/KO, 77.13s ± 2.263, n=8, One-way ANOVA, F(2, 35)=32.10, p=1.2 × 10−5, WT vs +/KO: p=0.0098, WT vs KO/KO: p=0.0015, +/KO vs KO/KO: p=0.014, Figure 2B). To assess their anxiety-like behavior, time spent in the center area (20×20cm) was measured. Homozygotes (KO/KO) spent less time in the center area than the control wild-type mice (WT, +/+) (WT (+/+), 55.13s ± 2.976, n=15: heterozygote (+/KO), 55.93s ± 3.209, n=15: homozygote (KO/KO), 34.75s ± 3.644, n=8, One-way ANOVA, F(2, 35)=11.01, p=0.00036, WT vs KO/KO: p=0.00097, +/KO vs KO/KO: p=0.00061, Figure 2C). However, we have not seen a significant difference between heterozygotes (+/KO) and the control mice (+/+) (WT vs +/KO: p>0.99). Although we found that the 14-3-3epsilon deficiency causes an increase in travel distance and rearing and a decrease in time spent in the center in the open field test, it is possible that these observations reflect that mice try to escape from the equipment or stereotyped/perseverative behaviors resulting in mice lapping the open field. Therefore, we adopted the ethological based assessment using a rapid time-sampling behavioral checklist technique, as described previously [14–17]. Mice were individually observed for 5-seconds at 1-minute intervals over 15 consecutive minutes, and this cycle (0–15 min) was repeated twice (20–35 and 40–55 min) over a total exploratory period of 60 minutes. The ethologically-based behavioral checklist covering the following behaviors was used: grooming (Gr, grooming of any form), locomotion (Lo, coordinated movement of all four limbs resulting in location change), total rearing (Rt, rearing of any form), rearing free (Rf, front paws reaching upwards away from the wall while standing), rearing to wall (Rw, front paws reaching upward onto a cage wall), rearing seated (Rs, front paws reaching upward with hind limbs on floor in sitting position), sniffing (Sn, flaring of nostrils with movements of vibrissae), and sifting (Si, characteristic sifting movements of the front paws through bedding material on the cage floor) (Figure 2D). We found increased locomotion in homozygotes (KO/KO) (WT(+/+), 35.6 ± 2.293, n=5: +/KO, 39.2 ± 1.685, n=5: KO/KO, 44.4 ± 2.112, n=5, One-way ANOVA, F(2, 12)=4.675, p=0.032, WT vs +/KO: p=0.71, WT vs KO/KO: p=0.0056, +/KO vs KO/KO: p=0.29). Also, we found that the heterozygotes (+/KO) and homozygotes (KO/KO) showed an increase in total rearing (WT (+/+), 13.6 ± 1.965, n=5: +/KO, 21.0 ± 1.924, n=5: KO/KO, 23.6 ± 3.059, n=5, One-way ANOVA, F(2, 12)=4.773, p=0.030, WT vs +/KO: p=0.024, WT vs KO/KO: p=0.00092, +/KO vs KO/KO: p>0.99, and rearing free (WT (+/+), 1.6 ± 0.400, n=5: +/KO, 7.6 ± 0.872, n=5: KO/KO, 7.6 ± 0.812, n=5, One-way ANOVA, F(2, 12)=22.78, p=8.2 × 10−5, WT vs +/KO: p=0.00024, WT vs KO/KO: p=0.00024, +/KO vs KO/KO: p>0.99, Figure 2D), but not rearing to wall (F(2, 12)=0.131, p=0.87) and rearing seated (F(2, 12)=2.42, p=0.13), suggesting that the increase rearing in the heterozygotes (+/KO) and homozygotes (KO/KO) are not the result of perseverative behaviors. These data strongly indicate that the deficiency in the 14-3-3epsilon protein causes hyperactivity. Also, these data suggest that it is unlikely that 14-3-3epsilon deficiency results in enhanced anxiety-like behaviors, however futher experiments are required.
Figure 2.
The Ywhae deficiency resulted in hyperactivity. Open field test revealed increased locomotor activity in the Ywhae heterozygous and homozygous knockout mice. Total travel distance (A) and vertical activity (B) were calculated. (C) Increased time spent in the center area in the homozygous Ywhae knockout mice (KO/KO). (D) Ethological assessment of spontaneous behaviors shows an increase in locomotion and a decrease in total rearing and realing free in Ywhae deficient mice. Significance was analyzed by one-way ANOVA with Bonferroni’s post-hoc test. * p<0.05, ** P<0.01 and *** P<0.001.
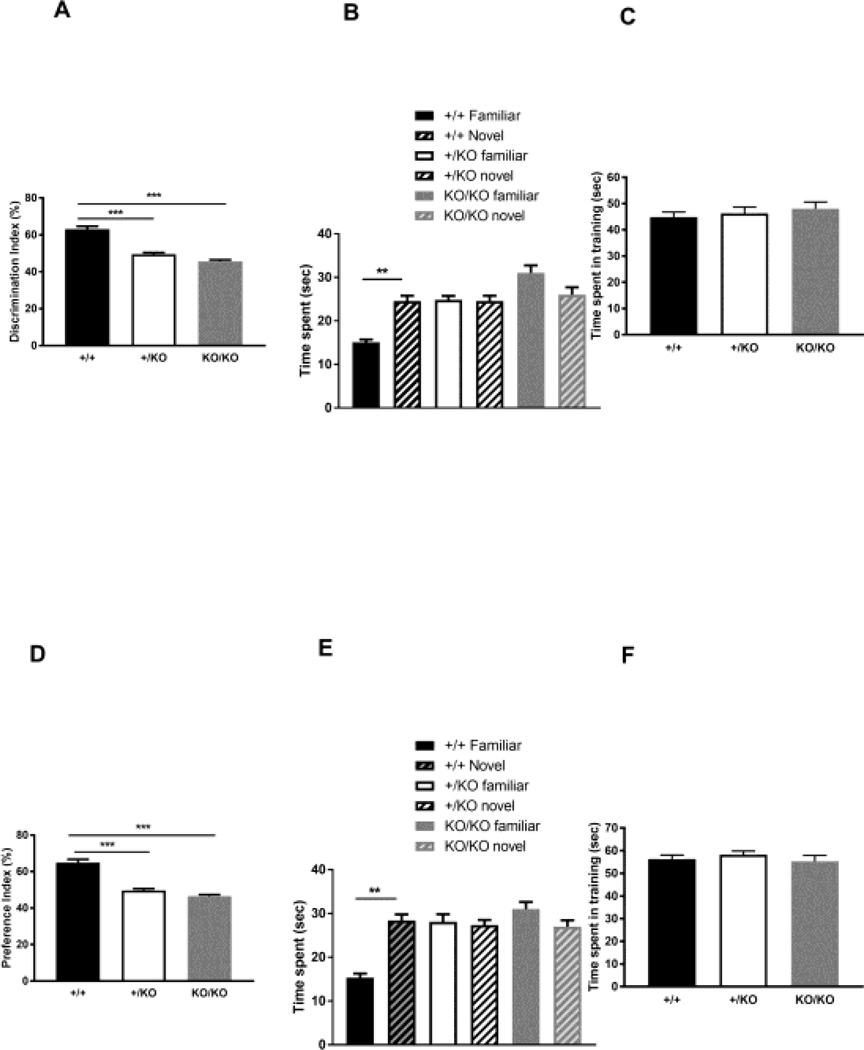
Next, since our previous histological and behavioral analyses indicated abnormal morphology in the hippocampus [12] and working memory defects [11], we tested if the Ywhae homozygous deficient mice (K/KO) show more severe defects in cognition using a visual recognition memory test (object recognition) and a spatial memory test (object placement). The tests were performed using the same arena (41×41×41cm) used for the open field test, and it was separated into nine areas (3 columns × 3 rows), and an object was placed in the center of the area. Before performing the test, each mouse was habituated to the arena for 10 minutes and allowed to freely explore. Then, two identical objects were placed in two out of the nine areas, and each animal was allowed to explore the objects for 10 minutes. Three hours later, we performed recording. For the visual memory test (object recognition, Figure 3 A–C), one object was replaced with a novel object with a different shape and color in the same location, and each mouse was allowed to explore for 10 minutes. We calculated the time spent with an object by measuring the period of time either sniffing or touching the object. There was no difference between genotypes in time spent with an object in the training sessions for the visual memory test and the spatial memory test (Figure 3C and F). Results were calculated and expressed as percentage of novel object exploration time (Discrimination Index) using the following formula: Discrimination Index = 100×[exploration duration of novel object/(exploration duration of novel object + exploration duration of familiar)] (WT (+/+), 63.11% ± 1.501, n=15: heterozygote (+/KO), 49.53% ± 0.8182, n=15: homozygote (KO/KO), 45.54% ± 0.9191, n=8, One-way ANOVA, F(2, 35)=56.03, p=1.2 × 10−6, WT vs +/KO: p=0.00014, WT vs KO/KO: p=0.00014, +/KO vs KO/KO: p=0.97, Figure 3A). In Figure 3B, time spent with familiar or novel objects is shown and used for calculating the Discrimination Index. As expected, wild-type mice(+/+) spent more time with the novel object than with the familiar object. However, heterozygotes (+/KO) and homozygotes (KO/KO) spent an identical time with both objects (Repeated mesures ANOVA, F(5, 35)=13.68, p=0.0017, WT Familiar vs WT Novel: p=0.0017, +/KO Familiar vs +/KO Novel: p>0.99, KO/KO Familiar vs KO/KO Novel: p=0.051). For the spatial memory test (object placement, Figure 3D–F), both of the same objects were placed in different areas which were randomly selected. The results are indicated as a preference score (%) (100% × exploration of relocated object/total exploration): WT (+/+), 64.87% ± 1.807, n=15: heterozygote (+/KO), 49.71% ± 0.9127, n=15: homozygote (KO/KO), 46.53% ± 0.8080, n=8, One-way ANOVA, F(2, 35)=47.87, p=9.6 × 10−6, WT vs +/KO: p=0.00014, WT vs KO/KO: p=0.00014, +/KO vs KO/KO: p>0.99, Figure 3D and Repeated mesures ANOVA, F(5, 35)=7.98, p=0.0056, WT Familiar vs WT Novel: p=0.0079, +/KO Familiar vs +/KO Novel: p>0.99, KO/KO Familiar vs KO/KO Novel: p=0.70, Figure 3E). As we expected, the heterozygotes (+/KO) and homozygotes (KO/KO) displayed both of visual and spatial memory defects. These data indicate that the 14-3-3epsilon protein is important for both visual and spatial memory.
Figure 3.
Novel object recognition (A, B and C) and object placement (D, E and F) tests revealed the importance of the 14-3-3epsilon protein in short visual and spatial memory. Discrimination Index and preference score were calculated at 3 hours after training. Significance was analyzed by one-way ANOVA and repeated measures ANOVA with Bonferroni’s post-hoc test. ** P<0.01 and ***P<0.001.
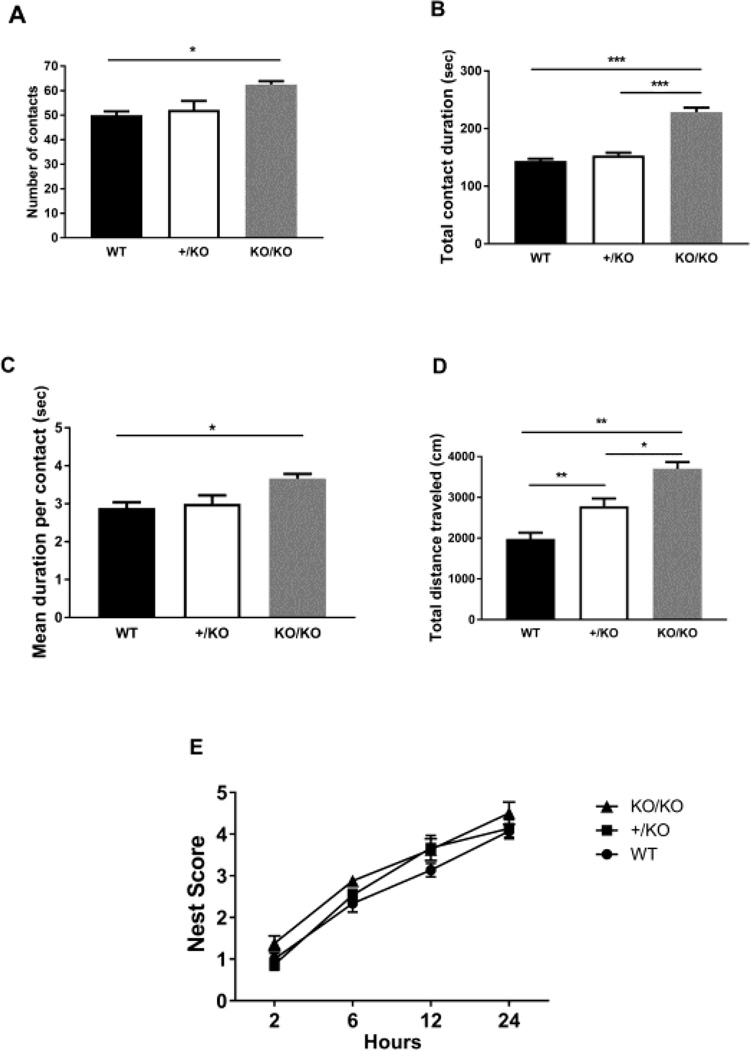
Finally, we tested if the Ywhae deficient mice show defects in sociability (Figure 4). To do this, we performed a social interaction test in a novel environment as well as examined nesting behavior. The novel environment test was performed using the arena used for the open field test. Two mice of identical genotypes (wild-type (+/+) vs wild-type (+/+), heterozygote (+/KO) vs heterozygote (+/KO) and homozygote (KO/KO) vs homozygote (KO/KO)) that were previously housed in different cages were placed in two corners diagonally from each other and allowed to freely explore for 10 minutes. 7 pairs for wild-type mice and heterozygotes (+/KO) and 4 pairs for homozygotes (KO/KO) were used, but 2 pairs of wild-type and heterozygotes (KO/KO) started fighting. Therefore, we excluded these mice from statistical analysis. The total number of contacts (WT (+/+), 50.00 ± 1.517, 5 pairs: heterozygote(+/KO), 52.20 ± 3.611, 5 pairs: homozygote (KO/KO), 64.50 ± 1.323, 4 pairs, One-way ANOVA, F(2, 11)=6.433, p=0.014, WT vs +/KO: p>0.99, WT vs KO/KO: p=0.017, +/KO vs KO/KO: p=0.051, Figure 4A), total contact duration (WT, 143.8s ± 3.787, 5 pairs: heterozygote, 153.4s ± 4.697, 5 pairs: homozygote, 228.5s ± 7.932, 4 pairs, One-way ANOVA, F(2, 11)=68.50, p=0.00012, WT vs +/KO: p=0.65, WT vs KO/KO: p=9.5 × 10−6, +/KO vs KO/KO: p=0.00032, Figure 4B), mean duration per contact (WT (+/+), 2.892s ± 0.1438, 5 pairs: heterozygote (+/KO), 2.997s ± 0.2229, 5 pairs: homozygote (KO/KO), 3.659s ± 0.1279, 4 pairs, One-way ANOVA, F(2, 11)=5.099, p=0.027, WT vs +/KO: p>0.99, WT vs KO/KO: p=0.037, +/KO vs KO/KO: p=0.076, Figure 4C), and total distance traveled (WT (+/+), 1,980cm ± 151.2, 5 pairs: heterozygote (+/KO), 2,789 ± 190.4, 5 pairs: homozygote (K/KO), 3,702 ± 163.5, 4 pairs, One-way ANOVA, F(2, 25)=24.18, p=0.00094, WT vs +/KO: p=0.0062, WT vs KO/KO: p=0.0012, +/KO vs KO/KO: p=0.030, Figure 4D) were calculated. The homozygotes (KO/KO) demonstrated an increased total number of contacts, total contact duration and mean duration per contact. Since the total travel distance was increased in homozygotes (KO/KO), suggesting hyperactivity in homozygotes (KO/KO), there is a possibility that the increase in the number of interactions in homozygotes (KO/KO) could potentially be caused by this hyper-activity. However, these data suggest that 14-3-3epsilon protein deficiency results in increased sociability because the mean duration per contact is increased. To assess nest building, a mouse was placed in a cage with a nest building white square cotton pad which was placed in the center of the cage (Figure 4E). Nest quality was scored on a scale of 0 to 5 as previously published [18]. Nest building was evaluated at 2, 6, 12 and 24 hours. There were no significant differencies between genotypes (Repeated measures ANOVA, F(6, 105)=0.837, p=0.64).
Figure 4.
The Ywhae knockout mice demonstrate a tendency toward increased sociability. In the social interaction test in a novel environment, the total number of contacts (A), total contact duration (B), mean duration per contact (C), and total distance traveled (D) were calculated. In the nest building test (E), the degree of nest building was indicated by a scale of 0 to 5 as described in the text. Significance was analyzed by one-way ANOVA and repeated measures ANOVA with Bonferroni’s posthoc test. * p<0.05, ** P<0.01 and *** P<0.001.
Thus, the complete ablation of 14-3-3epsilon resulted in more severe abnormalities in a variety of neurobehaviors including locomotor activity, cognition and sociability compared to both the control mice (+/+) and the heterozygous mice (+/KO). Although almost all of the results obtained in this study and our previous study are consistent, the results regarding anxiety-like behavior in this study were different from the previous study (Figure 2C). Also, although in both studies the Ywhae knockout mice with mixed genetic background were used, the slightly different genetic background (129/SVE × C57BL/6 mixed background here vs 129/S6 × NIH Black Swiss in the previous study) might affect the results. Further studies using additional tests will be needed to determine the functions of the 14-3-3epsilon protein in anxiety behavior. We scarcely obtained 13 homozygous male knockout mice (KO/KO), and this number is not enough to elucidate the precise functions of 14-3-3epsilon in the behaviors tested in this study. Therefore, further analyses using the Ywhae conditional knockout mice should be performed to analyze the precise functions of 14-3-3epsilon protein in neurobehavior.
Highlights.
The Ywhae homozygous knockout mice had a lower body weight than the wild-type mice.
The complete 14-3-3epsilon deficiency caused increased locomotor activity.
Ywhae homozygous deficient mice showed deficits in cognition.
14-3-3epsilon ablation resulted in increased sociability.
Acknowledgments
We thank Christina M. Stinger and Dr. Richard B. Huneke for their support in mouse breeding and maintenance. This study was supported by startup funds from the Department of Neurobiology and Anatomy at Drexel University College of Medicine and a research grant from the NIH (NS096098).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berg D, Holzmann C, Riess O. 14-3-3 proteins in the nervous system. Nat Rev Neurosci. 2003;4:752–762. doi: 10.1038/nrn1197. [DOI] [PubMed] [Google Scholar]
- 2.Aitken A. 14-3-3 proteins: a historic overview. Seminars in cancer biology. 2006;16:162–172. doi: 10.1016/j.semcancer.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Obsilova V, Kopecka M, Kosek D, Kacirova M, Kylarova S, Rezabkova L, et al. Mechanisms of the 14-3-3 protein function: regulation of protein function through conformational modulation. Physiol Res. 2014;63(Suppl 1):S155–S164. doi: 10.33549/physiolres.932659. [DOI] [PubMed] [Google Scholar]
- 4.Darling DL, Yingling J, Wynshaw-Boris A. Role of 14-3-3 proteins in eukaryotic signaling and development. Current topics in developmental biology. 2005;68:281–315. doi: 10.1016/S0070-2153(05)68010-6. [DOI] [PubMed] [Google Scholar]
- 5.Guenet JL, Salzgeber B, Tassin MT. Repeated epilation: a genetic epidermal syndrome in mice. J Hered. 1979;70:90–94. doi: 10.1093/oxfordjournals.jhered.a109223. [DOI] [PubMed] [Google Scholar]
- 6.Steinacker P, Schwarz P, Reim K, Brechlin P, Jahn O, Kratzin H, et al. Unchanged survival rates of 14-3-3gamma knockout mice after inoculation with pathological prion protein. Mol Cell Biol. 2005;25:1339–1346. doi: 10.1128/MCB.25.4.1339-1346.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wachi T, Cornell B, Marshall C, Zhukarev V, Baas PW, Toyo-Oka K. Ablation of the 14-3-3gamma Protein Results in Neuronal Migration Delay and Morphological Defects in the Developing Cerebral Cortex. Dev Neurobiol. 2016;76:600–614. doi: 10.1002/dneu.22335. [DOI] [PubMed] [Google Scholar]
- 8.Ramocki MB, Bartnik M, Szafranski P, Kołodziejska KE, Xia Z, Bravo J, et al. Recurrent distal 7q11.23 deletion including HIP1 and YWHAG identified in patients with intellectual disabilities, epilepsy, and neurobehavioral problems. Am J Hum Genet. 2010;87:857–865. doi: 10.1016/j.ajhg.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicita F, Garone G, Spalice A, Savasta S, Striano P, Pantaleoni C, et al. Epilepsy is a possible feature in Williams-Beuren syndrome patients harboring typical deletions of the 7q11.23 critical region. Am J Med Genet A. 2015 doi: 10.1002/ajmg.a.37410. [DOI] [PubMed] [Google Scholar]
- 10.Cheah PS, Ramshaw HS, Thomas PQ, Toyo-Oka K, Xu X, Martin S, et al. Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3ζ deficiency. Mol Psychiatry. 2012;17:451–466. doi: 10.1038/mp.2011.158. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda M, Hikita T, Taya S, Uraguchi-Asaki J, Toyo-oka K, Wynshaw-Boris A, et al. Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum Mol Genet. 2008;17:3212–3222. doi: 10.1093/hmg/ddn217. [DOI] [PubMed] [Google Scholar]
- 12.Toyo-oka K, Shionoya A, Gambello MJ, Cardoso C, Leventer R, Ward HL, et al. 14-3-3epsilon is important for neuronal migration by binding to NUDEL: a molecular explanation for Miller-Dieker syndrome. Nat Genet. 2003;34:274–285. doi: 10.1038/ng1169. [DOI] [PubMed] [Google Scholar]
- 13.Koshimizu H, Takao K, Matozaki T, Ohnishi H, Miyakawa T. Comprehensive behavioral analysis of cluster of differentiation 47 knockout mice. PloS one. 2014;9:e89584. doi: 10.1371/journal.pone.0089584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babovic D, Jiang L, Gantois I, Lawrence AJ, Ferreri V, Schütz G, et al. Age-related behavioural phenotype and cellular characterisation of mice with progressive ablation of D1 dopamine receptor-expressing cells. Behav Brain Res. 2010;206:78–87. doi: 10.1016/j.bbr.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Clifford JJ, Waddington JL. Topographically based search for an "Ethogram" among a series of novel D(4) dopamine receptor agonists and antagonists. Neuropsychopharmacology. 2000;22:538–544. doi: 10.1016/S0893-133X(99)00141-4. [DOI] [PubMed] [Google Scholar]
- 16.Walsh J, Desbonnet L, Clarke N, Waddington JL, O'Tuathaigh CMP. Disruption of exploratory and habituation behavior in mice with mutation of DISC1: an ethologically based analysis. J Neurosci Res. 2012;90:1445–1453. doi: 10.1002/jnr.23024. [DOI] [PubMed] [Google Scholar]
- 17.O'Sullivan GJ, Kinsella A, Grandy DK, Tighe O, Croke DT, Waddington JL. Ethological resolution of behavioral topography and D2-like vs. D1-like agonist responses in congenic D4 dopamine receptor "knockouts": identification of D4:D1-like interactions. Synapse. 2006;59:107–118. doi: 10.1002/syn.20225. [DOI] [PubMed] [Google Scholar]
- 18.Deacon RMJ. Assessing nest building in mice. Nat Protoc. 2006;1:1117–1119. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]