Abstract
Acute stress stimulates corticotrophin-releasing hormone (CRH)-expressing neurons in the hypothalamic paraventricular nucleus (PVN), which is an essential component of hypothalamic-pituitary-adrenal (HPA) axis. However, the cellular and molecular mechanisms remain unclear. The M-channel is a voltage-dependent K+ channel involved in stabilizing the neuronal membrane potential and regulating neuronal excitability. In this study, we tested our hypothesis that acute stress suppresses expression of Kv7 channels to stimulate PVN-CRH neurons and the HPA axis. Rat PVN-CRH neurons were identified by expressing enhanced green fluorescent protein driven by Crh promoter. Acute restraint stress attenuated the excitatory effect of Kv7 blocker XE-991 on the firing activity of PVN-CRH neurons and blunted the increase in plasma corticosterone (CORT) levels induced by microinjection of XE-991 into the PVN. Furthermore, acute stress significantly decreased the M-currents in PVN-CRH neurons and reduced PVN expression of Kv7.3 subunit in the membrane. In addition, acute stress significantly increased phosphorylated AMP-activated protein kinase (AMPK) levels in the PVN tissue. Intracerebroventricular injection of the AMPK inhibitor dorsomorphin restored acute stress-induced elevation of CORT levels and reduction of membrane Kv7.3 protein level in the PVN. Dorsomorphin treatment increased the M-currents and reduced the firing activity of PVN-CRH neurons in acutely stressed rats. Collectively, these data suggest that acute stress diminishes Kv7 channels to stimulate PVN-CRH neurons and the HPA axis potentially via increased AMPK activity.
Keywords: acute stress, corticosterone, paraventricular nucleus, voltage dependent K+ channel, AMPK
Graphical Abstract
Introduction
The hypothalamic–pituitary–adrenal (HPA) axis is critical in maintaining homeostasis as the body responds to environmental stressor (Lupien et al., 2009; McEwen, 2007; Pedersen et al., 2001). The HPA axis include the paraventricular nucleus (PVN) of the hypothalamus, which secretes corticotrophin-releasing hormone (CRH) and arginine vasopressin; the pituitary gland which releases corticotrophin (ACTH), a process triggered by CRH and arginine vasopressin; and the adrenal gland cortex, that secretes the glucocorticoids (Goncharova, 2013). As a key component of the HPA axis in basal conditions and in response to stress, the PVN-CRH neurons synthesize and release CRH, a peptide of 41 amino acid residues, and project to the median eminence (Vale et al., 1981), where CRH is released into the portal system of the pituitary (Aguilera and Liu, 2012). Acute restraint stress causes an increase in CRH mRNA levels and c-fos expression in the CRH neurons in the PVN (Day et al., 2005; Girotti et al., 2006; Imaki et al., 1998). However, the cellular mechanisms underlying the hyperactivity of the PVN-CRH neurons under stress conditions are not clear.
It has been challenging to functionally analyze CRH neuronal activity until recently, when a genetic approach to tagging CRH neuron by expressing green fluorescent protein (GFP) in transgenic mouse line was developed (Alon et al., 2009; Itoi et al., 2014; Martin et al., 2010; Wamsteeker Cusulin et al., 2013). To target CRH neurons in rat PVN, we used a recently developed approach for reliably express enhanced GFP (eGFP) driven by rat Crh promoter (Gao et al., 2016). The intrinsic neuronal excitability is tightly controlled by the transmembrane ionic currents including M-current, a voltage-gated and non-inactivating K+ current (Brown and Adams, 1980; Delmas and Brown, 2005; Marrion, 1997; Peters et al., 2005). The M-current stabilizes the membrane potential and helps maintain the resting membrane potential of neurons (Brown and Adams, 1980). Kcnq genes encode Kv7.1–7.5 K+ channel subunits, which form Kv7 channels (Brown and Yu, 2000; Brown and Adams, 1980). Genetic ablation of or acute inhibition of Kv7 channels leads to depolarization and excitation, whereas opening of Kv7-channels results in hyperpolarization and inhibition of neurons. The neuronal M-current is predominantly carried by heterotetrameric Kv7.2 and Kv7.3 subunits (Shah et al., 2002; Wang et al., 1998). Dysfunction of Kv7-channels results in several neuron-generated diseases including epilepsy, pain, memory deficit/decline, and depression (Cavaliere et al., 2013; Passmore et al., 2003; Qi et al., 2014; Zhang et al., 2013). The Kv7-channel is also involved in the regulation of a stress-related neuronal process. In this regard, activation of Kcnq/Kv7 channels prevents acute stress-induced impairments of hippocampal long-term potentiation and spatial memory retrieval in rats (Li et al., 2014).
AMP-activated protein kinase (AMPK) is a ubiquitous serine/threonine kinase which is involved in cellular responses to many metabolic stresses (Kim et al., 2009). AMPK is involved in many cellular processes, such as regulation of apoptosis, stimulation of autophagy and phagocytosis, inhibition of cell growth and proliferation, and counteraction of hypertrophy (Dermaku-Sopjani et al., 2014; Hardie, 2003). Acute restraint stress increases AMPK activity (Marques et al., 2012) and AMPK activation in the central nervous system mediates fructose-induced elevation of plasma corticosterone (CORT) levels (Kinote et al., 2012). Furthermore, AMPK activation decreases membrane expression of Kv7.1 and epithelial Na+ channel through promoting endocytosis and degradation in lysosomes via a Nedd2-4-dependent mechanism (Andersen et al., 2012; Bhalla et al., 2006). Nedd4-2 is an ubiquitin ligase that ubiquitylates membrane proteins to increase protein internalization and degradation (Abriel and Staub, 2005). Nedd4-2 suppresses Kv7.2/7.3-mediated M-currents (Ekberg et al., 2007), indicating that AMPK-Nedd4-2 is a potential pathway through which acute stress regulates Kv7 channels in PVN-CRH neuron. Thus, in this study, we tested the hypothesis that acute stress suppresses the Kv7 channels to stimulate CRH neurons through activation of AMPK.
Method and Materials
Male Sprague-Dawley rats (12-week old) were used in this study. The rats were group-housed (n = 3 rats per cage) in a 12-h light/dark cycle and maintained under controlled temperature (24 – 25°C) with food and water ad libitum. The surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals.
Acute restraint stress in rats
Restraint stress was designed to restrict the rat’s movement without promoting any apparent signs of pain. To avoid diurnal fluctuations of CORT, the restraint procedure was performed at 10:00 am in all rats. The acute restraint stress started with a 30-min equilibration period that allowed the rats adjust to their new surroundings. Upon completion of this period, each rat was placed into a 5-cm diameter Plexiglas tube and their movement was limited in its ability to turn around by an adjustable plastic plug that was secured behind the rat in the tube for a period of 2 h. Fresh air was accessible to the Plexiglas tube through holes on the sides of the tube. Food and water were not provided to the rats during the restraint procedure (Sweerts et al., 1999). Blood samples were collected from a saphenous vein before restraint and at multiple time points (0, 20, 40, 60, and 90 min) after restraint. To avoid the influence of blood volume loss on hormone regulation, small amounts (100 µl) of blood were collected. CORT levels were measured by using an ELISA kit from Enzo Life Science (Farmingdale, NY) according to the manufacturer’s instructions.
PVN injections
Under anesthesia with isoflurane (2%), the head of the rats were placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). A bilateral guide cannula (26-gauge, 1.0-mm spacing between cannula, and extending 6.8 mm from the pedestal) was implanted at the following coordinates: 1.8 mm caudal to the bregma, 0.5 mm lateral to the midline, and 6.8 mm ventral to the surface of the dura (1.0-mm dorsal to the intended drug injection site). The bilateral guide cannula for PVN injection was affixed to the skull by using dental acrylic, a dummy cannula was inserted into each side of the guide cannula, and a dust cap was then placed over the external end of the dummy cannula. After a 1-week recovery period, the dummy cannula was removed and a bilateral injection cannula with tips protruding 1.0 mm beyond the tip of the guide cannula was inserted into the guide cannula (Gao et al., 2016). Kv7 blocker 10, 10-bis (4-pyridinylmethyl)-9(10H)-anthracnose (XE-991, 0.5 pmol in 100 nl of aCSF) was dissolved in aCSF and injected bilaterally into the PVN. To mark the infusion sites, fluorescent microspheres (0.04 µm, wavelength 580 nm) were delivered through the cannula after XE-991 injection.
PVN-CRH neuron identification
We selectively identified CRH-expressing neurons in rat PVN by expressing enhanced green fluorescent protein (eGFP) driven by rat Crh promoter (Gao et al., 2016). The full-length promoter fragment (−2125/+94) and necessary components were subcloned into the adeno-associated virus (AAV, serotype 1/2) vector expression cassette. The packaging was performed by Gene detect, Ltd (Auckland, New Zealand) with a titer of 10^13 genome copies per ml for the packaged AAV-Crh promoter eGFP vector. This viral construct was delivered into the PVN through microinjection (100 nl) following the coordinates targeting the PVN. After the injection, the muscle and skin were sutured, and the wound was closed. A 3- to 4-week period was allowed for the Crh promoter-driven eGFP to be specifically expressed in the PVN-CRH neurons.
Immunohistochemical staining for CRH and Kv7.2/7.3
To determine the distribution of Kv7.2 or Kv7.3 channels in the PVN-CRH neurons, we performed double fluorescent labeling by using antibodies against CRH and Kv7.2 or Kv7.3. To enhance CRH immunoreactivity in the PVN, rats were injected intracerebroventricularly with 50–60 mg of colchicine (Gao et al., 2016; Sawchenko et al., 1984). Briefly, rats were deeply anesthetized (with sodium pentobarbital 50 mg/kg by intraperitoneal injection) and rapidly perfused transcardially with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). The brains were removed and sectioned into 30-µm-thick slices (Zhou et al., 2015). After tissue sections had been treated with blocking agent, they were incubated with the primary antibody mixture (rabbit anti-Kv7.2 from Sigma or rabbit anti-Kv7.3 from Alomone Labs, for CRH: guinea pig anti-corticotrophin-releasing factor antibody, Peninsula Laboratories International, San Carlos, Calif., USA). Then, the sections were incubated with a secondary antibody (goat anti-rabbit Alexa 488 with goat anti-guinea pig Alexa 594; Invitrogen, Carlsbad, Calif., USA). Confocal microscopy was used to visualize co-localization of the fluorescent markers.
Electrophysiological recordings in brain slices
Hypothalamic slices were prepared from the AAV-CRH-eGFP viral vector-injected rats. Briefly, the rats were decapitated under 2% isoflurane anesthesia, and the brain was quickly removed and placed in ice-cold aCSF containing (in mM) 124.0 NaCl, 3.0 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.4 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3 (continually gassed with a mixture of 95% O2 and 5% CO2). A tissue block containing the PVN was trimmed and fixed with tissue glue on the stage of a vibrating microtome (VT1000, Leica Biosystems Inc., Buffalo Grove, IL). Coronal slices were cut 300 µm thick, as described previously (Gao et al., 2016; Li and Pan, 2005). Then, the slices were transferred to an incubation chamber containing aCSF continuously gassed with a mixture of 95% O2 and 5% CO2 at 34°C for at least 1 h, the slices were then transferred to the recording chamber for electrophysiological recordings.
The slices in the recording chamber were perfused (3 ml/min) with aCSF (gassed with 95% O2 and 5% CO2) at a temperature of 34°C that was maintained by using an in-line solution heater. We first identified eGFP-tagged neurons with use of an upright microscope (BX51WI; Olympus) equipped with epifluorescence illumination and differential interference contrast optics. The recording electrodes were pulled by using a micropipette puller (P-97; Sutter Instruments) from borosilicate capillaries (1.2 mm outer diameter, 0.68 mm inner diameter; World Precision Instruments). The resistance of the pipette was 3–6 MΩ when it was filled by an internal solution containing (in mM) 140.0 K gluconate, 2.0 MgCl2, 0.1 CaCl2, 10.0 HEPES, 1.1 EGTA, 0.3 Na2-GTP, and 2.0 Na2-ATP, adjusted to pH 7.25 with 1 M KOH (270–290 mOsm). The junction potential in voltage recording was determined by calculating ion components of intracellular and perfused solutions and was compensated during the recording. Electrical signals were processed by using a Multiclamp 700B amplifier (Molecular Devices), filtered at 1–2 kHz, and digitized at 20 kHz by using Digidata 1440 (Molecular Devices).
The spontaneous action potentials were recorded in current-clamp mode at a holding current of 0 pA before and after blocking the M-current with 3 µM XE-991. M-currents were recorded at voltage-clamp mode and series resistance and membrane captaincies were compensated before recording. M-currents were evoked by a hyperpolarizing voltage pulse from −20 to −50 mV followed by −20 mV for 700 ms. M-currents were defined as the deactivation tail currents sensitive to 3 µM XE-991. All M-current recordings were carried out in the presence of tetrodotoxin (0.5 µM) to block Na+ currents, CdCl2 (100 µM) to block Ca2+ currents, DTX-K (100 nM) to block Kv1.1 currents, and Cs+ (1 mM) to block hyperpolarization-activated currents to minimize the contamination of other currents. Retigabine (10 µM) were used to determine the total M-current. Drugs were prepared in the external solution and delivered by pump at a perfusion speed of 3 ml/min. The recordings were abandoned if input resistance changed more than 30% during recordings. To minimize any potential interference of previously applied drugs, data were collected from only one neuron per slice.
Western blot analysis of membrane and cytosolic protein levels in PVN
Immediately after completion of acute restraint stress, rats were decapitated under isoflurane anesthesia. The PVN tissues were obtained by micro-punch method (Li et al., 2015). Membrane proteins were extracted with a radioimmunoprecipitation assay buffer in the presence of a mixture of inhibitors for proteases (serine proteases, cysteine proteases, aspartic proteases, and metalloproteases). Membrane protein (PVN tissues from 3 rats were pooled as 1 sample) was obtained by using a ProteoExtract Subcellular Proteome Extraction Kit (Calbiochem, San Diego, CA) according to the manufacturer’s instruction (Butcher et al., 2001; Zhang and Insel, 2004). The protein concentrations were determined by using the Bradford protein assay. The samples were subjected to 4%-12% SDS-PAGE grade gels and transferred to polyvinylidene difluoride membrane (Millipore). The immunoblots were probed with a rabbit anti-Kv7.2 antibody (1:1000, Alomone Labs) and rabbit anti-Kv7.3 antibody (1:1000, Alomone Labs). For the AMPK detection, the immunoblots were blocked with 2% BSA for 1 h at room temperature; and then incubated with anti-phosphorylated AMPK (1:1000, Millipore) and rabbit anti-AMPK (1:1000, Millipore) antibodies diluted with 2% albumin (Sigma). The cytosolic fraction protein and membrane protein were normalized by GAPDH and Na+/K+-ATPase, respectively. An ECL kit (GE Healthcare Life Sciences) was used to detect the protein bands quantified by the Image J software. The mean values of Kv7.2, Kv7.3, phosphorylated AMPK, and AMPK in respective groups in unstressed rats were considered to be 1.0.
Data Analysis
Data are presented as means ± SEM. For electrophysiological experiments, one neuron was recorded from one brain slice and at least 3 rats were included in each group. The junction potential was corrected on the basis of various different potentials by using internal and external solutions. For comparisons of more than two data sets, two-way ANOVA with repeated measurement and Bonferroni post-hoc test were used. Unpaired t-test was used to compare between 2 groups. p < 0.05 was considered statistically significant.
Results
Acute stress blunted the excitatory effect of Kv7 channel blocker on firing activity in PVN-CRH neurons
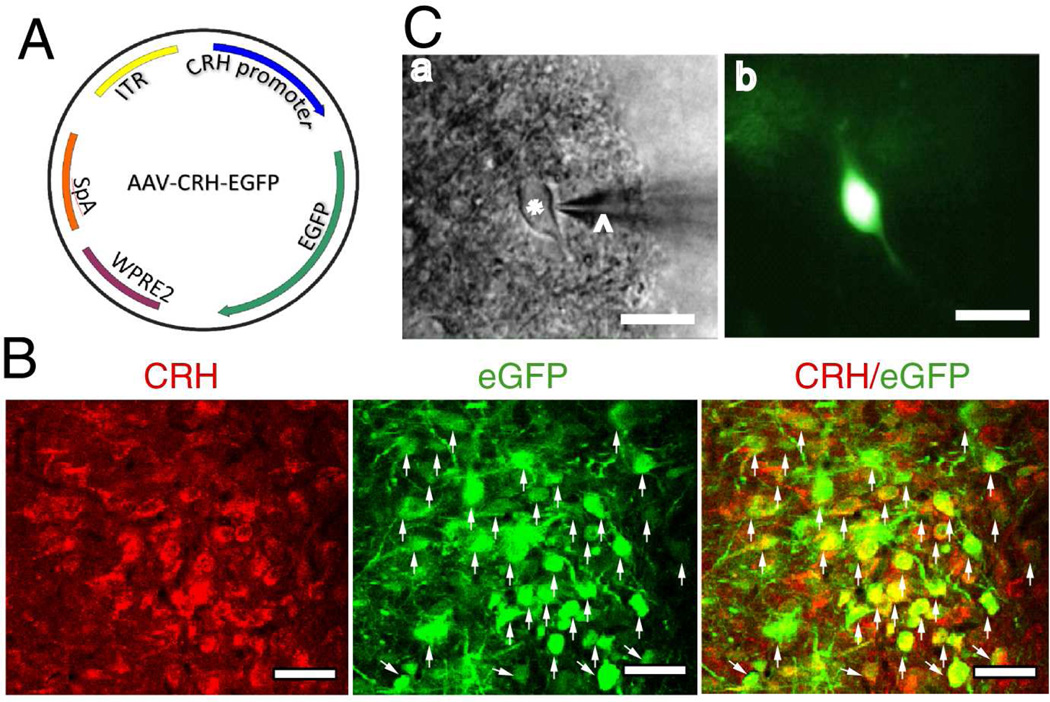
We first identified PVN-CRH neurons expressing eGFP drive by AAV2-Crh promoter (Fig. 1A). All eGFP-tagged neurons (green) were CRH immunoreactivity positive (red) (Fig. 1B). The eGFP-tagged neurons in brain slices were viewed by using fluorescent microscope (Fig. 1C). To determine the distribution of Kv7.2 and Kv7.3 channels in the PVN-CRH neurons, we performing immunohistochemical staining with antibodies against CRH and Kv7.2 or Kv7.3. All negative controls displayed no detectable staining. All CRH-positive neurons were identified to be Kv7.2-positive and Kv7.3-positive (Fig. 2A).
Figure 1. Identification of PVN-CRH neuron.
(A): Construct of AAV2 viral vector. WPRE = Woodchuck postregulatory element; ITR = inverted terminal repeat; bGH = bovine growth hormone; polyA = polyadenylation. (B): Immunocytochemical staining shows that eGFP-tagged neurons (indicated by white arrows) were positive for CRH-immunoreactivity. (C): eGFP-tagged PVN neurons (*) with an attached recording electrode (^) viewed with infrared differential interference contrast optics (a) and fluorescence illumination (b) in the brain slice. Scale bars indicate 20 µm in B and 50 µm in C.
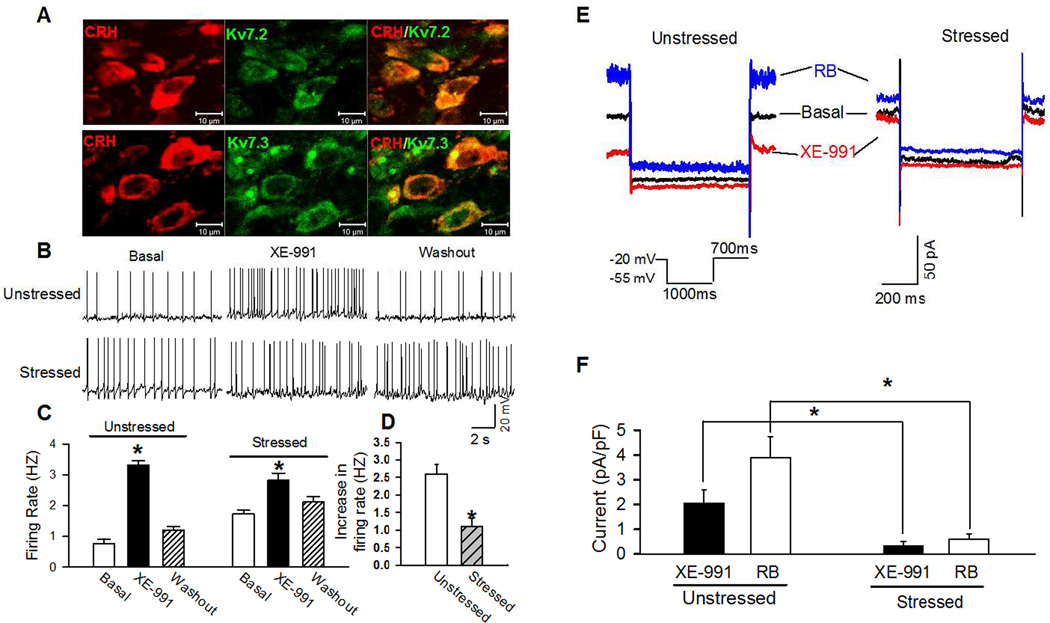
Figure 2. Acute stress diminished excitatory effect of M-current blocker and reduced M-current in PVN-CRH neurons.
(A): Confocal images show the distribution of Kv7.2 or Kv7.3 in the PVN-CRH neurons. B and C: Original recordings (B) and summary data (C) show the firing activity of eGFP-tagged PVN neurons in basal condition, the presence of Kv7 channel blocker XE-991 (3 µM), and washout in unstressed and acutely stressed rats (n = 20 neurons in unstressed and stressed rats, respectively). (D): Acute stress significantly reduced the increase in firing activity induced by XE-991. (E): Representative traces show deactivation tail currents in the absence (basal) or presence of Kv7 opener retigabine (10 µM) and blocker XE-991 (3 µM) in eGFP-tagged PVN neurons in stressed and unstressed rats. The deactivation tail currents were elicited by the voltage protocol shown in the insert. (F): summary data shows the basal and retigabine-induced M-currents, which were defined as XE-991-sensitive tail currents in basal and the presence of retigabine, were significantly diminished in the PVN-CRH neurons in stressed rats (n =15 neurons in 4 rats) compared with unstressed rats (n = 12 neurons in 4 rats).* p < 0.05 compared with the basal value of each group, repeated-measures ANOVA with Dunnett’s post hoc test. The scale bars in A indicate 10 µm.
Acute restraint stress significantly increased the basal firing activity of PVN-CRH neurons from 0.8 ± 0.1 to 1.7 ± 0.1 Hz (n = 20 neurons in 5 unstressed rat and n = 20 in 4 stressed rats, p < 0.05; Fig. 2A and B). Then, bath application of Kv7-channel specific blocker XE-991 (3 µM) (Romero et al., 2004) significantly depolarized membrane potential in PVN-CRH neurons from −56.7 ± 3.5 to −44.1 ± 2.2 mV (p < 0.05) and increased firing rate from 0.8 ± 0.1 to 3.3 ± 0.2 Hz (n = 20, p < 0.05; Fig. 2B and C) in unstressed rats. In acute restraint stress rats, XE-991 (3 µM) depolarized membrane potential from −47.2 ± 1.0 to −43.3 ± 0.9 mV (n = 20, p < 0.05) and increased the firing rate from 1.7 ± 0.1 to 2.8 ± 0.2 Hz (p < 0.05; Fig. 2B and C). XE-991-induced depolarization and increase in firing rate in PVN-CRH neurons were significantly greater in unstressed than in stressed rat (Fig. 2D).
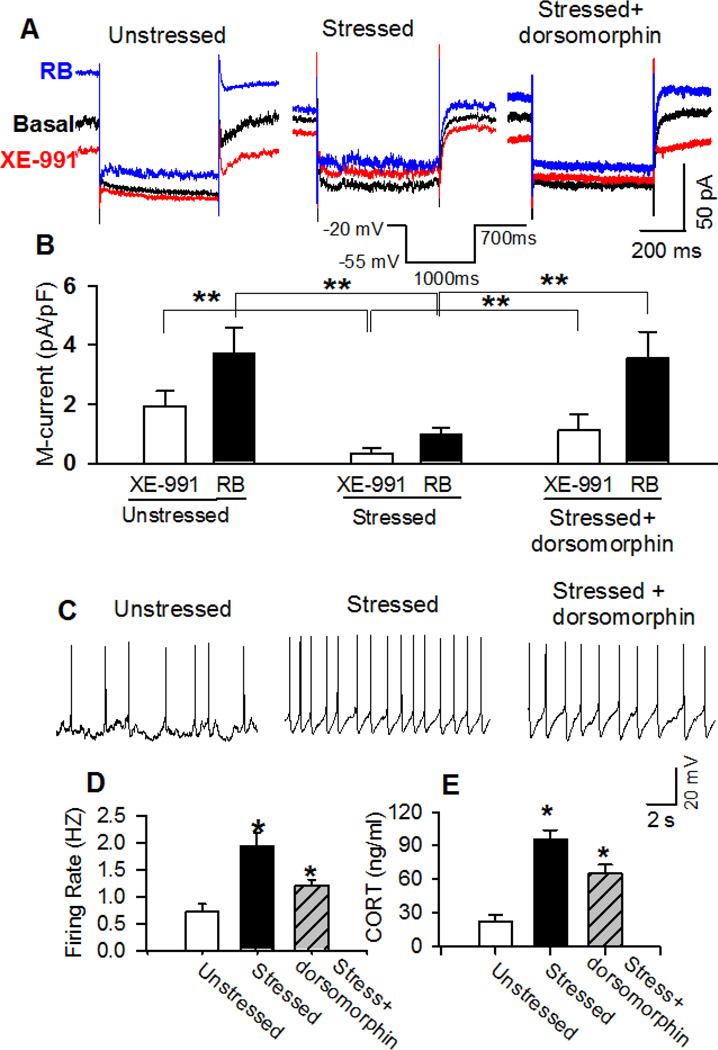
Acute stress suppressed M-currents in PVN-CRH neurons
We next directly assessed the impact of acute stress on M-currents in PVN-CRH neurons. Depolarization of membrane potentials opens Kv7-channels to generate steady outward K+ currents characterized with slowly closed tail-current (Brown and Adams, 1980; Filippov and Brown, 2013). The M-currents were recorded in the presence of retigabine and XE-991 when the neuron was depolarized to −20 mV and hyperpolarized to −50 mV for 1 s before being depolarized to −20 mV again (Passmore et al., 2003; Qi et al., 2014). Basal M-current was defined as XE-991-sensitive current under basal condition and the total M-current was defined as XE-991-sensitive current in the presence of Kv7-channel opener retigabine (10 µM). Both basal and total M-current were significantly decreased in acutely stressed rats (n = 15 neurons in 4 rats) compared with unstressed rats (n = 12 neurons in 4 rats, p < 0.05; Fig. 2E and F).
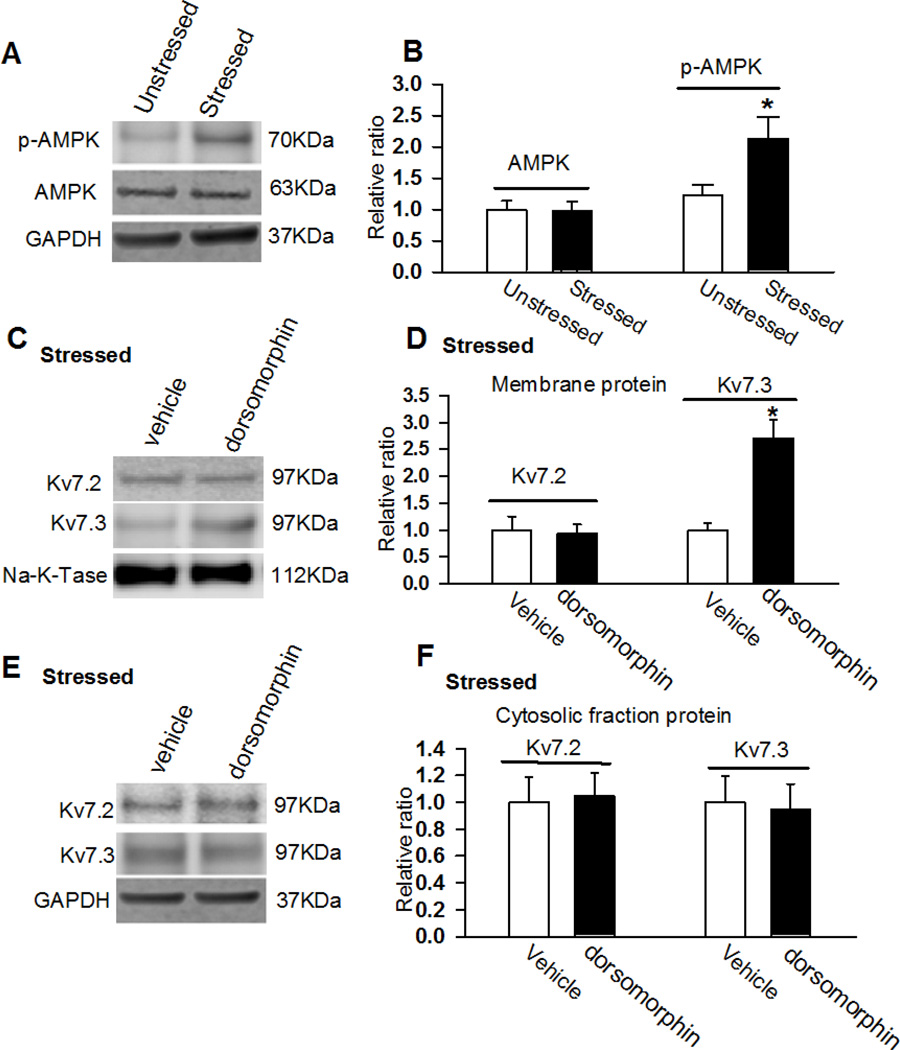
Acute stress decreased Kv7.3 subunit expression in PVN
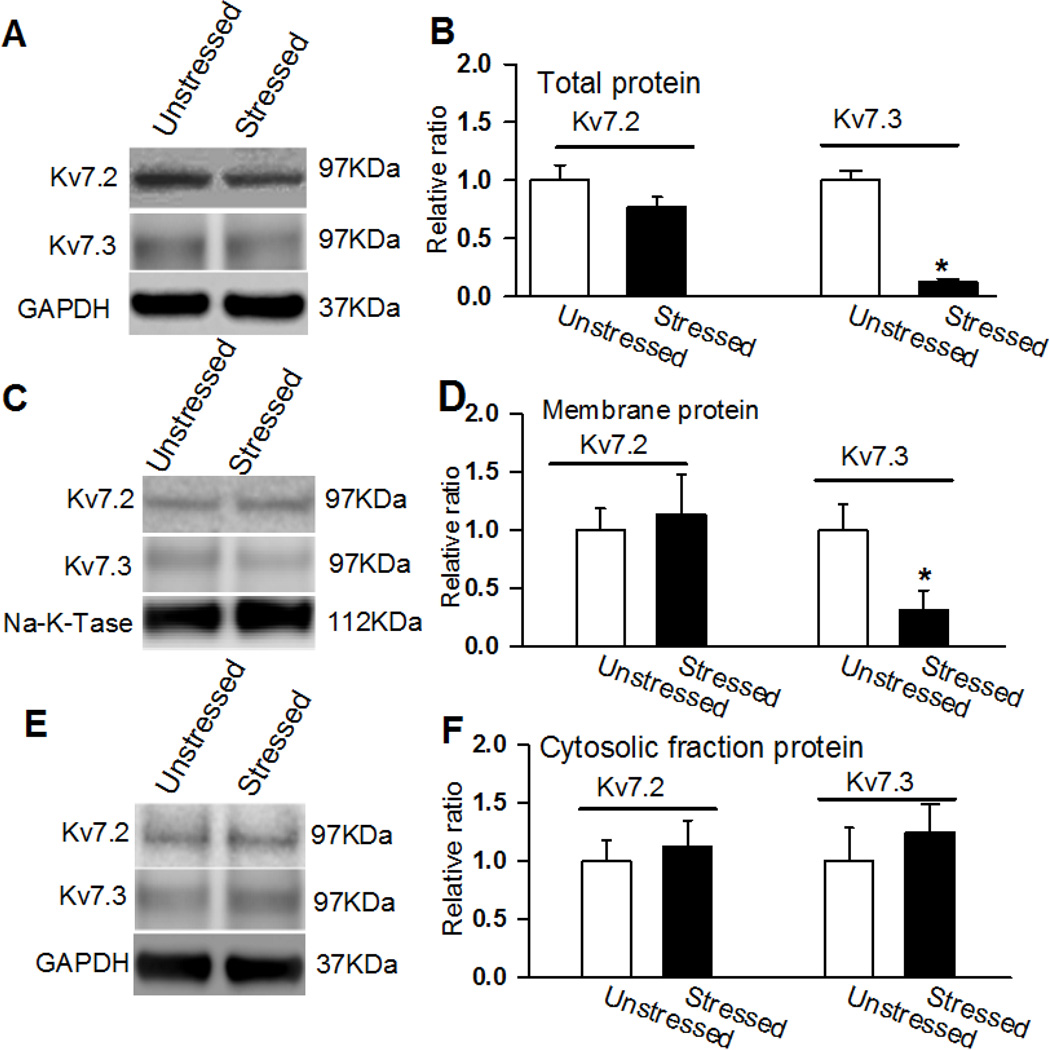
Neuronal Kv7-channels are composed of Kv7.2 and Kv7.3 subunits encoded by Kcnq2 and Kcnq3, respectively (Wang et al., 1998). We measured Kv7.2 and Kv7.3 subunit expression levels in the PVN tissue in unstressed and acutely stressed rats. In all the protein assays, 4 samples (each sample contains PVN tissues of 3 rats) were used. Both the total and membrane portions of the Kv7.3 subunit expression levels were significantly decreased in the PVN tissue in acutely stressed rats compared with unstressed rats (p < 0.05; Fig. 3A – D). However, the cytosolic fraction expression levels of Kv7.3 subunit in the PVN tissue did not differ between acutely stressed and unstressed rats (Fig. 3E and F). Neither the membrane nor the cytosolic fraction of the Kv7.2 subunits in the PVN tissues differed between acutely stressed and unstressed rats (p > 0.05; Fig. 3).
Figure 3. Kv7.2 and Kv7.3 protein expression levels in the PVN in stressed and unstressed rats.
A and B, Representative blots (A) and quantification (B) show the total protein levels of Kv7.2 and Kv7.3 in the PVN in unstressed and stressed rats. C and D, Original gel images (C) and quantification (D) show the membrane fraction protein levels of Kv7.2 and Kv7.3 in the PVN in unstressed and stressed rats. E and F, Original gel images (E) and quantification (F) show the cytosolic fraction protein levels of Kv7.2 and Kv7.3 in the PVN in unstressed and stressed rats (n = 4 samples, each sample contains PVN tissues of 3 rats) were used in each group. *p < 0.05, compared with the unstressed rat group by using ANOVA with Dunnett’s post hoc test.
Acute stress impaired the Kv7-channel function in the control of circulating CORT levels
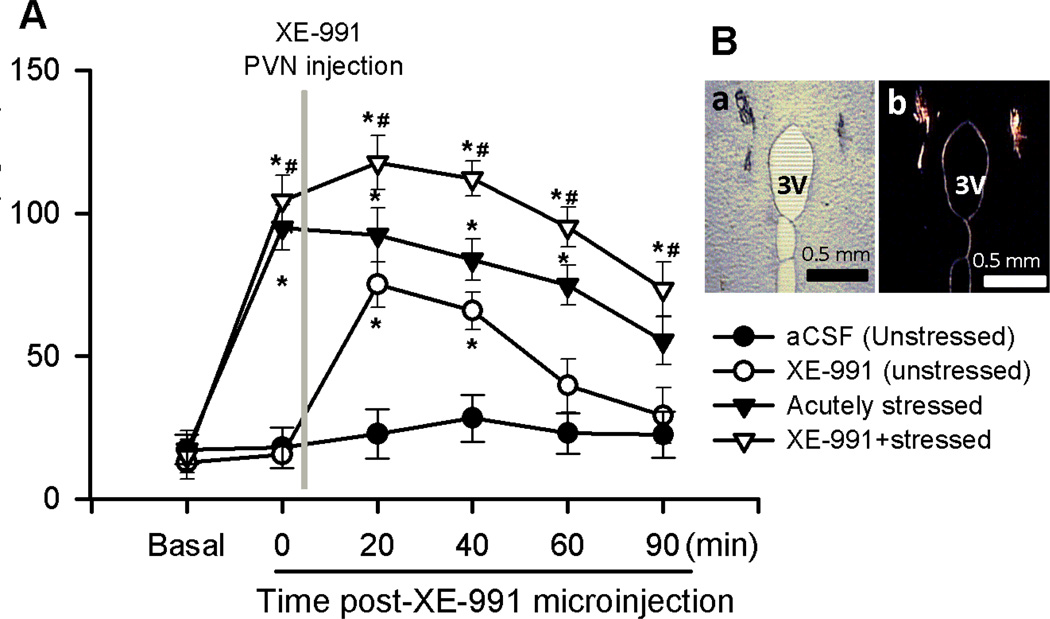
To determine the role of Kv7-channels in the regulation of HPA axis activity and circulating CORT levels during acute stress, we microinjected aCSF (vehicle) or XE-991 into PVN through implanted cannula in unstressed and acutely stressed rats (Fig. 4B). In 9 unstressed rats, microinjection of XE-991 (0.5 pmol in 100 nl of aCSF) into the bilateral PVN significantly increased plasma CORT levels from 22.8 ± 8.7 to 74.9 ± 7.8 ng/ml (p < 0.05; Fig. 4A) and reached a peak response at 20 min after XE-991 administration. The plasma CORT levels returned to basal levels 90 min after XE-991 injection. Microinjection of the same amount of vehicle (100 nl aCSF) through the implanted cannula had no significant effect on CORT levels. Acute restraint stress (2 h) significantly elevated plasma CORT levels to 94.9 ± 7.9 ng/ml (n = 8 rats; p < 0.05; Fig. 4A). The elevated CORT levels returned to basal level after 90 to120 min recovery from the acute restraint stress procedure. In other group of 8 rats subjected to 2 h-acute restraint stress, microinjection of XE-991 (0.5 pmol in 100 nl of aCSF) into the PVN immediately after completion of the acute restraint stress procedure significantly increased plasma CORT levels to 117.6 ± 9.5 ng/ml (Fig. 4A).
Figure 4. Acute stress impaired the M-channel function in the control of circulating CORT levels.
A: Summary data shows plasma CORT levels during basal condition, before and after microinjection of XE-991 into the bilateral PVN in unstressed (n = 9) and acutely stressed rats (n = 8). B: Representative microphotography shows the injection sites (a) of PVN identified by red fluorescence (b). * p < 0.05 compared with the basal value, repeated-measures ANOVA with Dunnett’s post hoc test, # p < 0.05 two-way ANOVA with Bonferroni’s post hoc test, ^ p < 0.05, unpaired t test.
AMP kinase (AMPK) activation contributes to stress-induced downregulation of Kv7-channel
Previous studies have shown that acute restraint stress increases hypothalamic AMPK activity and that central administration of AMPK activator elevates plasma CORT levels (Kinote et al., 2012). Furthermore, AMPK activation decreases membrane Kv7.1 subunit expression by promoting its endocytosis and degradation (Andersen et al., 2012). We hypothesized that acute stress downregulates Kv7.3 expression in the PVN through activation of AMPK. AMPK expression levels in the PVN tissue did not differ between unstressed and acutely stressed rats (n = 4 samples, each sample contains PVN tissues of 3 rats; p > 0.05; Figs. 5A and B). However, the phosphorylated AMPK levels were significantly increased in acutely stressed rats compared with unstressed rats (2.13 ± 0.4 vs 1.0 ± 0.13, n = 4 samples; p < 0.05; Figs. 5A and B). To determine the interaction between downregulation of Kv7.3 and upregulation of phosphorylated AMPK, we inhibited AMPK activity by ICV injection of dorsomorphin (100 nmol in 10 µl aCSF), a selective AMPK inhibitor (Han et al., 2005), through ICV cannula 1h before restraint stress. Dorsomorphin treatment significantly restored Kv7.3 membrane expression levels in stressed rats but had no effect on cytosolic fraction expression of Kv7.3 level (n = 4 samples, p > 0.05; Fig. 5C and D). In addition, dorsomorphin treatment did not affect either membrane fraction or cytosolic fraction Kv7.2 expression levels in stressed rats (Fig. 5E and F).
Figure 5. Inhibition of AMPK activity restored acute stress-induced decrease in membrane fraction of Kv7.3 subunit.
A and B: Gel images (A) and quantification of band density normalized by GAPDH (B) show the total protein levels of AMPK in the PVN in unstressed and stressed rats. C and D, Original gel images (C) and quantification (D) of GAPDH-normalized band density of membrane fraction of Kv7.2 and Kv7.3 in the PVN in stressed rats treated with ICV injection of vehicle and dorsomorphin (100 nmol in 10 µl aCSF). E and F, Original gel images (E) and quantification (F) of GAPDH-normalized band density of cytosolic fraction of Kv7.2 and Kv7.3 in the PVN in stressed rats treated with vehicle and dorsomorphin. The molecular weight was indicated on the right side of the gel images. In all the protein assays, 4 samples (each sample contains PVN tissues of 3 rats) were used in each group. *p < 0.05, compared with the unstressed rat group by using ANOVA with Dunnett’s post hoc test.
Next, we determined if inhibition of AMPK activity recovers blunted Kv7-channel function. XE-991-senstive M-currents in basal condition and in the presence of retigabine were significantly reduced in acutely stressed rats (n = 8 neurons from 3 rats) compared with the M-currents in unstressed rats (n = 9 neurons from 4 rats, p < 0.05; Fig. 6A and B). In another group of 12 neurons from 5 stressed rats, pretreatment with the AMPK inhibitor dorsomorphin (ICV, 100 nmol in 10 µl aCSF) for 1 h significantly increased XE-991-senstive M-currents in basal condition and in the presence of retigabine (Fig. 6B).
Figure 6. Inhibition of AMPK activity restored M-currents and firing activity in PVN-CRH neurons and recovered circulating CORT levels in acutely stressed rats.
A: Raw tracings show deactivation tail currents in the basal condition and in the presence of Kv7 opener retigabine (RB) (10 µM) or blocker XE-991 (3 µM) in eGFP-tagged PVN neurons from unstressed, acutely stressed, and dorsomorphin-treated stressed rats. A voltage protocol shown in the insert was used to evoke the deactivation tail currents. B: Summary data shows that pretreatment of AMPK inhibitor dorsomorphin restored M-currents in basal condition and in the presence of retigabine in PVN-CRH neurons in acutely stressed rats. C and D: Raw tracings (C) and summary data (D) show that AMPK inhibitor dorsomorphin significantly decreased firing activity of PVN-CRH neurons in acutely stressed rats. E: Summary data show that ICV administration of dorsomorphin (100 nmol in 10 µl aCSF) significantly decreased plasma CORT levels in 10 acutely stressed rats. * p < 0.05 compared with the basal value of each group, repeated-measures ANOVA with Dunnett’s post hoc test.
In addition, we determined if inhibition of AMPK activity decreased firing activity of PVN-CRH neurons in acutely stressed rats. Dorsomorphin (100 nmol in 10 µl aCSF) was microinjected through ICV cannula in stressed rats. Brain slices were prepared 1 h after dorsomorphin treatment in acutely stressed rats. The firing rate of PVN-CRH neurons (1.20 ± 0.11 Hz, n = 17 neurons) in 4 stressed rats treated with dorsomorphin was significantly lower than the firing rate of CRH neurons (1.9 ± 0.2 Hz; n = 15 neurons) in 4 stressed rats (p < 0.05, Fig. 6C and D). In addition, we measured plasma CORT levels in stressed rats treated with ICV administration of dorsomorphin. Dorsomorphin pretreatment significantly reduced CORT level in 10 acutely stressed rats from 95.6 ± 8.2 to 65.5 ± 7.5 ng/ml (p < 0.05; Fig. 6E).
Discussion
This study determined the cellular mechanism underlying hyperactivity of PVN-CRH neuron in acute stress. We found that acute restraint stress reduced the M-current in identified PVN-CRH neurons and downregulated the expression level of Kv7.3, a subunit consisting of heterotetrameric neuronal Kv7-channels, in the PVN. Furthermore, we found that acute stress blunted Kv7-channels function in the control of circulating CORT levels and neuronal activity of PVN-CRH neurons. These data suggest that diminished M-currents contribute to hyperactivity of PVN-CRH neurons under acute stress condition. We also found that acute stress increased phosphorylated AMPK levels in the PVN. Inhibition of AMPK activity restored the diminished M-currents and Kv7.3 expression levels. In addition, AMPK inhibitor decreased acute stress-induced hyperactivity of PVN-CRH neurons and elevated circulating CORT levels.
Many stressors, including social defeat, heat, noise, electric foot shock, and restraint stress cause elevation of circulating CORT levels in mice (Hu et al., 2014; Ito et al., 2015; Jaroenporn et al., 2007; Montagud-Romero et al., 2015; Yamano et al., 2004). Acute stress leads to CRH releases into the pituitary portal circulation and a rapid increase in CRH mRNA and c-fos expression in the PVN (Alexander et al., 1994; Day et al., 2005; Girotti et al., 2006; Imaki et al., 1998; Plotsky, 1988). The findings that CRH antagonists or antibodies attenuate about 70% of the ACTH response to acute stress (Rivier and Plotsky, 1986) and that CRH-deficient mice have severely impaired adrenal responses to acute stress (Muglia et al., 2000) suggest that CRH is largely responsible for HPA axis activation during stress. Previous studies suggest that several mechanisms are responsible for the hyperactivity of neuroendocrine neurons in the PVN under stress conditions. For example, acute stress causes a loss of GABAergic inhibition of the PVN endocrine neurons through shifting the GABAA reversal potential to depolarization induced by a downregulation of cation chloride co-transporter K+-2Cl− (Hewitt et al., 2009). Also, repeated restraint stress enhances glutamatergic synaptic inputs to the PVN parvocellular neurons (Kusek et al., 2013). However, the aforementioned studies were carried out on neuroendocrine PVN neurons without further determining if the suggested mechanisms applied to CRH neurons in the PVN. In this study, we used a recently developed transgenic approach to identify CRH neurons by specifically expressing eGFP driven by Crh promoter (Gao et al., 2016). Consistently with the elevation of circulating CORT levels induced by acute restraint stress, we found that acute stress significantly increased the firing activity of eGFP-tagged PVN-CRH neurons.
It has been shown that M-currents are crucial in the regulation of neuronal activity since inhibition of M-currents can lead to depolarization and increase neuronal excitability (Brown and Adams, 1980; Delmas and Brown, 2005). We found that acute stress reduced the excitatory effect induced by Kv7-channel blocker XE-991 on PVN neurons, suggesting that Kv7-channel function was impaired in controlling PVN-CRH neurons under acute stress condition. Then, we measured M-currents to directly assess the effect of acute stress on the Kv7-channel function in PVN-CRH neurons. Both basal and retigabine-sensitive M-currents were significantly decreased in the PVN-CRH neurons in stressed rats compared with unstressed rats. Consistent with these in vitro findings, blockade of Kv7-channels in the PVN remarkably increased plasma CORT levels in unstressed rats, whereas this effect was significantly attenuated in acutely stressed rats. We cannot rule out the possibility that the ceiling effect of XE-991 on circulating CORT levels limited the increase in CORT levels in stressed rats. Both in vivo and in vitro data suggest that acute stress blunted Kv7-channel function in the control of PVN-CRH neuron activity and circulating CORT levels.
In support of the notion that Kv7-channel function is blunted in the PVN by acute stress, both the total and membrane fraction of Kv7.3 expression levels in the PVN tissue were significantly decreased in acutely stressed rats, whereas the cytosolic fraction of Kv7.3 levels in the PVN did not differ between unstressed and stressed rats. However, the member fraction of the Kv7.2 protein levels in the PVN tissue did not differ between unstressed and stressed rats. It has been shown that neuronal M-currents are predominantly carried by heterotetrameric Kv7.2/7.3 (Lerche et al., 2000; Roche et al., 2002; Schroeder et al., 2000; Shah et al., 2002; Wang et al., 1998). Furthermore, homogenic Kv7.2 alone generates an M-current amplitude that is 5 to 10 times smaller than that of heterogenic Kv7.2/Kv7.3 channels (Etxeberria et al., 2004). Thus, reduction of Kv7.3 expression levels in the PVN would efficiently cause significant M-currents to diminish in the PVN-CRH neurons. Although acute stress reduced Kv7.3 membrane fraction expression levels, the cytosolic fraction of Kv7.3 levels remained unchanged. The reason for this diverse alteration of Kv7.3 levels between membrane and cytosolic fraction is not clear. It has been shown that acute stress alters receptor trafficking including NMDA and AMPA receptors (Yuen et al., 2011) as well as the neurokinin-3 receptor (Miklos et al., 2014). Thus, the inconsistent alteration of Kv7.3 levels may be caused by acute stress-induced protein degradation of Kv7.3 during the protein recycling process. The hypothalamic PVN is heterogeneous and contains many types of neurons including PVN-CRH neurons, the changes of Kv7.2/7.3 protein levels in the PVN tissue may not completely represent the alteration of Kv7.2/7.3 expression levels in PVN-CRH neurons. Our immunocytochemical staining revealed that Kv7.2 and Kv7.3 immunoreactivities were colocalized with CRH immunoreactivties in the PVN neurons, together with our electrophysiological data showing that acute stress blunted Kv7 channel function in PVN-CRH neurons, these data suggest that Kv7 channels are downregulated in PVN-CRH neurons in acute stress. However, we cannot rule out the possibility that acute stress downregulates Kv7 channels in non-CRH neurons.
Previous studies show that acute stress may rapidly downregulate protein expression levels to induce behavioral change. In this regard, acute stress decreases the expression of Kv7.2 and Kv7.3 in the hippocampus, resulting in impaired spatial memory and the generation of hippocampal long-term potentiation (Li et al., 2014). Although the signaling pathways mediating downregulation of membrane expression of Kv7.3 in acute stress are not completely understood, it seems that AMPK is involved in this process. AMPK regulates cellular energy homeostasis by sensing the intracellular AMP/ATP ratio and is involved in regulation of ion channel expression and activities. For example, Kv7.1 membrane expression and Kv7.1-mediated currents are decreased by activation of AMPK through an ubiquitin ligase Nedd4-2-dependent mechanism (Andersen et al., 2012). Furthermore, epithelial Na+ channel membrane expression is reduced by activation of AMPK through the AMPK-Nedd4-2 pathway in Xenopus oocytes (Bhalla et al., 2006). Consistent with previous findings that acute stress increases hypothalamic AMPK activity (Marques et al., 2012), acute stress profoundly increased phosphorylated AMPK expression levels, although the total AMPK expression level was unchanged. We also found that inhibition of AMPK activity with its specific inhibitor restored acute stress-induced reduction of membrane Kv7.3 expression in the PVN tissue and recovered the decreased M-currents in the PVN-CRH neurons induced by stress. These data suggest that activation of AMPK is required to reduce membrane Kv7.3 expression in acute stress. On the basis of this finding, inhibition of central AMPK activity by ICV administration of AMPK inhibitor significantly decreased firing activity of PVN-CRH neurons and circulating CORT levels in acutely stressed rats. However, central inhibition of AMPK activity does not alter CORT levels in unstressed condition (Kinote et al., 2012), suggesting that activation of AMPK is responsible for elevated CORT levels in stress condition. ICV injection of dorsomorphin would be expected to inhibit AMPK throughout the brain since AMPK is widely expressed. The most likely target of dorsomorphin is the hypothalamic PVN, since PVN is the key brain region responsible for acute stress-induced elevation of circulating CORT levels. However, we cannot completely exclude contributions of other nucleus in the elevation of CORT levels during acute stress.
Collectively, this study reveals a new mechanism underlying hyperactivity of PVN-CRH neurons under acute stress condition. Our data suggest that acute stress-caused hyperactivity of HPA axis is due to a dysfunction of Kv7-channels, which may be caused by elevated AMPK activity. Thus, inhibition of AMPK activity may be a novel potential therapeutic strategy to limit hyper-responsiveness of PVN-CRH neurons during an acute stress response. Since 17β-estradiol suppresses Kv7 channels to stimulate neuropeptide Y neurons in female arcuate nucleus (Roepke et al., 2011), we speculate that the activity of PVN neurons is relatively high due to a suppression of Kv7 channels in females. This is the possible reason that female rats display higher basal CORT levels and greater CORT responses to novel stressors than males (Kant et al., 1983). Thus, future studies are warranty to study if AMPK-Kv7 mechanism is involved in the stress response in females.
Highlights.
Acute restraint stress blunted M-current activity in PVN-CRH neurons
Acute restraint stress reduced the expression level of membrane fraction of Kv7.3 subunit.
Acute restraint stress increased AMPK activity in the PVN tissue.
Inhibition o AMPK activity restored acute stress-induced reduction of M-current function and increased protein level of membrane Kv7.3.
Acknowledgments
Funding and Disclosure
This study was supported by National Institute of Mental Health (NIMH) grants MH096086, and by an IRG grant from MD Anderson Cancer Center. This project was also supported by the NIH/NCI under award number P30CA016672.
List of Nonstandard Abbreviations
- AAV
adeno-associated virus
- aCSF
artificial cerebrospinal fluid
- AMPK
AMP-activated protein kinase
- CORT
corticosterone
- CRH
corticotrophin-releasing hormone
- eGFP
enhanced green fluorescent protein
- HPA
hypothalamic–pituitary–adrenal
- ICV
intracerebroventricular
- PVN
paraventricular nucleus
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
D-P. L., Z., Z., and T.A. K. designed the study. Y-G. G, J-J. Z, Z. Z, and D-P. L. performed the experiments and analyzed the data. J-J. Z, and D-P. L wrote the manuscript. T.A. K. and Z.Z revised and commented on the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- Abriel H, Staub O. Ubiquitylation of ion channels. Physiology (Bethesda) 2005;20:398–407. doi: 10.1152/physiol.00033.2005. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33:67–84. doi: 10.1016/j.yfrne.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SL, Irvine CH, Donald RA. Short-term secretion patterns of corticotropin-releasing hormone, arginine vasopressin and ACTH as shown by intensive sampling of pituitary venous blood from horses. Neuroendocrinology. 1994;60:225–236. doi: 10.1159/000126755. [DOI] [PubMed] [Google Scholar]
- Alon T, Zhou L, Perez CA, Garfield AS, Friedman JM, Heisler LK. Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter. Endocrinology. 2009;150:5626–5632. doi: 10.1210/en.2009-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen MN, Krzystanek K, Jespersen T, Olesen SP, Rasmussen HB. AMP-activated protein kinase downregulates Kv7.1 cell surface expression. Traffic. 2012;13:143–156. doi: 10.1111/j.1600-0854.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem. 2006;281:26159–26169. doi: 10.1074/jbc.M606045200. [DOI] [PubMed] [Google Scholar]
- Brown BS, Yu SP. Modulation and genetic identification of the M channel. Prog Biophys Mol Biol. 2000;73:135–166. doi: 10.1016/s0079-6107(00)00004-3. [DOI] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Butcher BA, Kim L, Johnson PF, Denkers EY. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kappa B. J Immunol. 2001;167:2193–2201. doi: 10.4049/jimmunol.167.4.2193. [DOI] [PubMed] [Google Scholar]
- Cavaliere S, Malik BR, Hodge JJ. KCNQ channels regulate age-related memory impairment. PLoS One. 2013;8:e62445. doi: 10.1371/journal.pone.0062445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Nebel S, Sasse S, Campeau S. Inhibition of the central extended amygdala by loud noise and restraint stress. Eur J Neurosci. 2005;21:441–454. doi: 10.1111/j.1460-9568.2005.03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Dermaku-Sopjani M, Abazi S, Faggio C, Kolgeci J, Sopjani M. AMPK-sensitive cellular transport. J Biochem. 2014;155:147–158. doi: 10.1093/jb/mvu002. [DOI] [PubMed] [Google Scholar]
- Ekberg J, Schuetz F, Boase NA, Conroy SJ, Manning J, Kumar S, Poronnik P, Adams DJ. Regulation of the voltage-gated K(+) channels KCNQ2/3 and KCNQ3/5 by ubiquitination. Novel role for Nedd4-2. J Biol Chem. 2007;282:12135–12142. doi: 10.1074/jbc.M609385200. [DOI] [PubMed] [Google Scholar]
- Etxeberria A, Santana-Castro I, Regalado MP, Aivar P, Villarroel A. Three mechanisms underlie KCNQ2/3 heteromeric potassium M-channel potentiation. J Neurosci. 2004;24:9146–9152. doi: 10.1523/JNEUROSCI.3194-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Brown DA. A mechanism for nerve cell excitation by norepinephrine via alpha-1 adrenoceptors: inhibition of potassium M-current. Cell Mol Neurobiol. 2013;33:1–4. doi: 10.1007/s10571-012-9870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhou JJ, Zhu Y, Kosten T, Li DP. Chronic Unpredictable Mild Stress Induces Loss of GABA Inhibition in Corticotrophin-Releasing Hormone-Expressing Neurons Through NKCC1 Upregulation. Neuroendocrinology. 2016 doi: 10.1159/000446114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Goncharova ND. Stress responsiveness of the hypothalamic-pituitary-adrenal axis: age-related features of the vasopressinergic regulation. Front Endocrinol (Lausanne) 2013;4:26. doi: 10.3389/fendo.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SM, Namkoong C, Jang PG, Park IS, Hong SW, Katakami H, Chun S, Kim SW, Park JY, Lee KU, Kim MS. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. 2005;48:2170–2178. doi: 10.1007/s00125-005-1913-1. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- Hewitt SA, Wamsteeker JI, Kurz EU, Bains JS. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat Neurosci. 2009;12:438–443. doi: 10.1038/nn.2274. [DOI] [PubMed] [Google Scholar]
- Hu L, Yang J, Song T, Hou N, Liu Y, Zhao X, Zhang D, Wang L, Wang T, Huang C. A new stress model, a scream sound, alters learning and monoamine levels in rat brain. Physiol Behav. 2014;123:105–113. doi: 10.1016/j.physbeh.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Imaki T, Naruse M, Harada S, Chikada N, Nakajima K, Yoshimoto T, Demura H. Stress-induced changes of gene expression in the paraventricular nucleus are enhanced in spontaneously hypertensive rats. J Neuroendocrinol. 1998;10:635–643. doi: 10.1046/j.1365-2826.1998.00249.x. [DOI] [PubMed] [Google Scholar]
- Ito K, Bahry MA, Hui Y, Furuse M, Chowdhury VS. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp Biochem Physiol A Mol Integr Physiol. 2015;187:13–19. doi: 10.1016/j.cbpa.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Itoi K, Talukder AH, Fuse T, Kaneko T, Ozawa R, Sato T, Sugaya T, Uchida K, Yamazaki M, Abe M, Natsume R, Sakimura K. Visualization of corticotropin-releasing factor neurons by fluorescent proteins in the mouse brain and characterization of labeled neurons in the paraventricular nucleus of the hypothalamus. Endocrinology. 2014;155:4054–4060. doi: 10.1210/en.2014-1182. [DOI] [PubMed] [Google Scholar]
- Jaroenporn S, Nagaoka K, Kasahara C, Ohta R, Watanabe G, Taya K. Physiological roles of prolactin in the adrenocortical response to acute restraint stress. Endocr J. 2007;54:703–711. doi: 10.1507/endocrj.k07-003. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Lenox RH, Bunnell BN, Mougey EH, Pennington LL, Meyerhoff JL. Comparison of stress response in male and female rats: pituitary cyclic AMP and plasma prolactin, growth hormone and corticosterone. Psychoneuroendocrinology. 1983;8:421–428. doi: 10.1016/0306-4530(83)90021-5. [DOI] [PubMed] [Google Scholar]
- Kim AS, Miller EJ, Young LH. AMP-activated protein kinase: a core signalling pathway in the heart. Acta Physiol (Oxf) 2009;196:37–53. doi: 10.1111/j.1748-1716.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- Kinote A, Faria JA, Roman EA, Solon C, Razolli DS, Ignacio-Souza LM, Sollon CS, Nascimento LF, de Araujo TM, Barbosa AP, Lellis-Santos C, Velloso LA, Bordin S, Anhe GF. Fructose-induced hypothalamic AMPK activation stimulates hepatic PEPCK and gluconeogenesis due to increased corticosterone levels. Endocrinology. 2012;153:3633–3645. doi: 10.1210/en.2012-1341. [DOI] [PubMed] [Google Scholar]
- Kusek M, Tokarski K, Hess G. Repeated restraint stress enhances glutamatergic transmission in the paraventricular nucleus of the rat hypothalamus. J Physiol Pharmacol. 2013;64:565–570. [PubMed] [Google Scholar]
- Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, Steinmeyer K. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J Biol Chem. 2000;275:22395–22400. doi: 10.1074/jbc.M002378200. [DOI] [PubMed] [Google Scholar]
- Li C, Huang P, Lu Q, Zhou M, Guo L, Xu X. KCNQ/Kv7 channel activator flupirtine protects against acute stress-induced impairments of spatial memory retrieval and hippocampal LTP in rats. Neuroscience. 2014;280:19–30. doi: 10.1016/j.neuroscience.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther. 2005;313:1035–1045. doi: 10.1124/jpet.104.082495. [DOI] [PubMed] [Google Scholar]
- Li DP, Zhou JJ, Pan HL. Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Physiol. 2015;593:4439–4452. doi: 10.1113/JP270831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Marques MB, Ribeiro-Oliveira A, Jr, Guimaraes J, Nascimento GF, Anjos AP, Vilas-Boas WW, Santos RA, Thomas JD, Igreja SM, Grossman AB, Kola B, Korbonits M. Modifications in basal and stress-induced hypothalamic AMP-activated protein kinase (AMPK) activity in rats chronically treated with an angiotensin II receptor blocker. Stress. 2012;15:554–561. doi: 10.3109/10253890.2011.648673. [DOI] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, Rainnie DG, Nemeroff CB, Owens MJ. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol Psychiatry. 2010;67:1212–1216. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- Miklos Z, Flynn FW, Lessard A. Stress-induced dendritic internalization and nuclear translocation of the neurokinin-3 (NK3) receptor in vasopressinergic profiles of the rat paraventricular nucleus of the hypothalamus. Brain Res. 2014;1590:31–44. doi: 10.1016/j.brainres.2014.09.043. [DOI] [PubMed] [Google Scholar]
- Montagud-Romero S, Aguilar MA, Maldonado C, Manzanedo C, Minarro J, Rodriguez-Arias M. Acute social defeat stress increases the conditioned rewarding effects of cocaine in adult but not in adolescent mice. Pharmacol Biochem Behav. 2015;135:1–12. doi: 10.1016/j.pbb.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Bethin KE, Jacobson L, Vogt SK, Majzoub JA. Pituitary-adrenal axis regulation in CRH-deficient mice. Endocr Res. 2000;26:1057–1066. doi: 10.3109/07435800009048638. [DOI] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, Brown DA. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WA, Wan R, Mattson MP. Impact of aging on stress-responsive neuroendocrine systems. Mech Ageing Dev. 2001;122:963–983. doi: 10.1016/s0047-6374(01)00250-0. [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Hypophysiotropic regulation of stress-induced ACTH secretion. Adv Exp Med Biol. 1988;245:65–81. doi: 10.1007/978-1-4899-2064-5_6. [DOI] [PubMed] [Google Scholar]
- Qi Y, Wang J, Bomben VC, Li DP, Chen SR, Sun H, Xi Y, Reed JG, Cheng J, Pan HL, Noebels JL, Yeh ET. Hyper-SUMOylation of the Kv7 potassium channel diminishes the M-current leading to seizures and sudden death. Neuron. 2014;83:1159–1171. doi: 10.1016/j.neuron.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier CL, Plotsky PM. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Annu Rev Physiol. 1986;48:475–494. doi: 10.1146/annurev.ph.48.030186.002355. [DOI] [PubMed] [Google Scholar]
- Roche JP, Westenbroek R, Sorom AJ, Hille B, Mackie K, Shapiro MS. Antibodies and a cysteine-modifying reagent show correspondence of M current in neurons to KCNQ2 and KCNQ3 K+ channels. Br J Pharmacol. 2002;137:1173–1186. doi: 10.1038/sj.bjp.0704989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Qiu J, Smith AW, Ronnekleiv OK, Kelly MJ. Fasting and 17beta-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci. 2011;31:11825–11835. doi: 10.1523/JNEUROSCI.1395-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M, Reboreda A, Sanchez E, Lamas JA. Newly developed blockers of the M-current do not reduce spike frequency adaptation in cultured mouse sympathetic neurons. Eur J Neurosci. 2004;19:2693–2702. doi: 10.1111/j.1460-9568.2004.03363.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984;81:1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Shah M, Mistry M, Marsh SJ, Brown DA, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol. 2002;544:29–37. doi: 10.1113/jphysiol.2002.028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweerts BW, Jarrott B, Lawrence AJ. Expression of preprogalanin mRNA following acute and chronic restraint stress in brains of normotensive and hypertensive rats. Brain Res Mol Brain Res. 1999;69:113–123. doi: 10.1016/s0169-328x(99)00095-9. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wamsteeker Cusulin JI, Fuzesi T, Watts AG, Bains JS. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS One. 2013;8:e64943. doi: 10.1371/journal.pone.0064943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, Morishima I. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. J Vet Med Sci. 2004;66:1323–1327. doi: 10.1292/jvms.66.1323. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Insel PA. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem. 2004;279:20858–20865. doi: 10.1074/jbc.M310643200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jakubowski M, Buettner C, Kainz V, Gold M, Burstein R. Ezogabine (KCNQ2/3 channel opener) prevents delayed activation of meningeal nociceptors if given before but not after the occurrence of cortical spreading depression. Epilepsy Behav. 2013;28:243–248. doi: 10.1016/j.yebeh.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JJ, Yuan F, Zhang Y, Li DP. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology. 2015;99:481–490. doi: 10.1016/j.neuropharm.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]