Abstract
Introduction
To evaluate how experienced dual users used cigarettes and e-cigarettes in real-world use and under different levels of cigarette availability.
Methods
Dual users (cigarettes + e-cigarettes; n=74) and a smoke-only group (just cigarettes; n=74) engaged in a 26-day study with two ad lib use intervals, a week of 75% cigarette reduction and three days of 100% cigarette reduction. After a week of ad lib use of products, all participants were asked to reduce smoking by 75% (dual users were free to use their e-cigarettes as they wished), followed by another week of ad lib use. All participants were then asked to reduce smoking by 100% (cessation) for three days. Primary outcomes were biological samples (carbon monoxide, urinary nicotine and cotinine). Participants also provided real-time reports of product use, craving, and withdrawal symptoms using a smartphone app.
Results
Dual users did not smoke fewer cigarettes than smoke-only participants during ad lib periods, but quadrupled their use of e-cigarettes during smoking reduction periods. Dual users were significantly more likely to maintain 100% reduction (97.1% vs. 81.2%). Amongst women, dual use was associated with higher nicotine levels and withdrawal suppression.
Discussion
Among a group of experienced dual users, e-cigarettes helped maintain smoking reduction and reduced some withdrawal symptoms, although both withdrawal symptoms and nicotine levels varied as a function of gender.
Keywords: cigarette smoking, e-cigarettes, dual use, nicotine, nicotine dependence, gender differences
1. Introduction
Ever-use of electronic cigarettes (e-cigarettes) in a US probability sample increased from 1.8% in 2010 to 13% in 2013, while current-use (use on some days or every day) climbed from 0.3% to 6.8% over the same time period (McMillen, et al., 2015). Current e-cigarette use is highest amongst daily cigarette smokers – one-third reporting use in 2014 (McMillen et al., 2015; Brown et al., 2014; Centers for Disease Control, 2013; Dockrell, et al., 2013; King, et al., 2015).
Tobacco use is a causal factor in about 6 million deaths annually worldwide (World Health Organization, 2011), with the majority attributable to smoking (Prabhat, 2012). E-cigarettes likely have less severe direct health effects than do combustible cigarettes (Farsalinos & Polosa, 2014; Hecht et al., 2015; Polosa, 2015). If e-cigarettes can substitute for cigarettes, they can potentially produce public health benefit.
Whether e-cigarettes will substitute for cigarettes depends, in part, on if they yield effects approximating the cigarette effects thought to cause dependent cigarette use (reduce tobacco withdrawal symptoms, deliver meaningful levels of nicotine; Institute of Medicine, 2012). This study examines whether e-cigarettes appear to produce effects similar to cigarettes (as noted above), and whether e-cigarette use was associated with reduced cigarette use.
This study comprised both “dual users” (DUs: users of both e-cigarettes and cigarettes) and those who smoke only (SOs). This permitted analysis of the extent to which conjoint e-cigarette use was associated with different patterns of cigarette use and other related outcomes. We were interested in whether DUs 1) smoke fewer cigarettes and have lower carbon monoxide (CO) levels than SOs; 2) show elevated levels of nicotine relative to SOs, especially during periods of smoking reduction when they could use e-cigarettes ad libitum; 3) report lower levels of withdrawal symptoms during periods of smoking reduction; and 4) are more able than SOs to reduce and/or stop their cigarette use. We sought to determine whether any observed differences between DUs and SOs were related to gender and nicotine dependence, factors implicated in smoking motivation and cessation success (Perkins, Donny, & Caggiula, 1999; Piper, McCarthy, et al., 2008; Wray et al., 2015).
Survey and laboratory research has addressed the effects of e-cigarettes on the outcomes listed above. Survey research (Etter & Bullen, 2011) shows most e-cigarette users report that e-cigarettes are helpful for reducing withdrawal symptoms, craving, and smoking heaviness. Obviously, survey studies provide neither real-time data associated with e-cigarette use, nor data arising from experimental manipulations such as smoking deprivation.
Laboratory research has yielded data on the potential of e-cigarettes to displace or substitute for cigarette use (Bullen et al., 2010; Dawkins & Corcoran, 2014; Dawkins, et al., 2012; Nides, et al., 2014; Vansickel, et al., 2010; Vansickel & Eissenberg, 2013). Such studies suggest that e-cigarettes, especially when used by experienced users, can reduce tobacco withdrawal symptoms, exert appetitive effects, and reduce urges to smoke cigarettes (Farsalinos et al., 2014). Some studies (Bullen et al., 2010; Vansickel et al., 2010) did not use experienced e-cigarette users using their own e-cigarette brands. Evidence shows that experienced users obtain stronger effects from e-cigarettes than do inexperienced users (Farsalinos et al., 2014; Nides et al., 2014; Vansickel & Eissenberg, 2013). Most studies involved only acute use of e-cigarettes and did not observe their effects over extended periods of time in real-world use (Bullen et al., 2010; Dawkins & Corcoran, 2014; Farsalinos et al., 2014; Nides et al., 2014; Vansickel et al., 2010; Vansickel & Eissenberg, 2013).
2. Methods
2.1 Study sample and data collection
This was a 26-day study, conducted March 2013 to May 2014, in the Madison and Milwaukee, WI, metropolitan areas. All participants provided written informed consent and the study received approval from the University of Wisconsin Health Sciences Institutional Review Board.
Eligibility for study participation included: minimum 18 years old; able to read and write English; smoking at least five cigarettes per day for the past six months; not currently using any smoking cessation medication; planning to remain in the area for the study duration; no history of psychosis or bipolar disorder; not planning to quit tobacco use in the next 30 days; willing to follow study procedures; and if female, not be pregnant or nursing and willing to use acceptable methods of birth control during the study. For the Smoke Only group, participants could not have used a single type of alternate tobacco products (e.g., e-cigarettes, snus, or chewing tobacco) more than five times in their life and not have used alternate tobacco products in the past six months. For the Dual Use group, participants had to have used e-cigarettes at least three times per week for the past three months. Recruitment occurred through point-of-purchase displays at convenience stores in southern Wisconsin and the Milwaukee metropolitan area and through a context-sensitive Facebook ad seeking both smokers and dual users. Interested people completed a brief screening interview and if cleared were invited to attend an in-person initial study visit.
Once informed consent was obtained and eligibility criteria confirmed at Visit 1 (V1, Day 1), participants provided a carbon monoxide (CO) breath sample and a urine sample for nicotine analysis. They also completed a baseline survey including demographics, smoking history, the Fagerstrom Test of Cigarette Dependence (FTCD: Fagerstrom, 2012), and the Wisconsin Inventory of Smoking Dependence Motives (WISDM: Piper, Bolt, et al., 2008). Participants were then given a study cellphone with the app preinstalled. They were trained in the use of the app. Participants were asked to use the app to log each time they smoked or vaped throughout the day. DU participants were instructed that a vaping episode meant taking two or more puffs on an e-cigarette close together and isolated in time from other vaping episodes. Four hours after waking, and at two subsequent four hour intervals, the app prompted participants to complete a brief assessment of withdrawal symptoms, the amount of smoking (and/or vaping) in the past four hours, time since last cigarette (or e-cigarette), and environmental factors related to last use. Participants were asked to complete the surveys as soon as possible following the notification.
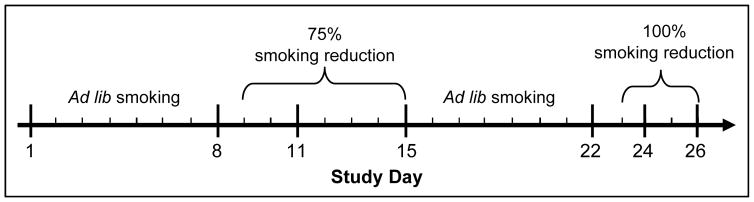
Participants were instructed to continue smoking and vaping normally over the next week, and to use the smartphone app as directed (see Figure 1 for study timeline). On Study Day 8 (V2), participants provided CO and urine samples, and completed another survey. Their average daily smoking during the previous week was calculated. All participants were then asked to reduce their smoking to 75% of baseline (rounded to the nearest whole cigarette) for the next week. Participants in the DU group were told they were free to use their e-cigarettes as they wished. Participants made another in-person visit (V3) on Study Day 11 to confirm adherence to reduction by CO level. On Study Day 15 (V4), participants once again provided biological samples and completed a survey. Following that visit, they were instructed to return to their normal cigarette smoking rate over the next week. On Study Day 22 (V5), participants completed regular study visit assessments. Starting the next day, they were instructed to not smoke at all until their visit on Day 26 (100% reduction; DU participants were again told they were free to use their e-cigarettes as they wished). Both 75% and 100% reduction periods were used in order to determine whether hypothesized e-cigarette effects occurred during either partial or full smoking deprivation. Participants returned to assess adherence on Study Days 24 (V6) and 26 (V7).
Figure 1.
Study Timeline
Participants received financial incentives for attending study visits, completing at least 80% of their smartphone assessments, meeting CO targets during the reduction and cessation intervals, and for returning study smartphones at the end of the study (total possible study compensation = $560).
2.2 Measures
Key outcome measures included biological measures (urinary nicotine, CO), self-reported e-cigarette and cigarette use, and self-reported ratings of craving and negative affect. Biological measures were collected at each of the seven study visits. Daily symptom ratings and cigarette/e-cigarette use were collected via the smartphone app. The craving and negative affect symptom measures were derived from the Wisconsin Smoking Withdrawal Scale (WSWS: Welsch et al., 1999) with items rated on a 0–4 scale from 0=Strongly Disagree to 4 = Strongly Agree. Craving was measured with a single item: “I have been bothered by the desire to smoke a cigarette.” Negative Affect was measured as the mean of three items: “I have been tense or anxious,” “I have been irritable, easily angered” and “I have felt sad or depressed.”
Urine samples were transferred to storage tubes and frozen immediately following collection. Samples were stored in −20°C freezers and shipped at two-week intervals to Weck Laboratories (City of Industry, CA). Nicotine levels in each sample were determined using liquid chromatography-mass spectrometry electro-spray positive ionization methods with minimum reporting limits of 20 μg/l.
(2.2.1) Ad Libitum Period
observations collected during each ad lib period (V1, V2, and V5 for nicotine; Days 1–8 and 16–21 for craving and negative affect app measures) were combined into a mean ad lib value for each participant; this was done because analyses showed similar patterns of results within the two ad lib periods and to obtain more stable estimates (Nunnally & Bernstein, 1994).
(2.2.2) 75% Reduction Period
observations collected during the 75% reduction period (V3 and V4; Days 9–14) were combined into a mean 75% reduction value.
(2.2.3) 100% Cessation Period
observations collected during the 100% cessation period (V6, V7; Days 24–25) were combined into a mean 100% cessation value.
2.3 Data analysis
Group differences (DU vs. SO) in baseline characteristics were tested via t-tests for continuous variables and chi-square tests for categorical variables. Key study outcomes were analyzed via mixed ANOVA with Period (Ad Lib vs. 100% Reduction Period) as a within-subjects (repeated measures) factor and Group (DU vs. SO) as a between-subjects factor. We also tested additional between-subjects factors including gender and cigarette dependence level (low vs. high, as measured by scores on the FTCD with a cut-score of 5). Mixed ANOVAs yielded tests of main effects and interactions; partial eta-squared ( )was computed as a measure of effect size. All tests were two-tailed tests with alpha set at .05 and conducted using SPSS (IBM Corporation, 2013).
3. Results
3.1 Sample characteristics
A total of 148 participants enrolled in the study, divided evenly between the SO and DU groups (Table 1). Compared to the DU group, the typical SO participant was more likely to be female, a member of a minority group, older, to have smoked longer, to have made fewer quit attempts, and to have higher levels of Primary and Secondary dependence as assessed by the WISDM (Piper et al., 2008). Table 2 characterizes the e-cigarettes used by participants in the DU group by Device Configuration and Nicotine Concentration (percent nicotine in “e-juice”). Device type and nicotine concentration were unrelated to gender.
Table 1.
Participant Characteristics by Study Group
| Smoke Only (n=74) | Dual Use (n=74) | Statistical Test of Group Difference | |
|---|---|---|---|
| Gender (% Female) | 58.1% | 40.5% | χ2(1)=4.57, p=.03 |
|
| |||
| Race: | χ2(4)=12.98, p=.01 | ||
| % White | 79.7% | 90.5% | |
| % African-American | 13.5% | 0% | |
| % American Indian/Native Alaskan | 1.4% | 2.7% | |
| % Mixed | 5.4% | 4.1% | |
| % Other | 0% | 2.7% | |
|
| |||
| Ethnicity (% Hispanic) | 4.5% | 4.1% | χ2(1)=0.12, p=.91 |
|
| |||
| Mean Age (SD) | 43.4 (12.8) | 33 (12.4) | t(146)=5, p<.0001 |
|
| |||
| % with Post-High School Education | 62.2% | 75.7% | χ2(1)=3.154, p=.076 |
|
| |||
| Mean Years Smoked (SD) | 25.4 (13.4) | 17.2 (12.6) | t(146)=3.8, p<.0001 |
|
| |||
| Mean Quit Attempts (SD) | 2.0 (1.9) | 3.1 (2.8) | t(144)= −2.8, p=.006 |
|
| |||
| Mean FTCD1 Score (SD) | 4.9 (2.1) | 4.5 (2.1) | t(145)=1.1, p=.28 |
|
| |||
| Mean WISDM2 Primary Dependence Motives Scale (SD) | 4.8 (1.4) | 2.3 (1.1) | t(146)=12.0, p<.0001 |
|
| |||
| Mean WISDM2 Secondary Dependence Motives Scale (SD) | 4.3 (1.3) | 2.7 (1.0) | t(146)=8.5, p<.0001 |
|
| |||
| Mean Age at Daily Smoking (SD) | 17.2 (3.3) | 16.3 (2.1) | t(146)=1.9, p=.062 |
|
| |||
| Live with a Smoker (Other than Spouse or Partner) (% Yes) | 38.0% | 45.1% | χ2(1)=0.52, p=.47 |
|
| |||
| Ban on Smoking Indoors in the Home (%Yes) | 44.6% | 69.9% | χ2(1)=9.58, p=.002 |
|
| |||
| Live with a Spouse or Partner Who Smokes (% Yes) | 56.1% | 40.0% | χ2(1)=1.96, p=.16 |
FTCD = Fagerstrom Test of Cigarette Dependence (Fagerstrom, 2014).
WISDM = Wisconsin Inventory of Smoking Dependence Motives (Piper, Bolt, Kim, et al., 2008).
Table 2.
E-Cigarette Device Information (Dual Use Group Only)
| Device Configuration | N | Percentage of Total Sample of Dual User Participants |
|---|---|---|
| Disposable | 25 | 33.8% |
| Replaceable cartridge | 12 | 16.2% |
| Tank system | 10 | 13.5% |
| Unknown | 27 | 36.5% |
| Nicotine Concentration | N | Percentage of Total Sample of Dual User Participants |
| 0.1–0.3% | 3 | 4% |
| 0.4–0.6% | 6 | 8.1% |
| 0.7–1.2% | 5 | 6.7% |
| 1.3–1.8% | 6 | 8.1% |
| 1.9+% | 1 | 1.3% |
| Unspecified | 53 | 71.6% |
3.2 Smoking rates and intensity
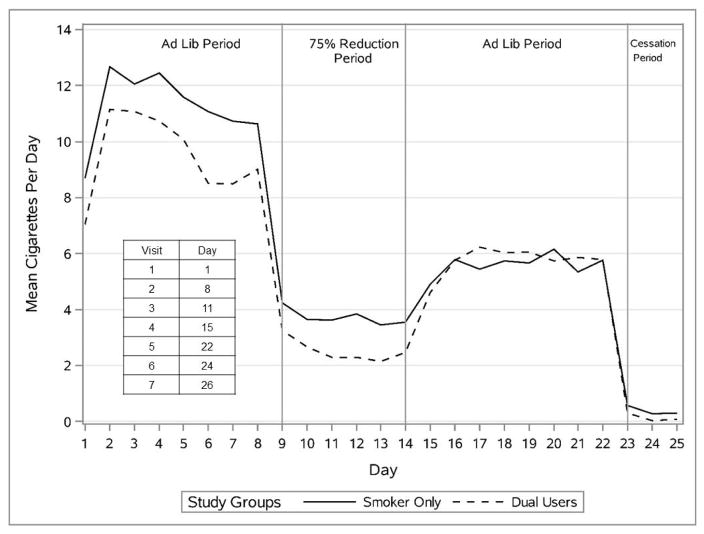
Figure 2 displays the mean smoking rates of participants across the experimental phases. The smoking rates of DUs and SOs were quite similar across these phases, with the SO participants showing a slight tendency to smoke more in the initial baseline phase. No group effects were present, and the pattern of effects was similar in both genders.
Figure 2.
Mean Smoking Rates of Participants Across the Experimental Phases by Group.
Carbon monoxide levels were analyzed to evaluate the intensity of smoking. Groups did not differ on CO when it was modeled as a continuous variable (p’s > .05). The mean CO values for the Ad Libitum smoking periods were nonsignificantly lower for DUs than SOs (14.4 ppm [SE = .94] vs. 15.3 ppm [SE = .93], respectively).
3.3 Vaping episodes
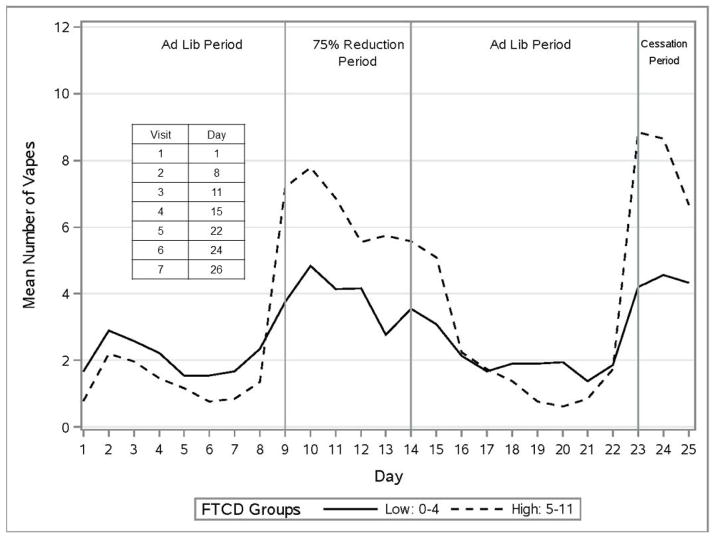
Figure 3 displays the pattern of self-reported vaping episodes for DUs across the four experimental periods by dependence level (low vs. high FTCD score). For DU participants, e-cigarette use was fairly low during the Ad Libitum smoking periods (M’s = 1.69 & 1.58 uses/day for the first and second Ad Libitum periods, respectively), but rose substantially in the 75% (M = 5.00) and the 100% Reduction periods (M = 6.21), respectively; these increases from the combined Ad Libitum periods were both significant in analyses with and without covariates: (F (1, 72) = 35.4, p < .05; ) and (F (1, 72) = 29.1, p < .05; ), respectively.
Figure 3.
Mean Number of Vaping Episodes for Dual Users by Dependence Level.
Analyses of vaping episodes revealed that women showed greater escalation of vaping during Smoking Reduction than did men. Women increased vapes/day from 1.3 during Ad Libitum use to 6.3 during 75% Reduction; the corresponding figures for men were 1.9 and 4.4 yielding a Period X Gender interaction (F (1, 72) = 4.0, p < .05; ). Moreover, there was a Period X FTCD interaction, as more dependent smokers escalated their use of e-cigarettes more than did less dependent smokers: from 1.3 to 6.6 versus 1.7 to 3.4 (F (1, 67) = 6.6, p < .05; ). Higher dependence consistently predicted greater escalation in vaping during smoking reduction (see Figure 3). Highly dependent women increased their vaping rate by a factor approaching 10 when they transitioned to 100% Reduction, a much greater increase than seen in other participant groups.
3.4 Adherence, nicotine, craving, and negative affect during smoking reduction
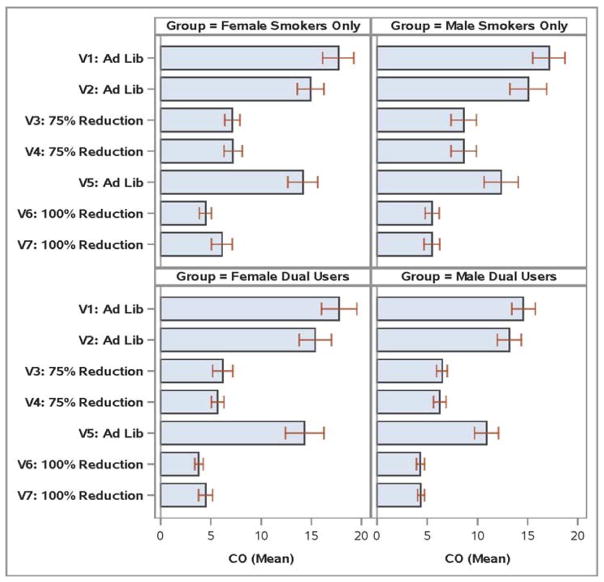
Adherence to the smoking reduction instructions was assessed by expired CO level (highly consistent with app reported smoking rates). For the 75% Reduction period, the percentages of participants meeting the CO criterion for adherence (CO ≤ baseline CO) were 59.7% and 66.2% for the SO and DU participants, respectively (χ2=.64, p=.42). Participants in the DU Group were more likely to meet the 100% Reduction abstinence criterion (CO≤8 ppm) than those in the SO Group: 97.1% versus 81.2%, respectively (χ2=9.05, p=.003). Figure 4 displays visit CO values by Gender and Group.
Figure 4.
Mean Carbon Monoxide (CO) Values by Study Visit, Group, and Gender.
The main analyses of urinary nicotine contrasted nicotine levels (ng/ml) in the Ad Libitum smoking periods separately with levels in the 75% and 100% Reduction periods. Effects were examined in models both with and without gender and FTCD covariates. Models were also run with all participants and with only those meeting the CO criteria for smoking reduction in the Reduction periods.
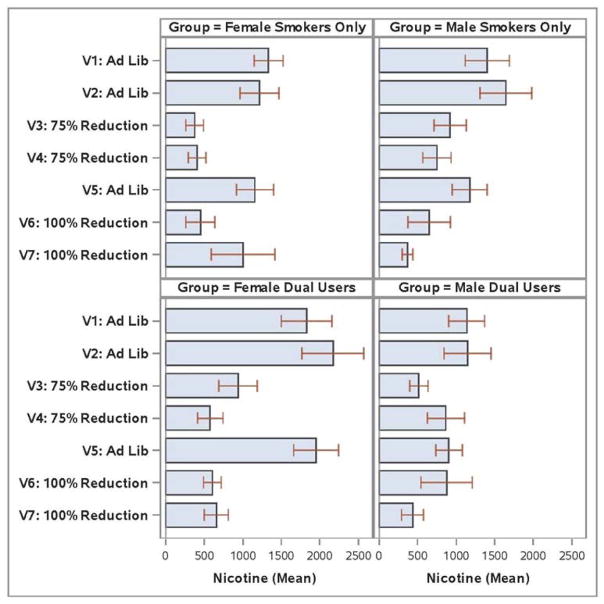
A Period X Group X Gender effect was found when analyzed with and without the FTCD covariate (F’s (1, 97/1,93) = 4.2/4.4; p’s < .05; ). Female DUs had especially high levels of nicotine during the Ad Libitum periods and showed especially large drops during the 100% Reduction period (also true for the 75% Reduction period; see Figure 5). Females’ reduction-related drop in nicotine (1413 μg/l) was especially great relative to the drop seen in DU males (535 μg/l). However, even after this drop, DU females had nicotine levels higher than those of female SOs during the 100% Reduction period (p < .05). DU males were nonsignificantly higher than SO males. When concentration of nicotine in the e-cigarette nicotine solution was correlated with urinary nicotine level at each visit amongst the DUs for whom data were available, all correlations > .29.
Figure 5.
Mean Urinary Nicotine Values by Study Visit, Group, and Gender.
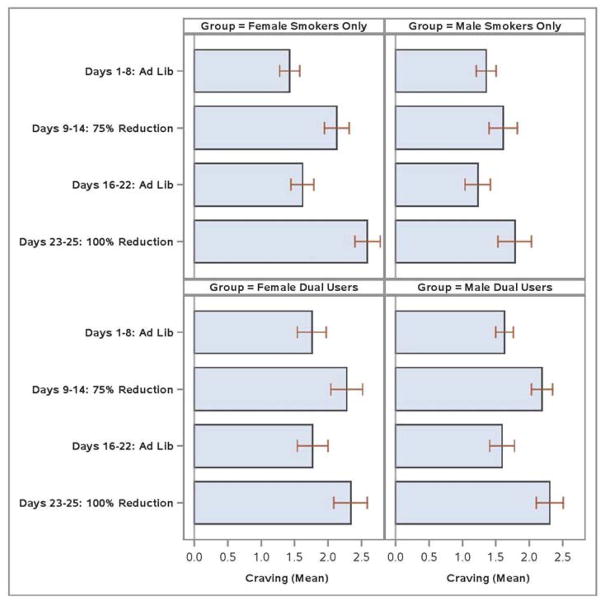
Consistent effects were found in analyses that compared craving in the Ad Libitum versus 100% Smoking Reduction periods. Analyses with all participants yielded a Period X Group X Gender interaction (F (1, 133) = 6.3, p < .05; ; this effect remained significant when the FTCD was included as a covariate). This interaction reflects, in part, that women SOs showed larger increases in craving from Ad Libitum periods to 100% Reduction than did DU women (see Figure 6). The mean increase in craving was 1.03 for SO women and 0.56 for DU women. Male DUs tended to report greater increases in craving than did male SOs.
Figure 6.
Mean Craving by Study Period, Group, and Gender.
Group effects were present in covariate adjusted analyses comparing negative affect in the Ad Libitum versus 100% Reduction periods. With all participants in the analysis, a 4-way interaction was found [Period X Group X Gender X FTCD (F (1, 128) = 4.8, p < .05; )]. Highly dependent SO women showed an increase in negative affect from Ad Libitum to 100% Smoking Reduction = 0.9, while the respective figure for highly dependent DU women = 0.54. However, amongst women low in dependence, DU women showed larger withdrawal related increases (an increase of 1.0 vs. 0.25 respectively, for DU and SO women low in dependence). The 4-way interaction approached significance when the analysis was restricted to those who met the 100% Reduction criterion (p = .055) and was significant amongst all participants in comparisons of the Ad Libitum and 75% Reduction periods, (F (1, 132) = 5.0, p < .05; ). When the FTCD was not included in the analysis, there were no Group effects.
3.5 Motivation, confidence, and likelihood of smoking cessation
At the final study visit, participants were asked how motivated they were to quit, how confident they were of being able to quit successfully, and how likely they were to quit in the coming year. SOs were significantly more motivated to quit than were DUs (mean rating on a 10-point scale = 6.51 vs. 3.97, t(138)=5.65, p<.001; Cohen’s d=.96). The two groups did not differ on confidence or likelihood estimates. Amongst those in the DU group, 70% thought e-cigarettes made it more likely they would quit smoking, 18.6% thought e-cigarettes would have no effect on their quitting, and 10% thought e-cigarettes would make them less likely to quit smoking.
4. Discussion
This research explored the extent to which e-cigarettes have the potential to substitute for cigarettes during periods of ab libitum smoking and smoking reduction. Neither self-report nor CO data showed reliable differences in cigarette consumption between the groups across any of the experimental periods. One study noted such reduction (McRobbie et al., 2015), but did not study long-term DUs; this study was conducted using participants quitting smoking. The DUs used e-cigarettes at a fairly low level during the Ad Libitum Smoking periods, but, on average, quadrupled their use of e-cigarettes during Smoking Reduction periods. Highly dependent women DUs showed large increases in reduction-related vaping.
DU women had higher nicotine levels during Ad Libitum smoking than did other participants. The causes of this are unknown, but could reflect their use of two nicotine delivery systems, their smaller body masses (than men), and differences in smoking efficiency. Interestingly, these DU women attained the highest nicotine levels despite the fact that DUs as a group were significantly lower in cigarette dependence than SO participants (Table 1). While DU women showed especially large drops in nicotine during Reduction periods, they still had significantly higher nicotine levels than SO women during the 100% Smoking Reduction period.
SO women showed the largest increases in craving in the 100% Smoking Reduction period, relative to both men and DU women. In women, dual use was associated with reduced craving; this was not true for DU males. There was no evidence that DU males experienced less craving than SO males. Highly dependent SO women, but not highly dependent DU women, showed large jumps in negative affect due to attempted cessation. Amongst women low in dependence, DU women showed a greater increase in negative affect during abstinence than did SO women. The results provide some support for the hypothesis that e-cigarette use may suppress withdrawal symptoms, especially craving, amongst women. Prior data show e-cigarettes reduce acute withdrawal in smokers (Dawkins & Corcoran, 2014; Farsalinos et al., 2014; Nides et al., 2014; Vansickel et al., 2010); the current research suggests that, at least in DU women, extended real-world use of e-cigarettes may reduce withdrawal tonically during periods of smoking reduction.
DUs were less motivated to quit smoking than were SOs. DUs may have tried e-cigarettes for harm reduction purposes. Also, use of e-cigarettes may have led them to view smoking as less harmful. DUs reported more quit attempts than SOs; perhaps e-cigarette use was a fall back strategy in response to failed quit attempts. On the other hand, a large majority of DUs believed e-cigarettes would help them quit smoking. At present, it is unclear how this pattern of findings is best reconciled.
Several findings suggested that e-cigarettes could, in theory, effectively substitute for cigarettes. At least amongst women, e-cigarettes were associated with higher, tonic levels of nicotine, suggesting that they could be a meaningful alternative source of nicotine in real-world use. Although much of this effect appeared to occur during ad libitum use, there was some evidence of carry-over to periods of smoking reduction. The substitution potential of e-cigarettes is partially supported by evidence that women DUs reported less reduction-related craving than did women SOs. That DUs escalated their use of e-cigarettes during periods of smoking reduction suggests that they used e-cigarettes to cope with smoking reduction. The fact that DUs were more successful than SOs in achieving brief abstinence from smoking suggests that such coping was effective (Bullen et al., 2013; Nides et al., 2014; Polosa et al., 2011; although cf. Caponnetto et al., 2013). Finally, DUs showed less cigarette dependence on the WISDM than did SOs; it would be interesting to see if e-cigarette use preceded or followed their reduced cigarette dependence.
Some findings challenge the hypothesis that e-cigarettes might substitute effectively for cigarettes. It was primarily women who showed some “substitution” effects: the relative suppression of withdrawal symptoms when abstinent. The pattern observed in DU women of high levels of nicotine during ad libitum use, and plunging levels during smoking reduction, could actually sustain cigarette use and dependence. There was no clear evidence that e-cigarettes led DUs to smoke fewer cigarettes or reduce CO levels. This mixed pattern of findings demonstrates that additional research is needed to establish how well e-cigarettes substitute for smoking, for which smokers e-cigarettes substitute most effectively, and which effects of e-cigarettes are critical to substitution.
The most dramatic differences between DUs and SOs were found amongst women: in vaping rates during smoking reduction, in nicotine levels, and in reduction-related craving. Such observations are consistent with data showing that women report greater satisfaction with e-cigarettes (Grace, Kivell, & Laugesen, 2015) and show especially rapid uptake of e-cigarette use (Strong et al., 2015). This pattern may be related to gender differences in nicotine motivation. Women smokers appear to differ from men smokers in: nicotine reinforcement (Perkins et al., 1999), sensitivity to the nicotine content of cigarettes as reflected by withdrawal alleviation (Perkins & Karelitz, 2015), sensitivity to stressors, smoking cues, and abstinence as manifested in increased smoking motivation (Perkins, et al., 2013; Doran, 2014; Wray et al., 2015; although cf. Ferguson, et al., 2015); and negative reinforcement expectancies for smoking (Ferguson, Shiffman, & Gwaltney, 2006; Pang, et al., 2015; Perkins et al., 2006). Any of these could yield gender differences in the use of, and response to, e-cigarettes. If women are more motivated to smoke because of nonpharmacologic motives (Perkins et al., 1999) than for nicotine receipt per se, then the former might motivate their e-cigarettes use and they would not be deterred by the relatively low nicotine yield of e-cigarettes (Farsalinos et al., 2014; Goniewicz, et al., 2013; Vansickel et al., 2010).
4.1 Limitations
This study had several limitations, including the low frequency of use of e-cigarettes, limited data on type of e-cigarette and strength of the e-juice (Farsalinos et al., 2014; Vansickel & Eissenberg, 2013). DUs and SOs differed on numerous variables (Table 1) because they reflect naturally occurring groups. Differences between the groups may result from factors other than their e-cigarette use per se. The greater dependence of SO participants may have negatively affected their withdrawal symptoms and smoking reduction success. Attempts to covary out such differences could result in uninterpretable results, however (Miller & Chapman, 2001; Vansickel & Eissenberg, 2013). By recruiting people engaged in ongoing dual use, this sample did not include DUs who had previously transitioned to the exclusive use of e-cigarettes, or to nicotine abstinence. Finally, the tests of the ability of participants to reduce or quit smoking during the study in exchange for payment may have little external validity with regard to real-world reduction or cessation attempts.
5. Conclusion
In summary, this research compared two samples: DUs and SOs, on their real-world use of cigarettes and e-cigarettes, including during conditions of smoking reduction. Results showed that DUs increased their use of e-cigarettes when trying to reduce their smoking, and that DUs were more able than SOs to achieve brief abstinence from smoking. Women DUs displayed: 1) relatively high nicotine levels, especially during ad libitum smoking; 2) steep declines in nicotine during smoking reduction; and 3) reduced craving in response to smoking reduction and abstinence. Compared to SOs, DUs did not smoke significantly fewer cigarettes during either periods of ab libitum use or during periods of smoking restriction, nor did they produce lower CO levels. Thus, this research yielded a mixed pattern of results with regard to how effective e-cigarettes might be at displacing cigarette use based upon effects that are thought to underlie cigarette dependence.
Acknowledgments
Role of Funding Source
This research was sponsored by grant 3P50CA143188-15S1 from the National Cancer Institute and the Food and Drug Administration to the University of Wisconsin Center for Tobacco Research and Intervention. The sponsors had no role in study design; collection, analysis, and interpretation of the data; in the writing of the report; nor the decision to submit the article.
Footnotes
Conflict of Interest
No conflict declared.
Author Disclosures
Contributors
All authors contributed to conceptualizing and designing the study. Michael C. Fiore and Timothy B. Baker obtained the funding for this research. Stevens S. Smith conducted the analyses. Douglas E. Jorenby and Timothy B. Baker led the manuscript writing; Stevens S. Smith and Michael C. Fiore provided substantial edits to the manuscript. All authors reviewed and approved the final manuscript.
References
- Brown J, West R, Beard E, Michie S, Shahab L, McNeill A. Prevalence and characteristics of e-cigarette users in Great Britain: Findings from a general population survey of smokers. Addict Behav. 2014;39:1120–1125. doi: 10.1016/j.addbeh.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, Walker N. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;19:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS ONE. 2013;8:e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease, C. a. P. Notes from the field: electronic cigarette use among middle and high school students - United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2013;62:729–730. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24005229. [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology (Berl) 2014;231:401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addict Behav. 2012;37:970–973. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Dockrell M, Morrison R, Bauld L, McNeill A. E-cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob Res. 2013;15:1737–1744. doi: 10.1093/ntr/ntt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N. Sex differences in smoking cue reactivity: craving, negative affect, and preference for immediate smoking. Am J Addict. 2014;23:211–217. doi: 10.1111/j.1521-0391.2014.12094.x. [DOI] [PubMed] [Google Scholar]
- Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K, Russ C, Yu CR, Yunis C, Foulds J. The Fagerstrom Test for Nicotine Dependence as a predictor of smoking abstinence: A pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14:1476–1473. doi: 10.1093/ntr/nts018. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf. 2014;5:67–86. doi: 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Frandsen M, Dunbar MS, Shiffman S. Gender and stimulus control of smoking behavior. Nicotine Tob Res. 2015;17:431–437. doi: 10.1093/ntr/ntu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S, Gwaltney CJ. Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol. 2006;74:1153–1161. doi: 10.1037/0022-006X.74.6.1153. [DOI] [PubMed] [Google Scholar]
- Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob Res. 2013;15:158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- Grace RC, Kivell BM, Laugesen M. Gender differences in satisfaction ratings for nicotine electronic cigarettes by first-time users. Addict Behav. 2015;50:140–143. doi: 10.1016/j.addbeh.2015.06.027. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1932883. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Kotandeniya D, Pillsbury ME, Chen M, Ransom BW, … Hatsukami DK. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res. 2015;17:704–709. doi: 10.1093/ntr/ntu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corporation. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corporation; 2013. [Google Scholar]
- Institute of Medicine. Methods for studying risk perception and risk communication (Chapt 5) Scientific standards for studies on modified risk tobacco products. Washington, D.C: The National Academies Press; 2012. [Google Scholar]
- King BA, Patel R, Nguyen KH, Dube SR. Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine Tob Res. 2015;17:219–227. doi: 10.1093/ntr/ntu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RM, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob Res. 2015;17:1195–1202. doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- McRobbie H, Phillips A, Goniewicz ML, Smith KM, Knight-West O, Przulj D, Hajek P. Switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prev Res. 2015;8:873–878. doi: 10.1158/1940-6207.CAPR-15-0058. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11261398. [DOI] [PubMed] [Google Scholar]
- Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am J Health Behav. 2014;38:265–274. doi: 10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric Theory. 3. New York: McGraw Hill; 1994. [Google Scholar]
- Pang RD, Zvolensky MJ, Schmidt NB, Leventhal AM. Gender differences in negative reinforcement smoking expectancies. Nicotine Tob Res. 2015;17:750–754. doi: 10.1093/ntr/ntu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: review of human and animal evidence. Nicotine Tob Res. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, Conklin C, Sayette M, Caggiula A. Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology (Berl) 2006;184:600–607. doi: 10.1007/s00213-005-0103-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nicotine Tob Res. 2015;17:443–448. doi: 10.1093/ntr/ntu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. Negative mood effects on craving to smoke in women versus men. Addict Behav. 2013;38:1527–1531. doi: 10.1016/j.addbeh.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Bolt DM, Kim SY, Japuntich SJ, Smith SS, Niederdeppe J, … Baker TB. Refining the tobacco dependence phenotype using the Wisconsin Inventory of Smoking Dependence Motives. J Abnorm Psychol. 2008;117:747–761. doi: 10.1037/a0013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, McCarthy DE, Bolt DM, Smith SS, Lerman C, Benowitz N, … Baker TB. Assessing dimensions of nicotine dependence: an evaluation of the Nicotine Dependence Syndrome Scale (NDSS) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM) Nicotine Tob Res. 2008;10:1009–1020. doi: 10.1080/14622200802097563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R. Electronic cigarette use and harm reversal: emerging evidence in the lung. BMC Med. 2015;13:54. doi: 10.1186/s12916-015-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhat J. Avoidable deaths from smoking: a global perspective. Public Health Rev. 2012;33:569–600. [Google Scholar]
- Strong DR, Myers M, Linke S, Leas E, Hofstetter R, Edland S, Al-Delaimy WK. Gender differences influence overweight smokers’ experimentation with electronic nicotine delivery systems. Addict Behav. 2015;49:20–25. doi: 10.1016/j.addbeh.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob Res. 2013;15:267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7:354–361. doi: 10.1037/1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic. 2011 http://www.who.int/tobacco/global_report/2011/en/
- Wray JM, Gray KM, McClure EA, Carpenter MJ, Tiffany ST, Saladin ME. Gender differences in responses to cues presented in the natural environment of cigarette smokers. Nicotine Tob Res. 2015;17:438–442. doi: 10.1093/ntr/ntu248. [DOI] [PMC free article] [PubMed] [Google Scholar]