Abstract
Purpose
To develop an efficient MRI approach to estimate the non-water proton fraction (f) in human brain.
Methods
We implement a brief, efficient MT pulse that selectively saturates the magnetization of the (semi-) solid protons, and monitor the transfer of this saturation to the water protons as a function of delay after saturation.
Results
Analysis of the transient MT effect with two-pool model allowed robust extraction of f at both 3 T and 7 T. This required estimating the longitudinal relaxation rate constant (R1,MP and R1,WP) for both proton pools, which was achieved with the assumption of uniform R1,MP and R1,WP across brain tissues. Resulting values of f were approximately 50% higher than reported previously, which is partly attributed to MP pulse efficiency and R1,MP being higher than assumed previously.
Conclusion
Experiments performed on human brain in-vivo at 3 T and 7 T demonstrate the ability of the method to robustly determine f in a scan time of about 5 minutes.
Keywords: brain tissue, white matter, myelin, T1 relaxation, magnetization transfer, macromolecular proton fraction
INTRODUCTION
Although MRI almost exclusively measures the signal of water hydrogen protons (WPs), a substantial fraction (‘f’) of tissue hydrogen protons resides in molecules other than water, predominantly protein and lipid (here, for simplicity, these are categorically indicated by “macromolecular hydrogen protons” or MPs). While MPs are generally not directly visible because of their rapid transverse relaxation owing to restricted mobility, they can dramatically affect the MRI signal and the apparent longitudinal and transverse relaxation time constants (T1 and T2 respectively) through interaction with WPs.
In human brain, a relatively high fraction of MP (f ~0.2–0.3) is found in white matter (WM)(1–5), primarily because of its high content of myelin. Myelin, which is important for nerve conduction, is rich in proteins and lipids, and may contain up to 60% of MPs in white matter (6–8). Study of the effect of MPs on WP T1 and T2 relaxation therefore provides an opportunity to indirectly detect myelin loss (9).
One way to study myelin loss is through T2 relaxation. In WM, T2 relaxation has been shown to be multi-exponential, with the most rapid relaxation (shortest T2) attributed to a pool of water trapped between the myelin layers, and strongly interacting with MPs in these layers (9,10). The size of this pool has been shown to correlate with brain myelin content (11). Similarly, the MPs in myelin are a strong contributor to T1 relaxation (12), and in fact it has been argued that outside the iron rich subcortical grey matter, f is the main determinant of T1 (4,13–15). Thus, changes in f related to myelin loss may be sensitively detected by T1-weighted techniques. Nevertheless, it should be realized that NMR relaxation processes are generally complex and that both changes in T1 relaxation and T2 relaxation may not be specific to changes in f. A further complication is a potential bias resulting from inter-compartmental exchange, which may lead to and underestimation of tissue myelin content (10).
Another approach to investigate variations or changes in brain myelination is by determining f through the classical MT experiment (16) in which radiofrequency (RF) irradiation is used to selectively reduce (saturate) the longitudinal magnetization of MPs and monitor the effect on the WP signal. This selectivity is based on the short T2 of MPs, which has been found to be generally below 100 μs for proteins and lipids based on super-Lorentzian lineshapes (6).
To maximize the effect on WP saturation, most modern MT methods used for studying pathological changes in f in human brain use the so called “steady state approach”, in which the MT effect is measured after long (relative to the T1 of WP) continuous or repeated pulsed irradiation. However, while steady state approaches allow large saturation effects and provide good sensitivity, the loss of information about transient aspects of the MT process complicates interpretation and quantitative measurement of f, as the MT effects become strongly dependent of various parameters, including irradiation specifics, T2 of the MP pool, and T1 of both WP and MP pools. Mitigation of these issues is possible using so-called quantitative MT (qMT) techniques (17–22), which have shown promise in detecting myelin loss in multiple sclerosis and other neurological diseases (22–24). While rapid approaches may be possible (22), accurate qMT techniques are generally time-consuming and require collection of several reference datasets to mitigate confounds, such as variations in T1 and RF amplitude.
An alternative to the steady-state approach, and a potentially faster way to measure f is to use of a “transient” MT approach which uses a single, brief irradiation pulse to differentially affect the longitudinal magnetization (Mz) of MPs and WPs (e.g. saturation of MP and inversion of WP magnetization) and monitors the equilibration process as a function of delay after the MT pulse. This approach was initially implemented on NMR spectrometers to study MT in tissue samples, and relied on direct measurement of signal from both the short T2 MP pool and the longer T2 WP pool (25–29). Combining this approach with a 2-pool model to fit to the MP and WP signal evolution allowed quantification of f as well as MT exchange rates. Subsequently, a number of studies has explored ways to use the transient MT approach to extract f without the need to detect the MP signal, which would make the method amendable for use on clinical MRI scanners (20,30–33).
One of the outstanding issues with measurement of f using transient MT approaches is the difficulty in estimating MP T1 (in the following referred to as R1,MP, equal to the inverse of MP T1), a parameter in the 2-pool model whose value significantly affects f. This is also an issue (possibly to a lesser extent) for steady state MT approaches, which typically assume R1,MP to be similar to R1,WP (around 1 s−1 in white matter). It has been pointed out however that actual values for R1,MP may be much higher, and that this would lead to an underestimation of f (34). To address this for the transient MT approach, we jointly analyzed MT and inversion recovery (IR) data from human brain using a 2-pool model of exchange and explored the validity of a number of simplifying assumptions. Based on this, we arrived at realistic estimates for R1,MP in human brain at 3 T and 7 T, allowing us to properly quantifying f from a transient MT experiment with a measurement time as short as 5 minutes.
METHODS
Our method for quantifying f is based on the notion that in most brain regions outside the iron-rich sub-cortical grey matter (e.g. globus pallidus, caudate and red nuclei, and substantia nigra), longitudinal WP relaxation has a dominant contribution from MT with MP and that tissue contrast primarily results from variations in f (13). Thus, rather than assuming R1,MP and R1,WP to be equal, as has generally been done in the analysis of MT experiments, we allowed them to differ but assumed them to constant across the brain. We then performed pulsed, transient MT using a highly efficient MP saturation pulse, as well as inversion recovery (IR) to facilitate estimation of R1,MP and R1,WP. For this purpose MT and IR data were jointly analyzed with a 2-pool model of exchange, during which R1,MP and R1,WP values were determined that led to realistic values of MP saturation and allowed estimation of f. Additionally, we evaluated the feasibility of estimating f from MT data only by introducing additional constraints.
MRI Scanning
Experiments were performed on 3 T and 7 T Siemens MRI scanners (Erlangen, Germany; Skyra and Magnetom platforms respectively). Eleven subjects (6 female, ages 19–60, average 30) were scanned at both field strengths under an IRB-approved protocol to investigate the robustness in determining f. On 5 subjects at the 3 T only, a second scan was performed to assess test-retest reproducibility.
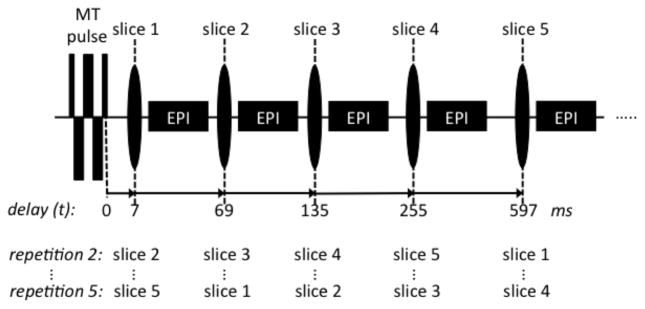
Our transient MT experiment (Fig. 1) used a brief, T2-selective composite RF saturation pulse (33,35,36) to saturate MP, after which multiple image slices at variable delay t were acquired with EPI. We first determined the appropriate RF pulse (in the following called “MT pulse”) parameters to achieve optimal saturation characteristics, and then studied the feasibility to robustly extract f using a two-pool model of exchange.
Figure 1.
Image acquisition for the pulsed transient MT experiment. Five image slices are acquired at incrementally increasing delay times t after an MT pulse. By shifting the order of the slices in subsequent repetitions, all five delay times are sampled for each slice location.
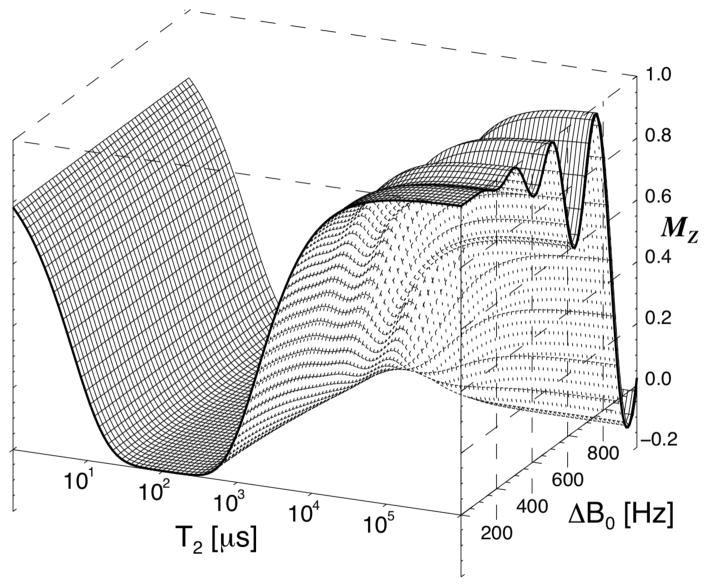
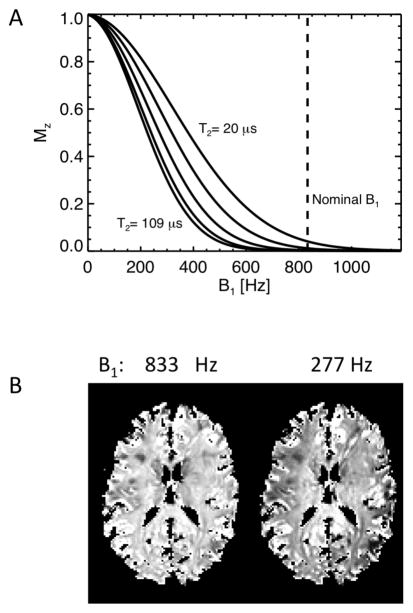
The composite, phase modulated MT pulse had a constant B1 amplitude of 19.6 μT and consisted of 16 sub-pulses of with nominal flip angles of 60°, −120°,120°,−120°….. 120°,−60°. Its duration of 6 ms was sufficient to achieve a near optimal (about 90% of maximum, see below) saturation of MP, as judged from the delayed effect on the WP signal. This was based on initial experiments (n=6) that measured dependence of the saturation effect on increasing the MT pulse duration from 6 up to 11 ms (by adding −120°,120° pulse segments). Numerical simulation of the effects of the 6 ms pulse (based on the Bloch equations), shown in Fig. 2, confirmed the efficient saturation of MPs assuming a Lorentzian lineshape with T2 in the range of 20–400 μs. This range roughly covers the range of values reported in literature (6,7,37). A Lorentzian line shape was used in order to allow simulations using the time domain Bloch equations. In terms of the saturation effect of our MT pulse, the applied Lorentzian T2 range is roughly equivalent to the T2 range of 5–20 μs reported for super-Lorentzian MP lineshapes (17,18,38–42). At the same time, the pulse had an only minor (<10%) effect on T2 species > 20 ms (WPs), as long as it was applied close enough (<500Hz) to resonance (Fig. 2). A simulation of the MP saturation as function of B1 amplitude for a range of T2 values in shown in Fig. 3a, demonstrating that MP saturation is nearly complete for the expected range of T2 and B1 values. At high B1 amplitude, only minimal sensitivity of the saturation level to variations in B1 amplitude is observed. This is further illustrated with the experimental data shown in Fig. 3b, confirming uniform MP saturation at high B1.
Figure 2.
Mz after a 6 ms composite MT pulse, as function of T2 and frequency offset (B0), based on simulation of the Bloch equations. The MT pulse effectively saturates spins with a T2 in the range from 20 to 400 μs at all offset frequencies (for a Lorentzian lineshape). Long T2 spins suffer only a small perturbation on resonance (< 5% for T2 > 36 ms), with a larger effect for frequencies > 500 Hz.
Figure 3.
Robustness of transient MT approach to variations in B1. A) Simulated MP saturation levels (FSMP(0)) after a 6 ms MT pulse for (Lorentzian) MP T2 values of 20, 32, 48, 72 and 109 μs. The curves for T2’s up to 400 μs fall between the 32 and 109 μs lines. The dashed line represents the nominal (brain-averaged) B1 of 833 Hz used in the experiments. The plots show that MP magnetization is effectively saturated for a range of B1 and T2 values, reducing the sensitivity of the experiments to variations in B1 amplitude. B) Experimental (7 T) demonstration of B1 dependence of MP saturation. FSMP(0) was calculated with approach 1 from data at actual (833 Hz) B1 and a strongly reduced (277 Hz) B1. Incomplete MP saturation is only seen at strongly reduced B1, in particular towards the edges of the brain where B1 is lowest. The images were normalized to level in the SCC.
The order of the five EPI slices acquired sequentially after each repetition of the MT pulse (Fig. 1) was cycled to allow time-efficient collection of the five delay times (43). Delay (t) values of 7, 69, 135, 255 and 597 ms were chosen to cover most of the saturation dynamics. EPI scan parameters were: echo spacing 0.77 ms, bandwidth 250 kHz, 45% ramp-sampling, matrix size 144×108, FOV 240×180 mm2, SENSE rate 2, 2 mm slice thickness, 5.4 mm slice spacing (center to center), TE 30 ms (3 T) and 24 ms (7 T), TR = 3 s. The slice spacing was chosen relatively large to reduce potential MT effects of the excitation pulses, see supplementary material (Fig. S1). Artifacts from scalp lipids were suppressed by acquiring every other repetition with a shifted EPI echo train (1.15 ms and 0.48 ms at 3 T and 7 T respectively), which resulted in a phase-inverted lipid signal. Addition of the two repetitions allowed elimination of this lipid signal, assuming the T2 is small compared to the applied shift in echo time. Twenty scan repetitions were performed at each slice location, taking a total scan time of 5 minutes. Four of the 20 repetitions were acquired without MT pulse to serve as reference signal.
IR scans were performed by replacing the MT pulse with a hyperbolic-secant inversion pulse (5.12 ms, 19.6 μT maximum B1 amplitude, 830 Hz maximum frequency modulation, β = 1400 s−1 (44) with an adjustment of the amplitude to start at zero B1). Delay times t (also called inversion times (TIs)) of 6, 69, 135, 282 and 1197 ms were used. The TR for these scans was 4 s, the number of repetitions was 18 for 3 T and 22 for 7 T. Again, four of the repetitions were acquired without inversion pulse to serve as reference signal to calculate the fractional saturation resulting from the MT (or IR) pulse.
Estimation of model parameters
The extraction of f and other model parameters was based on fitting the delay-dependent signal to a two-pool model of MT (26,29,45,46). The same model was used for both preparation pulses (MT and IR pulses) albeit with different initial magnetization levels for the MP and WP pools. The calculations were based on the fractional saturation FS derived from the MT weighted signal S measured at delay t and reference signal Sref: . For both WP and MP, FS(t) can be written as the sum of 2 exponentials (29):
| [1] |
| [2] |
with:
| [3] |
In these equations, R1,MP, R1,WP are the longitudinal relaxation rates of both pools in the absence of exchange; kMW and kWM refer to the rates of change in FSMP and FSWP due to MT. These rates are the volume-fraction normalized equivalent of the often used cross-relaxation rate constant k, i.e. kMW = k/f, and kWM = k/(1-f) (see e.g ref. (47)).
Both MT and IR data can be analyzed by this model, and Eq. [1] will fit with the same exponential rate constants (λ1 and λ2) but with different coefficients (a and b). After determining a, b, λ1 and λ2 from fitting of Eq. [1] to the experimental data, one can proceed with solving for kMW, f, R1,MP and R1,WP using [3], and the initial saturation levels FSWP(0) and FSMP(0) from Eqs. [1] and [2]. This requires two additional pieces of information, as we have only four equations for 6 unknowns.
To solve this problem, and to investigate the possibility of omitting some of the data from the analysis in order to shorten the measurement protocol, four analysis approaches with different simplifying assumptions were evaluated. Briefly, four different combinations of parameters were fixed to (field specific) values common to all brain voxels: 1) R1,MP and R1,WP; 2) R1,MP and FSMP(0); 3) R1,MP, R1,WP and FSMP(0); 4) R1,MP, R1,WP, FSMP(0) and FSWP(0). The extracted values for kMW and f were then compared between the four approaches, as well as their consistency across field strengths.
Global, fixed values for R1,MP and R1,WP under approach 1 were estimated from joint analysis of MT and IR data. Fixing R1,MP and R1,WP was motivated by a previously proposed model of T1 relaxation, in which MT between WP and MP is the dominant source of T1 contrast (4,13–15). This model has been shown to accurately describe T1 relaxation in most brain regions, perhaps with exception of iron rich grey matter areas (e.g. globus pallidus, caudate and red nuclei, substantia nigra) (13). To estimate R1,MP and R1,WP, first the MT and IR data were fitted jointly in every voxel with Eq. [1], resulting in one set of rate constants (λ1 and λ2) and two sets of amplitudes (a and b) for every voxel. Then R1,MP and R1,WP were varied to obtain reasonable values FSMP(0) values for both the MT and IR data. That is, for each combination of R1,MP and R1,WP values (within a realistic range), FSMP(0) for both the MT and IR experiments were calculated (together with corresponding values kMW and f ) in each voxel and the number of voxels with permissible values was used as a criterion to select R1,MP and R1,WP that best described the data. The permissible range was set as follows: FSMP(0) for both IR and MT experiments could not exceed 1.0 (i.e. complete saturation), while the value for IR data should be between 0.7 and 1.0 times that for MT (that is, the MP saturation after the inversion pulse is somewhere between 70 and 100% of the saturation after the MT pulse). The data from all subjects was combined for this analysis, resulting in one global R1,MP and one global R1,WP value for each field strength.
Under approach 2, we used the fixed value for R1,MP found with approach 1, and as well as a fixed value for the FSMP(0) for the MT experiment. Again, as in approach 1, one set of rate constants (λ1 and λ2) and two sets of amplitudes (a and b) extracted from the MT and IR data were used. The motivation of this approach was to evaluate the variation in R1,WP, based on the notion that variation in FSMP(0) for the MT experiment was constrained within a rather restricted range (between about 0.8 and 1.0, judged from simulations (see above) and experiments (see RESULTS, first paragraph).
Under approach 3, fixing both R1,MP and R1,WP, as well as FSMP(0) allowed omission of the IR data from the analysis. Now, only the MT data was fitted with Eq. 1, which represented only three degrees of freedom due to the dependence introduced between a, b, λ1 and λ2 by fixing the three parameters.
Finally, under approach 4, fixing a fourth parameter (FSWP(0)) obviated the need for measuring Sref and reduced the 4 parameter model represented in Eq. 1 to a 3 parameter model:
| [4] |
where S0 is a scaling factor for the signal level, λ1 and λ2 are defined as above, and two amplitudes a and b are now constants derived from the fixed average saturation levels of the two pools at t = 0 (FSWP(0) and FSMP(0)). From the fitted parameters S0, λ1 and λ2, both f and kMW can be derived when applying Eq. [3] with fixed values for R1,MP and R1,WP.
All fitting was performed on both a pixel-by-pixel basis and on the average signal in four region of interest (ROI): in white matter of the splenium of the corpus callosum (SCC), in grey matter in the globus pallidus (GP), the putamen (Put) and the head of the caudate nucleus (NC). The ROIs were manually selected directly on the EPI images using both the implicit T2* contrast and the T1 contrast from the IR data. The SCC-ROI encompassed 33–86 voxels (average 61), the GP-ROI size was 18–110 (67), the Put-ROI 86–245 (150) and the NC-ROI 73–143 (103). Fitting was based on a simple and robust iterative grid search and refinement of the non-linear parameters to be optimized, in combination with linear least squares (LLS) optimization for the linear parameters (either a,b or S0)(48). That is, for each choice of non-linear parameters, the linear parameters (amplitude factors) were estimated using LLS, and the residual was calculated. For each iteration, all combinations of parameters were tested in a search grid centered on the initial values. After selection of the best set (the one with the lowest residual), the step size was reduced to refine the search grid and next iteration was started using the current best fit as initial values. During joint fitting of MT and IR data, a single set of decay rates was used (λ1 and λ2) while the amplitudes (a,b) were allowed to be different to accommodate for the different saturation levels resulting from the MT and IR pulse. For both field strengths, average and standard deviation (SD) of the resulting parameter values was calculated. Results obtained from the ROI analysis were reported as averages and standard deviation over subjects. To investigate a potential bias related to magnetic field strength, differences of the results on the same subjects at the two field strengths were calculated for the SCC. The averages of the differences show a potential field related bias, while the SD of the differences reflects variability excluding inter subject variations. Approach 1 was only used to analyze data from white matter, as the grey matter ROIs are known to be high in iron and as a result have an R1,WP substantially different from the global average. For the fixed R1,WP needed for approaches 3 and 4, the average over subjects of the values fitted in approach 2 were used instead.
To estimate the precision (reproducibility) of the fitted parameters under influence of noise in the input data, simulated noise was incrementally added to 3 T and 7 T model curves, based on the average parameters found for the SCC. The resulting simulated data were fitted for each noise realization and the SD of the extracted f and kMW values were determined. The sensitivity of the fitted parameters to the variations in R1,MP and FSMP(0) is shown in the supplementary material. The plots were derived by recalculating the fitted parameters for a range of values for R1,MP and FSMP(0).
Image reconstruction and pre-processing
All image analysis was performed off-line using in-house IDL (Harris Geospatial Solutions, Boulder, CO, USA) based software and C code. The SENSE unfolding matrix, required for image reconstruction, was calculated from (multi–echo) GRE reference data acquired at the same slice position and resolution as the EPI data. This reference data were also used to derive field maps to calculate the geometric distortion corrections for the EPI. All resulting images in each scan session were spatially registered to the first volume to correct for in-plane motion. Through-plane motion was not corrected, as this proved difficult with the limited number of slices and large inter-slice gaps. Image registration included a fixed contrast adjustment to allow registration between reference, IR and MT data.
The addition of TE-shifted and non-shifted images for scalp lipid suppression was performed after calculating signal magnitude from the complex valued data; this proved sufficient and reduced problems with phase instabilities occasionally encountered at 7 T. However, for the IR scans, images acquired at the longest delay t required complex addition, due to the sign inversion of the longitudinal magnetization in some tissues. In this case, occasional manual phase adjustment was necessary. For earlier TIs, data were combined in magnitude mode and inverted (to reflect the negative polarity of the magnetization).
For both MT and reference data, signals were averaged over repetitions. In addition, for the reference signal (i.e. signal without inversion or MT), further averaging was performed over the acquisition with the different delay times to further improve image signal-to-noise ratio (SNR). Prior to multi-parametric fitting, voxels with a reference signal below 5% were eliminated, and signal intensities (S(t)) were converted to fractional saturation levels according to . With this normalization, MT and IR signals decay from 1 to 0 and 2 to 0 with increasing delay t respectively.
RESULTS
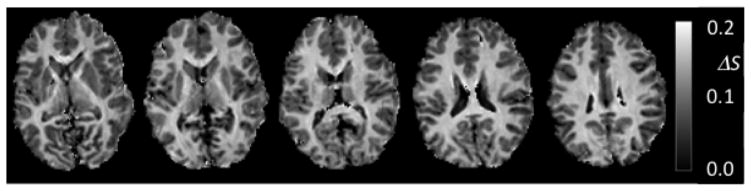
At both 3 T and 7 T, saturation of MP with a single, 6 ms MT pulse led to a delay dependent reduction in WP signal. As expected, this reduction was strongest in white matter and reached a maximum of around 15–20% at a delay between 150 and 250 ms (see Figs. 4, 5). Fig. 4 shows the averages and SD of FSWP(t) obtained for SCC at the two field strengths. The plots show high reproducibility over subjects, and furthermore indicate that the MT effect is more pronounced at 7 T. This is attributed to the slower T1-relaxation at high field. Fig. 5 shows the difference in saturation level between t = 7 ms and t = 255 ms, providing a model-free measure indicative of the MP fraction in human brain. Comparison of the fractional saturation levels (FSWP(0) in equations above) at t = 255 ms between the 6 ms and the 11 ms MT pulse (n=6) suggested effective saturation of macromolecular protons for the 6 ms pulse (FSMP(0) = 0.88 ±0.03 and 0.93 ±0.02 at 3 T and 7 T respectively, this assumed FSMP(0) = 1.0 for the 11 ms pulse). These values were subsequently used for the extraction of kMW and f with fitting approaches 2–4 (see METHODS).
Figure 4.
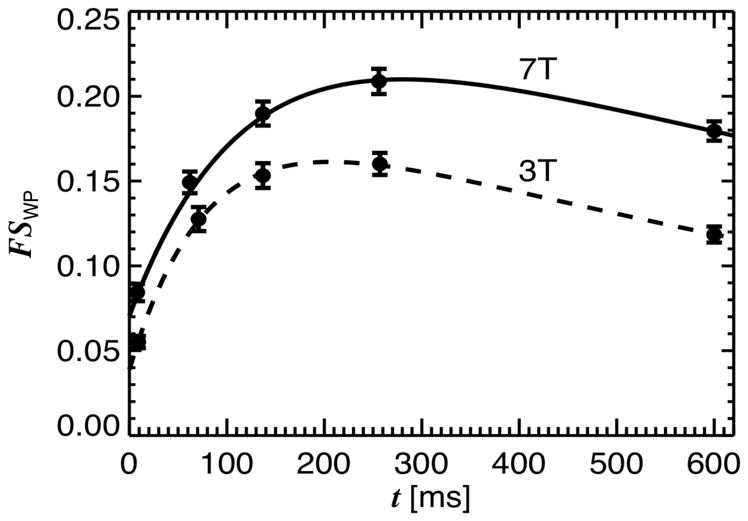
Fractional saturation of water protons (FSWP) at delay time t after MT pulse, for both 3 T and 7 T experiments. FSWP was averaged over ROI’s in the splenium of the corpus callosum; error bars reflect the SD over subjects (n=11). Solid lines represent 2-pool model fit with subject-averaged parameters.
Figure 5.
Fractional MT-related signal change ΔS (at 7 T) calculated from signal difference between images acquired at t = 7 ms and .
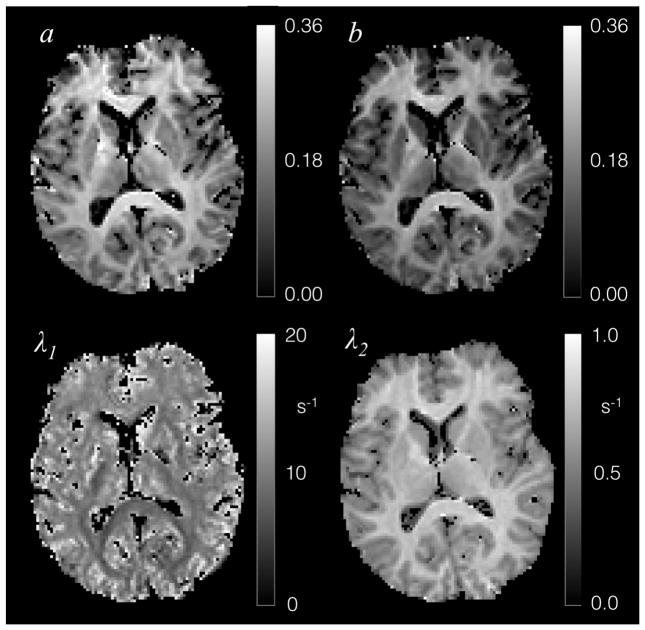
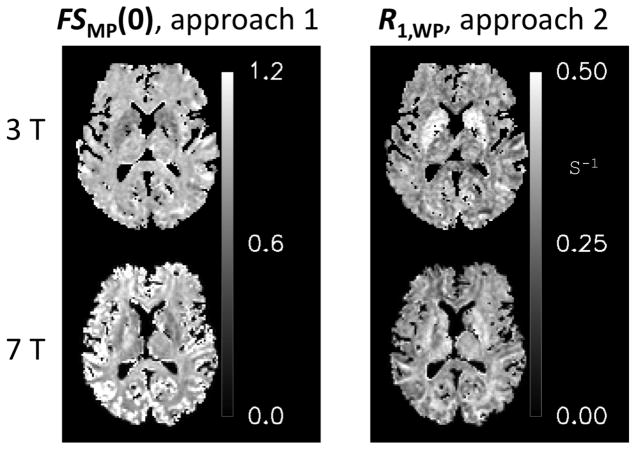
Conjoint fitting of the IR and MT data using fitting approach 1 and 2 consistently produced maps of the four fitted parameters a, b, λ1 and λ2 as exemplified in Fig. 6. Estimation of appropriate R1,MP and R1,WP values common to all brain voxels with approach 1 resulted in estimates of 4.0 s−1 and 2.05 s−1 for R1,MP 3 T and 7 T, and 0.40 s−1 and 0.35 s−1 for R1,WP at 3 T and 7 T. These were the values used for approaches 3 and 4. At these values, the brain distribution of FSWP(0) derived from approach 1 (Fig. 7) showed minimal variation at both field strengths, with values ranging between 0.8 and 1.0, confirming the validity of fixing this parameter in approaches 3 and 4. Similarly, R1,WP (derived from approach 2) was rather uniform across the brain (Fig. 7), with exception of the subcortical grey matter regions.
Figure 6.
Single slice example of fitted parameters of the 2-pool model (Eq. [1]) for 7 T data.
Figure 7.
Variation in FSMP(0) and R1,WP over the brain, deduced with analysis approaches 1 and 2 respectively.
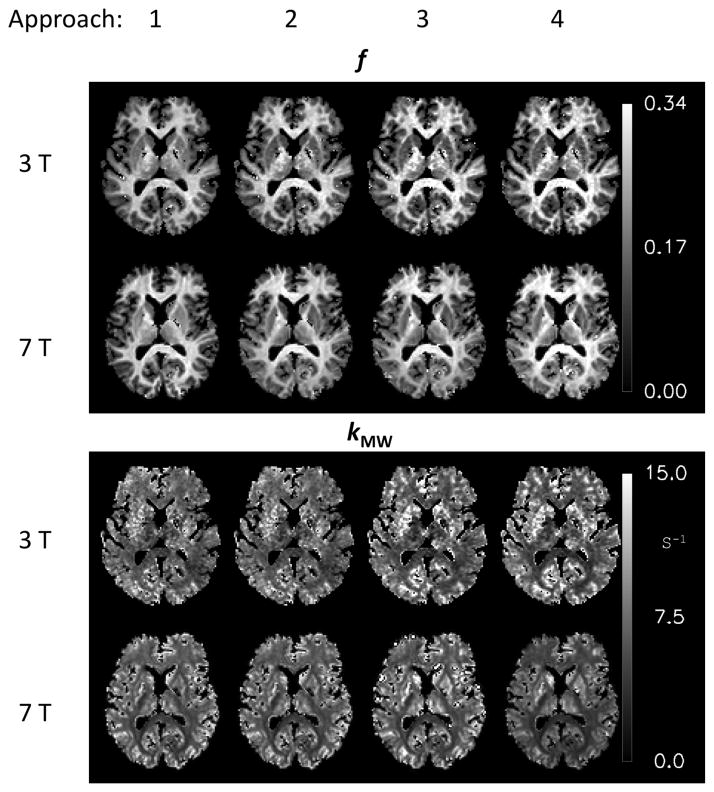
All approaches allowed reliable extraction of f and kMW in most brain regions (Fig. 8, Table 1). Values for f were in the range of 5–30% across the brain, and in the range of 20–30% in white matter. Exchange rate constant kMW varied in the range of 4–12 s−1. Similar values for f were found between 3 T and 7 T, whereas values for kMW were somewhat (5–20%) lower at 7 T (Table 1). Fit residuals (Table 1) indicate a somewhat more robust fit at 7 T; this is attributed to the lower R1,MP and consequently a larger available MT signal at the higher field strength. The average values for the WP exchange rate kWM were 2.3 s−1 (3 T) and 1.9 s−1 (7 T). The SDs of the differences were similar to SDs of the data acquired at each field, suggesting the inter-subject variability is small compared to the measurement induced SD and so these SDs are dominated by the reproducibility of the experiments. The observed differences between field strengths are not significant given the SDs of the data, with exception of the values for f and kMW in approach 1. Analysis results of the grey matter ROIs are reported in Table 2. The R1,WP values for these regions resulting from analysis approach 2 are given in Table 3. Slice coverage in one subject did not allow creation of a GP ROI and this data was therefore not included in this part of the study. The average kMW values in grey matter were higher than the value found in SCC white matter. However, it should be realized that the SD of the grey matter kMW values was relatively high (up to 20%), and kMW values themselves were not consistent between field strengths, suggesting they should be interpreted with caution.
Figure 8.
MP fraction f and exchange rate constant kMW extracted with the four analysis approaches for 3 T and 7 T.
Table 1.
Comparison of the four fitting approaches based on the 2-pool model, progressively fixing more parameters. Averages (Av) and SD (n=11) are shown for f and kMW for the SCC ROI, together with fit residue. The SD of the differences the 3 and 7 T data (diff) is an indication of the intra-subject reproducibility, the residue is reflects the fitting of the 2-pool model, which is the same for approaches 1 and 2 (both contain only 2 assumptions and therefore the same free fit of Eq. 1 to the data).
| Approach 1: Fixed R1,MP, R1,WP |
Approach 2: Fixed R1,MP, FSMP(0) |
Approach 3: + fixed R1,WP |
Approach 4: + fixed FSWP(0) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Av | SD | Av | SD | Av | SD | Av | SD | ||
| f | 3 T | 0.262 | 0.012 | 0.274 | 0.014 | 0.287 | 0.021 | 0.282 | 0.022 |
| 7 T | 0.293 | 0.010 | 0.273 | 0.012 | 0.267 | 0.013 | 0.266 | 0.013 | |
| diff | −0.031 | 0.008 | 0.002 | 0.015 | 0.020 | 0.022 | 0.016 | 0.023 | |
| kMW [s−1] | 3 T | 6.11 | 0.56 | 6.04 | 0.54 | 5.41 | 0.57 | 5.55 | 0.63 |
| 7 T | 5.01 | 0.29 | 5.13 | 0.32 | 5.30 | 0.45 | 5.30 | 0.46 | |
| diff | 1.09 | 0.66 | 0.91 | 0.68 | 0.12 | 0.75 | 0.25 | 0.81 | |
| Residue | 3 T | 1.7E-5 | 2.0E-5 | 1.8E-5 | |||||
| 7 T | 0.6E-5 | 0.5E-5 | 0.5E-5 | ||||||
Table 2.
Results of three grey matter ROIs: Globus Pallidus (GP), Putamen (Put) and Head of the Caudate Nucleus (NC), shown as the average and SD over 10 subjects. The R1,WP used in approaches 3 and 4 was taken from the result of approach 2, as use of the global value for R1,WP is not suitable for these iron rich regions.
| B0 | ROI | Approach 2: Fixed R1,MP, FSMP(0) |
Approach 3: + fixed R1,WP |
Approach 4: + fixed FSWP(0) |
||||
|---|---|---|---|---|---|---|---|---|
| Av | SD | Av | SD | Av | SD | |||
| f | 3 | GP | 0.172 | 0.010 | 0.176 | 0.012 | 0.172 | 0.013 |
| Put | 0.124 | 0.009 | 0.125 | 0.010 | 0.122 | 0.010 | ||
| NC | 0.113 | 0.005 | 0.113 | 0.006 | 0.111 | 0.008 | ||
| 7 | GP | 0.181 | 0.013 | 0.185 | 0.015 | 0.182 | 0.015 | |
| Put | 0.122 | 0.014 | 0.126 | 0.017 | 0.124 | 0.016 | ||
| NC | 0.114 | 0.011 | 0.116 | 0.015 | 0.114 | 0.013 | ||
| kMW [s−1] | 3 | GP | 9.3 | 1.9 | 8.3 | 1.7 | 8.6 | 1.2 |
| Put | 10.2 | 1.3 | 9.8 | 1.3 | 9.9 | 1.2 | ||
| NC | 10.4 | 1.6 | 9.8 | 1.6 | 9.9 | 0.7 | ||
| 7 | GP | 7.1 | 1.3 | 6.3 | 1.3 | 6.4 | 1.2 | |
| Put | 8.9 | 1.3 | 7.6 | 1.5 | 7.7 | 1.6 | ||
| NC | 8.4 | 1.3 | 7.8 | 1.7 | 7.9 | 1.7 | ||
Table 3.
The average and SD over ten subjects of the R1,WP values for the 3 grey matter ROIs following from approach 2 as used in approaches 3 and 4.
| B0 | ROI | R1,WP [s−1] | |
|---|---|---|---|
| Av | SD | ||
| 3 | GP | 0.505 | 0.035 |
| Put | 0.466 | 0.018 | |
| NC | 0.449 | 0.016 | |
| 7 | GP | 0.448 | 0.032 |
| Put | 0.444 | 0.018 | |
| NC | 0.426 | 0.021 | |
The test-retest results (repeated scans at 3 T, n=5) indicated a 0.5% error for the ROI average f values in SCC, and a 5% error for the corresponding kMW values at 3 T. Voxel-by-voxel test-retest analysis showed the error in f and kMW in white matter to be around 0.015 and 1.1 s−1 respectively equivalent to 6% and 18% errors. The signal to noise ratio (SNR) in the (baseline) images was in the range of 200–400 at 7 T and 100–250 for 3 T. The image stability, based on the average variance in the SCC ROI over the repetitions within one scan series, was 2.9 (±0.4)% at 3 T and 1.7 (±0.4)% at 7 T. Omission of the IR data from approaches 1 and 2 strongly affected the fitting procedure, resulting in poor convergence and widely varying values for f and kMW. This was not the case for approaches 3 and 4, in which the fixing of additional parameters improved fitting stability, and which led to similar results as approaches 1 and 2 (Fig. 8 and Table 1). This indicates that IR data can be omitted from the analysis when fixing R1,WP and FSMP(0) to values common to all brain pixels.
The estimated precision (the variability due to measurement noise) of the various fitting results is shown in Table 4 for an input SNR (i.e. input to the fitting program) of 500. This estimate was derived from the stability of the SCC-ROI averaged signals over repetitions, in combination with the effects of averaging over time and dividing by the reference data. It was found that fitting errors simply scale (inversely) with SNR. The test-retest errors for f and kMW were consistent with the numbers shown in the Table 4. The simulation shows that in spite of the high input SNR, the precision of kMW is limited, and the results need to be interpreted with some care.
Table 4.
Estimated precision for f and kMW for an (ROI-) SNR of 500 (for both saturation and reference scans), based on fitting of model data with simulated noise; the model data was based on average values found for f and kMW in the SCC (Table 1). The relative precision is given in parenthesis.
| Approach 1,2: Fixed R1,MP, FSMP(0) |
Approach 3: + fixed R1,WP |
Approach 4: + fixed FSWP(0) |
||
|---|---|---|---|---|
| f | 3T | 0.0081 (3%) | 0.011 (4%) | 0.0084 (3%) |
| 7T | 0.0054 (2%) | 0.0064 (2.5%) | 0.0054 (2%) | |
| kMP [s−1] | 3T | 0.32 (6%) | 0.39 (7%) | 0.25(5%) |
| 7T | 0.22 (4%) | 0.26 (5%) | 0.17 (3%) |
DISCUSSION
The experiments described in this work indicate the feasibility to measure the macromolecular proton (MP) fraction in human brain in scan times as short as 5 minutes. The measurement approach is based in instantaneous MP saturation, and analyzing the transfer of this saturation to WP as function of delay after the MT pulse using a 2-pool model of magnetization exchange. The feasibility of this approach relies on the high (~90%) saturation efficiency of the composite MT pulse, as well as several simplifying assumptions, including the notion that R1 relaxivity in human brain white matter is linearly dependent on MP fraction.
Strongly saturating MP while minimally (~10%) saturating WP is difficult to achieve with conventional MT approaches that use continuous off-resonance RF irradiation. A major advantage of the strong MP saturation achieved here is an excellent sensitivity in extracting f, owing to the large MT effect. In addition, strongly saturating MP reduces sensitivity to B1 inhomogeneities as confirmed with simulations and experiments (Fig. 3). This property enabled reliable extraction of f, even at 7 T where B1 inhomogeneity is substantial. In contrast, poorly designed MT pulses may show reduced saturation in areas with low B1, which, if not accounted for, will cause f to be underestimated.
Another advantage of the type of MT pulse used here is its minimal affect on WP, by virtue of their long T2 WP, even in the presence of off-resonance effects due to B0 inhomogeneities. Sensitivity to the latter can be adjusted by changing the number of sub-pulses, while keeping pulse duration constant. The overall pulse energy (time integral of B1 amplitude squared) determines the T2 value below which MPs are fully saturated, while the combination of B1 amplitude and number of sub-pulses determines the T2 values above which WP magnetization is left untouched. Within the limits of the scanner hardware and allowable tissue heating, this gives sufficient flexibility to efficiently implement the pulsed MT approach at both 3 T and 7 T.
Quantifying f and kMW with the 2-pool model fitting approach required simplifying assumptions which, in this study, involved fixing two or more parameters to values common to all brain voxels and all subjects (n=11). These parameters included R1,MP, R1,WP and the saturation levels of MP and WP resulting for the direct effect of the MT pulse. Similar values for f and kMW were obtained for different sets of parameter combinations, and the similarity of the results between 3 T and 7 T supported the validity of this approach. Importantly, fixing 3 or more parameters (approaches 3 and 4, see METHODS) obviated the need for inclusion of IR data in the fitting process, reducing the measurement time to only 5 minutes. The use of four fixed parameters (approach 4) allows further scan time reduction as it removes the necessity of acquiring a reference scan without MT pulse.
The excellent precision of the MP-pool fraction estimate, as determined from simulations and the test-retest results, demonstrates the sensitivity of the proposed transient MT approach. This is further supported by the small SD over subjects of the difference of the measurements at 7 T and 3 T. The short, 5-minute scan time compares favorably to the more traditional steady-state qMT methods, which generally require longer scan times and may be difficult to perform at high field (7 T and above) due to the significant RF power deposition associated by conventional saturation approaches. In addition, in contrast to steady-state MT approaches, the transient MT approach proposed here is minimally sensitive to variations in R1,WP, therefore obviating the need for additional IR experiments. This is because of the assumed relationship between f and λ2, and the relative low saturation level of WP resulting from the pulsed saturation. Although resulting in a less direct measurement of f, the proposed method may be more practical than approaches based on direct detection of MP signal at very short TE (6,7,49,50), which suffer from limited sensitivity and are technically challenging.
The white matter MP fractions reported here (f~15–25%) are substantially larger than the range of 9–16% found previously with quantitative MT methods (18,19,22,23,38,51). This may in part relate to methodological differences, for example the efficiency of the MT pulses used to saturate MP between the different methods. Nevertheless, our values seem reasonable considering the water content of white matter, which has been reported to be around 70% (1–5). Since the proton density of the 30% non-water fraction is at least similar to that of water (e.g. proton density of myelin is 35% higher than that of water (7), while in proteins it may be somewhat lower than in water), one would expect that the MP fraction in white matter to not be far below 30%. This of course assumes that all of the saturated MPs participate in the MT process. As in white matter, the grey matter MP fractions found here are somewhat higher than estimates from qMT measurements: 8–9% for GP, 7% for Put, and 5.3–6.7 % for NC (18,19,52). On the other hand, the kWM are similar to literature values (1.4–2 s−1) (19). Compared to estimates based on the non-water content of tissue (23–26% for GP, 18–21% for Put and 17–19% for NC (1–5)), our MT-based estimates are somewhat low but not entirely unreasonable given the uncertainty in grey matter 1H content.
In addition to differences in MP saturation levels, a reason for the relatively high values of f reported here as compared to much of the MT literature may be differences in assumed values for R1,MP. Previous MT studies have generally assumed R1 to be similar between MP and WP (e.g. around 1 s−1 at 1.5 T), and it has been pointed out that this value may be too low and lead to under-estimation of f (34). In the current work much higher values of 3.8 s−1 and 2 s−1 were estimated for R1,MP at 3 T and 7 T respectively, based on fitting of the 2-pool model to the MT and IR data. Since much of the R1 relaxivity of WM is thought to originate from exchange with MP (12,13,15), such high values for R1,MP are not unexpected. A further indication that R1,MP may previously have been underestimated comes from the observation that fixing R1,MP to 1 s−1 during the fitting procedure indeed led to lower values of f: reductions of about 20% and 10% were estimated at 3 T and 7 T respectively (see supplementary material, Fig. S2). However, under this condition, the fits led to inconsistent values for k and f between the two field strengths.
The finding of R1,MP being substantially higher than R1,WP is consistent with earlier analysis of the relationship between apparent R1 and f based on of large collection of MRI brain data (13). Further support for a relatively high R1,MP comes from measurements on membrane lipids in model systems, which have found values ranging from 1.6 s−1 to 4.6 s−1 for R1,MP at fields ranging from 8 to 1.4 T (53–55). Nevertheless, it should be realized that R1,MP is difficult to measure directly and dependent on experimental conditions (e.g. temperature). Thus, the precise value of R1,MP remains uncertain and likely is not uniform across and within molecular species: for example it may vary substantially between different lipids.
In order to be able to estimate kMW and f without the use of IR data, we assumed R1,WP to be constant over the brain. This was motivated by the notion that much of T1 relaxation in brain tissue is mediated by MT between MP and WP. Nevertheless, there are other contributions to T1 relaxation, most notably those from paramagnetic substances such as iron. In fact, in regions richest in iron such as the basal ganglia (concentration up to 0.2 mg/g (56)), R1,WP may increase by as much as 0.15 s−1 at 7 T (13). This was also observed in the results of analysis approach 2, in which R1,WP was allowed to vary (Figure 6). Failure to take this into account will affect the values of kMW and f: simulations indicate that it will bias their estimates by about 15% (data not shown). Thus, accurate determination of kMW and f throughout all of the brain may require collection of additional data (e.g. T2*-weighted MRI (57)) from which the local concentration of iron can be inferred, and then used to adjust R1,WP. Alternatively, R1,WP can be estimated using joint analysis of IR and MT data, as was done here with analysis approach 2. The resulting R1,WP values were indeed higher than the global values, consistent with the effect of iron on R1 relaxivity (13).
The values for exchange rates kMW and kWM found here are somewhat lower than those reported in literature. For example, the value of ~2 s−1 for here for kWM found here is somewhat below the range of 2.5–3.9 from previous studies (19,51,58). Again (as with f), a potential source for this discrepancy is the value used for R1,MP which we estimated higher than assumed previously. It is also interesting to compare the exchange rates found here with those from fitting of transient MT data to a 4-pool model in a previous study (32). This study reported on cross-relaxation time constants TCR, a measure of exchange rates defined by TCR= kMW −1 + kWM −1. In SCC, TCR representing exchange between MP in myelin to WP outside the myelin sheath (i.e. the water visible in our study) was reported to be at least 1280 ms and limited by exchange between water compartments within and outside the myelin sheath (32). A similar calculation based on a 3-pool exchange model for rat optic nerve data yields a TCR of 890 ms (10). These values compare to a value of about 600 ms calculated from Table 1. Thus, while our exchange rates appear somewhat slower than reported in previous MT studies, they are higher than those suggested by cross-relaxation studies that take into account a myelin water compartment. Due to the short T2* of myelin water, our measurements were dominated by signal from axonal and interstitial water, and therefore could not account for the effect of inter-compartmental water exchange on kWM.
Although the fitting results suggest that both kMW and f can be robustly estimated with the proposed method, there are several factors that can affect the accuracy of the estimated values. We will discuss a few of them, realizing that our list may be incomplete.
First, it is possible that a sizeable fraction of MP is incompletely saturated, which would lead to a commensurate underestimation of f. If not properly taken into account, this can bias f and therefore also kMW. From experiments with varying MT pulse durations, in particular the comparison of the 6 ms saturation to the 11 ms pulse, it is apparent that the longer pulse is only 10% more effective, suggesting these pulses saturate the MP-pool nearly completely (if not, dependence on pulse duration should be stronger). This notion of nearly complete saturation with the 6 ms pulse is only valid for MP with T2 < 400 μs, i.e. those that are relatively immobile; protons on freely rotating end-groups of larger molecules are not included in the measured MP-fraction as they are not efficiently saturated by the MT pulse. Fortunately, such mobile protons form only a small fraction of the total MP pool, and will therefore not substantially affect the estimate of f.
Secondly, a small error is introduced due of the incomplete signal recovery associated with the finite TR in our experiments. The TR of 3 s for the MT experiment was substantially longer than the effective T1 in tissue, but not sufficiently long to ignore incomplete signal recovery: ideally this should be incorporated in the analysis model. Fortunately, because this issue affects both the MT and the reference data, simulations indicated that associated errors were small, i.e. lower than 2% for f and 7% for kWM and kMW,. Nevertheless, when using the proposed method with a shorter TR to increase time efficiency, it may become necessary to account for incomplete signal recovery effects in the model equations.
Third, the precision of the exchange rates kMW and kWM estimate depends on the available signal differences and therefore on the size of the MP-pool. If the MP-pool fraction f is low, the precisions of kMW and kWM will be low too. This effect and possibly partial volume effects with CSF explain the brighter pixels towards the edge of the brain slices shown in Fig. 8, especially for the (lower SNR) 3 T images.
Fourth, the two-pool model applied here is a gross simplification and may cause significant systematic errors in the estimates of f and the exchange rates. For example, accurately representing magnetization transfer through multiple myelin layers and between white matter water compartments (e.g. intra-axonal versus interstitial) may require a many-pool model, or the modeling of a diffusion process. This problem may be exacerbated when the actual MP and WP R1 strongly deviate from their assumed average values, or have a distribution that is not accurately represented by an average. The values of kMW and f also depend somewhat on the measurement approach, and the extent to which TE and TR affect the visibility of the different water pools. For example, about 15% of WP in WM may be situated between the myelin layers (10,11,59) and only marginally visible at the long TEs used in our experiments. This biases f, because of an underestimation of WP volume. Similarly, limited visibility of WP between the myelin layers biases kMW, as its determination, in our experiments, depends on mixing of the magnetization between the various WP pools.
Fifth, as indicated above, the assumption of single value of R1,WP and R1,MP for all brain tissues may not be valid, and lead to inaccuracies in kMW and f. For example, in disease, R1,WP and R1,MP could change due to iron accumulation or changes in tissue molecular structure respectively; also, it is possible that pathological conditions could render kMW too low to cause WP saturation levels sufficient for accurate quantification of f. It remains to be seen to what extent these issue arise in practice.
Our approach compares favorably with previous methods proposed for rapid measurement of f. An interesting comparison is with IR-based methods (29) which have the potential in providing improved sensitivity (compared to out MT approach) owing to a potentially 2-fold increase in initial saturation difference between MP and WP. However, this improvement may not be realized in practice due to the difficulty in inverting WP without substantial saturation of MP. In addition this MP saturation will be dependent on B1 power, rendering the quantification of f sensitive to B1 inhomogeneities. Alternatively, rapid measurement of f can be performed with a transient MT method based on a stimulated echo preparation (30): this approach however suffers from a 2-fold sensitivity reduction associated with stimulated echoes, and furthermore has substantial sensitivity to B1 inhomogeneities.
In the presented experiments, only few slices were acquired, the number of which was tied to the number of delay times. One way to extend slice coverage is to move the selected set of slices with successive repetitions of the MT pulse, while shortening the MT repetition time. When keeping the slice repetition time (i.e. time between successive excitations of the same slice) constant, this should only minimally affect the sensitivity of the experiment, as the WP saturation caused by the MT pulse is a small fraction of the total magnetization. Alternatively, or additionally one can perform simultaneous multi-slice acquisitions, or acquire data in 3D fashion, where the excitation is performed over the entire brain and phase encoding is performed over the through-plane direction (perpendicular to the plane encoded by EPI). This would allow large brain coverage in clinically feasible scan times, in particular when reducing the number of delay times to three (or even two) by fixing an increasing number of model parameters. The practical benefits of these approaches are currently being investigated in our laboratory.
CONCLUSION
We implemented a rapid, transient MT approach to measure the fraction of macromolecular protons f. Because of its insensitivity to B1 inhomogeneities, and its minimal RF power deposition, the approach can be readily applied at high field, where its sensitivity benefits substantially from the slower T1 relaxation of macromolecular protons. Values of f in white matter, obtained by fitting the MT data to a 2-pool model, and assuming a dominant contribution of MT to longitudinal relaxation, were found to be about 50% higher than previous estimates. This is partly attributed to discrepancies in the estimates of R1 of macromolecular protons, which was much higher here than reported previously.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NS 002990-15).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests
References
- 1.Fatouros PP, Marmarou A. Use of magnetic resonance imaging for in vivo measurements of water content in human brain: method and normal values. Journal of neurosurgery. 1999;90:109–115. doi: 10.3171/jns.1999.90.1.0109. [DOI] [PubMed] [Google Scholar]
- 2.Randall LO. Chemical topography of the brain. J Biol Chem. 1938;124:0481–0488. [Google Scholar]
- 3.Volz S, Noth U, Jurcoane A, Ziemann U, Hattingen E, Deichmann R. Quantitative proton density mapping: correcting the receiver sensitivity bias via pseudo proton densities. NeuroImage. 2012;63:540–552. doi: 10.1016/j.neuroimage.2012.06.076. [DOI] [PubMed] [Google Scholar]
- 4.Gelman N, Ewing JR, Gorell JM, Spickler EM, Solomon EG. Interregional variation of longitudinal relaxation rates in human brain at 3. 0 T: relation to estimated iron and water contents. Magn Reson Med. 2001;45:71–79. doi: 10.1002/1522-2594(200101)45:1<71::aid-mrm1011>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Abbas Z, Gras V, Mollenhoff K, Oros-Peusquens AM, Shah NJ. Quantitative water content mapping at clinically relevant field strengths: a comparative study at 1. 5 T and 3 T. Neuroimage. 2015;106:404–413. doi: 10.1016/j.neuroimage.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm MJ, Ong HH, Wehrli SL, Li C, Tsai PH, Hackney DB, Wehrli FW. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc Natl Acad Sci U S A. 2012;109:9605–9610. doi: 10.1073/pnas.1115107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horch RA, Gore JC, Does MD. Origins of the ultrashort-T2 1H NMR signals in myelinated nerve: a direct measure of myelin content? Magn Reson Med. 2011;66:24–31. doi: 10.1002/mrm.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res. 1965;6:537–544. [PubMed] [Google Scholar]
- 9.MacKay AL, Vavasour IM, Rauscher A, Kolind SH, Madler B, Moore GR, Traboulsee AL, Li DK, Laule C. MR relaxation in multiple sclerosis. Neuroimaging Clin N Am. 2009;19:1–26. doi: 10.1016/j.nic.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Dortch RD, Harkins KD, Juttukonda MR, Gore JC, Does MD. Characterizing inter-compartmental water exchange in myelinated tissue using relaxation exchange spectroscopy. Magn Reson Med. 2013;70:1450–1459. doi: 10.1002/mrm.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laule C, Vavasour IM, Kolind SH, Li DK, Traboulsee TL, Moore GR, MacKay AL. Magnetic resonance imaging of myelin. Neurotherapeutics. 2007;4:460–484. doi: 10.1016/j.nurt.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koenig SH, Brown RD, 3rd, Spiller M, Lundbom N. Relaxometry of brain: why white matter appears bright in MRI. Magn Reson Med. 1990;14:482–495. doi: 10.1002/mrm.1910140306. [DOI] [PubMed] [Google Scholar]
- 13.Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS., Jr Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med. 2007;57:308–318. doi: 10.1002/mrm.21122. [DOI] [PubMed] [Google Scholar]
- 14.Fatouros PP, Marmarou A, Kraft KA, Inao S, Schwarz FP. In vivo brain water determination by T1 measurements: effect of total water content, hydration fraction, and field strength. Magn Reson Med. 1991;17:402–413. doi: 10.1002/mrm.1910170212. [DOI] [PubMed] [Google Scholar]
- 15.Kamman RL, Go KG, Brouwer W, Berendsen HJ. Nuclear magnetic resonance relaxation in experimental brain edema: effects of water concentration, protein concentration, and temperature. Magn Reson Med. 1988;6:265–274. doi: 10.1002/mrm.1910060304. [DOI] [PubMed] [Google Scholar]
- 16.Wolff SD, Balaban RS. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10:135–144. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 17.Henkelman RM, Huang X, Xiang QS, Stanisz GJ, Swanson SD, Bronskill MJ. Quantitative interpretation of magnetization transfer. Magn Reson Med. 1993;29:759–766. doi: 10.1002/mrm.1910290607. [DOI] [PubMed] [Google Scholar]
- 18.Sled JG, Pike GB. Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magn Reson Med. 2001;46:923–931. doi: 10.1002/mrm.1278. [DOI] [PubMed] [Google Scholar]
- 19.Yarnykh VL, Yuan C. Cross-relaxation imaging reveals detailed anatomy of white matter fiber tracts in the human brain. Neuroimage. 2004;23:409–424. doi: 10.1016/j.neuroimage.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Dortch RD, Li K, Gochberg DF, Welch EB, Dula AN, Tamhane AA, Gore JC, Smith SA. Quantitative magnetization transfer imaging in human brain at 3 T via selective inversion recovery. Magn Reson Med. 2011;66:1346–1352. doi: 10.1002/mrm.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tozer DJ, Rees JH, Benton CE, Waldman AD, Jager HR, Tofts PS. Quantitative magnetisation transfer imaging in glioma: preliminary results. NMR in biomedicine. 2011;24:492–498. doi: 10.1002/nbm.1614. [DOI] [PubMed] [Google Scholar]
- 22.Yarnykh VL, Bowen JD, Samsonov A, Repovic P, Mayadev A, Qian P, Gangadharan B, Keogh BP, Maravilla KR, Jung Henson LK. Fast Whole-Brain Three-dimensional Macromolecular Proton Fraction Mapping in Multiple Sclerosis. Radiology. 2015;274:210–220. doi: 10.1148/radiol.14140528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies GR, Tozer DJ, Cercignani M, Ramani A, Dalton CM, Thompson AJ, Barker GJ, Tofts PS, Miller DH. Estimation of the macromolecular proton fraction and bound pool T2 in multiple sclerosis. Multiple sclerosis. 2004;10:607–613. doi: 10.1191/1352458504ms1105oa. [DOI] [PubMed] [Google Scholar]
- 24.Underhill HR, Rostomily RC, Mikheev AM, Yuan C, Yarnykh VL. Fast bound pool fraction imaging of the in vivo rat brain: association with myelin content and validation in the C6 glioma model. Neuroimage. 2011;54:2052–2065. doi: 10.1016/j.neuroimage.2010.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobol WT, Cameron IG, Inch WR, Pintar MM. Modeling of proton spin relaxation in muscle tissue using nuclear magnetic resonance spin grouping and exchange analysis. Biophysical journal. 1986;50:181–191. doi: 10.1016/S0006-3495(86)83450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsen S, Hoffman RA. Study of Moderately Rapid Chemical Exchange Reactions by Means of Nuclear Magnetic Double Resonance. J Chem Phys. 1963;39:2892–2901. [Google Scholar]
- 27.Morris GA, Freemont AJ. Direct observation of the magnetization exchange dynamics responsible for magnetization transfer contrast in human cartilage in vitro. Magn Reson Med. 1992;28:97–104. doi: 10.1002/mrm.1910280110. [DOI] [PubMed] [Google Scholar]
- 28.Goldman M, Shen L. Spin-Spin Relaxation in Laf3. Phys Rev. 1966;144:321–331. [Google Scholar]
- 29.Gochberg DF, Kennan RP, Gore JC. Quantitative studies of magnetization transfer by selective excitation and T1 recovery. Magn Reson Med. 1997;38:224–231. doi: 10.1002/mrm.1910380210. [DOI] [PubMed] [Google Scholar]
- 30.Soellinger M, Langkammer C, Seifert-Held T, Fazekas F, Ropele S. Fast bound pool fraction mapping using stimulated echoes. Magn Reson Med. 2011;66:717–724. doi: 10.1002/mrm.22846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gochberg DF, Gore JC. Quantitative imaging of magnetization transfer using an inversion recovery sequence. Magn Reson Med. 2003;49:501–505. doi: 10.1002/mrm.10386. [DOI] [PubMed] [Google Scholar]
- 32.Kalantari S, Laule C, Bjarnason TA, Vavasour IM, MacKay AL. Insight into in vivo magnetization exchange in human white matter regions. Magn Reson Med. 2011;66:1142–1151. doi: 10.1002/mrm.22873. [DOI] [PubMed] [Google Scholar]
- 33.Hu BS, Conolly SM, Wright GA, Nishimura DG, Macovski A. Pulsed saturation transfer contrast. Magn Reson Med. 1992;26:231–240. doi: 10.1002/mrm.1910260205. [DOI] [PubMed] [Google Scholar]
- 34.Helms G, Hagberg GE. In vivo quantification of the bound pool T1 in human white matter using the binary spin-bath model of progressive magnetization transfer saturation. Physics in medicine and biology. 2009;54:N529–540. doi: 10.1088/0031-9155/54/23/N01. [DOI] [PubMed] [Google Scholar]
- 35.Forster J, Schick F, Pfeffer M, Lutz O. Magnetization-Transfer by Simple Sequences of Rectangular Pulses. Magn Reson Mater Phy. 1995;3:83–93. doi: 10.1007/BF01709851. [DOI] [PubMed] [Google Scholar]
- 36.Pike GB, Glover GH, Hu BS, Enzmann DR. Pulsed magnetization transfer spin-echo MR imaging. Journal of magnetic resonance imaging : JMRI. 1993;3:531–539. doi: 10.1002/jmri.1880030316. [DOI] [PubMed] [Google Scholar]
- 37.Ramani A, Aliev AE, Barker GJ, Tofts PS. Another approach to protons with constricted mobility in white matter: pilot studies using wideline and high-resolution NMR spectroscopy. Magn Reson Imaging. 2003;21:1039–1043. doi: 10.1016/s0730-725x(03)00207-8. [DOI] [PubMed] [Google Scholar]
- 38.Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 39.Morrison C, Stanisz G, Henkelman RM. Modeling magnetization transfer for biological-like systems using a semi-solid pool with a super-Lorentzian lineshape and dipolar reservoir. Journal of magnetic resonance Series B. 1995;108:103–113. doi: 10.1006/jmrb.1995.1111. [DOI] [PubMed] [Google Scholar]
- 40.Malyarenko DI, Zimmermann EM, Adler J, Swanson SD. Magnetization Transfer in Lamellar Liquid Crystals. Magnet Reson Med. 2014;72:1427–1434. doi: 10.1002/mrm.25034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lattanzio PJ, Marshall KW, Damyanovich AZ, Peemoeller H. Macromolecule and water magnetization exchange modeling in articular cartilage. Magnet Reson Med. 2000;44:840–851. doi: 10.1002/1522-2594(200012)44:6<840::aid-mrm4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 42.Pampel A, Muller DK, Anwander A, Marschner H, Moller HE. Orientation dependence of magnetization transfer parameters in human white matter. Neuroimage. 2015;114:136–146. doi: 10.1016/j.neuroimage.2015.03.068. [DOI] [PubMed] [Google Scholar]
- 43.Ordidge RJ, Gibbs P, Chapman B, Stehling MK, Mansfield P. High-speed multislice T1 mapping using inversion-recovery echo-planar imaging. Magn Reson Med. 1990;16:238–245. doi: 10.1002/mrm.1910160205. [DOI] [PubMed] [Google Scholar]
- 44.Tannus A, Garwood M. Adiabatic pulses. NMR in biomedicine. 1997;10:423–434. doi: 10.1002/(sici)1099-1492(199712)10:8<423::aid-nbm488>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Edzes HT, Samulski ET. Cross relaxation and spin diffusion in the proton NMR or hydrated collagen. Nature. 1977;265:521–523. doi: 10.1038/265521a0. [DOI] [PubMed] [Google Scholar]
- 46.Helms G, Hagberg GE. Quantification of magnetization transfer by sampling the transient signal using MT-prepared single-shot EPI. Concept Magn Reson A. 2003;19A:149–152. [Google Scholar]
- 47.Kalantari S, Laule C, Bjarnason TA, Vavasour IM, MacKay AL. Insight into in vivo magnetization exchange in human white matter regions. Magnetic resonance in medicine. 2011;66:1142–1151. doi: 10.1002/mrm.22873. [DOI] [PubMed] [Google Scholar]
- 48.van den Bos A. Application of statistical parameter estimation methods to physicsl measurements. J Physics E. 1977;10:753–760. [Google Scholar]
- 49.Waldman A, Rees JH, Brock CS, Robson MD, Gatehouse PD, Bydder GM. MRI of the brain with ultra-short echo-time pulse sequences. Neuroradiology. 2003;45:887–892. doi: 10.1007/s00234-003-1076-z. [DOI] [PubMed] [Google Scholar]
- 50.Du J, Ma G, Li S, Carl M, Szeverenyi NM, VandenBerg S, Corey-Bloom J, Bydder GM. Ultrashort echo time (UTE) magnetic resonance imaging of the short T2 components in white matter of the brain using a clinical 3T scanner. Neuroimage. 2014;87:32–41. doi: 10.1016/j.neuroimage.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sled JG, Levesque I, Santos AC, Francis SJ, Narayanan S, Brass SD, Arnold DL, Pike GB. Regional variations in normal brain shown by quantitative magnetization transfer imaging. Magn Reson Med. 2004;51:299–303. doi: 10.1002/mrm.10701. [DOI] [PubMed] [Google Scholar]
- 52.Underhill HR, Yuan C, Yarnykh VL. Direct quantitative comparison between crossrelaxation imaging and diffusion tensor imaging of the human brain at 3. 0 T. Neuroimage. 2009;47:1568–1578. doi: 10.1016/j.neuroimage.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 53.Deese AJ, Dratz EA, Hymel L, Fleischer S. Proton Nmr T1, T2, and T1-Rho Relaxation Studies of Native and Reconstituted Sarcoplasmic-Reticulum and Phospholipid-Vesicles. Biophysical journal. 1982;37:207–216. doi: 10.1016/S0006-3495(82)84670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan SI, Feigenson GW, Seiter CH. Nuclear relaxation studies of lecithin bilayers. Nature. 1971;231:110–112. doi: 10.1038/231110a0. [DOI] [PubMed] [Google Scholar]
- 55.Feigenson GW, Chan SI. Nuclear magnetic relaxation behavior of lecithin multilayers. Journal of the American Chemical Society. 1974;96:1312–1319. doi: 10.1021/ja00812a009. [DOI] [PubMed] [Google Scholar]
- 56.Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- 57.Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Samsonov A, Alexander AL, Mossahebi P, Wu YC, Duncan ID, Field AS. Quantitative MR imaging of two-pool magnetization transfer model parameters in myelin mutant shaking pup. Neuroimage. 2012;62:1390–1398. doi: 10.1016/j.neuroimage.2012.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sati P, van Gelderen P, Silva AC, Reich DS, Merkle H, de Zwart JA, Duyn JH. Micro-compartment specific T2* relaxation in the brain. NeuroImage. 2013;77:268–278. doi: 10.1016/j.neuroimage.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.