Abstract
Respiratory syncytial virus (RSV) remains a significant cause of morbidity and mortality in infants and young children, immunocompromised patients and the elderly. Despite the high disease burden, an effective and safe vaccine is lacking, although several candidates are currently in development. Current treatment for RSV infection remains largely supportive and RSV-specific options for prophylaxis are limited to palivizumab. In the past few years, novel therapeutic options including nanobodies, polyclonal and monoclonal antibodies have emerged and there are several products in preclinical and Phase-I, -II or -III clinical trials. The major target for antiviral drug development is the surface fusion (F) glycoprotein, which is crucial for the infectivity and pathogenesis of the virus. Solving the structures of the two conformations of the RSV F protein, the prefusion and postfusion forms, has revolutionized RSV research. It is now known that prefusion F is highly superior in inducing neutralizing antibodies. In this section we will review the stages of development and availability of different antibodies directed against RSV for the prevention and also for treatment of acute RSV infections. Some of these newer anti-RSV agents have shown enhanced potency, are being explored through alternative routes of administration, have improved pharmacokinetic profiles with an extended half-life, and may reduce design and manufacturing costs. Management strategies will require targeting not only high-risk populations (including adults or immunocompromised patients), but also previously healthy children who, in fact, represent the majority of children hospitalized with RSV infection. Following treated patients longitudinally is essential for determining the impact of these strategies on the acute disease as well as their possible long-term benefits on lung morbidity.
INTRODUCTION
Respiratory syncytial virus (RSV) is the main cause of bronchiolitis and pneumonia, in infants and toddlers, accounting for ~ 60% of all lower respiratory tract infections (LRTI) in preschool-aged children worldwide. Globally, it is estimated that RSV causes about 34 million episodes of acute LRTIs in children under five years of age, resulting in ~ 3.4 million hospitalizations per year [1, 2]. In the developing world, RSV is associated with significant morbidity and represents the second most common cause of infant mortality [3]. In addition, RSV also causes significant disease in immunocompromised hosts and in the elderly [4, 5].
By their first birthday nearly 70% of infants have been infected with RSV at least once. Seropositivity is ~ 100% by 2 years of age. Despite the high disease burden, an effective vaccine or specific therapy is lacking, although there are several products at different stages of development [6]. Epidemiologic studies have identified specific groups of infants at high-risk for severe disease and mortality including premature birth, compromised cardiopulmonary function (chronic lung disease or congenital heart disease), Down syndrome, and immunodeficiencies. However, the majority of infants requiring hospitalization for RSV LRTI do not have any risk factors and are previously healthy [7, 8]. Of those, up to 20% will be treated in the pediatric intensive care unit (PICU). [9, 10]
RSV has also been associated with the development of persistent wheezing and asthma inception [11–13]. Thus, it is possible that interventions aimed at reducing the acute burden of RSV disease may also have an impact on the development of long-term pulmonary sequela [14].
VIRAL STRUCTURE AND TARGETS FOR MONOCLONAL ANTIBODIES
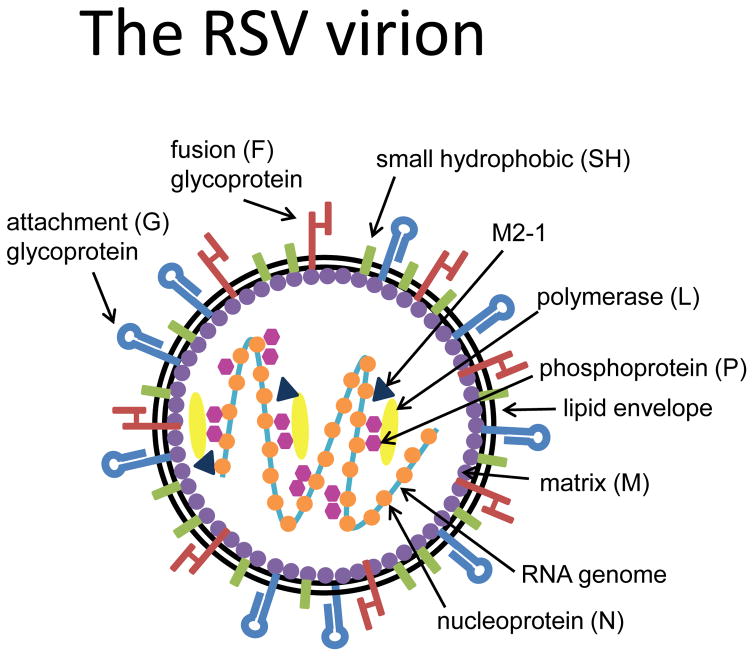
RSV is an enveloped, negative sense, single strand RNA virus that belongs to the family Paramyxoviridae, subfamily Pneumovirinae. Human RSV includes two antigenic subgroups, A and B, which can co-circulate during the same season and exhibit genome-wide sequence divergence. The RSV genome contains 15,222 nucleotides that encode eleven proteins (Figure 1). The RSV envelope contains three viral transmembrane surface glycoproteins: the attachment (G) protein, the fusion (F) protein, and the small hydrophobic (SH) protein. Of these proteins, SH is not required for initiating virus infection. On the other hand, the F and G proteins are crucial for the infectivity and pathogenesis of the virus, and carry the antigenic determinants that elicit the production of neutralizing antibodies by the host [15]. The F protein performs a dramatic structural rearrangement to cause membrane fusion, and is much more highly conserved among RSV strains than the G protein (Figure 2). For these reasons, the F protein represents the major target for antiviral drug development, both small molecules and neutralizing antibodies [6, 16].
Figure 1. Anatomy of RSV.
RSV is an enveloped, negative sense, single strand RNA virus whose genome contains 10 genes (15,222 nucleotides) encoding eleven proteins. Of the three transmembrane surface glycoproteins, the attachment (G) and fusion (F) proteins, are crucial for the infectivity and pathogenesis of the virus, and are the targets for neutralizing antibodies.
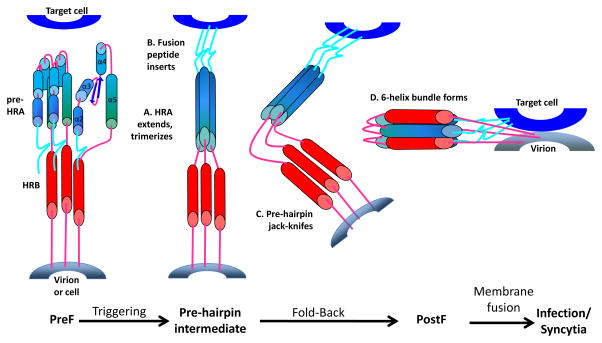
Figure 2. Refolding of the RSV F protein.
The F glycoprotein is present in two forms, a metastable prefusion F (preF), the active form on the virion membrane, and the postfusion F (postF) after triggering, refolding and bringing the virus and target cell membrane together to initiate fusion and infection. It is not clear what causes the F protein to trigger.
The G protein initiates infection by targeting the apical surface of the ciliated cells of the airways and mediates adherence of the virus to these host cells [17]. Heparan sulfate, surfactant protein A, annexin II and CX3CR1 have all been shown to bind the G protein and proposed as cellular receptors for RSV [18–21]. Recently, CX3CR1 was identified on the cilia of well differentiated human ciliated airway cells, acting as a cellular receptor for the G protein [22–24]. The F protein is essential for viral entry and initiates viral penetration by fusing the viral and cellular membranes [16]. In non-polarized laboratory cells, the F protein also promotes cell-to- cell fusion producing the characteristic syncytia. However, in polarized well differentiated human airway cells, the F protein does not cause cell-cell fusion probably because the F protein is limited to the apical domain [17, 25]. RSV lacking its G gene is infectious, though poorly, suggesting that the F protein may also have attachment activity [26]. Heparan sulfate, ICAM-1, TLR-4 and nucleolin have been proposed as cellular receptors for the F protein [26–30].
Our understanding of the RSV F protein was revolutionized in 2013 when McLellan et al. solved the crystal structure of its metastable, prefusion F (preF) conformation. Together with the crystal structure of the postfusion F (postF) protein [31], the beginning and end points of the F protein refolding became clear (Figure 2). The preF form is the active form of the F protein on the virion membrane surface. Antibodies that uniquely bind to the preF protein are much more efficient at neutralizing RSV compared to antibodies against the postF form [16, 32, 33]. In fact, some prefusion-specific monoclonal antibodies (mAbs) are able to stabilize the F protein in its prefusion state and in some cases this stabilization may be the mechanism by which the mAb prevents infection: they prevent the F protein from refolding [34]. The stages of development and the clinical applications for these antibodies will be discussed below.
POLYCLONAL ANTIBODIES
The field of antiviral therapy for RSV radically changed during the 1980s when prophylactic use of an intravenous (IV) polyclonal immunoglobulin preparation with high titers of neutralizing antibodies against RSV (RSV IVIG; Respigam) demonstrated efficacy in reducing hospitalizations due to severe RSV infections in high-risk children (PREVENT trial) [35, 36] (Table 1). Despite these positive results, RSV IVIG was associated with several limitations including fluid overload, adverse events in children with congenital heart diseases (CHD), need for intravenous access, development of hypoxemia or cyanosis, and the need to delay vaccination with live vaccines for ~ 9 months after the last dose. RSV IVIG was withdrawn from the market in 2004 but this preparation established the proof of principle that anti-RSV antibodies delivered IV can prevent severe RSV LRTI and prompted the development of mAbs protective against RSV. Subsequently, another IVIG preparation obtained from pooled plasma from donors with high titers of RSV (RI-001; ADMA Biologicals) was developed. RI-001 was tested in Phase-II clinical trials for the prevention of RSV LRTI in immunocompromised 2 to 65 year old patients who presented with upper respiratory tract symptoms with promising results [37]. Recently, the RI-001 formulation was replaced by RI-002, which in addition to high levels of RSV antibodies has naturally polyclonal antibodies against Streptococcus pneumoniae and Haemophilus influenza type b). A Phase-III open label clinical trial (www.clinicaltrials.gov; NCT01814800) has been completed using this second generation IVIG for the prevention of serious bacterial infections in patients with primary immunodeficiencies. The role of RI-002 in preventing RSV infection in this population has not been reported.
Table 1.
Anti-RSV antibodies at different stages of development
| Antibody Class |
Name/ Company |
Development phase |
Route | Target | Class | Endpoints/ Target Population |
Comments |
|---|---|---|---|---|---|---|---|
| (1) Nanobodies | ALX0171 (Ablynx) | Phase-IIa RCT completed | Inhaled | F | Antibody nanobody |
Treatment of infants and toddlers with RSV LRTI |
|
| (2) Monoclonal antibodies (mAb) | Palivizumab (Medimmune) | Phase-IV Marketed | IM | F | Humanized mAb | Prevention of RSV LRTI in high-risk infants |
|
| Motavizumab (Medimmune) | Phase-III RCT completed Not-licensed (no FDA approval) |
IM | F | Humanized mAb | Prevention of RSV LRTI in high-risk infants |
|
|
| Motavizumab-YTE (MEDI-557; Medimmune) | Phase-II RCT interrupted | IV | F | Humanized mAb derived from motavizumab with YTE technology | Prevention of RSV LRTI in high-risk infants |
|
|
| MEDI-8897 (Medimmune) | Phase-II RCT ongoing | IM | Prefusion F |
Human mAb derived from D25 | Prevention of RSV LRTI in healthy and high-risk infants. |
|
|
| REGN2222 (Regeneron pharmaceutics) | Phase-III RCT ongoing | IM | F | Human mAb anti- RSV | Prevention of RSV LRTI in premature healthy infants not candidates for palivizumab |
|
|
| (3) Polyclonal antibodies | RI-001 (ADMA) | Phase-II RCT completed | IV | Various viral epitopes | Polyclonal IG | Prevention of progression from RSV URI to LRTI in immunocompromised patients | Decreased RSV titers at day 18 from baseline. 4-fold rise in antibody titers. |
| RI-002 (ADMA) | Phase-III RCT completed | IV | Various viral epitopes | Polyclonal IG | Prevention of RSV and serious bacterial infections in patients with PIDD | No adverse events and no serious bacterial infections. Unclear benefits for RSV specifically |
DISCOVERY: (1) EV-046120 and EV-046135 (Evec, Inc): also targeting RSV and MPV; (2) Monoclonal antibodies (vanderBilt Univ): human mAb anti-F protein and human mAb anti G-protein.
PRECLINICAL: (1) AR-201 (Aridis Pharm) and Palivizumab Biosimilar (bioXPRESS Therapeutic, Celltrion, iBio, mAbxience & UCAB): mAb anti-F protein; (2) MPE-8 (Humabs BioMed): mAb anti prefusion-F protein. Possible prophylaxis and therapy of severe HRSV and HMPV infections; (3) Sym-003 (Symphogen): polyclonal antibody targeting various non- overlapping RSV epitopes (F and G proteins).
RCT: Randomized clinical trial; LRTI: Lower respiratory tract infection; FDA: Food and Drug Administration; MALRI: medically attended lower respiratory tract infections; URI: upper respiratory infection; IG: immunoglobulin; PIDD: Primary immunodeficiency diseases.
MONOCLONAL ANTIBODIES
The main advantages of mAbs against RSV, compared to polyclonal preparations, are their greatly superior neutralizing activity with practically no risk of fluid overload or interference with vaccine schedules, and no possibility of blood-borne pathogen transmission. Most of the original anti-RSV mAbs that were developed and tested clinically are IgG antibodies directed against viral epitopes in the F protein (Table 1). During the early 1990s three mAbs were evaluated in clinical studies in humans. Two of them were IgG formulations that were administered systemically (SB209763 and MEDI-493/palivizumab) [38, 39]. Palivizumab was shown to be clearly superior to SB209763 and, since 1998, it has been the only mAb licensed for passive immunization in high-risk children [40–42]. A third neutralizing mAb, HNK20, was an IgA mAb administered intranasally. Although HNK20 appeared promising in non-human primates, it failed to provide clinical benefit [43].
Other novel mAbs have emerged and several products are currently in preclinical, or Phase I, II or III clinical trials. Of particular interest is MPE8, a human mAb directed against the preF protein, that potently neutralizes both human RSV and human metapneumovirus (hMPV), as well as two animal paramyxoviruses [44]. MPE8 has shown potent prophylactic efficacy in animal models of RSV and hMPV infection, and both a prophylactic and a therapeutic effect against pneumonia virus of mice. The conservation of this neutralizing epitope in the F protein of such diverse viruses suggests that this epitope is important to the function or structure of the F protein.
Targeting the F Protein
1. Palivizumab
In 1998 the US Food and Drug Administration (FDA) licensed the use of palivizumab, a humanized mAb directed against the F glycoprotein, for the prevention of serious LRTI caused by RSV in children at high risk for RSV disease. Palivizumab is a humanized IgG1 mAb composed of the complement determining region from a murine mAb developed at the National Institutes of Health (NIH) and, transplanted into a human IgG1 mAb using recombinant DNA technology [39]. It is > 95% human and recognizes the antigenic site II on the F protein of both RSV A and B subtypes. Palivizumab is significantly more potent than the polyclonal preparations. It is now known that palivizumab binds to both the preF and postF forms of the F protein, because it recognizes a site on the central region of preF whose structure does not change during the preF to postF transition [45].
In the original large, randomized placebo-controlled studies, the safety and efficacy of palivizumab (the primary endpoint was a reduction in RSV-related hospitalization rates) were established in infants with a history of premature birth (≤35 weeks’ gestational age), children with chronic lung disease of prematurity (CLD), and children with hemodynamically significant congenital heart disease (CHD) [40, 41]. Since palivizumab approval in 1998, to provide guidance regarding the groups of infants at highest risk, the American Academy of Pediatrics (AAP) has updated the guidance on palivizumab prophylaxis, and increasingly limited its use [46]. Palivizumab has a half-life of 28 days and thus it is administered once a month during the RSV season at 15 mg/kg intramuscularly. It should be initiated immediately before the start of the RSV season and continued monthly until the end. Although there is significant regional and year-to-year variability in the beginning and end of the season, administration of five monthly doses starting in November usually ensures adequate serum concentrations throughout the season.
2. Motavizumab
Subsequently, MEDI-524 (motavizumab), a second-generation recombinant IgG1 mAb based on palivizumab and thus also directed against site II, was developed by directed mutagenesis of the complement determining regions and selection of the most effective version. It differed from palivizumab in 13 amino acid positions, selected for their 70 and 20- fold increased affinity and in vitro neutralizing activities respectively, as well as greater inhibition of RSV in the upper respiratory tract compared to palivizumab [47]. A large Phase III double blind active control study, in which 6,600 preterm infants with CLD were randomized to either motavizumab or palivizumab, at a dose of 15 mg/kg once a month for 5 months intramuscularly, showed that motavizumab was non-inferior to palivizumab at reducing RSV hospitalizations. However, it was associated with a 2.1% increase in cutaneous hypersensitivity reactions (7.2% vs. 5.1%) [48]. At the same dose, this second generation mAb was superior to palivizumab in further reducing RSV LRTI outpatients visits by 50%. The clinical efficacy of motavizumab was subsequently confirmed in two additional randomized clinical trials conducted in healthy US Native American Indians infants, and in children with hemodynamically significant CHD [49, 50]. Nevertheless, in 2010 the US FDA Antiviral Drug Advisory Committee did not grant motavizumab licensure and its development has been discontinued.
None of these antibody preparations, RSV IVIG, palivizumab or motavizumab, have been shown to be effective as treatments for acute RSV LRTI in previously healthy children [51, 52].
3. Motavizumab-YTE
Palivizumab and motavizumab have a half-life of 3–4 weeks and thus monthly injections are required to maintain protective levels during the RSV season. MEDI-557 (motavizumab-YTE) is a third generation, humanized mAb derived from motavizumab, in which three amino acid substitutions (M252Y/S254T/T256E [YTE]) were introduced in the IgG1 Fc region, extending the serum IgG half-life [53]. A Phase-I randomized double blind, dose escalation study to evaluate the safety, tolerability and pharmacokinetics of this mAb showed that the clearance of motavizumab-YTE was significantly lower and the half-life up to 4-fold longer than motavizumab [54]. Further development of this mAb has also been discontinued.
4. MEDI-8897
MEDI-8897 is an investigational recombinant human IgG1 mAb derived from D25, a human antibody with greater neutralizing potency than palivizumab, in which half-life has been extended using YTE technology. D25 targets antigenic site 0 that is unique to the preF conformation of the RSV F protein [32, 44]. In vitro, this drug has been shown to be 150-fold more potent than palivizumab, and 10-fold more potent in vivo. Phase-I clinical trials in adults confirmed its prolonged half-life [55]. Currently MEDI-8897 is undergoing Phase-II clinical trials in premature infants born between 32 and 35 weeks gestation. The long-term objective for MEDI-8897 would be to provide passive immunization for prevention of LRTI caused by RSV not only to children with chronic lung disease (CLD) or CHD but also to all infants (preterm and full term) entering their first RSV season, using a fixed, single intramuscular (IM) dose.
Extended half-life mAbs represent a promising advance for the prevention of RSV by enhancing efficacy, avoiding compliance issues since repeated dosing would not be required, and lowering costs. In addition, this strategy represents a potential opportunity for application to other patient populations, including otherwise healthy children or adults.
5. REGN-2222
REGN-2222 is a fully human IgG1 mAb that targets a well-conserved epitope of the F protein, different from site 0 or site II. In vitro, REGN2222 was 36-fold more potent than palivizumab at inhibiting RSV fusion to the cells, and 10 to 40-fold more potent than palivizumab in reducing lung and nasal viral loads in cotton rats. It is not clear if REGN-2222 is specific for preF. Phase-I ascending dose studies conducted in adults 18–60 years old demonstrated that the half-life of this mAb is longer that a typical IgG1 mAb, with low immunogenicity [56]. A Phase-III randomized, double-blind, placebo-controlled clinical trials (www.clinicaltrials.gov; NCT02325791) is currently underway in premature infants born at ≤ 35 weeks of gestation who are ≤ 6 months of age at enrollment in whom palivizumab is not recommended. The primary endpoint is the prevention of serious lower respiratory tract infections associated with RSV. Similar to other mAbs, REGN-2222 is administered IM, however it is expected that one or two doses will be sufficient to provide coverage for one RSV season.
Targeting the Attachment (G) Protein
The G protein is the primary RSV attachment protein. The sequence identity of the G protein between RSV isolates can be as little as 60%, therefore most of the efforts in antiviral drug development has focused on the F protein which is well conserved among RSV A and B strains. Nevertheless, the G protein has also been implicated in the pathogenesis of RSV disease due to its ability to modulate the virus-induced immune response and lung pathology.
Studies in animal models using the RSV G mAb 131-2G, either as prophylaxis or in a treatment mode, showed that it decreased both RSV loads and lung inflammation when administered early in the disease course [57, 58]. This result was originally interpreted as being due to antibody dependent cellular cytotoxicity since 131-2G is a “non-neutralizing mAb” in HEp-2 cells. However, the G receptor in HEp-2 cells is heparan sulfate while the receptor on human ciliated airway cells, the natural target in the airway epithelium, is CX3CR1. Indeed, mAb 31-2G showed to prevent the G protein from binding to CX3CR1 and inhibited infection of well differentiated human airway epithelial cells, suggesting that it might also have neutralized RSV in the mouse experiments [22]. However, a prophylactic antibody against the G protein would need to recognize both RSV A and B strains. Despite the overall widely variable sequences of the G protein its central region contains a completely conserved 13 amino acid sequence followed by a cysteine noose also with conserved elements. MAbs, like 131-2G, that bind to this region may be able to neutralize both A and B strains of RSV.
NANOBODIES
Nanobodies are small (12–15 kDa) antibody fragments consisting of a single monomeric variable domain (heavy chain) that retain full antigen-binding capacity [59, 60]. The affinity of nanobodies is similar to that of conventional antibodies, however, their size and stability allowing intranasal delivery, their formatting flexibility into multivalent constructs, and their relatively straightforward production make these small proteins an attractive approach for both prophylaxis and treatment of respiratory viral infections.
ALX-0171 (Ablynx) is a nanobody, composed of three identical single variable chain antibody domains linked end to end by flexible peptides. Like palivizumab, this trivalent nanobody binds to antigenic site II in the preF and postF configurations of the F protein, and likely inhibits the conformational changes in the F protein that are required for membrane fusion (Figure 2). ALX-0171 design has allowed its delivery via the intranasal route (inhalation) for the treatment of RSV infection [60]. Its multivalent formatting has provided ALX-0171 with improved activity and coverage for RSV A and B strains, as well as neutralizing activity that is superior to palivizumab [60]. In the neonatal lamb model, treatment with nebulized ALX-0171 showed a significant reduction of RSV loads in bronchoalveolar lavage samples, decreased lung histological changes, and improvement of clinical symptoms in RSV-infected animals [61]. Preliminary results from a Phase-I/IIa randomized clinical trial (www.clinicaltrials.gov; NCT02309320) conducted in healthy infants and toddlers hospitalized with RSV infection showed that inhaled ALX-0171 administered once a day for 3 consecutive days was safe and well tolerated, had an antiviral effect with a reduction of viral loads compared to placebo, and showed a trend towards improvement of the clinical severity score [62, 63].
LONG TERM BENEFITS
Observational studies have shown that at least 30% of children who develop RSV LRTI as infants will develop recurrent wheezing during childhood [11, 13]. In the mouse model, RSV infection alone was associated with lung inflammatory changes and airway hyperresponsiveness that persisted for months after the acute infection [64]. In this model, administration of prophylactic mAbs against RSV was protective against the acute disease and significantly reduced the long-term pulmonary morbidity and the development of airway hyperresponsiveness [65, 66]. Prospective non-controlled studies followed by a randomized, double blind, placebo-controlled clinical trial in preterm infants who received prophylaxis with palivizumab, demonstrated a decrease in the development of persistent wheezing, that was independent of the atopic background [14, 67, 68]. Thus, data derived from animal and clinical studies suggest that the prevention of acute RSV LRTI may have implications in the development of recurrent wheezing.
SUMMARY
There have been recent advances in the pipeline for both treatment and preventive strategies for RSV infection. There are several newer RSV antibodies in different phases of development that have shown enhanced potency, improved pharmacokinetic profiles and extended half-life, and these antibodies are being explored through alternative routes of administration. While the main barriers for mAb administration will likely be derived from their associated costs and the inconvenience of intramuscular dosing, inhaled nanobodies will need to be administered with caution in infants with an already inflamed airway. Nevertheless, the benefits associated with these interventions may well outweigh their potential side effects. Management strategies will require targeting not only high-risk populations, but also previously healthy children that represent the majority of hospitalized patients with RSV LRTI. In addition, ongoing studies should include the ability to follow these patients and determine the acute and possibly long term benefits associated with these compounds.
HIGHLIGHTS.
Several promising candidates including monoclonal antibodies and nanobodies are in different stages of development for the prevention and treatment of acute RSV infection in the main target populations.
Newer human monoclonal antibodies with enhanced potency and extended half-life have been developed, and represent a promising strategy for the prevention of RSV.
Nanobodies delivered via the intranasal route represent an attractive approach for the treatment of acute RSV infections in young children.
Evaluation of strategies aimed at preventing or treating RSV infections should include patient follow up to determine their impact on acute and long-term lung morbidity.
Acknowledgments
Funding
AM, OR and MEP have received support from the National Institutes of Health (NIH grant AI112524). Funding sources had no role in study design, data collection, data analysis, data interpretation, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interests
AM reports personal fees from Abbvie and Novartis, grants from Gilead and from Alios and grants and personal fees from Janssen. OR reports personal fees from HuMabs, Abbvie, Janssen, Medimmune and Regeneron, and grants from Janssen. MEP reports grants from Janssen, Medimmune, Abbott, Reviral and Agilvax. None of these fees or grants are related to the current work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. Jama. 1999;282:1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh EE, Falsey AR. Respiratory syncytial virus infection in adult populations. Infectious disorders drug targets. 2012;12:98–102. doi: 10.2174/187152612800100116. [DOI] [PubMed] [Google Scholar]
- 5.Campbell AP, Chien JW, Kuypers J, Englund JA, Wald A, Guthrie KA, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples, and clinical outcomes of HCT. J Infect Dis. 2010;201:1404–13. doi: 10.1086/651662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazur NI, Martinon-Torres F, Baraldi E, Fauroux B, Greenough A, Heikkinen T, et al. Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. The Lancet Respiratory medicine. 2015;3:888–900. doi: 10.1016/S2213-2600(15)00255-6. [DOI] [PubMed] [Google Scholar]
- 7.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia CG, Bhore R, Soriano-Fallas A, Trost M, Chason R, Ramilo O, et al. Risk factors in children hospitalized with RSV bronchiolitis versus non-RSV bronchiolitis. Pediatrics. 2010;126:e1453–60. doi: 10.1542/peds.2010-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, et al. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J. 1999;18:115–22. doi: 10.1097/00006454-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Mejias A, Dimo B, Suarez NM, Garcia C, Suarez-Arrabal MC, Jartti T, et al. Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10:e1001549. doi: 10.1371/journal.pmed.1001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 12.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65:1045–52. doi: 10.1136/thx.2009.121582. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Yarza EG, Moreno A, Lazaro P, Mejias A, Ramilo O. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr Infect Dis J. 2007;26:733–9. doi: 10.1097/INF.0b013e3180618c42. [DOI] [PubMed] [Google Scholar]
- 14.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JL, et al. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368:1791–9. doi: 10.1056/NEJMoa1211917. [DOI] [PubMed] [Google Scholar]
- 15.Mejias A, Ramilo O. New options in the treatment of respiratory syncytial virus disease. The Journal of infection. 2015;71(Suppl 1):S80–7. doi: 10.1016/j.jinf.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 16.McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013;372:83–104. doi: 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–66. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallak LK, Collins PL, Knudson W, Peeples ME. Iduronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology. 2000;271:264–75. doi: 10.1006/viro.2000.0293. [DOI] [PubMed] [Google Scholar]
- 19.Hickling TP, Malhotra R, Bright H, McDowell W, Blair ED, Sim RB. Lung surfactant protein A provides a route of entry for respiratory syncytial virus into host cells. Viral Immunol. 2000;13:125–35. doi: 10.1089/vim.2000.13.125. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra R, Ward M, Bright H, Priest R, Foster MR, Hurle M, et al. Isolation and characterisation of potential respiratory syncytial virus receptor(s) on epithelial cells. Microbes and infection / Institut Pasteur. 2003;5:123–33. doi: 10.1016/s1286-4579(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 21.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2:732–8. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SM, McNally BA, Ioannidis I, Flano E, Teng MN, Oomens AG, et al. Respiratory Syncytial Virus Uses CX3CR1 as a Receptor on Primary Human Airway Epithelial Cultures. PLoS Pathog. 2015;11:e1005318. doi: 10.1371/journal.ppat.1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chirkova T, Lin S, Oomens AG, Gaston KA, Boyoglu-Barnum S, Meng J, et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J Gen Virol. 2015;96:2543–56. doi: 10.1099/vir.0.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong KI, Piepenhagen PA, Kishko M, DiNapoli JM, Groppo RP, Zhang L, et al. CX3CR1 Is Expressed in Differentiated Human Ciliated Airway Cells and Co-Localizes with Respiratory Syncytial Virus on Cilia in a G Protein-Dependent Manner. PloS one. 2015;10:e0130517. doi: 10.1371/journal.pone.0130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts SR, Compans RW, Wertz GW. Respiratory syncytial virus matures at the apical surfaces of polarized epithelial cells. J Virol. 1995;69:2667–73. doi: 10.1128/jvi.69.4.2667-2673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Techaarpornkul S, Barretto N, Peeples ME. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J Virol. 2001;75:6825–34. doi: 10.1128/JVI.75.15.6825-6834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–7. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med. 2011;17:1132–5. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- 29.Techaarpornkul S, Collins PL, Peeples ME. Respiratory syncytial virus with the fusion protein as its only viral glycoprotein is less dependent on cellular glycosaminoglycans for attachment than complete virus. Virology. 2002;294:296–304. doi: 10.1006/viro.2001.1340. [DOI] [PubMed] [Google Scholar]
- 30.Behera AK, Matsuse H, Kumar M, Kong X, Lockey RF, Mohapatra SS. Blocking intercellular adhesion molecule-1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem Biophys Res Commun. 2001;280:188–95. doi: 10.1006/bbrc.2000.4093. [DOI] [PubMed] [Google Scholar]
- 31.McLellan JS, Yang Y, Graham BS, Kwong PD. Structure of respiratory syncytial virus fusion glycoprotein in the postfusion conformation reveals preservation of neutralizing epitopes. J Virol. 2011;85:7788–96. doi: 10.1128/JVI.00555-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113–7. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palomo C, Mas V, Vazquez M, Cano O, Luque D, Terron MC, et al. Polyclonal and monoclonal antibodies specific for the six-helix bundle of the human respiratory syncytial virus fusion glycoprotein as probes of the protein post-fusion conformation. Virology. 2014;460–461:119–27. doi: 10.1016/j.virol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Gilman MS, Moin SM, Mas V, Chen M, Patel NK, Kramer K, et al. Characterization of a Prefusion-Specific Antibody That Recognizes a Quaternary, Cleavage-Dependent Epitope on the RSV Fusion Glycoprotein. PLoS Pathog. 2015;11:e1005035. doi: 10.1371/journal.ppat.1005035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. The Respiratory Syncytial Virus Immune Globulin Study Group. N Engl J Med. 1993;329:1524–30. doi: 10.1056/NEJM199311183292102. [DOI] [PubMed] [Google Scholar]
- 36.Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. The PREVENT Study Group. Pediatrics. 1997;99:93–9. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 37. [accessed August 3 2016];RI-001 in Immunosuppressed Respiratory Syncytial Virus (RSV) Infected Patients at Risk of Lower Tract RSV Illness. 2013 http://www.clinicaltrials.gov. NCT00632463; updated March 12, 2013.
- 38.Meissner HC, Groothuis JR, Rodriguez WJ, Welliver RC, Hogg G, Gray PH, et al. Safety and pharmacokinetics of an intramuscular monoclonal antibody (SB 209763) against respiratory syncytial virus (RSV) in infants and young children at risk for severe RSV disease. Antimicrobial agents and chemotherapy. 1999;43:1183–8. doi: 10.1128/aac.43.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–24. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 40.Palivizumab a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics. 1998;102:531–7. [PubMed] [Google Scholar]
- 41.Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, Jr, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–40. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 42.Johnson S, Griego SD, Pfarr DS, Doyle ML, Woods R, Carlin D, et al. A direct comparison of the activities of two humanized respiratory syncytial virus monoclonal antibodies: MEDI-493 and RSHZl9. J Infect Dis. 1999;180:35–40. doi: 10.1086/314846. [DOI] [PubMed] [Google Scholar]
- 43.Weltzin R, Monath TP. Intranasal antibody prophylaxis for protection against viral disease. Clinical microbiology reviews. 1999;12:383–93. doi: 10.1128/cmr.12.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corti D, Bianchi S, Vanzetta F, Minola A, Perez L, Agatic G, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501:439–43. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 45.McLellan JS, Chen M, Kim A, Yang Y, Graham BS, Kwong PD. Structural basis of respiratory syncytial virus neutralization by motavizumab. Nat Struct Mol Biol. 2010;17:248–50. doi: 10.1038/nsmb.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Academy of Pediatrics Committee on Infectious D, American Academy of Pediatrics Bronchiolitis Guidelines C. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–20. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 47.Wu H, Pfarr DS, Johnson S, Brewah YA, Woods RM, Patel NK, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368:652–65. doi: 10.1016/j.jmb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 48.Carbonell-Estrany X, Simoes EA, Dagan R, Hall CB, Harris B, Hultquist M, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–51. doi: 10.1542/peds.2008-1036. [DOI] [PubMed] [Google Scholar]
- 49.O'Brien KL, Chandran A, Weatherholtz R, Jafri HS, Griffin MP, Bellamy T, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis. 2015;15:1398–408. doi: 10.1016/S1473-3099(15)00247-9. [DOI] [PubMed] [Google Scholar]
- 50.Feltes TF, Sondheimer HM, Tulloh RM, Harris BS, Jensen KM, Losonsky GA, et al. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatric research. 2011;70:186–91. doi: 10.1203/PDR.0b013e318220a553. [DOI] [PubMed] [Google Scholar]
- 51.Ramilo O, Lagos R, Saez-Llorens X, Suzich J, Wang CK, Jensen KM, et al. Motavizumab treatment of infants hospitalized with respiratory syncytial virus infection does not decrease viral load or severity of illness. Pediatr Infect Dis J. 2014;33:703–9. doi: 10.1097/INF.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 52.Committee on Infectious D. From the American Academy of Pediatrics: Policy statements-- Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 53.Weisman LE. Respiratory syncytial virus (RSV) prevention and treatment: past, present, and future. Cardiovasc Hematol Agents Med Chem. 2009;7:223–33. doi: 10.2174/187152509789105471. [DOI] [PubMed] [Google Scholar]
- 54.Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, et al. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrobial agents and chemotherapy. 2013;57:6147–53. doi: 10.1128/AAC.01285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulbrandt N, Kallewaard N, Palaszynski S, Zhang J, Svabek C, McAuliffe J, et al. A Next Generation Anti-RSV F Monoclonal Antibody As Passive Immunization for Prevention of RSV Disease in All Infants. 9th International Respiratpry Syncytial Virus Symposium; Cape Town, South Africa. 2014. Abstract OP 27. [Google Scholar]
- 56.Sivapalasingam S, Caballero-Perez D, Houghton M, Yang F, Davis J, Gao B, et al. Phase 1 study evaluating safety, tolerability, pharmacokinetics and immunogenicity of REGN2222 in healthy adults: a new human monoclonal RSV-F antibody for RSV prevention. Infectious Diseases Society of America 53th Annual Meeting ID Week; San Diego, CA. 2015. Abstract #912. [Google Scholar]
- 57.Haynes LM, Caidi H, Radu GU, Miao C, Harcourt JL, Tripp RA, et al. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect Dis. 2009;200:439–47. doi: 10.1086/600108. [DOI] [PubMed] [Google Scholar]
- 58.Radu GU, Caidi H, Miao C, Tripp RA, Anderson LJ, Haynes LM. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J Virol. 2010;84:9632–6. doi: 10.1128/JVI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schepens B, Ibanez LI, De Baets S, Hultberg A, Bogaert P, De Bleser P, et al. Nanobodies(R) specific for respiratory syncytial virus fusion protein protect against infection by inhibition of fusion. The Journal of infectious diseases. 2011;204:1692–701. doi: 10.1093/infdis/jir622. [DOI] [PubMed] [Google Scholar]
- 60.Detalle L, Stohr T, Palomo C, Piedra PA, Gilbert BE, Mas V, et al. Generation and Characterization of ALX-0171, a Potent Novel Therapeutic Nanobody for the Treatment of Respiratory Syncytial Virus Infection. Antimicrobial agents and chemotherapy. 2016;60:6–13. doi: 10.1128/AAC.01802-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.LD Delivery of ALX-0171 by inhalation greatly reduces disease burden in a neonatal lamb RSV infection model. 9th International Respiratory Syncytial Virus Symposium; 2014. [Google Scholar]
- 62.Germani M, Niederalt C, Kanacher T, Stohr T, Detalle L, Wendl T, et al. A physiologically-based Pharmacokinetic (PB-PK) model to explore ALX-0171 PK in infants following inhalation. Fifth American Conference on Pharmacometrics; 2014. [Google Scholar]
- 63.Ablynx reports positive top line results for its inhaled anti-RSV Nanobody (ALX-0171) in a Phase I/IIa study in infants hospitalised with an RSV infection. Vol. 2016 Zwijnaarde, Belgium: May 3, 2016. [Google Scholar]
- 64.Jafri HS, Chavez-Bueno S, Mejias A, Gomez AM, Rios AM, Nassi SS, et al. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis. 2004;189:1856–65. doi: 10.1086/386372. [DOI] [PubMed] [Google Scholar]
- 65.Mejias A, Chavez-Bueno S, Rios AM, Saavedra-Lozano J, Fonseca Aten M, Hatfield J, et al. Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model. Antimicrob Agents Chemother. 2004;48:1811–22. doi: 10.1128/AAC.48.5.1811-1822.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mejias A, Chavez-Bueno S, Rios AM, Aten MF, Raynor B, Peromingo E, et al. Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency. Antimicrob Agents Chemother. 2005;49:4700–7. doi: 10.1128/AAC.49.11.4700-4707.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. e1. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 68.Simoes EA, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick L, Groothuis JR, et al. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126:256–62. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]