Abstract
Purpose
Clarify which DNA double strand break repair pathway, non-homologous end-joining (NHEJ), homologous recombination repair (HRR) or both, plays a key role in potentially lethal damage repair (PLDR).
Methods and materials
Combining published data and our new potentially lethal damage repair (PLDR) data, we explain whether similar to sublethal damage repair (SLDR), PLDR also mainly depends on NHEJ versus HRR. The PLDR data were used the same cell lines: wild type, HRR or NHEJ deficient fibroblast cells, as those SLDR data published by our laboratory previously. The PLDR condition that we used was as commonly described by many other groups: the cells were collected immediately or overnight post ionizing radiation for colony formation after cultured to a plateau phase with a low concentration of serum medium.
Results
Enough data from other groups and our lab showed that wild type or HRR deficient cells had efficient PLDR, but NHEJ deficient cell lines did not.
Conclusion
NHEJ contributes more to PLDR than HRR in mammalian (including human) cells, which is similar to SLDR. Since both SLDR and PLDR are relevant to clinical tumor status while undergoing radiotherapy, such clarification may benefit radiotherapy in the near future.
Keywords: Ionizing radiation, DNA DSBs, DNA repair, NHEJ, HRR, SLDR, PLDR, Heavy ion
Introduction
When targeted by ionizing radiation (IR), DNA generates base damage, single strand breaks (SSB) and double strand breaks (DSB). DSB are much less than IR-induced base damage and SSB; however, DSB is the major cause for IR-induced cell killing. Irradiated cell survival depends mainly on IR dose and the efficient DSB repair. In general, 10 Gy is the lethal dose for all types of mammalian cells. By adjusting the cell culture conditions post IR or splitting one dose into two exposures below a lethal dose; however, more cells can survive. The former is termed “potentially lethal damage repair (PLDR)” (Belli et al. 1969, Phillips et al. 1966), and the latter is termed “sublethal damage repair (SLDR)” (Elkind et al. 1959). Adjusting cell culture conditions post IR for PLDR mainly occurs by affecting the normal cell cycle progression, which includes treating cells with certain metabolic inhibitors, culturing cells in a low concentration of serum medium or growing the cells to a plateau phase, etc. (Bedford 1991, Iliakis 1988, Little 1969). The majority of SLDR completes within 2 h post first dose of IR exposure (Elkind et al. 1959). Both PLDR and SLDR reflect a conditional promoted repair since both cases result in greater cell survival after the same dose that represent the same yield of DNA DSB.
There are two main DNA DSB repair pathways: non-homologous end-joining (NHEJ) and homologous recombination repair (HRR) in mammalian cells. Comparing the two pathways, NHEJ does not need a homologue template at the DNA DSB end, and is fast as well as independent of the cell cycle (Rothkamm et al. 2003). Different from NHEJ, HRR needs an extended template for a sister chromatin exchange and is more efficient in the S/G2 phases (Jackson et al. 2009, Rothkamm et al. 2003). A recent study reported that SLDR could not be observed in NHEJ deficient CHO cells (Somaiah et al. 2013), suggesting that NHEJ is the major pathway for SLDR, and we used different repair deficient mouse or human cell lines to further demonstrate that SLDR depends mainly on NHEJ (Liu et al. 2015). Since it remains unclear which repair pathway, NHEJ or HRR, or both contributes to PLDR (Hall et al. 2010), although different groups reported that some DNA repair related factors affect the efficiency of PLDR (Arlett et al. 1984, Autsavapromporn et al. 2013, Boothman et al. 1989, Riballo et al. 2004, Veuger et al. 2003, Wilson et al. 1989), it is also necessary to clarify this issue. Combining the published data from other group (Veuger et al. 2003) and our recent data strongly support the conclusion that similar to SLDR, PLDR also uses NHEJ as its main repair pathway and is independent of HRR. We briefly discuss here, possible reasons why NHEJ may play an essential role in SLDR and PLDR. Clinical radiotherapy is closely associated with SLDR (for fractionated IR) and PLDR (hypoxia in big solid tumors is a resistant factor to radiotherapy); therefore, clarifying the major repair pathway for SLDR and PLDR will improve radiotherapy.
Both SLDR and PLDR depend mainly on NHEJ
Recently our SLDR survival experiments not only confirmed previously published CHO cell results (Somaiah et al. 2013), but also verified the results in mouse and human cells (Liu et al. 2015). These results clarify that NHEJ is the major pathway for SLDR (Liu et al. 2015). Using the same cell lines (wild type, HRR deficient and NHEJ deficient CHO, mouse and human cells), we performed PLDR survival experiments and obtained similar results to SLDR. The data regarding NHEJ deficient cell lines did not show efficient PLDR, whichconfirmed the results previously reported by another group (Veuger et al. 2003).
Interestingly, it is known that both SLDR and PLDR dramatically decreased in heavy ion (high-linear energy transfer (LET) IR)-irradiated cells as compared to low-LET irradiated cells (Hall et al. 1975, Tsujii et al. 2014). Previously our group as well as other groups have shown that the enhanced relative biological effectiveness (RBE) on survival in high-LET irradiated cells is via interfering with NHEJ (Lind et al. 2003, Okayasu 2006, Wang et al. 2008), but not via affecting HRR (Wang et al. 2008, Wang et al. 2010, Zafar et al. 2010). These results indicate that high-LET irradiated cells depend more on HRR to repair DNA DSB and maintain their survival. We have observed that cells deficient in HRR are more sensitive to high-LET IR and showed higher RBE on their survival versus wild type cells (Wang et al. 2008). These results provide additional evidence to support that HRR does not affect PLDR. Based on these data, we conclude that both NHEJ but not HRR is the major pathway for both SLDR and PLDR (Figure 1).
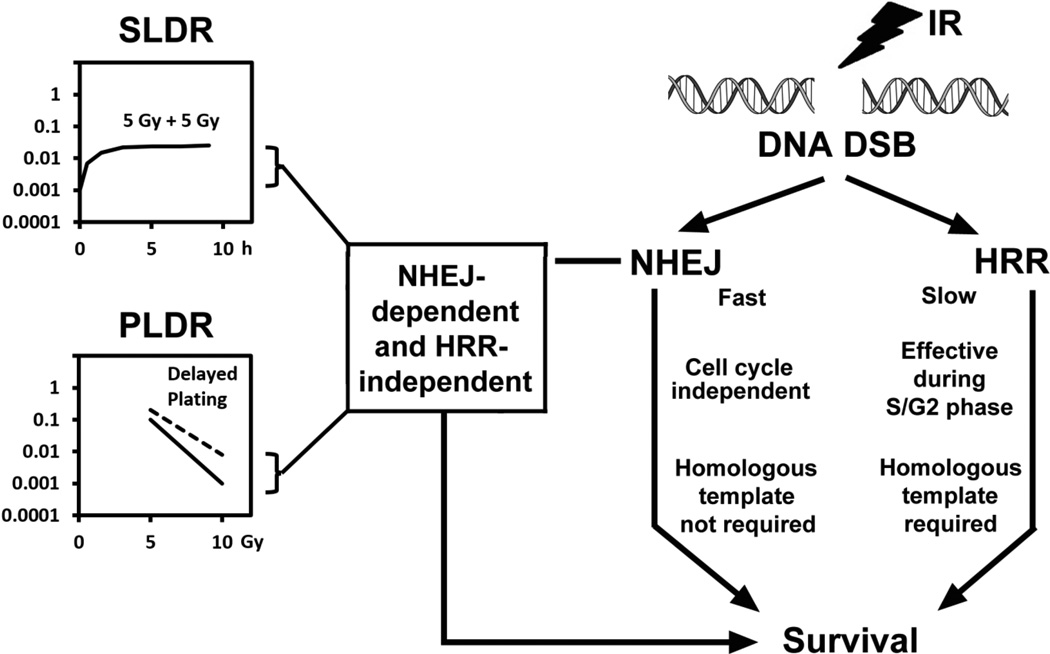
Figure 1.
Outline the choice of the DNA DSB repair pathway for SLDR and PLDR. Sublethal Damage Repair (SLDR) denotes the phenomenon that cell survival increases when irradiated with split doses compared to a single dose of irradiation. Potential Lethal Damage Repair (PLDR) denotes the phenomenon that survival of stationary-phase cells increases under delayed plating cells after irradiation, compared to immediate plating cells. Both SLDR and PLDR effects depend on the fast, cell cycle independent and simple NHEJ pathway but not the slow, S/G2 phase-dependent and complex HRR pathway.
Possible reasons why NHEJ repairs SLD and PLD
Although it is impossible to clearly elucidate why NHEJ is the major repair pathway for SLD and PLD without further studies, below we provide two possible reasons to explain the relationship between SLDR/PLDR and NHEJ based on the published data and our experience.
1. G1 phase cells prefer to use NHEJ immediately post-IR
It is known that HRR requires DNA templates and is efficient in the S/G2 phase but NHEJ is independent of the cell cycle (Jackson et al. 2009, Rothkamm et al. 2003). In general, mammalian cells under normal conditions have ≥ 70% in the G1 phase. It is efficient for mammalian cells using the NHEJ pathway to repair DNA DSB immediately post-IR exposure. In addition, although both NHEJ and HRR are required to maintain irradiated cell survival (Couedel et al. 2004, Mills et al. 2004), NHEJ and HRR compete as well as inhibit each other (Allen et al. 2002, Kim et al. 2005, Sonoda et al. 2006), suggesting that a preferred choice between the two repair pathways under varying conditions and time points post-IR exposure exists in irradiated cells. It has also been known for many years that IR reduces DNA replication initiation (Little 1968), which reflects an active cell response to help irradiated cell survival (Hartwell et al. 1989). These results indicate that immediately following IR, mammalian cells reduce their entrance the S phase and increase their G1 phase ratio, which also benefits cells that choose NHEJ. Therefore, maximizing cell survival following radiation, it is reasonable that mammalian cells prefer using NHEJ to HRR immediately following IR exposure.
2. Reduced disturbance of irradiated cells is a key requirement for SLDR and PLDR
Although SLDR and PLDR reflect different repair conditions post-IR exposure, one common feature is that both SLDR and PLDR occur when plated cells for a clonogenic assay is delayed. In addition, we observed a significant increase in survival during the delay plating with wild type or HRR deficient cells but not NHEJ deficient cells at exponential growth conditions, which is similar to that observed for PLDR (at dense-growth conditions) (our unpublished data). Therefore, the key requirement for SLDR and PLDR is actually to “delay plating cells for a clonogenic assay post-IR exposure”. More specifically, reduced disturbance to irradiated cells is essential for SLDR and PLDR, which could be due to NHEJ occurring more efficiently in consistent conditions although nutrition deficiencies affect NHEJ less than HRR. Immediate plating changes the cell environment, cell shape, chromatin structure and the relationship between non-rejoined DNA ends, which reduces the efficiency of NHEJ. In addition, immediate plating stimulates more cells to enter the S phase, which may induce more inhibitory effects on NHEJ from the HRR process during the S phase since it is known that NHEJ efficiency is inhibited by HRR (Allen et al. 2002). Of course, additional studies are needed to explore other possible reasons for this.
The relevance of SLDR and PLDR to clinical radiotherapy
Delayed plating cells (reduce disturbance of cell condition) post-IR exposure is essential for SLDR and PLDR. However, immediately plating the cells (also reducing disturbance of cell condition) post-IR exposure reflects a human-controlled in vitro process. In fact, irradiated patient tumors are not immediately affected by such disturbances, which results in repair including SLDR and PLDR that occur within a few hours post-IR exposure. Although IR-induced re-oxygenation and re-assortment (redistribution of the cell cycle) occurs a few hours post-IR, as well as repopulation that occurs several weeks post-IR exposure can also affect the repair efficiency, such effects are much different than the effects after immediately plating in vitro. As we mentioned above, immediate plating affects the cell shape, chromatin structure and relationship between non-rejoined DNA ends, which reduces the efficiency of NHEJ. Therefore, in general, SLDR and PLDR should exist in tumors during radiotherapy, which mainly depends on NHEJ. Blocking NHEJ will inhibit SLDR and PLDR, which can sensitize tumors to radiotherapy although the final radiotherapy results on locally controlled tumors not only depends on NHEJ-mediated SLDR and PLDR, but also depends on the HRR efficiency as well as the genetic background of tumors, which may affect NHEJ, HRR or both.
Taken together, NHEJ is the preferred choice for immediate repair of DNA DSB post-IR exposure. To clarify the important role that NHEJ plays in SLDR and PLDR will help to improve radiotherapy.
Acknowledgments
We thank Ms. Doreen Theune for editing the manuscript. This work is supported by grants from the National Aeronautics and Space Administration (NNX11AC30G to Y.W.) and the National Cancer Institute (CA186129, CA185882, to Y.W. and P30CA138292 to the Institute).
Abbreviation
- IR
Ionizing radiation
- DSB
double strand break
- NHEJ
non-homologous end-joining
- HRR
homologous recombination repair
- SLDR
Sublethal damage repair
- PLDR
Potentially lethal damage repair
- LET
Linear energy transfer
- CHO
Chinese hamster ovary
Footnotes
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the contents and writing of the paper.
References
- Allen C, Kurimasa A, Brennema MA, Chen DJ, Nickoloff JA. DNA-dependent protein kinase suppresses double-strand break-induced and spontaneous homologous recombination. Proc Natl Acad Sci USA. 2002;99:3758–3763. doi: 10.1073/pnas.052545899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlett CF, Priestley A. Deficient recovery from potentially lethal damage in some gamma-irradiated human fibroblast cell strains. Brit J Cancer. 1984;49:227–232. [PMC free article] [PubMed] [Google Scholar]
- Autsavapromporn N, Suzuki M, Plante I, Liu C, Uchihori Y, Hei TK, Azzam EI, Murakami T. Participation of gap junction communication in potentially lethal damage repair and DNA damage in human fibroblasts exposed to low- or high-LET radiation. Mutat Res. 2013;756:78–85. doi: 10.1016/j.mrgentox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford JS. Sublethal damage, potentially lethal damage, and chromosomal aberrations in mammalian cells exposed to ionizing radiations. Int J Radiat Oncol Biol Phys. 1991;21:1457–1469. doi: 10.1016/0360-3016(91)90320-4. [DOI] [PubMed] [Google Scholar]
- Belli JA, Shelton M. Potentially lethal radiation damage: Repair by mammalian cells in culture. Science. 1969;165:440–442. doi: 10.1126/science.165.3892.490. [DOI] [PubMed] [Google Scholar]
- Boothman DA, Trask DK, Pardee B. Inhibition of potentially lethal DNA damagea repair in human tumor cells by Beta-lapachone, an activator of topoisomerase I. Cancer Res. 1989;49:605–612. [PubMed] [Google Scholar]
- Couedel C, Mills KD, Barchi M, Shen L, Olshen A, Johnson RD, Nussenzweig A, Essers J, Kanaar R, Li GC, et al. Collaboration of homologous recombination and nonhomologous end-joining factors for the survival and integrity of mice and cells. Genes Dev. 2004;18:1293–1304. doi: 10.1101/gad.1209204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind M, Sutton H. X-ray damage and recovery in mammalian cells in culture. Nature. 1959;184:1293–1295. doi: 10.1038/1841293a0. [DOI] [PubMed] [Google Scholar]
- Hall E, Giaccia A. Radiobiology for the Radiologist. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- Hall EJ, Roizin-Towle L, Theus RB, August LS. Radiobiological Properties of High—Energy Cyclotron-Produced Neutrons Used for Radiotherapy. Radiology. 1975;117:173–178. doi: 10.1148/117.1.173. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: Controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Iliakis G. Radiation induced potentially lethal damage: DNA lesions susceptible to fixation. (Review Article) Int J Radiat Biol. 1988;53:541–584. doi: 10.1080/09553008814550901. [DOI] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, Krasieva TB, Kurumizaka H, Chen DJ, Taylor AMR, Yokomori K. Independent and sequential recruitment of NHEJ and HR factors to DNA damage sites in mammalian cells. J Cell Biol. 2005;170:341–347. doi: 10.1083/jcb.200411083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind B, Persson L, Edgren M, Hedlöf I, Brahme A. Repairable–Conditionally Repairable Damage Model Based on Dual Poisson Processes. Radiat Res. 2003;160:366–375. doi: 10.1667/0033-7587(2003)160[0366:rrdmbo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Little JB. Delayed initiation of DNA synthesis in irradiated human diploid cells. Nature. 1968;218:1064–1065. doi: 10.1038/2181064a0. [DOI] [PubMed] [Google Scholar]
- Little JB. Repair of sub-lethal and potentially lethal radiation damage in plateau phase cultures of human cells. Nature. 1969;224:804–806. doi: 10.1038/224804a0. [DOI] [PubMed] [Google Scholar]
- Liu M, Lee S, Liu B, Wang H, Dong L, Wang Y. Ku-dependent Non homologous End-joining as the Major Pathway Contributes to Sublethal Damage Repair. Int J Radiat Biol. 2015;91:867–871. doi: 10.3109/09553002.2015.1075178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KD, Ferguson DO, Essers J, Eckersdorff M, Kanaar R, Alt FW. Rad54 and DNA Ligase IV cooperate to maintain mammalian chromatid stability. Genes Dev. 2004;18:1283–1292. doi: 10.1101/gad.1204304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayasu R, Okada M, Okabe A, Noguchi M, Takakura K, Takahashi S. Repair of DNA Damage Induced by Accelerated Heavy Ions in Mammalian Cells Proficient and Deficient in the Non-homologous End-Joining Pathway. Radiat Res. 2006;165:59–67. doi: 10.1667/rr3489.1. [DOI] [PubMed] [Google Scholar]
- Phillips RA, Tolmach LJ. Repair of potentially lethal damage in x-irradiated hela cells. Radiation Research. 1966;29:413–432. [PubMed] [Google Scholar]
- Riballo E, Kuhne M, Rief N, Doherty A, Smith GC, Recio MJ, Reis C, Dahm K, Fricke A, Krempler A, et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol Cell Biol. 2003;23:5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaiah N, Yarnold J, Lagerqvist A, Rothkamm K, Helleday T. Homologous recombination mediates cellular resistance and fraction size sensitivity to radiation therapy. Radiother Oncol. 2013;108:155–161. doi: 10.1016/j.radonc.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair. 2006;5:1021–1029. doi: 10.1016/j.dnarep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Tsujii H, Kamada T, Shirai T, Noda K, Tsuji H, Karasaga K. Carbon-Ion Radiotherapy, Principles, Practices, and Treatment Planning. Springer; 2014. [Google Scholar]
- Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 2003;63:6008–6015. [PubMed] [Google Scholar]
- Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 2003;63:6008–6015. [PubMed] [Google Scholar]
- Wang H, Wang Y. Checkpoint response plays a more protective role in HZE particle-irradiated cells than in X ray-irradiated cells. Cell Cycle. 2008;7:2444–2445. doi: 10.4161/cc.6552. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang X, Zhang P-Y, Wang Y. The Ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair. 2008;7:725–733. doi: 10.1016/j.dnarep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang X, Wang P, Yu X, Essers J, Chen D, Kanaar R, Takeda S, Wang Y. Characteristics of DNA-binding proteins determine the biological sensitivity to high-linear energy transfer radiation. Nucleic Acids Res. 2010;38:3245–3251. doi: 10.1093/nar/gkq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KM, Keng PC. Radiation-induced DNA damage and repair in quiescent and proliferating human tumor cells in vitro. Int J Radiat Biol. 1989;55:385–395. doi: 10.1080/09553008914550431. [DOI] [PubMed] [Google Scholar]
- Zafar F, Seidler SB, Kronenberg A, Schild D, Wiese C. Homologous Recombination Contributes to the Repair of DNA Double-Strand Breaks Induced by High-Energy Iron Ions. Radiat Res. 2010;173:27–39. doi: 10.1667/RR1910.1. [DOI] [PubMed] [Google Scholar]