Abstract
Objective
Up to 12 % of all endometrial-carcinomas (EC) harbor DNA-polymerase-ε-(POLE) mutations. It is currently unknown whether the favorable prognosis of POLE-mutated EC is derived from their low metastatic capability, extraordinary number of somatic mutations thus imparting immunogenicity, or a high sensitivity to chemotherapy.
Methods
Polymerase-chain-reaction-amplification and Sanger-sequencing were used to test for POLE exonuclease-domain-mutations (exons 9–14) 131 EC. Infiltration of CD4+ and CD8+ T-lymphocytes (TIL) and PD-1-expression in POLE-mutated vs POLE wild-type EC was studied by immunohistochemistry (IHC) and the correlations between survival and molecular features were investigated. Finally, primary POLE-mutated and POLE-wild-type EC cell lines were established and compared in-vitro for their sensitivity to chemotherapy.
Results
Eleven POLE-mutated EC (8.5%) were identified. POLE-mutated tumors were associated with improved progression-free-survival (P<0.05) and displayed increased numbers of CD4+ (44.5 vs 21.8; P = .001) and CD8+ (32.8 vs 13.5; P < .001) TILs when compared to wild-type POLE EC. PD-1 receptor was overexpressed in TILs from POLE-mutated vs wild-type-tumors (81% vs 28%; P < .001). Primary POLE tumor cell lines were significantly more resistant to platinum-chemotherapy in-vitro when compared to POLE-wild-type tumors (P < 0.004).
Conclusions
POLE ultra-mutated EC are heavily infiltrated with CD4+/CD8+ TIL, overexpress PD-1 immune-check-point (i.e., features consistent with chronic antigen-exposure), and have a better prognosis when compared to other molecular subtypes of EC patients. POLE-mutated tumor-cell lines are resistant to platinum-chemotherapy in-vitro suggesting that the better prognosis of POLE-patients is not secondary to a higher sensitivity to chemotherapy but likely linked to enhanced immunogenicity.
Keywords: polymerase ε, endometrial carcinoma, uterine serous papillary cancer, cisplatin, whole exome sequencing
Introduction
Endometrial cancer (EC) represents a major cause of morbidity and mortality in the US with over 10,000 estimated deaths in 2016 [1]. The standard treatment modalities consist of surgery, post-operative radiotherapy and/or chemotherapy. While the majority of patients with EC have improved outcomes with these regimens, many patients fail to improve with the conventional treatments, and are thus exposed to substantial toxicities without much benefit.
The Cancer Genome Atlas (TCGA) network [2] has recently redefined the molecular classification of EC. This new molecular classification provides evidence that these tumors result from heterogeneous somatic mutations. The TCGA classifies EC into four categories: 1) polymerase epsilon (POLE)-ultra-mutated, 2) microsatellite instability hyper-mutated, 3) copy-number low and 4) copy-number high, serous-like [2]. Of great interest, mutations in the proofreading POLE gene were detected in 7–12% of EC patients [2–9] and thus identified a group of ultra-mutated tumors (ie, 232 × 10−6 mutations per Megabase). These POLE mutated tumors, although with high grade features (i.e., G3), have a favorable prognosis [2–9].
POLE constitutes a nuclear DNA polymerase endowed with intrinsic proofreading activity [2–9]. Together with polymerase δ (POLD), POLE is responsible for the bulk of chromosomal DNA synthesis during cell division. Multiple studies in yeast and mammalian cells have shown that polymerase proofreading and post-replication mismatch repair (MMR) represent the primary guardians of DNA replication fidelity [2–12]. Consistent with this view, loss of function in one or both of these genes is known to dramatically increase the number of spontaneous mutations [2–12]. Most of the POLE mutations detected in EC [2] cluster at invariate/highly conserved residues within, or near to, exo motifs required for exonuclease activity. Structural mapping demonstrates that most POLE mutated tumors are likely to compromise proofreading through perturbation of DNA binding.
It is currently not understood why patients with a POLE ultra-mutated phenotype may have improved outcomes [2–9]. Along these lines, multiple non-mutually exclusive hypotheses have been used to explain the favorable prognosis of POLE mutated EC patients. First, these tumors may be less fit than other cancers, as a result of their extremely high number of mutations, to quickly metastasize in the body. Second, due to their extraordinary number of somatic mutations these cancers may be highly immunogenic for the host. Finally, because of their defective DNA repair, they may be more sensitive to standard anti-cancer treatments such as chemotherapy [13].
In this study, in an attempt to further increase our knowledge of the biological properties of POLE mutated EC we have: a) sequenced the exonuclease domain of POLE (ie, exons 9 to 14) in 131 Type I (endometrioid) and Type II (non-endometrioid) EC patients and evaluated the association of POLE exonuclease mutations with survival; b) analyzed the infiltration of CD4+ and CD8+ T lymphocytes (TIL) and programmed cell death protein 1 (PD-1) expression in POLE-mutated vs POLE wild type EC and lastly, c) established primary POLE-mutated and POLE wild type EC cell lines and determined whether POLE-mutated tumors may differ in their sensitivity to platinum-based chemotherapy in vitro. We report evidence that POLE ultra-mutated tumors have a better prognosis when compared to POLE wild type tumors and they are significantly more infiltrated with T cells. These tumors also demonstrated a stronger up-regulation of the PD-1 immune check point receptors. Importantly, primary POLE ultramutated EC cell lines were found significantly more resistant to platinum but not paclitaxel when compared to primary POLE wild type EC in vitro. These results further support the hypothesis that the better prognosis of POLE patients is not related to a higher sensitivity to chemotherapy, but likely linked to their enhanced immunogenicity.
Patients and Methods
Specimens were obtained through the Gynecologic Oncology Section of the Obstetrics and Gynecology Department and the Pathology Department at the University of Brescia School of Medicine and at Yale University School of Medicine under approval of the Institutional Review Board. Tumor DNA was extracted from fresh frozen tissue from 131 EC patients. Haematoxylin and eosin-stained slides were reviewed and DNA extracted from consecutive sections containing >75% cancer cells by standard methods. Clinico-pathological information was obtained from medical records. Most patients (n = 96) had endometrioid (Type I) tumors, while 35 patients had serous (Type II) or mixed endometrioid/serous histology and the remainder had less common subtypes. Patient characteristics and molecular features of tumors included in the study are found in Table 1.
Table 1.
Demographic and clinic-pathologic features by POLE mutation status
| Patients | POLE wild-type (n=120) No. (%) 120 (91.6) |
POLE mutant (n=11) No. (%) 11 (8.4) |
|
|---|---|---|---|
| Age | |||
| Mean | 59.7 | 56.8 | |
| Range | 31–84 | 31–87 | |
| <60 | 66 (55.0) | 6 (54.5) | |
| 60–70 | 33 (27.5) | 4 (36.4) | |
| >70 | 21 (17.5) | 1 (9.1) | |
| Tumor type | |||
| EEC | 89 (74.1) | 7 (63.6) | |
| NECC | 31 (25.8) | 4 (36.4) | |
| Grade | |||
| G1 | 13 (10.8) | 3 (27.3) | |
| G2 | 41 (34.2) | 1 (9.1) | |
| G3 | 66 (55) | 7 (63.6) | |
| FIGO | |||
| I | 54 (45.0) | 8 (72.7) | |
| II | 22 (18.3) | 1 (9.1) | |
| III | 33 (27.5) | 1 (9.1) | |
| IV | 11 (9.2) | 1 (9.1) | |
| Rad/Chem | |||
| No Treatment | 28 (23.3) | 1 (9.1) | |
| Radiotherapy | 48 (40.0) | 6 (54.5) | |
| Chemotherapy | 19 (15.8) | 2 (18.2) | |
| Radio+Chemo | 25 (20.8) | 2 (18.1) |
*ECC: Endometrioid Endometrial Cancer.
*NEEC: Non-Endometrioid Endometrial Cancer.
Primary tumor cell lines
Fresh endometrial tumor cells were obtained from surgical specimens. Single cell suspensions were obtained by processing solid tumor samples under sterile conditions at room temperature as previously described [9]. Briefly, viable tumor tissue was mechanically minced in 5 ml of enzyme solution [0.14% collagenase Type I (Sigma, St. Louis, MO) and 0.01% DNAse (Sigma, 2000 KU/mg)] in RPMI 1640 to portions no larger than 1–3 mm3 and placed into 250 ml trypsinizing flasks containing 20 ml more of enzyme solution. This mix was then incubated on a magnetic stirring apparatus for 45 minutes at RT. Enzymatically dissociated tumor was then filtered through 150 µm nylon mesh to generate a single cell suspension. The resultant cell suspension was then washed twice in RPMI 1640 plus 10% fetal bovine serum (FBS)(Sigma). Fresh tumor cell lines were maintained initially in RPMI 1640, supplemented with 10% FBS at 37 °C, 5% CO2. Characteristics of the POLE wild type and POLE-mutated EC cell lines used in this study are found in Table 2. In the in vitro experiments described below we used both primary EC cell lines from early-passage (<5) as well as late-passage cultures (> 50). During the in vitro growth we did not notice any significant change in the doubling time or morphology of the cell line studied. All experiments were performed with fresh or cryopreserved tumor cultures which had at least 90% viability and contained >99% tumor cells.
Table 2.
Characteristics of primary POLE wild type and POLE-mutated EC cell lines.
| Sample ID |
Histology | Grade | Stage | Age | Race | Somatic missense POLE mutations |
|---|---|---|---|---|---|---|
| UTE4 | END* | G3 | IVA | 65 | W^ | A957V*** |
| ARK6 | USC** | G3 | IB | 48 | W | H76N-D368Y-A832T-C1642Y- A1967B-G2076V-L2207I |
| UTE 3 | END | G3 | IIIA | 66 | B~ | not present |
| ARK2 | USC | G3 | IVB | 63 | B | not present |
| ARK7 | USC | G3 | IIC | 75 | W | not present |
| ARK11 | USC/END | G3 | IIIC | 80 | B | not present |
END: endometrioid adenocarcinoma,
USC: uterine serous carcinoma,
A957V: polymerase domain mutation identified by whole exome sequencing (WES)[22].
White;
Black.
Molecular Analysis
The exonuclease domain of POLE (ie, exons 9–14) was assessed for mutations using PCR amplification (AmpliTaq DNA Polymerase Applied Biosystems) and Sanger sequencing. Primers are provided in supplementary Table A. Briefly, PCR products were treated with QIAquick PCR Purification Kit (cat. 28104, Qiagen) and sequenced (Applied Biosystems 3730xl DNA Analyzer) at the Keck DNA sequencing facility at Yale University School of Medicine. Sequences were analyzed and all variants were tested in matched normal DNA to determine if they were somatic or germline alterations.
Microsatellite instability (MSI) analysis
Primary cell lines MSI status was determined using a panel of five markers (BAT25, BAT26, D2S123, D5S346 and BAT40) for Polymerase Chain Reaction (PCR) as well as standard IHC techniques. Briefly, evaluation of MMR protein expression was performed on 5-µm sections of the tissue or cell blocks using antibodies to MLH1 (clone G168-15, 1:75 dilution, Biocare Medical, Concord, CA), PMS2 (clone A16-4, 1:300 dilution, Biocare Medical), MSH6 (clone BC/44, 1:100 dilution, Biocare Medical) and MSH2 (clone FE11, 1:100 dilution, Biocare Medical). Detection was performed using the MACH 3 Mouse HRP-Polymer Detection Kit (Biocare Medical). Chromogenic detection was achieved with diamniobenzidine (Dako) and sections were counterstained with hematoxylin (Dako). Results were evaluated by a board certified gynecologic pathologist experienced in MMR protein immunohistochemical interpretation. Staining of any tumor nuclei was interpreted as positive, with expression by lymphocytes and/or stromal cells considered a positive internal control. Tumors were classified as MSI-low if they had one unstable marker, MSI-high if they had two or more unstable markers and MSI-negative/MSS if all markers were stable by PCR.
Immunohistochemistry and evaluation of tumor associated lymphocytes (TIL)
Immunohistochemistry (IHC) was performed for CD3, CD4, CD8 and PD-1 on formalin fixed paraffin embedded (FFPE) tissue samples using standard protocols. Briefly, a tissue microarray (TMAs) was created from 48 formalin-fixed, paraffin-embedded EC and 25 normal endometrial (NE) tissues at the Department of Pathology, University of Brescia, Italy. TMAs were built using an automated tissue microarrayer (TMA Master, 3DHistech, Budapest, Hungary). Representative areas were chosen for sampling from haematoxylin and eosin (H&E) stained sections of selected NE and EC cases. Four 0.6-mm cores were collected from different areas of each tumor block in order to overcome sample heterogeneity and the possible loss of tissue due to cutting. Four micron sections were cut from TMAs and H&E staining was used for confirmation of tumor tissue. TMA serial sections were subjected to double immunohistochemistry for PD-1 combined with CD3 or CD8. Briefly, after antigen retrieval (microwave oven 3×5’ at 750 W in citrate buffer pH6.0), sections were incubated 60’ at RT with anti-PD-1 mouse monoclonal antibody (clone NAT 105/e3J, dilution 1:800, kindly provided by Dr. T. Marafioti, Department of Cellular Pathology, University College London, London, UK). The antibody was revealed by using horseradish peroxidase-conjugated Novolink polymer (Leica Biosystems, Newcastle upon Tyne, UK), followed by diaminobenzidine (DAB) as chromogen. After completing the first immune reaction, sections were incubated with anti-CD3 rabbit monoclonal (clone SP7, dilution 1:100, Thermo Scientific, Fremont, CA) or -CD8 mouse monoclonal (clone C8/144B, dilution 1:30, Dako, Glostrup, Denmark) antibodies. The second immune reaction was visualized using alkaline phosphatase-conjugated Mach4 MR (Biocare Medical, Concord, CA) followed by Ferangi Blue (Biocare Medical) as chromogen. Slight hematoxylin was used for counterstaining. For evaluation of TILs, we focused only on intraepithelial lymphocytes, i.e. lymphocytes located within the tumor epithelium, rather than in the peri-tumoral stroma. The immunostainings on TIL were performed on serial sections, which were scanned and digitalized using the Aperio Scanscope CS (Nikon). Two brands of CD4 antibodies (4B12 from Dako and from Thermo Scientific) were verified, but the reactivity of CD4+ cells was not bright enough to allow PD-1+CD4+ scoring. PD-1+CD4+ were therefore inferred from the total count of PD-1+CD3+ cells minus PD-1+CD8+ cells on sequential section. The mean area analyzed was 1.1 mm2 in EC and 0.56 mm2 in control tissues. Digital images were resized by using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA). Two independent gynecological pathologists examined the TMA immunoreactivity. Peri-tumoral lymphocytes (lymphocytes in the stroma immediately adjacent to the tumor epithelium) were scored using a semi-quantitative method [none (0), mild (1+), moderate (2+), marked (3+)].
Flow Cytometry
Briefly, 0.1 g of fresh tumor tissue obtained from two patients harboring endometrial cancer (ie, one harboring a POLE-ultra-mutated tumor and one harboring a POLE wild type cancer) where reduced in single cell suspensions after the processing of the solid tumor samples under sterile conditions as previously described [9]. Cell suspensions containing tumor cells and TIL were then washed twice in RPMI 1640 plus 10% autologous plasma, placed on discontinuous Ficoll–Hypaque (75/100%) density gradients, and centrifuged again to separate and harvest TIL and tumor cells. Enriched TIL preparations were then washed twice in RPMI 1640 plus 10% autologous plasma before being stained for superficial antigen expression analysis by flow cytometry using directly conjugated MAbs against CD4 (Leu-3, T helper/inducer); CD8, (Leu-2a, T cytotoxic/suppressor), (both from Becton–Dickinson, San Jose, CA) and anti-PD-1/CD279 (clone MIH4, from BD Pharmingen). The entire population of TIL from both patients was divided in the staining tubes and counted using flow cytometry. All analyses were conducted with a FACSCalibur, utilizing Cell Quest software (Becton–Dickinson).
Drug
Carboplatin and paclitaxel were purchased from Sigma (Sigma-Aldrich, St. Louis, MO). Carboplatin was dissolved in 0.9% sodium chloride while paclitaxel was dissolved in dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) as stock solutions protected from light exposure and stored at −20°C.
Chemo-response assay
The effect of carboplatin and paclitaxel on the viability and IC50 of cells was determined using flow cytometry assays as previously described [14]. Briefly, tumor cells derived from 5 primary EC cell lines established as long term cultures in vitro (i.e., 3 cell lines harboring wild type POLE versus 2 harboring POLE hot-spot mutations) were plated in six-well tissue culture plates and when in exponential growth treated with carboplatin and paclitaxel at scalar concentrations. After 72 hours of additional incubation, well contents were harvested in their entirety, centrifuged then stained with propidium iodide (2 µL of a 500 µg/mL stock solution in PBS with 0.1% sodium azide and 2% fetal bovine serum) for flow cytometric counts. Viable cells were then quantified using flow cytometry as percent of viable cells (mean +/− SEM) after exposure to different concentrations of chemotherapy agents relative to vehicle-treated cells taken as 100% viable. A minimum of 3 independent experiments per EC cell line were performed
Statistics
Data were analyzed in Excel and graphs were plotted using Prism6 (Graphpad software). Comparisons of tumor-infiltrating T lymphocyte counts and other categorical variables were carried out by using Wilcoxon-Mann-Whitney, Fisher's exact test and continuous variables using the t test. Statistical analysis was performed using SPSS version 18. A P-value < 0.05 was considered as the level of statistical significance.
Results
POLE Mutations in EC
Mutations were identified in 11 of 131 (8.5%) EC analyzed. All eleven mutations identified have previously been described (Supplementary Table B). The “hot spots” P286R and V411L mutations were each present in 2 and 4 tumors, respectively. Mutations A456P were detected in 2 tumors while S459F and S297F mutations were detected in one tumor each. None of the mutations were detected in the germline DNA of the POLE mutated patients.
POLE mutation is associated with increased survival
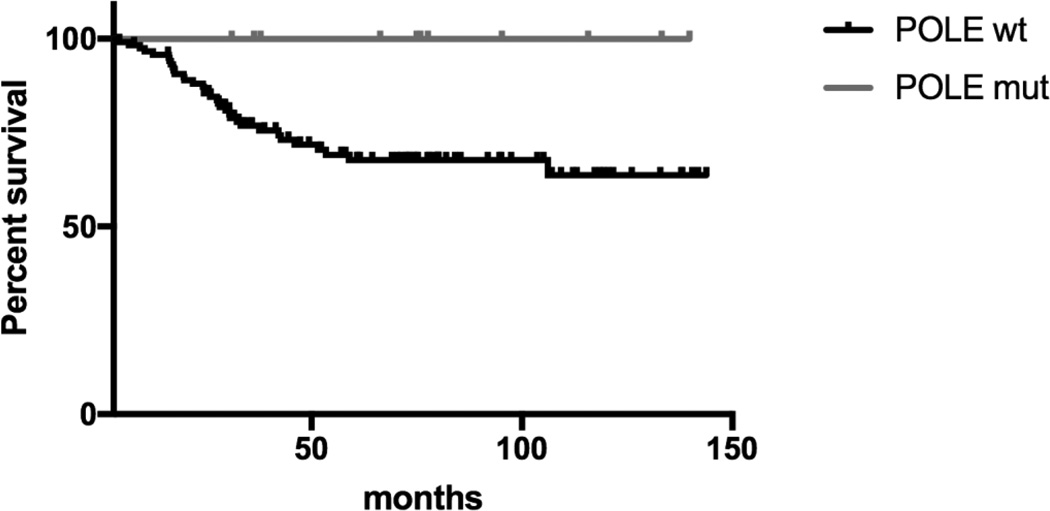
We next investigated whether POLE mutation was associated with survival. As demonstrated in Figure 1, Kaplan-Meier curves demonstrated POLE mutations to be associated with improved overall survival when compared to non-POLE EC patients (p = 0.04). We found no cancer related deaths among the 11 patients (8.4%) whose tumors had a POLE mutation (mean follow-up time = 80.4 months, median follow-up time 75.9 months, range follow-up time = 108.8 months). In contrast, cancer related mortality of wild-type patients was 28.3 % (mean follow-up time =55.5 months, median follow-up time 41.8 months, range follow-up time = 140.2 months) (Figure 1)
Figure 1.
Kaplan-Meier estimates for overall survival according to POLE mutational status. P value calculated using log-rank test = 0.04).
POLE-mutated EC are microsatellite-stable
Although a few cases of POLE MSI-high EC have recently been reported, the majority of previous studies have shown that POLE-mutated EC are microsatellite-stable (MSS) [15, 16]. To evaluate MSI status in POLE-mutated tumor cell lines and whether microsatellite instability may change after prolonged in vitro cultures we tested the tumor tissue blocks and the matched cell blocks of 2 POLE patients for MSI. We found the tumor tissue blocks of the POLE-mutated tumors (ie, UTE4 and ARK6) to be MSS (no instability in any of the evaluated markers) and MSI-L (instability in one out of five of the evaluated markers), respectively, when tested by PCR. In contrast, when the tumor cell blocks from the cell lines were tested after greater than 50 passages in vitro, we found ARK6 primary tumor cell line (but not UTE4 cell line) to have acquired a MSI-high phenotype (i.e., 4 unstable loci present in the cell line out of five evaluated vs one in the un-manipulated tumor tissue block) (data not shown).
TIL and PD-1 Expression in POLE mutated vs wild type EC
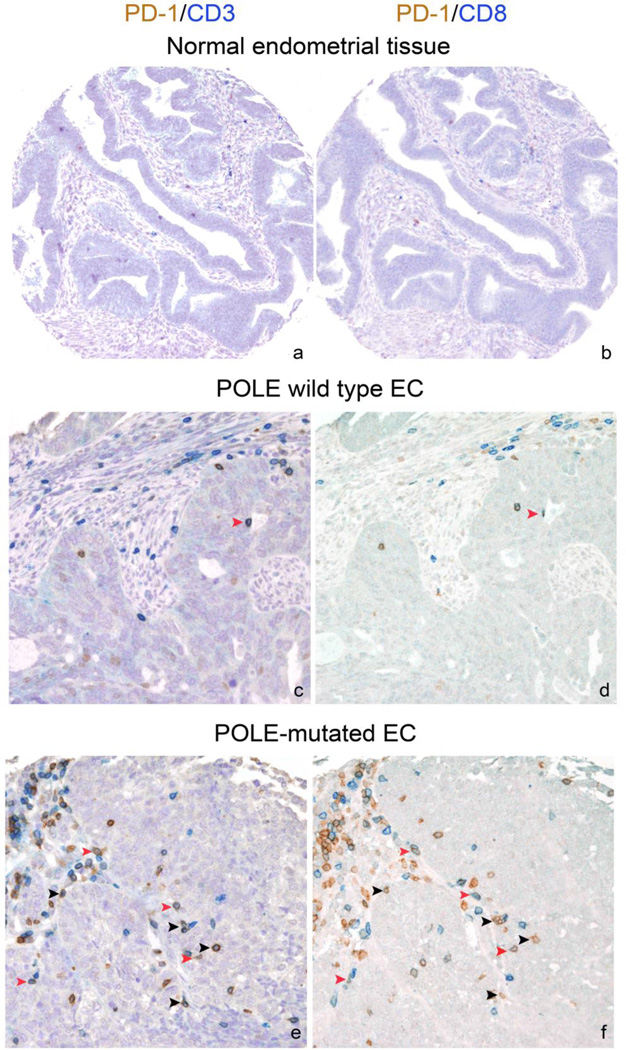
We used IHC co-staining to simultaneously detect the expression of PD-1, as well as CD3, CD8 and CD4, in formalin-fixed, paraffin-embedded (FFPE) TMAs. We initially compared patterns of PD-1+ TIL in tumor and control tissues. PD-1+ CD8+ TILs were significantly higher in EC compared to normal endometrial tissues (median: 21 vs 0, mean: 55 vs 1.56, p=0.0001, n = 25). A similar staining pattern was also observed for PD-1+ CD4+ TILs, that were found predominantly in tumor compared to control tissues (median: 11 vs 0, mean: 20 vs 0.2, p=0.0001). We next compared PD-1+TIL expression in POLE-mutated vs POLE wild type EC. The POLE mutated tumors exhibited a statistically significantly higher number of PD-1+ CD8+ TILs compared to POLE wild type ECs (median: 125 vs 15, mean: 106 vs 49, p=0.017, n = 48, Figure 2). POLE mutated cancer patients were also characterized by a higher number of PD-1+ CD4+ TILs compared to POLE wild type patients (median: 51 vs 10, mean: 56 vs 18, p=0.017, n = 48, data not shown). Flow cytometry results obtained using fresh TIL from two representative patients (ie, one harboring a POLE-ultra-mutated tumor and one harboring a POLE wild type endometrial cancer) were also performed and found confirmatory of the TMA results (Supplementary Figure 1).
Figure 2.
Representative photomicrographs of double immunostaining for PD-1 (brown) and CD3 (blue) (a,c,e) and for PD-1 (brown) and CD8 (blue) (b,d,f) on TMA sequential sections from representative FFPE normal endometrial tissues (a–b), POLE wild type (c–d) and POLE-mutated (e–f) EC cases. TILs are generally scant in POLE wild type but significantly increased in POLE-mutated tumors. Black arrowheads indicate PD-1+CD3+CD8-cells (inferred PD-1+CD4+); red arrowheads indicate PD-1+CD3+CD8+ cells. Sections are counterstained with Meyer’s haematoxylin. Original magnifications: 100× (a–b); 400× (c–f).
POLE mutated tumors are more resistant to carboplatin than POLE wild type EC
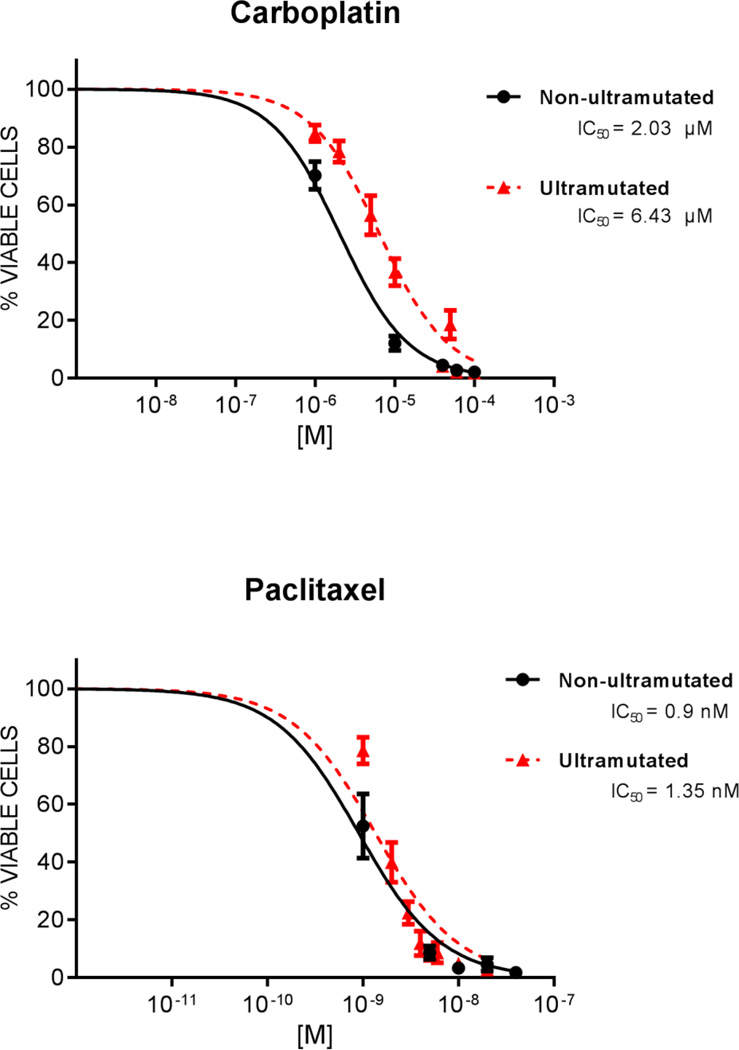
Next, we performed chemo-sensitivity testing of 5 primary EC cell lines (i.e., 2 primary POLE-mutated EC cell lines and 3 EC harboring wild type POLE). These EC cell lines were chosen on the basis of their similarities in growth rates and histology (Table 2). As determined by flow cytometric assays of cell viability, a significant difference in IC50 values for carboplatin among POLE-mutated vs POLE wild type cell lines was consistently detected [mean ± SEM: 6.43 ± 1.59 µM (range 3.36 – 10.83 µM) in POLE-mutated vs 2.03 ± 0.29 µM (range 1.12 – 3.00 µM) in POLE wild type (p=0.004)] (Table 3 and Figure 3). In contrast, no significant differences were noted when POLE-mutated vs POLE wild type were exposed to paclitaxel [mean ± SEM: 1.35 ± 0.16 nM (range 1.30 – 1.41 nM) vs 0.90 ± 0.24 nM (range 0.28 – 1.60 nM), respectively (p=0.140)] (Table 3 and Figure 3).
Table 3.
Chemo-sensitivity test of 2 POLE-mutated vs 3 POLE wild type EC cell lines
| POLE Wild Type (mean ± SEM) |
POLE-MUTATED (mean ± SEM) |
Pvalue | |
|---|---|---|---|
|
IC50 µM CARBOPLATIN |
2.03 ± 0.29 | 6.43 ± 1.59 | 0.004 * |
|
IC50 nM PACLITAXEL |
0.90 ±0.24 | 1.35 ±0.16 | 0.140 |
POLE Wild Type: ARK-7, ARK-11, UTE-3, POLE-Mutated: ARK-6, UTE-4 µM: microMolar, nM: nanoMolar, SEM: Standar Error of the Mean IC50: half maximal inhibitory concentration, POLE: Polymerase ε
P value < 0.05 statistically significant with two tailed t test
Figure 3.
Cumulative dose response curves of POLE wild type vs POLE-mutated EC cell lines after exposure to Carboplatin (upper panel) and Paclitaxel (lower panel). POLE mutated cell lines are significantly more resistant than POLE wild-type cell lines to Carboplatin (P =0.004), but not to Paclitaxel (P =0.140).
Discussion
Polymerase proofreading is vital to ensure replication fidelity, and in keeping with this, cancers with POLE exonuclease mutations display striking levels of mutations (i.e., ultramutator phenotype) [2– 12]. In this study we performed targeted POLE sequencing on 131 EC including both Type I and Type II tumors. We found POLE exonuclease domain mutations (EDMs) to be present in 8.5% of EC tested. All POLE mutations in our series were found to be somatic. Our data is consistent with previous reports from the TCGA network [2] where 17 Type I (endometrioid) ECs and 1 Type II (serous) EC with POLE EDMs were reported (7% overall rate). Other studies, such as that of Church et al. [5] and Billingsley et al., [16] are also congruent with our data. Church and Billingsley et al found 13 POLE EDMs among 173 tumors tested (7.5% overall rate) and 30 POLE EDMs among 544 tumors (5.6% overall rate), respectively. These studies combined with our current work and a recent report of the comprehensive whole exome sequencing (WES) analysis of 57 uterine serous carcinoma (i.e., 5 POLE EDMs among 57 tumors, 8.7% overall rate), [9] suggest that POLE EDMs can be detected in both type I and Type II EC.
Previous studies in ECs have suggested that while POLE mutated tumors are ultra-mutated, they are predominantly micro-satellite stable (MSS) tumors [2, 6, 8, 12, 13]. Our data are consistent with the literature in that POLE tumor tested in our study as FFPE tissue blocks were MSS or MSI-L. Of interest, however, we found one out of two POLE tumor primary cell lines established in vitro (i.e., ARK-6) to acquire high microsatellite instability after 50 passages in vitro [9]. These data support the speculation that POLE-mutated tumors, due to their defective polymerase proofreading capability, continue to acquire mutations through cell division in vitro. These additional mutations may eventually affect MMR genes controlling microsatellite stability. Supporting this hypothesis, the whole exome sequencing of POLE-ultra-mutated ARK-6 primary cell line, a high copy number uterine serous carcinoma, has recently been reported [9] and demonstrated mutations in multiple MMR gene (i.e., MLH1, MLH3 and MSH6).
Importantly, multiple reports in both Type I and Type II endometrial cancer patients have provided strong evidence that patients harboring tumors with POLE exonuclease-mutations may experience a significantly better prognosis when compared to the remaining group of endometrial cancer patients [2–13]. Our study results are consistent with the previous literature and demonstrates an association between POLE mutations and better overall survival in EC patients regardless of their Type I or Type II histology [2–13]. The biologic basis of improved outcome in POLE ultra-mutated cancers is currently poorly understood. Multiple non-mutually exclusive hypotheses have been used to explain the favorable prognosis of POLE mutated endometrial cancer patients. First, these tumors may be less fit than other cancers, possibly as a result of their extremely high number of mutations, to spread and metastasize in the body. We believe this hypothesis to be unlikely because a) POLE-mutated EC patients in our and other series may present with advanced, widespread metastatic disease (i.e., stage III-IV), b) POLE ultra-mutated tumors, although not as frequently as POLE wild type EC, may recur and such recurrences are generally lethal and c) POLE ultra-mutated tumors such as USC-ARK6 can be established as xenografts in SCID mice and such xenografts eventually lead to cancer-related death of the animals.
An alternative hypothesis is that POLE ultramutated tumors may potentially be significantly more sensitive to standard anti-cancer treatments such as platinum-based chemotherapy because of their defective DNA replication machinery. Accordingly, in this study we have established and compared the sensitivity of POLE mutated vs POLE wild type EC cell lines to carboplatin and paclitaxel (i.e., the gold standard chemotherapy agents used for the treatment of advanced/recurrent/metastatic EC). Surprisingly, we found a higher resistance to carboplatin of POLE mutated tumors vs wild type (i.e., 3.1 fold-difference). In contrast, no significant differences were observed in the sensitivity of the two groups of tumors to paclitaxel in vitro. While additional studies will be necessary to better understand the reason of the increased resistance of POLE mutated tumors to platinum chemotherapy, our results are consistent with previous reports demonstrating an increased resistance to cisplatin of hyper-mutated endometrial tumors harboring loss of DNA mismatch repair mechanisms [17,18].
Due to their extraordinary number of somatic mutations [(i.e., the number of somatic mutations in POLE-mutated tumors far exceed those found in endometrial and colorectal cancers with micro-satellite instability (MSI)] [4,11], POLE EC may be highly immunogenic for the host due to the presentation of large number of mutated epitopes. Accordingly, in this study we have examined the immune infiltration and the expression of the PD-1 immune check-point receptor in POLE-mutated vs POLE wild type EC. We found POLE-mutated EC to have a significant increase in both PD-1+CD8+ and PD-1+CD4+ T lymphocytes when compared to POLE wild type tumors, a features consistent with chronic antigen exposure. These results strongly suggest that POLE-mutated tumor may trigger activation of not only the cytotoxic arm of the immune system (i.e., CD8+ T cells) but also the helper arm of the immune system (i.e., CD4+ T cells). In this regard, previous studies have demonstrated that generation of potent cytotoxic T lymphocytes (CTL) against tumor-specific antigens (which are normally encountered outside an inflammatory context), requires the presence of CD4 helper T cells in addition to both helper and CTL determinants on the same antigen presenting cell (APC) [19–21]. Indeed, the inability to mount a potent antitumor immune response against tumors has often been attributed to the lack of generation of sufficient tumor-specific T cell help [19–21]. Our experimental IHC results in POLE EC, are consistent with a recent report from our group showing a potent activation of CD4+ T cells by autologous POLE-mutated tumor-antigen-loaded dendritic cells (DC) [22]. Taken together these data further suggest that POLE ultra-mutated tumors may potentially trigger strong immunity in vivo because of the combined activation of both the helper and the cytotoxic arms of the immune system.
In conclusion, our results demonstrate that although endowed with a higher resistance to platinum chemotherapy, POLE mutated EC patients are characterized by a high tumor infiltration of both CD4+ and CD8+ T cells and a better prognosis. Taken together, the high T cell infiltration of these tumors and their inborn resistance to platinum further support the hypothesis that the better prognosis of POLE-patients is most likely linked to tumor enhanced immunogenicity. Consistent with this view, a dramatic clinical response to nivolumab (ie, an immune check-point inhibitor) has recently been reported in a POLE-ultra-mutated MSI stable EC patient with widespread recurrent/metastatic disease resistant to chemotherapy (23). Clinical trials with immune check point inhibitors in patients with recurrent-chemotherapy resistant POLE EC are currently ongoing (https://clinicaltrials.gov/ct2/show/NCT02899793).
Supplementary Material
Highlights.
Up to 12% of endometrial cancers are ultra-mutated secondary to Polymerase-e (POLE) mutations
POLE (+) tumors are heavily infiltrated with CD4+/CD8+ TIL and overexpress PD-1 immune-checkpoint
POLE ultra-mutated tumors are significantly more resistant to platinum but not paclitaxel chemotherapy when compared to POLE wild type endometrial cancers.
Acknowledgments
This work was supported in part by U01 CA176067-01A1 grants from NIH, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, the Discovery to Cure Foundation and the Guido Berlucchi Foundation to ADS. This investigation was also supported by NIH Research Grant CA-16359 from the NCI.
Abbreviations
- POLE
Polymerase ε
- CTL
cytotoxic T lymphocytes.
- USC
uterine serous carcinoma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: The authors have no conflicts of interest to report.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature. 2013 May 2;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albertson TM, Ogawa M, Bugni JM, Hays LE, Chen Y, Wang Y, et al. DNA polymerase epsilon and delta proofreading suppress discrete mutator and cancer phenotypes in mice. Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):17101–17104. doi: 10.1073/pnas.0907147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briggs S, Tomlinson I. Germline and somatic polymerase ε and δ mutations define a new class of hypermutated colorectal and endometrial cancers. J Pathol. 2013 Jun;230(2):148–153. doi: 10.1002/path.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Church DN, Briggs S, Palles C. Hum Mol Genet [Internet] Oxford Univ Press; 2013. DNA polymerase ε and δ exonuclease domain mutations in endometrial cancer. Available from: http://hmg.oxfordjournals.org/content/22/14/2820.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palles C, Cazier J-B, Howarth KM, Domingo E, Jones AM, Broderick P, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013 Feb;45(2):136–144. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pursell ZF, Kunkel TA. DNA polymerase epsilon: a polymerase of unusual size (and complexity) Prog Nucleic Acid Res Mol Biol. 2008;82:101–145. doi: 10.1016/S0079-6603(08)00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church DN, Stelloo E, Nout RA, Valtcheva N, Depreeuw J, ter Haar N, et al. Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst. 2015 Jan;107(1):402. doi: 10.1093/jnci/dju402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng B, Hoang LN, McIntyre JB, Duggan MA, Nelson GS, Lee C-H, et al. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol. 2014 Jul;134(1):15–19. doi: 10.1016/j.ygyno.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Santin AD, Bellone S, Centritto F, Schlessinger J, Lifton R. Improved survival of patients with hypermutation in uterine serous carcinoma. Gynecologic Oncology Reports. doi: 10.1016/j.gore.2015.01.005. doi:10.1016/j.gore.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussein YR, Weigelt B, Levine DA, Schoolmeester JK, Dao LN, Balzer B, et al. Clinicopathologic Analysis of Endometrial Carcinomas with POLE Hotspot Mutation. Lab. Invest. 2014:287A–287A. [Google Scholar]
- 13.Rayner E, van Gool IC, Palles C, Kearsey SE, Bosse T, Tomlinson I, & Church DN. A panoply of errors: polymerase proofreading domain mutations in cancer. Nature Reviews Cancer. 2016;16:71–81. doi: 10.1038/nrc.2015.12. [DOI] [PubMed] [Google Scholar]
- 14.Roque DM1, Bellone S, Buza N, Romani C, Cocco E, Bignotti E, Ravaggi A, Rutherford TJ, Schwartz PE, Pecorelli S, Santin AD. Class III β-tubulin overexpression in ovarian clear cell and serous carcinoma as a maker for poor overall survival after platinum/taxane chemotherapy and sensitivity to patupilone. Am J Obstet Gynecol. 2013 Jul;209(1):62.e1–62.e9. doi: 10.1016/j.ajog.2013.04.017. Epub 2013 Apr 10. [DOI] [PubMed] [Google Scholar]
- 15.Konstantinopoulos PA, Matulonis UA. POLE Mutations as an Alternative Pathway for Microsatellite Instability in Endometrial Cancer: Implications for Lynch Syndrome Testing. Cancer. 2015;121:331–334. doi: 10.1002/cncr.29057. [DOI] [PubMed] [Google Scholar]
- 16.Billingsley CC, Cohn DE, Mutch DG, Stephens JA, Suarez AA, Goodfellow PJ. Polymerase ε (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer. 2015;121:386–394. doi: 10.1002/cncr.29046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyers M, Hwang A, Wagner MW, Boothman DA. Role of DNA mismatch repair in apoptotic responses to therapeutic agents. Environ. Mol. Mutagen. 2004;44:249–264. doi: 10.1002/em.20056. [DOI] [PubMed] [Google Scholar]
- 18.Fink D, Aebi S, Howell SB. The role of DNA mismatch repair in drug resistance. Clin. Cancer Res. 1998;4:1–6. [PubMed] [Google Scholar]
- 19.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997 Jul 7;186(1):65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ossendorp F, Mengedé E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998 Mar 2;187(5):693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzavecchia A. Immunology. Licence to kill. Nature. 1998 Jun 4;393(6684):413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 22.Bellone S, Centritto F, Black J, Schwab C, English D, Cocco E, Lopez S, Bonazzoli E, Predolini F, Ferrari F, Silasi DA, Ratner E, Azodi M, Schwartz PE, Santin AD. Polymerase ε (POLE) ultra-mutated tumors induce robust tumor-specific CD4+ T cell responses in endometrial cancer patients. Gynecol Oncol. 2015 Jul;138(1):11–17. doi: 10.1016/j.ygyno.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santin AD, Bellone S, Buza N, Choi J, Schwartz PE, Schlessinger J, Lifton RP. Regression of chemotherapy-resistant Polymerase epsilon (POLE) ultra-mutated and MSH6 hyper-mutated endometrial tumors with nivolumab. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-16-1031. Published August 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.