Abstract
Neutrophils are often viewed as non-specialized effector cells whose presence is a simple indicator of tissue inflammation. There is new evidence that neutrophils exist in subsets and have specialized effector functions that include extracellular trap generation and the stimulation of angiogenesis. The application of intravital imaging to transplanted organs has revealed novel requirements for neutrophil trafficking into graft tissue and illuminated direct interactions between neutrophils and other leukocytes that promote alloimmunity. Paradoxically, retaining some neutrophilia may be important to induce or maintain tolerance. Neutrophils can stimulate anti-inflammatory signals in other phagocytes and release molecules that inhibit T cell activation. Here we will review the available evidence of how neutrophils regulate acute and chronic inflammation in transplanted organs and discuss the possibility of targeting these cells to promote tolerance.
Introduction
Neutrophils are usually the first leukocytes to infiltrate transplanted organs and are a well-established marker of transplant injury (1). Most work on neutrophils in transplanted organs has focused on their destructive role during Ischemia Reperfusion Injury (IRI), a form of sterile tissue damage that is exacerbated by the massive release of oxidative and proteolytic effector activity by these cells. Advances in our understanding of the underlying mechanisms of sterile inflammation have revealed that both neutrophil infiltration and activation are augmented by the release of damage associated molecular patterns (DAMPs) from necrotic cells and the extracellular matrix (ECM) (2). Accordingly, DAMPs induce the expression of inflammatory cytokines via stimulating pattern recognition receptors (PRRs) on macrophages. These include ELR+ CXC chemokines (e.g., CXCL8 in humans & CXCL1, CXCL2 in rodents) and IL-1β that play a key role in neutrophil recruitment by activating vascular endothelium (3). Neutrophils also express PRRs, which when engaged by DAMPs, induce the generation of reactive oxygen species (ROS) and hydrolytic enzymes that exacerbate graft damage. From this perspective graft-infiltrating neutrophils respond they would during an infection since DAMPs contain the analogous structural features of pathogen associated molecular patterns.
However, this is a rather limited view of neutrophil function as there is accumulating evidence that they also play a critical role in adaptive immunity. Similar to professional antigen presenting cells (APCs), neutrophils have the capacity to leave peripheral sites and deliver antigen to lymph nodes (4). Additionally, neutrophils can induce the differentiation of T lymphocytes via expression of MHC and co-stimulatory molecules (5). Finally, and perhaps the least described role of neutrophils is their contribution to the resolution of inflammation. Through their own apoptotic death, neutrophils can induce the expression of anti-inflammatory molecules in other myeloid cells (6) while subsets of neutrophils can inhibit T cell activation (7) as well as promote angiogenesis (8). Here we will review neutrophil biology in the context of acute and chronic transplant inflammation as well as examine approaches to developing neutrophil targeted therapies to promote graft survival.
Acute Injury
Neutrophil recruitment begins as a passive process triggered initially by vascular endothelium. Neutrophils make contact with the vascular endothelium through a series of distinct dynamic behaviors known as tethering, rolling, adherence, and crawling before finally extravasating through the vessel (3). While these behaviors are likely common to all leukocytes, early studies suggested that targeting adhesion molecules had profound effects on neutrophil graft infiltration. For example, in a rat intestinal transplantation model treatment with a recombinant P-selectin glycoprotein ligand-1 reduced graft neutrophilia and ameliorated tissue injury (9).
Although these observations have illuminated some important signals neutrophils use to traffic into grafts only recently have there been direct observations of their dynamic behavior. These advancements have been forwarded by the use of intravital 2-photon (2P) microscopy for transplanted organs. In a beating mouse heart transplant model Li et al. visualized the effects of antibody-mediated blockade of the integrins Mac-1 and LFA-1 on neutrophil intravascular behavior and extravasation. Here LFA-1 blockade completely prevented adherence and crawling while Mac-1 blockade allowed adherence but slowed intravascular crawling and transendothelial migration speed (10). In a mouse lung transplant model intravital 2P microscopy revealed that neutrophils track behind inflammatory Ly6Chi monocytes while in the process of transendothelial migration (11). This relationship is likely critical for extravasation as clodronate-mediated depletion of monocytes induced neutrophils to accumulate along the luminal surfaces of vascular endothelium. Future studies will be required to determine how inflammatory monocytes promote neutrophil transendothelial migration and whether monocyte-dependent neutrophil extravasation occurs in other organs.
Neutrophils commit the bulk of their effector responses following exit from the vasculature. In the transplant setting one of the most destructive activities is the generation of reactive oxygen species (ROS) (12). Activated neutrophils primarily employ the oxidant-generating complex system NADPH oxidase to generate superoxide, which promotes macromolecule peroxidation and irreversible cellular damage. NADPH oxidase activity requires two membrane-spanning proteins (gp91phox, p22phox) to assemble with three cytosolic components of the complex (p40phox, p47phox, p67phox). In this regard, chronic granulomatous disease patients who encode hypofunctional mutations in gp91phox or p47phox exhibit significant protection from transiently induced upper limb IRI (13). Moreover, in canine heart and rat liver IRI models neutrophil NADPH oxidase-mediated ROS generation was the predominant contributor to tissue damage (14, 15). Neutrophil-mediated graft tissue damage is also driven by the release of tissue-digesting enzymes such as metalloproteinase-9 (MMP9) and neutrophil elastase (NE). MMP9 and NE, which have been observed to accumulate in acutely injured liver and lung transplants (16, 17), break down homeostatic barriers that promote graft function by hydrolyzing ECM proteins collagen, elastin and fibronectin. Accordingly, mice deficient in MMP9 are protected from steatotic liver IRI (18). The lack of MMP9 also promoted vascular integrity and inhibited the proteolysis of platelet endothelial cell adhesion protein, which is reported required for maintaining endothelial cell-cell junctions.
Neutrophils can also promote graft inflammation through undergoing a unique form of programmed cell death termed “NETosis” (19). These senescent neutrophils display extracellular chromatin (NETs) decorated with histones, antibacterial peptides and serine proteases. NETs were first described to have antimicrobial functions but later studies revealed that they cause extensive endothelial cell damage in response to sterile inflammation. NETs have been reported in the bronchoalveolar lavage fluid of human lung transplant recipients with primary graft dysfunction and in experimental lung transplant-mediated IRI and liver IRI mouse models (20, 21). In both studies the authors used DNAse to dissolve NETs, which led to improved organ function and attenuated acute inflammation measured up to one day. However, whether NETs exclusively promote inflammation remains controversial. Shauer et al reported that NETs in a chronic gout model can trap and degrade ELR+ chemokines and inflammatory cytokines (e.g., IL-6, IL-1β) due to their high concentrations of proteases (22). Neutrophil depletion, inhibition, or DNAse digestion of NETs actually worsened chronic tissue injury. Thus, future studies will be needed to determine the long-term consequences of NETs on graft survival.
Acute Cellular Rejection
Neutrophil depletion experiments have revealed the importance of neutrophils in promoting alloimmune responses. For example, in a mouse skin transplant model neutrophil depletion slowed acute rejection by attenuating the recruitment of alloreactive memory CD8+ T cells (23). Neutrophils may stimulate the recruitment of activated CD8+ T cells through their expression Fas ligand, which can induce expression of the T cell chemoattractants CCL1, CCL2 and CCL5 (24). Additionally, intravital imaging of allografts has uncovered the importance of neutrophil cross-talk with other graft-resident leukocytes in stimulating alloimmune responses. In a model orthotopic lung transplant model, neutrophil depletion was shown to promote immunosuppression-mediated allograft acceptance leading to less intragraft antigen-presenting cell (APC) IL-12 production and reduced Th1 cell alloimmunity (25). Intravital visualization of infiltrating neutrophils in acutely rejecting grafts showed that they made prolonged interactions with donor-derived graft-resident CD11c+ APCs. Additionally, when these infiltrating neutrophils were co-cultured with dendritic cells (DC) they induced IL-12 and MHC Class II expression in a manner that required contact and TNF-α on their plasma membrane. By contrast, neutrophils from resting mice had little plasma membrane TNF-α and did not activate DCs suggesting that IRI licenses neutrophils to promote alloimmune responses (25).
It remains conceivable that neutrophils additionally stimulate alloimmunity as also they infiltrate infected allografts. This possibility was recently investigated in a mouse lung transplant model where Pseudomonas aeruginosa infection prevented the maintenance of tolerance (26). Infiltrating neutrophils were visualized to interact with lung allograft resident T cells that were also in contact with CD11c+ APCs. These interactions were shown to stimulate alloimmune responses through Pseudomonas-dependent upregulation of neutrophil CD80 and CD86, which, in turn, provided a sufficient trans-costimulation signal to expand the intragraft Th1 and Th17 compartment (26). The interplay between infection and alloimmunity is of considerable interest and has been reviewed elsewhere (27).
Antibody-mediated Rejection
Unlike for T cell mediated-alloimmunity, there is comparatively little mechanistic data on how neutrophils regulate humoral responses to transplanted organs. Presently, there are no reports analyzing the effects of depleting neutrophils on antibody-mediated rejection. Clinical pathological findings show that graft neutrophilia is linked to antibody-mediated transplant rejection (28, 29). In mouse heart and lung transplant models, antibody-mediated rejection induced infiltrating neutrophil activity that played a significant role in tissue destruction (29, 30). In this regard neutrophils are conceivably being recruited to grafts by complement fixed antibodies or by platelets bound to vascular endothelium injured by alloantibodies (31). Accordingly, neutrophils express the complement receptor 1 which promotes adhesion to complement decorated immune complexes. Activated platelets can stimulate endothelial cell P-selectin upregulation that can additionally promote neutrophil recruitment. How alloantibodies contribute to neutrophil effector responses in grafts is even less clear. Early work in an anti-MHC Class I antibody-mediated acute lung injury model suggested that Fcγ receptor-mediated recognition of immunoglobulin complexes could lead to neutrophil effector responses within grafts (32). However, in a later report kidney transplant recipients with alloantibodies and a hypofunctional mutation in the inhibitory Fcγ IIB receptor, which negatively regulates Fcγ receptors that promote inflammation, there was no impact on graft survival (33). It is also possible that neutrophils control B cell differentiation following transplantation. Recent work has shown the existence of a neutrophil subset infiltrating the lymph node peri-marginal zones that secretes high levels of BAFF, APRIL, CD40L and IL-21, which are B cell stimulating factors involved in antibody class switching, plasma cell differentiation and survival (34). Future studies will need to be conducted to determine if B cell inducing neutrophils exist in transplant recipients and, if so, whether they play a role antibody-mediated rejection.
Chronic rejection
Infiltrating neutrophils are commonly observed in chronically rejecting allografts. The predominant view is that neutrophilia is an effector arm of IL-17 expression in chronic rejection, mainly through the accumulation of Th17 cells (35). IL-17 is a potent driver of both neutrophil infiltration and production by stimulating the production of ELR+ CXC chemokines and granulopoietic cytokines (e.g., G-CSF, GM-CSF), respectively. The impact of IL-17-mediated neutrophilia on chronic rejection was recently reported in lung transplant recipients where a mutation in the IL-17 receptor that promotes airway neutrophilia was shown to increase the risk for chronic rejection (36). How neutrophils promote chronic rejection remains poorly described. Studies linking TLR signaling pathways to chronic rejection suggest that, similar to acute settings, neutrophil-mediated injury is triggered by DAMPs. For example, the ECM DAMP hyaluronic acid (HA) accumulates in airway lesions of human lung recipients with bronchiolitis obliterans syndrome (BOS). Analysis of the low molecular weight form of HA in a mouse lung transplant model showed that it stimulated neutrophil infiltration in a TLR2/4 dependent manner in addition to ROS burst (37). Moreover, proteolytic enzymes commonly related to ECM degradation and neutrophils migration, such as metalloproteinases (e.g., MMP9, MMP8), have been found to be increased in patients affected by BOS (17). Neutrophils may also promote chronic inflammation by influencing the maintenance of parenchymal tissues. A recent report showed the accumulation of neutrophil-derived alpha and beta defensin peptides in BOS patients. These peptides induced airway epithelial cells to produce inflammatory cytokines (e.g., IL-8, IL-1β) and pro-fibrotic growth factors (e.g., VEGF, EGF) suggesting a link between neutrophil recruitment and the induction of gene expression patterns within parenchymal cells known to promote tissue remodeling (38).
Resolution of Inflammation
The resolution of graft inflammation is dependent on the efficient removal of neutrophils from inflamed tissue. This is largely controlled by induction of neutrophil apoptotic death (39). Importantly, neutrophil apoptosis not only terminates effector activity but also is a passive anti-inflammatory signal that is propagated to other immune cells. The externalization of phosphatidyl serine (PS) is a signal for the uptake or ‘efferocytosis’ of neutrophil carcasses by phagocytes (e.g., macrophages) and leads to several complementary mechanisms that can resolve inflammation (6, 39). First, the engulfment of apoptotic neutrophils prevents secondary necrosis, which can result in the uncontrolled release of damaging proteolytic and oxidative mediators. Second, efferocytosing phagocytes produce large amounts of the anti-inflammatory cytokines IL-10 and TGF-β, which not only act to inhibit inflammatory gene expression, but also help to enforce tolerance through generation and maintenance of peripheral regulatory CD4+ T cells (40). Lastly, efferocytosing phagocytes convert arachidonic acid, a lipid involved in the exacerbation of IRI, into pro-resolving lipid mediators that inhibit the activation and recruitment of neutrophils (41). In the latter case treatment with the pro-resolving lipid lipoxin A4 ameliorated hepatic tissue injury, reduced evidence of histopathological allograft rejection and improved liver function in a rat model of liver allotransplantation (42). Lipoxin A4 also increased the ratio of Th2 to Th1 cytokine expression, which is a CD4+ T cell helper cell profile often associated with allograft tolerance.
Apoptotic neutrophils can also modulate inflammation though the release of small extracellular vesicles called Neutrophils Derived Microvesicles (NDMV). NDMVs are small vesicles ranging in size from 0.1–1.0 µm that promote intercellular communication by allowing transfer of membrane and cytosolic proteins, lipids, and RNA. Notably, NDMV function as intercellular reservoirs of pro-resolving lipid mediator precursors which can be metabolized to an active form at the inflammation site (43). In addition, recognition of the PS expressed on NDMVs membranes stimulated macrophages to express the pro-resolving cytokine TGF-β and to be less responsive to LPS (44).
Perhaps the most unrecognized activity of neutrophils is the potential to inhibit alloimmunity by preventing T cell activation. For example, activated neutrophils can suppress T cell activation by the release of NE and cathepsin G (45). These serine proteases cleave and inactivate IL-2 and IL-6 and promote the shedding of their cognate receptors from T-cells. Neutrophils also may act preemptively to prevent T cell alloimmunity. Pillay et al demonstrated that CD16bbrightCD62lo neutrophil subsets in acutely injured patients could bind to T cells via Mac-1 and release hydrogen peroxide into the immunological synapses, resulting in the suppression of proliferation (7). Future studies will be required to determine if neutrophil subsets with regenerative or anti-inflammatory activity exist in transplant recipients.
Finally, neutrophils may also inhibit graft inflammation by promoting wound healing and tissue repair. Neutrophils use their integrin receptors to form dense clusters around necrotic tissue foci and seal them off from healthy tissue (46). Distinct neutrophil subsets then may help re-establish graft perfusion. Christoffersson et al identified a circulating pro-angiogenic CD11b+Gr-1+CXCR4hi neutrophil subset that was recruited in a vascular endothelial growth factor-A dependent manner to avascular mouse pancreatic islet transplants (8). Neovascularization was dependent on high levels of MMP9, a protease that is normally required for revascularization. Notably, a CD49+ VEGFR1hi CXCR4hi neutrophil subset with an analogous function was also found in humans (47).
Neutrophil-based therapy
At present, there are no clinically available agents that specifically inhibit neutrophil function. Given the immunosuppression status of transplant recipients, if such therapies are developed, rigorous infection monitoring will be needed because neutrophil impairment or neutropenia profoundly increases susceptibility to fungal and bacterial infections. However, it may be possible to target molecules on neutrophils that augment alloimmunity without detrimentally attenuating pathogen surveillance. In a mouse lung transplant model, CTLA4Ig was used to block the effects of Pseudomonas-mediated CD80/86 upregulation on neutrophils (26). Notably, CTLA4Ig treatment prevented acute cellular rejection and peribronchiolar fibrosis without inhibiting Pseudomonas clearance. It remains to be determined if CTLA4Ig modulates neutrophil function in humans. However, a recent 84-month study evaluating a humanized version of CTLA4Ig in kidney recipients showed improved graft function and survival without increasing the occurrence of serious infectious complications (48).
Blunting neutrophil chemotaxis may be a safe option for chronic rejection. In lung transplant recipients with no ongoing signs of infection, treatment with the macrolide antibiotic azithromycin inhibited expression of the ELR+ CXC chemokine IL-8, blunted airway neutrophilia and improved graft function (49). Of note azithromycin did not appear to alter rates of infection suggesting direct effects on neutrophil infiltration. However, application of this therapy remains controversial as a later data show its effectiveness is restricted to certain subtypes of neutrophilic chronic airway rejection (50).
Targeting neutrophil effector function may represent another therapeutic avenue to promote allograft survival. Sivelestat, a specific inhibitor of NE, has been shown to attenuate organ dysfunction in an experimental model of liver IRI (51). Several agents show promise to block NADPH-dependent ROS production. PR39, an anti-microbial peptide that prevents NADPH oxidase assembly is reported effective in protecting heart function and inhibiting neutrophil infiltration in a rat cardiac IRI model (52). Additionally, Apocynin has been shown to reduce neutrophilia, prevent signs of oxidative damage, and improve pulmonary function in a mouse lung IRI model (53). How Apocynin precisely functions is unclear, although it preferentially targets phagocytic NADPH oxidase over the vascular isoforms of this enzyme.
Finally, the induction of neutrophil apoptosis could promote tolerance. Extracorporeal Photophoresis (ECP) is an anti-inflammatory treatment in which circulating peripheral leukocytes are incubated with the DNA cross-linking agent psoralen, exposed to UV light, and then transferred back into the patient (54). Although the therapeutic mechanisms of ECP remain obscure a recent report has shown that it predominantly accelerates neutrophil apoptosis and results in the release of arginase-1 (55). Argininase-1 mediated arginine depletion inhibits T cell activation and has been reported to promote the tolerance of corneal allografts in mice (56). There also reports demonstrating therapeutic benefits of ECP for recipients with chronic rejection of lung, heart, liver and kidney transplants (54). As with ECP and other therapies that potentially involve neutrophils more studies will be necessary to determine what mechanisms should be targeted to promote transplant survival.
Conclusions
Neutrophils are increasingly recognized as important components of the inflammatory circuitry that regulate the link between innate and adaptive immunity. From the moment of engraftment, they exert a wide range of effector functions that result in the exacerbation of IRI and the promotion of rejection through bidirectional cross-talk with other immune cells. There is also substantial contrasting evidence that neutrophils have inducible anti-inflammatory properties or exist in regulatory subsets suggesting that they could be utilized to protect the allograft from injury and promote tolerance. However, it remains to be determined if targeting neutrophils is a workable approach to promote tolerance. When compared to other leukocyte populations few reports directly address the role of neutrophils in transplants. Future mechanistic studies will be required to better understand the role of neutrophils in transplanted organs.
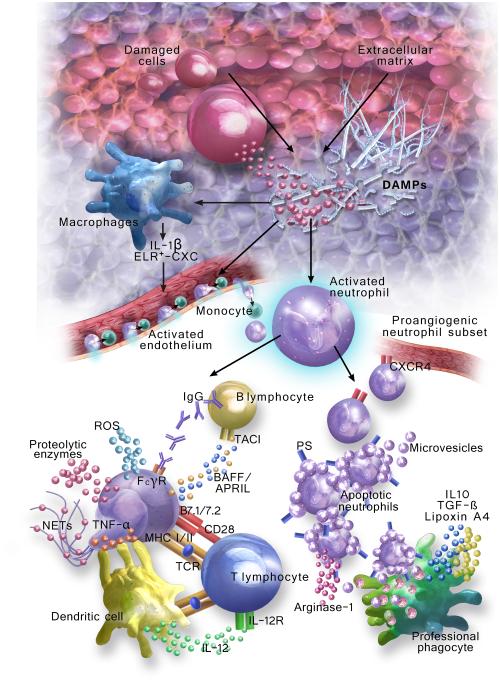
Figure. Pathways of neutrophil recruitment and activation that impact innate and adaptive immune responses to transplanted organs.
Early inflammatory events after transplantation lead to the release of DAMPs that can originate from the damaged cells or from the extra-cellular matrix. DAMPs, in turn, stimulate resident macrophages to secrete inflammatory mediators (e.g., ELR+-CXC, IL-1ß) that activate vascular endothelium to trigger the adherence of circulating neutrophils. Monocytes may also be required for neutrophil transendothelial migration. Once in extravascular spaces DAMPs contribute to neutrophil activation, which leads to graft injury through ROS burst, proteolytic enzyme release and NET generation. Neutrophils also engage in cross-talk with other leukocytes to stimulate alloimmunity. Neutrophil membrane TNF-α induces dendritic cells to augment MHC II and IL-12 expression. Neutrophils can also directly present antigens and co-stimulatory signals to T lymphocytes. Finally, they may amplify antibody-mediated rejection through FcΓ-receptor dependent mechanisms and release soluble factors (e.g., BAFF, APRIL) involved in shaping B lymphocyte maturation and differentiation through the TACI. Alternatively, neutrophils could help to resolve graft inflammation. Apoptotic neutrophils release Arginase-1, which metabolically suppresses T cell activation, and shed microvesicles containing anti-inflammatory mediators. Additionally, the externalization of phosphatidyl serine (PS) stimulates the clearance of apoptotic neutrophils by professional phagocytes, which induces the production of anti-inflammatory cytokines (e.g., IL10, TGF-ß) and pro-resolving lipids such as Lipoxin A4. Finally, a proangiogenic subset of CXCR4hi neutrophils has been shown to promote re-vascularization.
Table.
Neutrophil function in transplanted organs
| Organ | Species | Mechanisms of neutrophil trafficking into grafts | References |
|---|---|---|---|
| Intestine | Rat | P-selectin mediated intra-graft trafficking | (9) |
| Heart | Mouse | Mac-1 and LFA-1 mediated intragraft trafficking | (10) |
| Lung | Mouse | Monocyte dependent neutrophil extravasation | (11) |
|
| |||
| Organ | Species | Mechanisms of neutrophil-dependent graft injury | References |
|
| |||
| Heart, Liver | Dog, Rat | NADPH oxidase dependent tissue injury | (14,15) |
| Liver | Mouse | NE elastase dependent tissue injury | (16) |
| Lung, Liver | Human, Mouse | MMP 9 dependent digestion of ECM and disruption of vascular integrity | (17,18) |
| Lung, Liver | Human, Mouse | NET dependent tissue injury | (20,21) |
| Lung, Kidney | Mouse, Human | Fcy receptor-dependent tissue injury | (32,33) |
|
| |||
| Organ | Species | Mechanisms of molecular and cellular cross-talk | References |
|
| |||
| Skin | Mouse | Fas-ligand and perforin-dependent recruitment of activated CD8+ T cells | (24) |
| Lung | Mouse | Contact dependent dendritic cell maturation | (25) |
| Lung | Mouse | Th1 and Th17 compartment expansion through neutrophil costimulatory signals | (26) |
| Lung | Human | IL-17 dependent neutrophil infiltration | (36) |
| Lung | Human | Neutrophil defensin-mediated epithelial cell up-regulation offactors | (33) |
|
| |||
| Organ | Species | Mechanisms of neutrophil mediated wound healing | References |
|
| |||
| Pancreatic Islets | Human, Mouse | CXCR4 hi subset promoting revascularization | (8,47) |
References
- 1.Schofield ZV, Woodruff TM, Halai R, Wu MC, Cooper MA. Neutrophils--a key component of ischemia-reperfusion injury. Shock (Augusta, Ga) 2013;40(6):463–70. doi: 10.1097/SHK.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 2.Braza F, Brouard S, Chadban S, Goldstein DR. Role of TLRs and DAMPs in allograft inflammation and transplant outcomes. Nat Rev Nephrol. 2016 doi: 10.1038/nrneph.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–6. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 4.Beauvillain C, Cunin P, Doni A, Scotet M, Jaillon S, Loiry ML, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood. 2011;117(4):1196–204. doi: 10.1182/blood-2009-11-254490. [DOI] [PubMed] [Google Scholar]
- 5.Leliefeld P, Koenderman L, Pillay J. How neutrophils shape adaptive immune responses. Frontiers in Immunology. 2015:6. doi: 10.3389/fimmu.2015.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. The Journal of clinical investigation. 2002;109(1):41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. The Journal of clinical investigation. 2012;122(1):327–36. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christoffersson G, Vagesjo E, Vandooren J, Liden M, Massena S, Reinert RB, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120(23):4653–62. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farmer DG, Shen XD, Amersi F, Anselmo D, Ma JP, Ke B, et al. CD62 blockade with P-Selectin glycoprotein ligand-immunoglobulin fusion protein reduces ischemia-reperfusion injury after rat intestinal transplantation. Transplantation. 2005;79(1):44–51. doi: 10.1097/01.tp.0000146965.64706.e8. [DOI] [PubMed] [Google Scholar]
- 10.Li W, Nava RG, Bribriesco AC, Zinselmeyer BH, Spahn JH, Gelman AE, et al. Intravital 2-photon imaging of leukocyte trafficking in beating heart. The Journal of clinical investigation. 2012;122(7):2499–508. doi: 10.1172/JCI62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A. 2010;107(42):18073–8. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari RS, Andrade CF. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxidative medicine and cellular longevity. 2015;2015:590987. doi: 10.1155/2015/590987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loukogeorgakis SP, van den Berg MJ, Sofat R, Nitsch D, Charakida M, Haiyee B, et al. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation. 2010;121(21):2310–6. doi: 10.1161/CIRCULATIONAHA.108.814731. [DOI] [PubMed] [Google Scholar]
- 14.Duilio C, Ambrosio G, Kuppusamy P, DiPaula A, Becker LC, Zweier JL. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280(6):H2649–57. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- 15.Kimura K, Shirabe K, Yoshizumi T, Takeishi K, Itoh S, Harimoto N, et al. Ischemia-Reperfusion Injury in Fatty Liver Is Mediated by Activated NADPH Oxidase 2 in Rats. Transplantation. 2016;100(4):791–800. doi: 10.1097/TP.0000000000001130. [DOI] [PubMed] [Google Scholar]
- 16.Uchida Y, Freitas MCS, Zhao D, Busuttil RW, Kupiec-Weglinski JW. The Protective Function of Neutrophil Elastase Inhibitor in Liver Ischemia and Reperfusion Injury. Transplantation. 2010;89(9):1050–6. doi: 10.1097/TP.0b013e3181d45a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, et al. An Active Role for Matrix Degradation in Airway Inflammation Seen During Lung Transplantation Allograft Rejection. Journal of immunology (Baltimore, Md : 1950) 2009;182(7):4423–31. doi: 10.4049/jimmunol.0802457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato H, Kuriyama N, Duarte S, Clavien PA, Busuttil RW, Coito AJ. MMP-9 deficiency shelters endothelial PECAM-1 expression and enhances regeneration of steatotic livers after ischemia and reperfusion injury. J Hepatol. 2014;60(5):1032–9. doi: 10.1016/j.jhep.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu FC, Chuang YH, Tsai YF, Yu HP. Role of neutrophil extracellular traps following injury. Shock (Augusta, Ga) 2014;41(6):491–8. doi: 10.1097/SHK.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 20.Sayah DM, Mallavia B, Liu F, Ortiz-Munoz G, Caudrillier A, DerHovanessian A, et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. American journal of respiratory and critical care medicine. 2015;191(4):455–63. doi: 10.1164/rccm.201406-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H, Tohme S, Al-Khafaji AB, Tai S, Loughran P, Chen L, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62(2):600–14. doi: 10.1002/hep.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20(5):511–7. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 23.Jones ND, Brook MO, Carvalho-Gaspar M, Luo S, Wood KJ. Regulatory T cells can prevent memory CD8+ T-cell-mediated rejection following polymorphonuclear cell depletion. Eur J Immunol. 2010;40(11):3107–16. doi: 10.1002/eji.201040671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kish DD, Gorbachev AV, Parameswaran N, Gupta N, Fairchild RL. Neutrophil expression of Fas ligand and perforin directs effector CD8 T cell infiltration into antigen-challenged skin. J Immunol. 2012;189(5):2191–202. doi: 10.4049/jimmunol.1102729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreisel D, Sugimoto S, Zhu J, Nava R, Li W, Okazaki M, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118(23):6172–82. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto S, Nava RG, Zhu J, Huang HJ, Ibrahim M, Mohanakumar T, et al. Cutting edge: Pseudomonas aeruginosa abolishes established lung transplant tolerance by stimulating B7 expression on neutrophils. J Immunol. 2012;189(9):4221–5. doi: 10.4049/jimmunol.1201683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chong AS, Alegre ML. Transplantation tolerance and its outcome during infections and inflammation. Immunol Rev. 2014;258(1):80–101. doi: 10.1111/imr.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wasowska BA. Mechanisms involved in antibody- and complement-mediated allograft rejection. Immunologic research. 2010;47(1-3):25–44. doi: 10.1007/s12026-009-8136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saini D, Angaswamy N, Tiriveedhi V, Fukami N, Ramachandran S, Hachem R, et al. Synergistic effect of antibodies to human leukocyte antigens and defensins in pathogenesis of bronchiolitis obliterans syndrome after human lung transplantation. J Heart Lung Transplant. 2010;29(12):1330–6. doi: 10.1016/j.healun.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hattori Y, Bucy RP, Kubota Y, Baldwin WM, 3rd, Fairchild RL. Antibody-mediated rejection of single class I MHC-disparate cardiac allografts. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(8):2017–28. doi: 10.1111/j.1600-6143.2012.04073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrell CN, Murata K, Swaim AM, Mason E, Martin TV, Thompson LE, et al. In vivo platelet-endothelial cell interactions in response to major histocompatibility complex alloantibody. Circ Res. 2008;102(7):777–85. doi: 10.1161/CIRCRESAHA.107.170332. [DOI] [PubMed] [Google Scholar]
- 32.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. The Journal of clinical investigation. 2006;116(6):1615–23. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clatworthy MR, Matthews RJ, Doehler B, Willcocks LC, Opelz G, Smith KG. Defunctioning polymorphism in the immunoglobulin G inhibitory receptor (FcgammaRIIB-T/T232) does not impact on kidney transplant or recipient survival. Transplantation. 2014;98(3):285–91. doi: 10.1097/TP.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerutti A, Puga I, Magri G. The B cell helper side of neutrophils. Journal of leukocyte biology. 2013;94(4):677–82. doi: 10.1189/jlb.1112596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abadja F, Sarraj B, Ansari MJ. Significance of Th17 Immunity in Transplantation. Current opinion in organ transplantation. 2012;17(1):8–14. doi: 10.1097/MOT.0b013e32834ef4e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruttens D, Wauters E, Kicinski M, Verleden SE, Vandermeulen E, Vos R, et al. Genetic variation in interleukin-17 receptor A is functionally associated with chronic rejection after lung transplantation. J Heart Lung Transplant. 2013;32(12):1233–40. doi: 10.1016/j.healun.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, Pavlisko EN, et al. Hyaluronan Contributes to Bronchiolitis Obliterans Syndrome and Stimulates Lung Allograft Rejection through Activation of Innate Immunity. American journal of respiratory and critical care medicine. 2014;189(5):556–66. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiriveedhi V, Banan B, Deepti S, Nataraju A, Hachem R, Trulock E, et al. Role of defensins in the pathogenesis of chronic lung allograft rejection. Human immunology. 2014;75(4):370–7. doi: 10.1016/j.humimm.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO molecular medicine. 2013;5(5):661–74. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasagi S, Zhang P, Che L, Abbatiello B, Maruyama T, Nakatsukasa H, et al. In vivo-generated antigen-specific regulatory T cells treat autoimmunity without compromising antibacterial immune response. Sci Transl Med. 2014;6(241):241ra78. doi: 10.1126/scitranslmed.3008895. [DOI] [PubMed] [Google Scholar]
- 41.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews Immunology. 2008;8(5):349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao W, Zeng F, Kang K, Qi Y, Yao L, Yang H, et al. Lipoxin A4 attenuates acute rejection via shifting TH1/TH2 cytokine balance in rat liver transplantation. Transplantation proceedings. 2013;45(6):2451–4. doi: 10.1016/j.transproceed.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 43.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eken C, Martin PJ, Sadallah S, Treves S, Schaller M, Schifferli JA. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J Biol Chem. 2010;285(51):39914–21. doi: 10.1074/jbc.M110.126748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bank U, Reinhold D, Schneemilch C, Kunz D, Synowitz HJ, Ansorge S. Selective proteolytic cleavage of IL-2 receptor and IL-6 receptor ligand binding chains by neutrophil-derived serine proteases at foci of inflammation. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1999;19(11):1277–87. doi: 10.1089/107999099312957. [DOI] [PubMed] [Google Scholar]
- 46.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498(7454):371–5. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massena S, Christoffersson G, Vagesjo E, Seignez C, Gustafsson K, Binet F, et al. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126(17):2016–26. doi: 10.1182/blood-2015-03-631572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med. 2016;374(4):333–43. doi: 10.1056/NEJMoa1506027. [DOI] [PubMed] [Google Scholar]
- 49.Verleden GM, Vanaudenaerde BM, Dupont LJ, Van Raemdonck DE. Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. American journal of respiratory and critical care medicine. 2006;174(5):566–70. doi: 10.1164/rccm.200601-071OC. [DOI] [PubMed] [Google Scholar]
- 50.Verleden SE, Vandermeulen E, Ruttens D, Vos R, Vaneylen A, Dupont LJ, et al. Neutrophilic reversible allograft dysfunction (NRAD) and restrictive allograft syndrome (RAS) Seminars in respiratory and critical care medicine. 2013;34(3):352–60. doi: 10.1055/s-0033-1348463. [DOI] [PubMed] [Google Scholar]
- 51.Uchida Y, Freitas MC, Zhao D, Busuttil RW, Kupiec-Weglinski JW. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation. 2010;89(9):1050–6. doi: 10.1097/TP.0b013e3181d45a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ikeda Y, Young LH, Scalia R, Ross CR, Lefer AM. PR-39, a proline/arginine-rich antimicrobial peptide, exerts cardioprotective effects in myocardial ischemia-reperfusion. Cardiovascular research. 2001;49(1):69–77. doi: 10.1016/s0008-6363(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 53.Kim SY, Moon KA, Jo HY, Jeong S, Seon SH, Jung E, et al. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunology and cell biology. 2012;90(4):441–8. doi: 10.1038/icb.2011.60. [DOI] [PubMed] [Google Scholar]
- 54.Knobler R, Berlin G, Calzavara-Pinton P, Greinix H, Jaksch P, Laroche L, et al. Guidelines on the use of extracorporeal photopheresis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2014;28(Suppl 1):1–37. doi: 10.1111/jdv.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franklin C, Cesko E, Hillen U, Schilling B, Brandau S. Modulation and Apoptosis of Neutrophil Granulocytes by Extracorporeal Photopheresis in the Treatment of Chronic Graft-Versus-Host Disease. PloS one. 2015;10(8):e0134518. doi: 10.1371/journal.pone.0134518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu H, Khan A, Coe D, Zaher S, Chai JG, Kropf P, et al. Arginine depletion as a mechanism for the immune privilege of corneal allografts. Eur J Immunol. 2011;41(10):2997–3005. doi: 10.1002/eji.201141683. [DOI] [PMC free article] [PubMed] [Google Scholar]