It was recently reported the activation of the β-catenin pathway in dermal papilla (DP) cells achieved by Prom1CreERlacZ mediated deletion of the Catn1Ex3flox allele increased the number of DP cells per hair follicle (Kaushal et al., 2016). This result seemed to conflict with our observation that deletion of Catn1Ex3flox in dermal papilla cells had a dramatic effect on coat color but did not have a significant effect on the structure of the hair coat (Enshell-Seijffers et al., 2010b). We have reported that dermal papilla cell number per follicle correlates with hair size and shape (Chi et al., 2013) and would therefore expect that a change in the number of dermal papilla cells per follicle would cause a change in hair coat composition. We had not directly scored DP number in our previous work with the Catn1Ex3flox allele, but here report that stabilization of β-catenin in the DP had no effect on DP cell number in follicles producing zigzag hairs. Zigzag hair follicles comprise the majority of hair follicles in the mouse pelage and as such, our data stand in direct contradiction to the conclusion ” that stabilization of β-catenin in Prom1-expressing DP cells results in an increase in the size of anagen and telogen DP”.
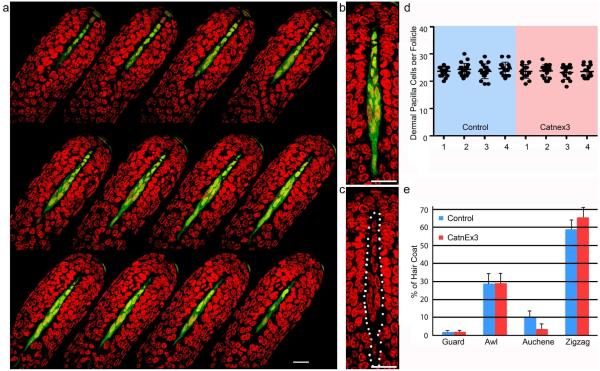
Our methods differ from those of Kaushal et al., but provide a more accurate characterization of DP cell number per follicle. Individual follicles in anagen stage V1 (Muller-Rover et al., 2001) were dissected from fixed skin from the same anatomical site for all samples and follicle types were identified by the hairs contained in them. These were stained and optical sections through the hair bulb were collected as described (Fig. 1a)(Chi et al., 2015). Corin cre/+; tyr c/c; rYFP/+ mice (Enshell-Seijffers et al., 2010a) with and without a single copy of the Catn1Ex3flox allele (Harada et al., 1999) were analyzed. In these mice, cre-mediated deletion of the floxed allele occurs in the vast majority of dermal papilla cells in all follicle types and the resultant deletion of exon3 leads to expression of an activated β-catenin protein (Enshell-Seijffers et al., 2010b). The lack of pigment in tyr c/c mice allows us to score DP cells that can otherwise be obscured by pigment, and the cre-dependent rYFP allele allows unambiguous identification of DP cells to facilitate accurate counts (Fig. 1b,c). Like Kaushal et al., we confined our analysis to zigzag hair follicles. We focused on the anagen VI follicle where the de-condensed state of the DP allows accurate counting of nuclei and unambiguous identification of DP cells, herein defined as any mesenchymal cell within the bulb above the plane defined by the lowermost edge of matrix keratinocytes. As shown in Fig. 1, this provides a superior method to score DP cell number. Analyzing 4 mutant and 4 control animals, we find no difference in the number of DP cells in zigzag follicles (Fig. 1d).
Figure 1. Dermal papilla cell number is unchanged in mice with β-catenin stabilized in the DP.
a. Consecutive optical sections through a zigzag hair follicle. DP cells are green, nuclei are red. b,c. Magnified image from a with (b) and without (c) the green channel to count nuclei in the DP (dotted line, c). d. DP number per zigzag follicle shown for four control mice (Control, Blue) and four mice with β-catenin activated in the DP (CatnEx3, red). Averages ± s.d. are shown. d. Frequency of each hair type in the second hair coat of control mice (blue) and mice with β-catenin activated in the DP (CatnEx3, red). Averages ± s.d. are shown. (n= 13 control mice, n=12 CatnEx3 mice, at least 250 hairs per mouse analyzed). Scale bar: 25 micrometers.
We surmise that the disputed conclusion arose from the methodology employed. Kaushal et al. used 60 micron sections of flash frozen and thawed skin from pigmented mice in which the allele was variably activated in 15-60% of the DP cells per follicle. The figures suggest tissue preservation is superior in our study, and this, the lack of pigment, and the lineage trace may explain the higher average number of DP cells we recorded. Kaushal et al. inferred follicle types based on size. Each hair type has a characteristic range of DP cell number per follicle (Chi et al., 2010). Therefore any incorrect identification of a follicle type will influence this analysis. Size alone is an inexact criteria to define follicle type, and the fact that follicle size correlates with DP number makes the identification of follicle type by size alone for inclusion in this study inherently problematic. DP cell number per follicle within the zigzag population is consistent between mice (Fig. 1d). However the fraction of the second hair coat comprised of zigzag hairs can range from 50-70% in wild type mice (note the s.d. of 7.3 in Fig. 1e control). Thus, without accurate identification of follicle type by reliable criteria, variation between mice due to differential coat composition within a treatment group can be substantial and n=2 mice is inadequate to attribute the differences reported in Kaushal et al. to the genetic manipulation. Furthermore, the analysis at p65, a time when most follicles are in telogen, implies either that the position of hair follicles was not controlled, or that follicles that selectively underwent anagen initiation were sampled. Hair size and DP cell number vary at different positions in the animal.
Our discrepant results could theoretically be reconciled if the relative proportion of zigzag follicles was decreased by activation of β-catenin in the DP. If Kaushal et. al. had inadvertently incorporated larger follicle types in their dataset, but these nevertheless reflected a shift towards larger DP size in the general population, we would not have detected this increase because only zigzag hair follicles were scored in our analysis. However, the second hair coat of mice with activated β-catenin in the DP shows a modest, but significant (p<0.5) increase in the frequency of zigzag hair follicles, so this does not explain the difference in our results (Fig. 1e).
Regardless of whether the discrepant results arise from misidentification of follicle types, inadequate sample depth, or differences in the frequency of β-catenin activation in DP cells, the clear conclusion of our work is that stabilization of β-catenin in the vast majority of DP cells does not result in a change in the number of DP cells per zigzag hair follicle. As this hair type comprises over half of the hair coat, we can conclude that stabilization of β-catenin in the DP does not change DP cell number in the majority of hair follicles. While activation of β-catenin signaling may yet prove an effective strategy to augment hair growth, we felt it important to clarify that de-regulated stabilization of β-catenin in the DP does not enlarge this niche population.
Acknowledgments
This work was supported by grant R01AR055256 from NIAMS to BAM. The authors state no conflict of interest. Animal experiments were approved by the MGH IUCAC.
Abreviations used
- DP
dermal papilla
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development. 2013;140:1676–83. doi: 10.1242/dev.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Wu E, Morgan BA. Earlier-born secondary hair follicles exhibit phenotypic plasticity. Exp Dermatol. 2015;24:265–68. doi: 10.1111/exd.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi WY, Enshell-Seijffers D, Morgan BA. De novo production of dermal papilla cells during the anagen phase of the hair cycle. J Invest Dermatol. 2010;130:2664–66. doi: 10.1038/jid.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010a;18:633–42. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D, Lindon C, Wu E, Taketo MM, Morgan BA. β-catenin activity in the dermal papilla of the hair follicle regulates pigment-type switching. Proc Natl Acad Sci U S A. 2010b;107:21564–69. doi: 10.1073/pnas.1007326107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal GS, Rognoni E, Lichtenberger BM, Driskell RR, Kretzschmar K, Hoste E, et al. Fate of Prominin-1 Expressing Dermal Papilla Cells during Homeostasis, Wound Healing and Wnt Activation. J Invest Dermatol. 2016;135:2926–34. doi: 10.1038/jid.2015.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]