Abstract
Background
The major inhibitory neurotransmitter, gamma-aminobutyric acid (GABA), modulates many of the behavioral effects of alcohol, including sedation, tolerance, and withdrawal. The α1 subunit of the benzodiazepine GABAA receptor is the most widely expressed alpha subunit in the brain, and has been implicated in the reinforcing- and abuse-related effects of alcohol. The aim of the present study was to examine whether treatment with a benzodiazepine GABAA α1-preferring ligand, 3-isopropoxy-β-carboline hydrochloride (3-ISOPBC), selectively decreases alcohol seeking and consumption.
Methods
Eight baboons self-administered alcohol (4% w/v; n=5; alcohol group) or a non-alcoholic beverage (n=3; control group) in Component 3 of a chained schedule of reinforcement. Responses in Component 2 provided indices of motivation to drink (seeking). Doses of 3-ISOPBC (5.0 – 30.0 mg/kg) and vehicle were administered before drinking sessions under both acute and chronic (5 day) conditions.
Results
Chronic, and not acute, administration of 3-ISOPBC significantly decreased self-administration responses, g/kg alcohol consumed, and the number of drinks in and duration of the first drinking bout in the alcohol group. In the control group, chronic administration of 3-ISOPBC did not significantly decrease any of these measures at any of the doses.
Conclusions
The GABAA α1-preferring ligand 3-ISOPBC may have therapeutic potential in the treatment of alcohol use disorder due to its ability to selectively reduce alcohol use.
Keywords: Alcohol, 3-isopropoxy-β-carboline hydrochloride, 3-ISOPBC, Self-Administration, Baboon
1. Introduction
Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the central nervous system and is an important target in the development of pharmacotherapies for alcohol use disorder. GABA binds to type A receptors, which have been implicated in the acute and chronic effects of alcohol, including sedation, tolerance, and withdrawal as well as the motivational effects of alcohol, including alcohol reinforcement and consumption (for reviews, see Enoch, 2008; Kumar et al., 2009; Lobo and Harris, 2008). GABAA receptors are composed of five subunits that form a central chloride channel and can belong to different subunit classes: α(1-6), β(1-3), γ(1-3), δ, ε, π, θ, and ρ(1-3). While many GABAA receptors are composed of one γ, two α, and two β subunits, the various subunit classes allow for extensive heterogeneity in receptor subunit composition. The subunit composition is a major determinant of the pharmacological profile of the receptor and the presence or absence of certain subunits may regulate specific behavioral effects of drugs such as alcohol (Olsen and Sieghart, 2009).
The α1 subunit of the GABAA receptor may play a role in the reinforcing- and abuse-related effects of alcohol. Knockout mice without the GABAA α1 receptor have been shown to consume less alcohol under a two-bottle alcohol versus water choice procedure and to respond less for alcohol under an operant self-administration procedure, although these were accompanied by reductions in saccharin and sucrose consumption (Blednov et al., 2003; June et al., 2007). In rodents bred for high alcohol intake, benzodiazepine GABAA α1-preferring antagonists – 3-propoxy-β-carboline hydrochloride (3-PBC) and β-carboline-3-carboxylate-tert-butyl ester (βCCT) – decreased alcohol intake when administered systemically or through microinfusions into the ventral palladium (Harvey et al., 2002; June et al., 2003). In primates, chronic administration of 3-PBC significantly decreased self-administration responses, volume consumed, and g/kg alcohol intake but also had some effects on self-administration of a non-alcoholic reinforcer in one study (Kaminski et al., 2013). In another study, chronic administration of 3-PBC and βCCT did not decrease alcohol intake or blood alcohol levels (Sawyer et al., 2014).
It is clear from the work of Licata et al (2009) in primates, June et al. (2003) and Harvey et al (2002) in rodents, and Platt et al. (2002) in rhesus macaques, that both βCCT and 3-PBC have been shown to be α1-preferring antagonists in vivo. Moreover, both βCCT and 3-PBC have been shown to be potent antagonists in vitro (Harvey et al., 2002; Yin et al., 2010). Based on these and the above data, the synthesis of an analog of 3-PBC, 3-isopropoxy-β-carboline hydrochloride (3-ISOPBC), was undertaken (Tiruveedhula et al., 2015). This choice was guided by molecular modeling (He et al., 2000; Huang et al., 2000) wherein it is well established that a major change in the structure of a benzodiazepine GABAA (α1-6β2/3γ2) receptor subunit selective ligand can dramatically alter the subunit selectivity (Clayton et al., 2007, 2015). 3-ISOPBC displays a 7-fold selectivity for the α1 subunit over the α2 and α3 subunits as well as a 30-fold selectivity over the α5 subunit (supplemental Table 1s1). 3-PBC binding affinities have been published previously (Harvey et al., 2002). 3-ISOPBC did not bind to any other receptors in the 43 receptor panel tested (supplemental Table 2s2) in the psychoactive drug screening program (UNC, B. Roth; Besnard et al., 2012; Huang et al., 2010), analogous to the ligand 3-PBC. In addition, 3-ISOPBC did not exhibit sedative nor ataxic activity, as illustrated by results from rotarod testing (supplemental Figure 1s3). Even though 3-ISOPBC binds more potently to α1 receptors (supplemental Table 1s4), it is clear from the rotarod data that it does not affect positive allosteric modulation at benzodiazepine α1 GABAA receptors. The ligand 3-PBC also had no agonist activity at α1 subunits, even though it binds more potently to the α1 subunit than other DS sites (Yin et al., 2010). The choice of 3-ISOPBC was also based on the structure of the branched isopropyl group, which would hinder metabolism by beta (omega-1) oxidation. Since 3-ISOPBC may undergo phase 1 metabolism by cytochrome P450 enzymes – potentially by beta (omega-1) oxidation of the linear n-propyl group in 3-PBC (Foye et al., 2013) – at a much slower rate than 3-PBC, it was hypothesized that this would increase the duration of action and provide a ligand more active in vivo than 3-PBC.
The present study investigated whether acute and chronic administration of 3-ISOPBC could selectively reduce alcohol seeking and self-administration in baboons. The baboons consumed alcohol daily under a chained schedule of reinforcement at levels that produce blood alcohol levels exceeding 0.08%. The use of the chain schedule allows for examination of drug effects on responding in the presence of alcohol-related cues that is maintained by conditioned reinforcement (i.e., responding that produces access to alcohol or “seeking”), as well as alcohol self-administration within the same session. To determine the specificity of effects on alcohol-related behaviors, chronic administration of 3-ISOPBC was also conducted with baboons that self-administered a preferred, non-alcoholic beverage under the chained schedule.
2. Material and Methods
2.1. Subjects
Eight singly-housed adult male baboons (Papio anubis; Southwest Foundation for Biomedical Research, San Antonio, TX) weighing on average 28.1 kg (+ 4.2 SD) served as subjects. For the alcohol group (N=5), the reinforcer delivered was 4% w/v alcohol. For the control group (N=3), the reinforcer delivered was a preferred non-alcohol beverage (orange-flavored, sugar-free Tang®), diluted to a concentration that functioned as a comparable reinforcer (Duke et al., 2014). All baboons had extensive histories of self-administration of either alcohol or the non-alcoholic beverage under the chained schedule of reinforcement as reported previously (Duke et al., 2014; Holtyn et al., 2014; Kaminski and Weerts, 2014; Kaminski et al., 2008, 2012, 2013). Each day, the baboons were fed standard primate chow that was adjusted to maintain sufficient caloric intake for normal baboons of their size, age, and activity level (about 50-73 kcals/kg); fresh fruit or vegetables; and a children's chewable multivitamin. Water was available ad libitum except during sessions. The housing room was maintained under a 12–hour light/dark cycle (lights on at 6:00 AM). Facilities were maintained in accordance with USDA and AAALAC standards. The protocol was approved by the JHU Animal Care and Use Committee and followed the Guide for the Care and Use of Laboratory Animals (2011).
2.2. Apparatus
Sessions were conducted in modified primate cages as described in detail previously (Kaminski et al., 2008; Weerts et al., 2006) and contained (1) a panel with three colored “cue” lights, (2) an intelligence panel with two vertically operated levers and two different colored “jewel” lights each located above one of the levers, (3) a “drinkometer” connected to a calibrated 1000-ml bottle, and (4) a speaker mounted above the cages for presentation of auditory stimuli (tones). A computer interfaced with Med Associates hardware and software remotely controlled the experimental conditions and data collection.
2.3. Chained Schedule of Reinforcement Procedure
Sessions were conducted seven days per week and began at the same time (8:30 AM) each day. The start of a session and the onset of Component 1 was signaled by a 3-s tone. During Component 1, a red cue light was illuminated and all instrumental responses were recorded but had no programmed consequence. After 20 min, Component 1 ended and Component 2 was initiated.
Component 2 was signaled by the illumination of a yellow cue light and consisted of two links. During the first link, the jewel light over the left lever was turned on, and a concurrent fixed interval 10 min, fixed time 20 min (FI 10-min FT 20-min) schedule was in effect. The first link ended either a) with the first response on the left lever after 10 min elapsed or b) automatically after 20 min, whichever occurred first. During the second link, the jewel light over the left lever flashed and a fixed-ratio (FR) 10 schedule was in effect on the left lever. Completion of the FR response requirement ended Component 2; the yellow cue light and the jewel light were turned off and Component 3 was initiated. If the FR 10 requirement was not completed within 90 min, the session terminated without transitioning to Component 3 (i.e., no access to alcohol or the non-alcoholic beverage for the day).
Component 3 was signaled by the illumination of the blue cue light. A blue jewel light over the right lever was also illuminated, and the opportunity to self-administer alcohol or the non-alcoholic beverage (depending on group assignment) was available under an FR 10 schedule on the right lever. Completion of each FR and subsequent contact with the drinkometer spout delivered fluid for the duration of spout contact or for a programmed duration (5 sec), whichever came first. This defined a single drink. Component 3 and the session ended after 120 min.
2.4. Drugs
All solutions for oral consumption were mixed using reverse osmosis (RO) purified drinking water. Ethyl alcohol (190 Proof, Pharmco-AAPER, Brookville CT) was diluted with RO water to 4% w/v alcohol. Orange-flavored, sugar-free, Tang® powder (Kraft Foods) was dissolved in RO water as described previously (Duke et al., 2014). The 3-ISOPBC was synthesized in the laboratory of Dr. James Cook (University of Wisconsin-Milwaukee; Tiruveedhula et al., 2015). Doses of 3-ISOPBC (5.0 – 30.0 mg/kg) were dissolved in a vehicle of 50% saline, 37.5% propylene glycol, and 12.5% ethanol and administered via the intramuscular route (2-3 mls/injection). Vehicle tests were completed using the same volume and procedures as detailed below.
2.5. 3-ISOPBC Test Procedures
The baseline stability criterion was defined as stable self-administration of alcohol or non-alcoholic beverage (i.e., ± 20%) for three consecutive sessions. To evaluate acute effects of 3-ISOPBC on alcohol-related behaviors and to verify the safety of the dose range, in Experiment 1 doses of 3-ISOPBC (10.0 – 30.0 mg/kg) or its vehicle were administered acutely in the alcohol group only. The baseline stability criterion was met before each test dose of 3-ISOPBC. Doses were tested in mixed order, with active doses tested no more than once per week. In Experiment 2 doses of 3-ISOPBC (5.0 – 20.0 mg/kg) or vehicle were administered daily for 5 consecutive days to baboons in both groups. For both experiments, doses of 3-ISOPBC were administered 30 min before sessions.
2.6. Data Analysis
The primary variables of interest included measures of seeking (Component 2: FI responses and latency to complete the FI requirement) and measures of consumption (Component 3: FR self-administration responses, drink contacts, and volume consumed). Total g/kg and ml/kg consumed were calculated based on individual body weights and the total volume of alcohol or non-alcoholic beverage consumed, respectively. The patterning of drinking was analyzed as a function of drinking “bouts” as in our previous study (Kaminski and Weerts, 2014). A drinking bout was defined as 2 or more drinks with less than 5 minutes between each drink, beginning with the first drink.
For each baboon, the mean of the 3 sessions that preceded each test condition was used as the baseline for comparison with doses of 3-ISOPBC and vehicle. To determine whether there were any differences in baseline responding in the alcohol and control groups, baseline responding in Experiment 2 was compared using independent-samples t-tests (baseline responding of the alcohol group in Experiment 1 is not included because corresponding control group sessions were not conducted). In Experiment 2, data analyzed were the last 3 of the 5 days of 3-ISOPBC or vehicle administration. Data were analyzed using separate statistical analysis of variance (ANOVA) for each group (Alcohol or Control) with 3-ISOPBC dose (BL, 0.0 – 30.0 mg/kg) as a repeated measure. Bonferroni post-hoc tests were used for pair-wise comparisons of vehicle with 3-ISOPBC doses.
3. Results
During baseline sessions, stable drinking was observed in all baboons, in both groups and few or no responses were recorded on the inactive operanda (all operanda in Component 1, right lever and drinkometer in Component 2, and left lever in Component 3). Systematic differences between the groups during baseline sessions were not observed for measures of seeking (Component 2: FI responses and latency to complete the FI requirement). During the baseline sessions preceding drug test sessions, the grand mean (+ SEM) latency to complete the FI schedule was 639.5 (20.2) seconds for the alcohol group and 609.7 (5.3) seconds for the control group [t(6) = 1.09, p = .317]. The grand mean (+ SEM) number of FI responses was 141.2 (68.4) for the alcohol group and 144.9 (96.2) for the control group [t(6) = 0.03, p = .975].
During baseline sessions, the grand mean (+ SEM) alcohol intake was 748.1 (78.6) ml and 1.07 (0.07) g/kg, comparable to intake which has previously been reported to produce blood-alcohol levels (BAL) of >0.08% in baboons (Holtyn et al., 2014; Kaminski et al., 2008). The grand mean non-alcoholic beverage intake during baseline sessions was 1000.0 (0.0) ml. While volume of intake of the non-alcoholic beverage was higher than the volume of intake of alcohol [t(6) = 2.40, p = .053], we have previously demonstrated that 4% w/v alcohol and the nonalcoholic beverage function as equivalent reinforcers (i.e., maintain similar breaking points under a progressive ratio procedure) despite the fact that they maintain different intake volumes (Duke et al., 2014).
3.1. Experiment 1: Effects of Acute Administration of 3-ISOPBC
Table 1 shows effects of acute administration of 3-ISOPBC on alcohol seeking and consumption under the chained schedule of reinforcement. Acute administration of 3-ISOPBC did not significantly change any of the measures of seeking (Component 2: FI responses and latency to complete the FI requirement) or consumption (Component 3: FR self-administration responses and g/kg alcohol consumed). Behavioral observations conducted by laboratory personnel that included the recording of any signs or symptoms of drug side effects (e.g., sedation, muscle relaxation, motor incoordination, gastro-intestinal symptoms, etc.) confirmed that administration of doses up to and including 30.0 mg/kg were safe and did not produce adverse effects. However, there was some difficulty with solubility at the 30.0 mg/kg dose. Because of this, in combination with the difficulty in synthesizing the large quantities needed for testing in baboons, the 30.0 mg/kg dose was not tested under chronic conditions. Acute administration of 3-ISOPBC was not conducted in the control group because it did not significantly reduce seeking or consumption in the alcohol group.
Table 1. Experiment 1.
Effects of acute administration of vehicle (V) and doses of 3-ISOPBC on seeking in Component 2 (C2) and consumption in Component 3 (C3) for alcohol under the chained schedule of reinforcement. Baseline (BL) is the grand mean of the 3 days preceding each acute administration.
| BL | V | 3-ISOPBC dose (mg/kg) | F(4,16) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 10.0 | 20.0 | 30.0 | ||||||
| C2 | Left Lever FI Resp | Mean | 125.5 | 574.8 | 582.8 | 272.0 | 417.0 | 1.91 |
| SEM | 48.0 | 270.1 | 307.3 | 166.6 | 236.6 | |||
|
| ||||||||
| Left Lever FI Resp Latency (s) | Mean | 632.3 | 625.4 | 615.1 | 629.9 | 613.6 | 0.46 | |
| SEM | 24.8 | 19.2 | 13.7 | 13.2 | 10.7 | |||
|
| ||||||||
| C3 | Right Lever FR Resp | Mean | 117.9 | 126.2 | 108.0 | 109.4 | 106.0 | 0.82 |
| SEM | 14.6 | 12.6 | 13.0 | 16.1 | 19.5 | |||
|
| ||||||||
| g/kg Alc Consumed | Mean | 1.1 | 1.2 | 1.1 | 1.0 | 1.0 | 0.60 | |
| SEM | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |||
Note: FI, Fixed Interval; FR, Fixed Ratio; Resp, Response; Alc, Alcohol.
3.2. Experiment 2: Effects of Chronic Administration of 3-ISOPBC
Table 2 shows effects of chronic administration of 3-ISOPBC on seeking for alcohol and the non-alcoholic beverage under the chained schedule of reinforcement. Chronic administration of 3-ISOPBC did not significantly change any of the measures of seeking (Component 2: FI responses and latency to complete the FI requirement) in both the alcohol and control groups.
Table 2. Experiment 2.
Effects of chronic (5 day) administration of vehicle (V) and doses of 3-ISOPBC on seeking in Component 2 (C2) for alcohol (Alcohol Group) and a non-alcoholic beverage (Control Group) under the chained schedule of reinforcement. Baseline (BL) is the grand mean of the 3 days preceding each chronic condition.
| Alcohol Group | BL | V | 3-ISOPBC dose (mg/kg) | F(4,16) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 5.0 | 10.0 | 20.0 | ||||||
| C2 | Left Lever FI Resp | Mean | 141. 2 | 394.5 | 212.3 | 161.5 | 198.2 | 1.93 |
| SEM | 68.4 | 217.4 | 118.1 | 107.4 | 113.1 | |||
|
| ||||||||
| Left Lever FI Resp Latency (s) | Mean | 639. 5 | 620.4 | 706.0 | 676.5 | 755.3 | 1.05 | |
| SEM | 20.2 | 10.8 | 58.1 | 52.0 | 93.2 | |||
|
| ||||||||
| Control Group | BL | V | 5.0 | 10.0 | 20.0 | F(4,8) | ||
|
| ||||||||
| C2 | Left Lever FI Resp | Mean | 144. 9 | 128.2 | 200.4 | 154.8 | 75.0 | 1.03 |
| SEM | 96.2 | 55.3 | 131.1 | 85.1 | 37.7 | |||
|
| ||||||||
| Left Lever FI Resp Latency (s) | Mean | 609. 7 | 674.6 | 612.8 | 624.7 | 735.0 | 1.33 | |
| SEM | 5.3 | 20.7 | 11.3 | 18.3 | 95.1 | |||
Note: FI, Fixed Interval; FR, Fixed Ratio; Resp, Response; Alc, Alcohol.
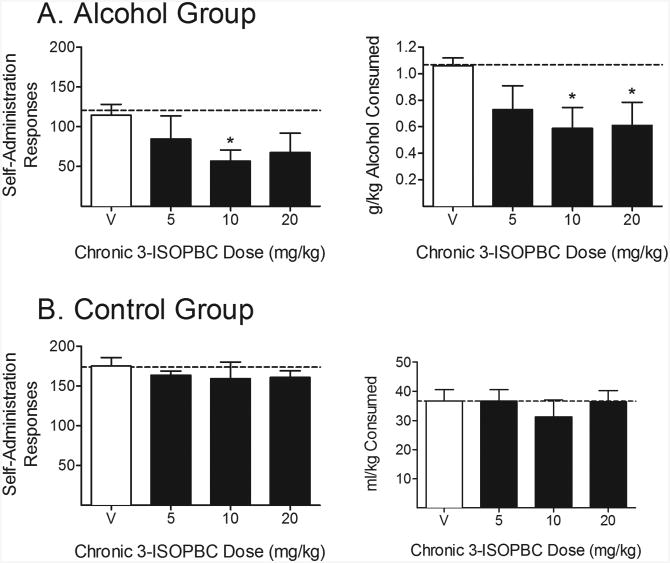
Figure 1 shows effects of chronic administration of 3-ISOPBC on consumption. In the alcohol group, chronic administration of 3-ISOPBC decreased the number of self-administration responses (Component 3: FR responses), with a significant decrease relative to vehicle at the 10.0 mg/kg dose. Chronic administration of 3-ISOPBC decreased g/kg alcohol consumed, with significant decreases relative to vehicle at the 10.0 and 20.0 mg/kg doses. In the control group, 3-ISOPBC did not reduce the number of self-administration responses or ml/kg consumed at any of the doses.
Figure 1. Experiment 2.
Effects of chronic (5 day) administration of 3-ISOPBC (5.0 – 20.0 mg/kg) on consumption in Component 3 of the chained schedule of reinforcement in the (A) Alcohol Group and (B) Control Group. Data shown are the group means (+ SEM) of self-administration responses (left panels), and g/kg alcohol consumed for the alcohol group and ml/kg consumed for the control group (right panel). Baseline responding is indicated by the horizontal, dashed lines. *indicates p < .05 for pair-wise comparison for each 3-ISOPBC dose vs. vehicle.
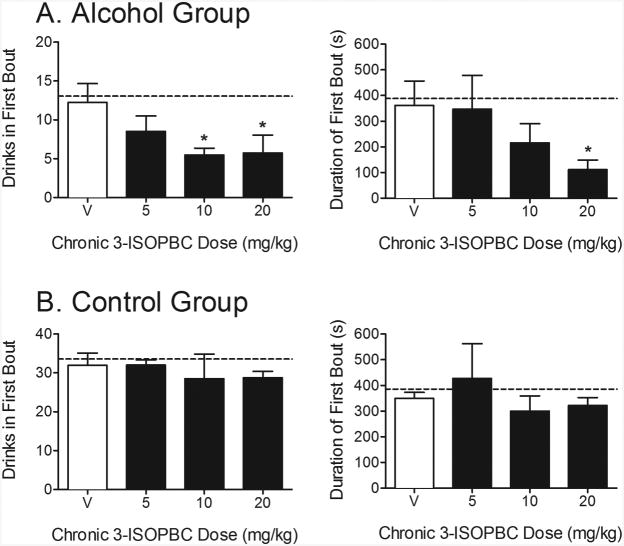
Figure 2 shows effects of chronic administration of 3-ISOPBC on the pattern of drinking in the first drinking bout. In the alcohol group, chronic administration of 3-ISOPBC decreased the number of drinks in the first drinking bout, with a significant decrease relative to vehicle at the 10.0 and 20.0 mg/kg doses. Chronic administration of 3-ISOPBC decreased the duration of the first drinking bout, with significant decreases relative to vehicle at the 20.0 mg/kg dose. In the control group, 3-ISOPBC did not reduce the number of drinks in or the duration of the first drinking bout at any of the doses.
Figure 2. Experiment 2.
Effects of chronic (5 day) administration of 3-ISOPBC (5.0 – 20.0 mg/kg) on the pattern of drinking in the first drinking bout in Component 3 of the chained schedule of reinforcement in the (A) Alcohol Group and (B) Control Group. Data shown are the group means (+ SEM) of the number of drinks in the first drinking bout (left panels), and the duration (seconds) of the first drinking bout (right panels). Baseline responding is indicated by the horizontal, dashed lines. *indicates p < .05 for pair-wise comparison for each 3-ISOPBC dose vs. vehicle.
4. Discussion
The α1 subunit of the GABAA receptor has been implicated in the reinforcing- and abuse-related effects of alcohol in some studies (Blednov et al., 2003; Harvey et al., 2002; June et al., 2007; Kaminski et al., 2013). The present study investigated whether acute and chronic administration of the benzodiazepine GABAA α1-preferring ligand 3-ISOPBC could selectively reduce alcohol seeking and self-administration in baboons. Pretreatment with 3-ISOPBC did not reduce alcohol seeking in a group that self-administered alcohol or in a control group that self-administered a non-alcoholic beverage. This is consistent with a prior similar study in which acute and chronic administration of 3-PBC did not reduce alcohol seeking in baboons responding under a chained schedule of alcohol reinforcement (Kaminski et al., 2013). Chronic, and not acute, administration of 3-ISOPBC reduced alcohol consumption in the alcohol group without reducing consumption of the non-alcoholic reinforcer in the control group. Thus, 3-ISOPBC may have therapeutic potential in the treatment of alcohol use disorder due to its ability to selectively reduce alcohol use.
Identification of the precise role of the GABAA α1 receptor in alcohol reinforcement and consumption is ongoing. Knockout mice without the GABAA α1 receptor have been shown to consume less alcohol under a two-bottle alcohol versus water choice procedure (Blednov et al., 2003) and an operant self-administration procedure (June et al., 2007). The GABAA α1-preferring antagonist 3-PBC has been shown to decrease alcohol self-administration in rodents bred for high alcohol intake (Harvey et al., 2002), as well as binge-like drinking in a maternally deprived rodent model (Gondre-Lewis et al., 2016). A similar reduction in alcohol maintained responding was observed following administration of 3-ISOPBC in the maternal deprivation model (Tiruveedhula et al., 2015). In baboons, 3-PBC reduced self-administration of alcohol but also had some effects on self-administration of a non-alcoholic reinforcer (Kaminski et al., 2013). In contrast to the findings in rodents and baboons, both 3-PBC and βCCT failed to attenuate alcohol drinking in rhesus macaques (Sawyer et al., 2014). The reason for this contradictory result is unclear; however, the highest dose of 3-PBC tested in Sawyer et al.'s study (10.0 mg/kg) was lower than that which reduced total g/kg alcohol intake in the baboon model (18.0 mg/kg). The choice of 3-ISOPBC for the present study was based on the structure of the branched isopropyl group, which would hinder metabolism by beta (omega-1) oxidation. It was hypothesized that this would increase the duration of action and provide a ligand more active in vivo than 3-PBC; this appears to be the case. In the present study, 3-ISOPBC selectively reduced alcohol drinking.
In both the alcohol and control groups, the majority of drinks occurred within the first drinking bout. Prior studies also have shown rats (Samson et al., 2000), baboons (Weerts et al., 2006), and other primates (Boyle et al., 1998) to engage in “loading,” wherein the highest rate of alcohol drinking occurs early in the alcohol self-administration period followed by lower rates of drinking for the remainder of the period. Chronic administration of 3-ISOPBC reduced the number of drinks in and the duration of the first drinking bout in the alcohol group, but not in the control group. This suggests that 3-ISOPBC may reduce alcohol intake once consumption is initiated, which could be important in preventing drinking episodes from becoming a full relapse to heavy drinking. Chronic administration of 3-PBC also has been shown to decrease the number of drinks during the first 20 minutes of alcohol availability in Component 3 of the chained schedule (Kaminski et al., 2013). In Kaminski et al.'s (2013) study, the highest doses of 3-PBC tested (10.0 and 18.0 mg/kg) also decreased the number of drinks in the first 20 minutes in a control group that self-administered a non-alcoholic beverage. Harvey et al. (2002) observed a decrease in saccharin-maintained responding when the highest dose of 3-PBC (20.0 mg/kg) was administered to rodents. The authors suggested that the non-selective effects at higher doses of 3-PBC may be due to a saturation of all α receptors as 3-PBC binds to other α receptors to some degree. It is worth emphasizing, however, that in both studies, responding maintained by the non-alcoholic reinforcer was reduced at higher doses than required to suppress alcohol self-administration. In the present study, effects of 3-ISOPBC were selective for alcohol. The mechanism by which 3-ISOPBC selectively attenuated alcohol response in the present study is not known. Possible differences between 3-PBC and 3-ISOPBC include differences in metabolism or ligand transport.
Although the mechanisms of action of the α1 -preferring antagonists are not well understood, one possibility rests on the tonic control in the central nervous system by opposing systems, including GABA. It is possible that these α1 -preferring antagonists simply stabilize the benzodiazepine α1 GABA receptor system in the antagonist conformation, the result of which would be to slightly decrease the normal flow of chloride ions through the α1β2/3γ2 ion channel. The effect via the projections from the ventral tegmental area (Harvey et al., 2002) to the nucleus accumbens would then effect the levels of dopamine release; this slight decrease may be why Warnock, June et al. (personal communication) did observe a decrease in alcohol self-administration in a binge drinking model (rodents) in the complete absence of anhedonia or depression. Tiruveedhula, et al. (2015) reported that 3-ISOPBC decreased alcohol consumption in a maternally deprived rodent model. Gondre-Lewis et al. (2016) reported a similar effect with 3-PBC and proposed some involvement of α2-receptor subunits; however, this effect could not be reversed by administration of flumazenil. Consequently, this cannot be due to an effect at α2β2/3γ2 benzodiazepine GABAA receptors. It is possible that the observed effect was mediated by a different set of α2-related receptors (Gondre-Lewis et al., 2016) or to the α1-preferring antagonist effect at benzodiazepine α1 GABAA receptors. Much work remains to understand this observation. Nevertheless, the real strength of the use of α1-preferring antagonists in the treatment of alcohol use disorder stems from the fact that this type of ligand lacks sedating, amnesic, and ataxic properties (Ator et al., 2010; Licata et al., 2009) because it is an antagonist at this α1 benzodiazepine GABAA site.
The present study examined whether the GABAA α1-preferring ligand 3-ISOPBC possesses therapeutic potential in regard to its ability to selectively reduce alcohol seeking and consumption. Alcohol use disorders are heterogeneous and development of more efficacious and safe pharmacotherapies is needed to expand the number of individuals who may benefit from treatment. In the present study, 3-ISOPBC did not decrease alcohol seeking, but did selectively reduce alcohol self-administration and consumption by primarily altering the pattern of drinking. 3-ISOPBC selectively reduced the number of drinks and the duration of drinks in the first alcohol drinking bout. No changes in drinking patterns were found for the non-alcoholic reinforcer. These data suggest that 3-ISOPBC may be clinically useful for reducing alcohol use.
Supplementary Material
Highlights.
Acute doses of 3-ISOPBC did not decrease alcohol seeking or consumption in baboons.
Chronic doses of 3-ISOPBC reduced alcohol consumption.
Chronic doses of 3-ISOPBC did not affect consumption of a non-alcoholic beverage.
Effects of chronic doses of 3-ISOPBC on consumption were specific to alcohol.
Acknowledgments
The authors thank Margot Ernst, Petra Scholze, and Xenia Simeone for providing data on the binding affinity of 3-ISOPBC. Psychoactive drug screening program data were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2013-00017-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth, MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda, MD.
Role of Funding Source. This work was supported by the National Institutes of Health under grant numbers R01AA015971 (Weerts), R01MH096463 (Cook), and R01NS076517 (Cook). The grants provided financial support for the conduct of the research and the preparation of the article. The funding sponsors were not involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributions. All authors have contributed to the research and the article preparation. Holtyn performed the data analyses, wrote the first draft of the manuscript, and incorporated edits from the co-authors. Weerts contributed to the original conceptualization and design of the experiments, directed the present experiments, and edited the manuscript. Cook directed the development of the novel compound used in these experiments and contributed to the original conceptualization and design of the experiments. Tiruveedhula and Stephen synthesized the novel compound used in the experiments and edited the manuscript. All authors have approved the final manuscript.
Conflict of Interest. The authors have no conflicts of interest to declare.
References
- Ator NA, Atack JR, Hargreaves RJ, Burns HD, Dawson GR. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at α1 and α2/3 subtypes. J Pharmacol Exp Ther. 2010;332:4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA. GABAA receptor α1 and β2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther. 2003;305:854–863. doi: 10.1124/jpet.103.049478. [DOI] [PubMed] [Google Scholar]
- Boyle AEL, Stewart RB, Macenski MJ, Spiga R, Johnson BA, Meisch RA. Effects of acute and chronic doses of naltrexone on ethanol self-administration in rhesus monkeys. Alcohol Clin Exp Res. 1998;22:359–366. [PubMed] [Google Scholar]
- Clayton T, Chen JL, Ernst M, Richter L, Cromer BA, Morton CJ, Ng H, Kaczorowski CC, Helmstetter FJ, Furtmuller R, Ecker G, Parker MW, Sieghart W, Cook JM. An updated unified pharmacophore model of the benzodiazepine binding site on gamma-aminobutyric acid(a) receptors: correlation with comparative models. Curr Med Chem. 2007;14:2755–2775. doi: 10.2174/092986707782360097. [DOI] [PubMed] [Google Scholar]
- Clayton T, Poe MM, Rallapalli S, Biawat P, Savić MM, Rowlett JK, Gallos G, Emala CW, Kaczorowski CC, Stafford DC, Arnold LA. A review of the updated pharmacophore for the alpha 5 GABA (A) benzodiazepine receptor model. Int J Med Chem. 2015;2015:1–54. doi: 10.1155/2015/430248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AN, Kaminski BJ, Weerts EM. Baclofen effects on alcohol seeking, self-administration and extinction of seeking responses in a within-session design in baboons. Addict Biol. 2014;19:16–26. doi: 10.1111/j.1369-1600.2012.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90:95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foye WO, Lemke TL, Williams DA. Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins; 2013. [Google Scholar]
- Gondré-Lewis MC, Warnock KT, Wang H, June HL, Jr, Bell KA, Rabe H, Tiruveedhula VP, Cook JM, Lüddens H, Aurelian L, June HL., Sr Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABAA mechanism. Stress. 2016;19:235–247. doi: 10.3109/10253890.2016.1160280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma CR, Cook JM, June HL. The GABA(A) receptor alpha(1) subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22:3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Huang Q, Ma C, Yu S, McKernan R, Cook JM. Pharmacophore/receptor models for GABA(A)/BzR alpha2beta3gamma2, alpha3beta3gamma2 and alpha4beta3gamma2 recombinant subtypes. Included volume analysis and comparison to alpha1beta3gamma2, alpha5beta3gamma2, and alpha6beta3gamma2 subtypes. Drug Des Discov. 2000;17:131–171. [PubMed] [Google Scholar]
- Holtyn AF, Kaminski BJ, Wand GS, Weerts EM. Differences in extinction of cue-maintained conditioned responses associated with self-administration: alcohol versus a nonalcoholic reinforcer. Alcohol Clin Exp Res. 2014;38:2639–2646. doi: 10.1111/acer.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, He X, Ma C, Liu R, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM. Pharmacophore/receptor models for GABAA/BzR subtypes (α1β3γ2, α5β3γ2, and α6β3γ2) via a comprehensive ligand-mapping approach. J Med Chem. 2000;43:71–95. doi: 10.1021/jm990341r. [DOI] [PubMed] [Google Scholar]
- Huang XP, Mangano T, Hufeisen S, Setola V, Roth BL. Identification of human ether-a-go-go related gene modulators by three screening platforms in an academic drug-discovery setting. Assay Drug Dev Technol. 2010;8:727–742. doi: 10.1089/adt.2010.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, II, Harvey SC, Eiler WJA, II, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacol. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- June HL, Foster KL, Eiler WJ, Goergen J, Cook JB, Johnson N, Mensah-Zoe B, Simmons JO, Yin W, Cook JM, Homanics GE. Dopamine and benzodiazepine-dependent mechanisms regulate the EtOH-enhanced locomotor stimulation in the GABAA α1 subunit null mutant mice. Neuropsychopharmacol. 2007;32:137–152. doi: 10.1038/sj.npp.1301097. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Duke AN, Weerts EM. Effects of naltrexone on alcohol drinking patterns and extinction of alcohol seeking in baboons. Psychopharmacol. 2012;223:55–66. doi: 10.1007/s00213-012-2688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Goodwin AK, Wand G, Weerts EM. Dissociation of alcohol-seeking and consumption under a chained schedule of oral alcohol reinforcement. Alcohol Clin Exp Res. 2008;32:1–9. doi: 10.1111/j.1530-0277.2008.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Van Linn ML, Cook JM, Yin W, Weerts EM. Effects of the benzodiazepine GABAA α1-preferring ligand, 3-propoxy-β-carboline hydrochloride (3-PBC), on alcohol seeking and self-administration in baboons. Psychopharmacology. 2013;227:127–36. doi: 10.1007/s00213-012-2946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Weerts EM. The effects of varenicline on alcohol seeking and self-administration in baboons. Alcohol Clin Exp Res. 2014;38:376–383. doi: 10.1111/acer.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Van Linn ML, Rowlett JK. Contribution of alpha1 subunit-containing gamma-aminobutyric acidA (GABAA) receptors to motor-impairing effects of benzodiazepines in squirrel monkeys. Psychopharmacol. 2009;203:539–546. doi: 10.1007/s00213-008-1401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo IA, Harris RA. GABA(A) receptors and alcohol. Pharmacol Biochem Behav. 2008;90:90–94. doi: 10.1016/j.pbb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide For The Care And Use Of Laboratory Animals. Eighth. The National Academies Press; Washington, DC: 2011. [Google Scholar]
- Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD, Cook J, Ma C. Selective antagonism of the ataxic effects of zolpidem and triazolam by the GABAA/alpha1-preferring antagonist beta-CCt in squirrel monkeys. Psychopharmacol. 2002;164:151–159. doi: 10.1007/s00213-002-1189-9. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcohol Clin Exp Res. 2000;24:766–773. [PubMed] [Google Scholar]
- Sawyer EK, Moran C, Sirbu MH, Szafir M, Linn M, Namjoshi O, Phani Babu Tiruveedhula VVN, Cook JM, Platt DM. Little evidence of a role for the α1GABAA subunit-containing receptor in a rhesus monkey model of alcohol drinking. Alcohol Clin Exp Res. 2014;38:1108–1117. doi: 10.1111/acer.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruveedhula VP, Methuku KR, Deschamps JR, Cook JM. Synthesis of aza and carbocyclic β-carbolines for the treatment of alcohol abuse. Regiospecific solution to the problem of 3, 6-disubstituted β-and aza-β-carboline specificity. Organic Biomol Chem. 2015;13:10705–10715. doi: 10.1039/c5ob01572c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Goodwin AK, Kaminski BJ, Hienz RD. Environmental cues, alcohol seeking, and consumption in baboons: effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res. 2006;30:2026–2036. doi: 10.1111/j.1530-0277.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Yin W, Majumder S, Clayton T, Petrou S, VanLinn ML, Namjoshi OA, Ma C, Cromer BA, Roth BL, Platt DM, Cook JM. Design, synthesis, and subtype selectivity of 3,6-disubstituted beta-carbolines at Bz/GABA(A)ergic receptors. SAR and studies directed toward agents for treatment of alcohol abuse. Bioorg Med Chem. 2010;8:7548–64. doi: 10.1016/j.bmc.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.