Abstract
Introduction
Efficacious chemotherapy does not exist for treatment or prevention of prostate, liver, and pancreatic carcinomas, and some other cancers that exhibit decreased zinc in malignancy. Zinc treatment offers a potential solution; but its support has been deterred by adverse bias.
Areas covered
1. The clinical and experimental evidence for the common ZIP transporter/Zn down regulation in these cancers. 2. The evidence for a zinc approach to prevent and/or treat these carcinomas. 3. The issues that introduce bias against support for the zinc approach.
Expert opinion
ZIP/Zn downregulation is a clinically established common event in prostate, hepatocellular and pancreatic cancers. 2. Compelling evidence supports the plausibility that a zinc treatment regimen will prevent development of malignancy and termination of progressing malignancy in these cancers; and likely other carcinomas that exhibit decreased zinc. 3. Scientifically-unfounded issues that oppose this ZIP/Zn relationship have introduced bias against support for research and funding of a zinc treatment approach. 4. The clinically-established and supporting experimental evidence provide the scientific credibility that should dictate the support for research and funding of a zinc approach for the treatment and possible prevention of these cancers. 5. This is in the best interest of the medical community and the public-at-large.
Keywords: ZIP transporters, decreased zinc carcinomas, prostate cancer, hepatocellular carcinoma, zinc treatment
1. Introduction
Cancer remains the second leading cause of death in the United States, with ~590,000 deaths and ~1.7 million new cases expected in 2016 [1]. This includes ~350,000 deaths and ~500,000 new cases of carcinomas that exhibit the common characteristic of decreased zinc in malignancy as represented in prostate cancer (PrCa), hepatocellular carcinoma (HCC), and pancreatic adenocarcinoma (PanCa). In most of these cancers, there exists no effective chemotherapy that will prevent the development of early malignancy and/or terminate progressing advanced-stage malignancy; in order to decease the incidence, morbidity, and mortality. This requires a better and more appropriate understanding of the important and required factors and events involved in the oncogenic process leading to premalignancy and its progression to malignancy. The decreased zinc relationship described below represents a required event in the development and progression of these carcinomas, and thereby presents a viable target for their prevention and treatment.
The interest and reported studies of the status and implications of zinc in cancer dates back nearly 100 years ago. Over this period, differences in the relationships of zinc in various cancers and the effects of zinc treatment on malignant cells have been reported. The results and conclusions of many, if not most, studies have been compromised by inappropriate and unphysiological experimental conditions and by accompanying confounding conditions. As such, a clearly established clinical status of zinc and its implications in the development and progression of cancers had not been apparent and established.
However, over time, the progressing and expanding clinical and experimental evidence had begun to identify the factors associated with the implications of zinc in the development of the individual cancers, which we have described in several recent reviews [2–7]. The evolving clinical and experimental evidence has now established the scientifically credible relationship that ‘decreased zinc and downregulation of ZIP-family zinc transporter (i.e. ZIP/Zn transformation) is a required common event in the early development and progression of malignancy in PrCa, HCC, and PanCa’. There exists no comparable scientifically credible evidence that contradicts or negates this ZIP/Zn relationship in these cancers. This leads to the relationship that ‘the decreased ZIP/Zn transformation is required to prevent the uptake and accumulation of the higher zinc levels that exists in the normal cells, which would be cytotoxic in the malignant cells’. This provides the basis for the concept that ‘a zinc treatment regimen that will facilitate the uptake and accumulation of zinc in the malignant cells will inhibit the development of malignancy and will terminate progressing malignancy in these cancers’. It is then plausible to propose that ‘the ZIP/Zn relationship will likely exist in other carcinomas (such as lung cancer, thyroid cancer, gall bladder cancer; and possibly breast cancer) that exhibit decreased zinc’.
This review will focus on the background and the clinical and experimental evidence that supports the above relationships and concepts. Equally important, we describe the validity of the issues that have impeded the support of necessary research and deterred progress in the development of a zinc treatment approach for these cancers. Since we have published several reviews of the relationships of zinc in normal and malignant cells and cancers, the reader can avail himself/herself of that information for an appropriate understanding of the implications of the zinc relationships. In addition, the extensive clinical and experimental data, which are available in published reports, will not be provided herein. Instead, we will present some representative data along with the references; so that the reader can readily access the additional supporting data and description.
The absence of the essential and correct understanding of the zinc relationships has resulted in unfounded and misguided criticisms and objections regarding the status and implications of zinc in cancer. We welcome clinically and scientifically credible discourse in response to the compelling evidence that will be presented in support of the ZIP/Zn relationship in carcinomas.
2. Important cellular and zinc relationships in mammalian cells
Before describing the clinical status of zinc in specific carcinomas, some fundamental cellular and zinc relationships are briefly presented.
2.1. Status of zinc in mammalian cells
Zinc is essential for the survival, growth, proliferation, metabolism, and functional activities of all cells. In each cell type, the status of zinc is maintained at the optimal range of the total cellular concentration and its intracellular distribution. Significant deviation (increase or decrease) from its optimal zinc status will result in cellular/metabolic dysfunction, and pathophysiological consequences that can result in death. The cells possess mechanisms that maintain their required zinc status in their in situ environment. Such mechanisms include plasma membrane-localized zinc transporters that are involved in the uptake of zinc from the extracellular environment (i.e. mainly ZIP-family transporters; Slc39A), intracellular zinc transporters that distribute zinc among the cytosol and the cellular organelles (i.e. mainly Znt-family transporters; Slc30A), and by the binding of zinc to zinc ligands.
Zinc exists in cells (and in extracellular fluids) in two forms: (1) tightly bound zinc (mainly to proteins) as the ‘immobile, non-exchangeable unreactive pool’, which comprises more than 90% of the total cellular zinc; and (2) loosely to moderately bound zinc as the ‘mobile, exchangeable, reactive pool’, which comprises the remaining less than 10% of the total cellular zinc. This pool of zinc includes low molecular weight Zn ligands such as Zn amino acids (aspartate, cysteine, histidine, etc.), Zn citrate, Zn metallothionein. These Zn ligands exhibit binding affinities (i.e. formation constants) of log Kf ~ 10 and lower. Zn Ligands with log Kf ~ 12 and greater are tightly bound, unreactive zinc compounds. Notably, the concentration of ‘free Zn2+ ions’ is negligible; with estimates being in the fM–nM range, whereas the total cellular concentration ranges from ~100 to 800 µM. These zinc relationships are described in our reviews, along with others [8–10].
2.2. Zinc cytotoxicity and malignant cells
We first identified in 1998 [11] that treatment of malignant prostate cells with physiological concentrations of zinc results in inhibition of proliferation, which results from its inhibition of the cell cycle, and from its induction of apoptosis. Since then numerous reports have confirmed and extended the cytotoxic effects of zinc on prostate and other malignant cells; and includes the inhibition of malignant activities such as cell migration and invasion (for reviews [3,4,12]). The reports also provide evidence that the concentrations that are cytotoxic in malignant cells are not cytotoxic in the corresponding normal cells; i.e. the malignant cells are susceptible to cytotoxicity at the cellular concentrations that exist in and are required by the normal cells.
Consequently, the malignant cells evolved with mechanisms that prevent the uptake and accumulation of the higher zinc levels of the normal cells, which would be cytotoxic in the malignant cells; and at the cellular concentration that is required for the proliferation, growth, and support of their malignant activities. One such mechanism is the downregulation of the functional zinc uptake transporter (ZIP transporter) during the transformation of the normal cell to the malignant cell (described later).
It is also important to note that some reports have described that zinc treatment promotes malignant cell proliferation and inhibits apoptosis. Many and possibly most of those reports employed experimentally zinc-depleted cells to determine the effects of zinc treatment. This especially applies to the widely employed use of cell permeable N,N,N′,N′-tetrakis(2-pyridylmethyl) ethane-1,2-diamine (TPEN) to decrease the cellular concentration of zinc. The assumptions of the levels of cellular zinc following treatment with TPEN are not appropriate or correct. TPEN has an extremely strong zinc binding affinity of log Kf ~ 15. Even at low concentrations, TPEN strongly binds the mobile reactive pool of zinc and also the immobile zinc proteins and zinc enzymes. The TPEN treatment results in zinc-depleted dying cells, a condition that does not exist in cells in their tissue environment. The effect of zinc treatment results in restoring the cellular zinc to a level that prevents the cell death imposed by the TPEN and other conditions that result in zinc-depleted dying cells. We discuss this issue in Franklin et al. [12].
2.3. The interdependence of cell metabolism and cell activity; and its application in oncogenesis
The activity of a cell and its metabolism is guided by the following relationships that we describe as ‘axioms’, that is, universal principles that are applicable to all cells [4,13]. (1) The existing intermediary metabolism of a cell provides the bioenergetic/synthetic/catabolic requirements that are essential for the manifestation of the cell’s current activity (e.g. function, growth, proliferation, and differentiation). (2) When the activity of a cell changes, its metabolism must also be altered to provide the bioenergetic/synthetic/catabolic requirements for the cell’s changing activity.
These principles must also be applied to the transformation of a normal cell to a malignant cell. Therefore, we have extended these principles as the following additional axioms associated with oncogenesis. (3) Malignant cells are derived from normal cells, which have undergone oncogenic transformation to neoplastic cells that have malignant potential. (4) Manifestation of the malignant potential of the neoplastic cell requires cellular metabolic alterations to provide the bioenergetic, synthetic, and catabolic requirements of malignancy. (5) In the absence of the metabolic transformation, the neoplastic cell will not progress to a malignant cell with its manifestation of malignancy. Conversely, the metabolic transformation, in the absence of the oncogenic transformation of the normal cell to a neoplastic cell, will not cause malignancy.
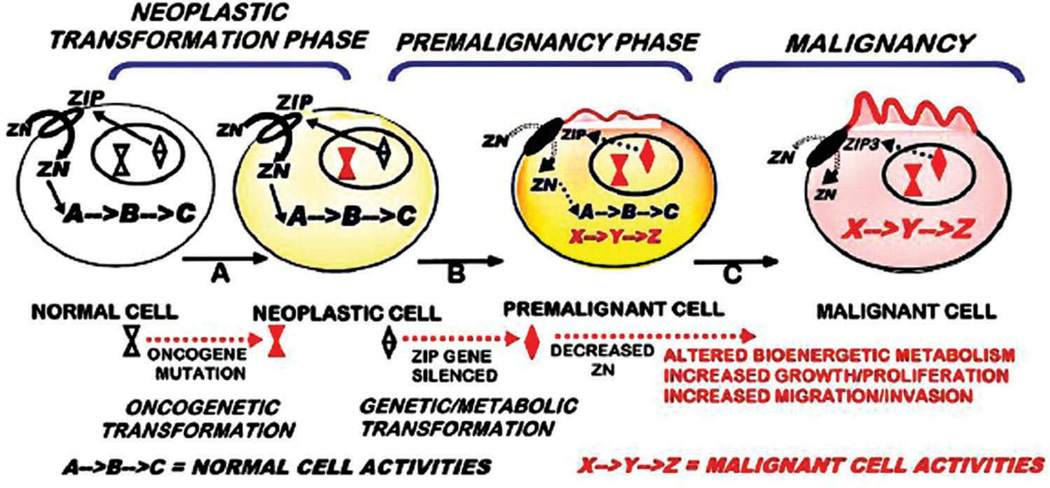
These relationships should be represented in the understanding of the oncogenic process resulting in the transformation of the normal cell to the malignant cell (Figure 1). As such, our concept focuses on three important phases during the oncogenic development of malignancy: (1) the ‘neoplastic transformation phase’, in which ‘oncogene’ initiation of oncogenesis transforms the normal cell to the neoplastic cell that has malignant potential; (2) the ‘premalignant phase’, during which the neoplastic cell is transformed to the premalignant cell which involves the genetic/metabolic transformations required for manifestation of malignancy; (3) the ‘malignancy phase’ during which the premalignant cell progresses to the malignant cell with capability of malignancy The important difference from most other representations of oncogenesis is the inclusion of the neoplastic cell resulting from the initiating oncogenic transformation of the normal cell, followed by its transformation to malignancy via premalignancy. We do not view that a malignant cell is the direct result of the oncogenic transformation of the normal cell, and that the ‘neoplastic cell’ and ‘malignant cell’ are synonymous. Instead, the downstream ‘genetic/metabolic’ transformations are required to transform the neoplastic cell to the malignant cell in accord with the axioms 1 and 2. Figure 1 presents these relationships in the context of the oncogenic development of carcinomas in which ZIP-family zinc uptake transporter zinc is downregulated and zinc is decreased to prevent the accumulation of cytotoxic levels of zinc in the malignant cells (described later).
Figure 1.
The concept of the genetic/metabolic transformation in the process of the oncogenic development of malignancy; and its application to the ZIP/Zn transformation in carcinomas.
3. The clinical status of zinc in carcinomas
Many reports exist regarding the zinc levels in cancer versus the corresponding normal/benign tissues. Of the cancers in which zinc levels have been reported, the zinc status is conclusively established for PrCa and HCC, both of which consistently exhibit marked decrease in zinc. In these carcinomas, decreased zinc in malignant versus normal epithelium was established by in situ zinc staining of human tissue sections; which confirmed the consistent reports of decreased zinc levels in cancer versus normal tissue preparations. It has also been recently established in PanCa by in situ zinc staining of human tissue sections. Collectively in these carcinomas, all or most of the cancer cases consistently exhibit marked decreased zinc and cases that exhibit an increased zinc in malignancy rarely, if ever, exist.
The status of zinc in other cancers is unsettled, but some evidence exists for decreased zinc in lung, thyroid, kidney, gall bladder, and ovarian cancers. The notable exception has been breast cancer in which several reports have consistently shown that the tissue zinc levels are increased in breast cancer versus normal breast. However, our in situ zinc staining of breast tissue sections identified a marked decrease in the invasive ductal malignant cells versus the normal epithelium (described below).
The above description is a reasonable representation and generalization of the studies of the status in cancers in which zinc levels have been reported. It becomes apparent that further studies are necessary to establish the clinical zinc status for many of the cancers. Considering the presence and differences of various tissue and cell types that comprise the tissue preparations employed for zinc level analyses, the only reliable and definitive approach is the in situ cellular determination of zinc levels in tissue sections; which is achieved by zinc staining methodology. This methodology visualizes, identifies, and establishes the comparative zinc levels specifically in the normal cells, premalignant cells, and malignant cells, as well as other components of the tissue section.
The evolving clinical evidence leads to a common relationship that ‘a decreased zinc in malignancy is common in many carcinomas.’ This will be further developed in the following sections, to the extent that it should be an expected relationship that is required to prevent the accumulation of increased zinc levels that are cytotoxic in the malignant cells.
3.1. The decreased zinc status in prostate cancer
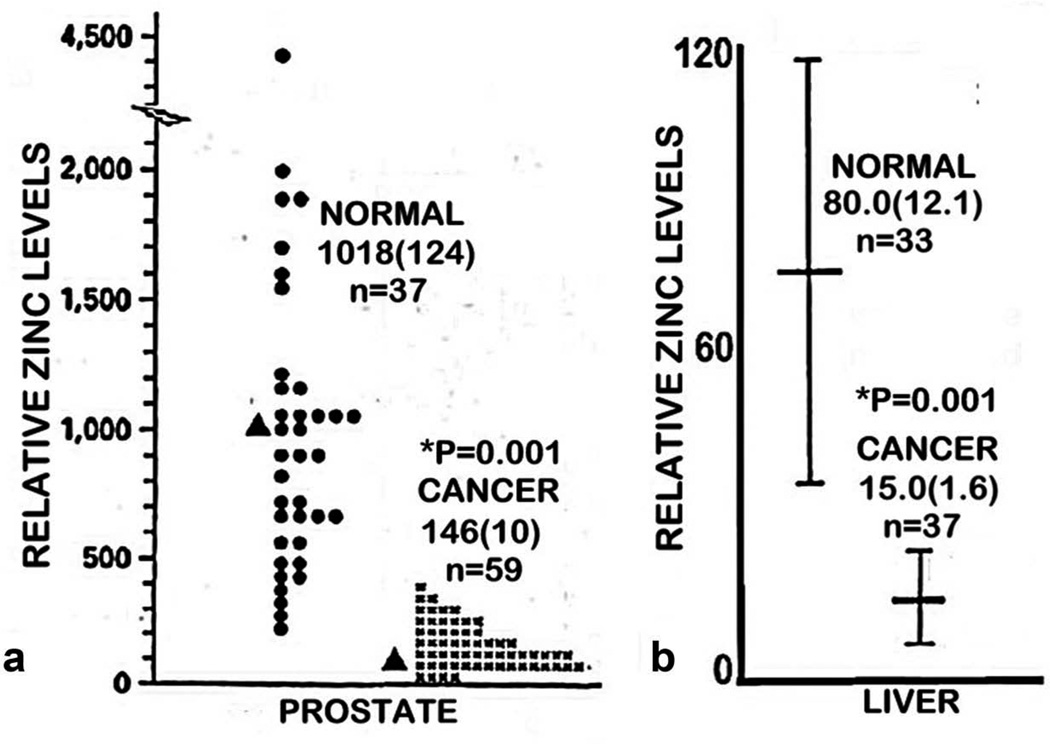
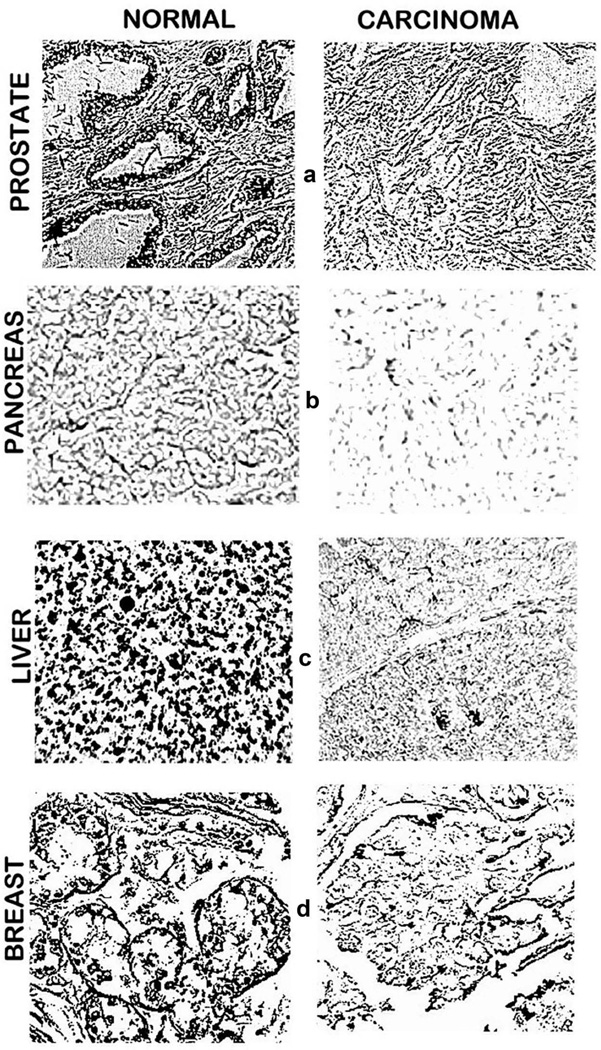
The most studied and established zinc status is for prostate cancer. Since the initial report in 1954 [14], more than 17 reports collectively involving several hundred prostate cancer cases have consistently shown that the concentration of zinc is markedly decreased in the cancer versus normal tissues [2]. The consistency of this relationship is revealed by the mean decrease of ~65% with a mean standard error of <10% for the composite of the reported studies, which involved different populations, various stages of cancer, differing tissue preparations, and other variables. This consistency is further revealed in the study [15] shown in Figure 2, in which a mean decrease of ~90% characterized the cancer cases, and no case exhibited the mean zinc level that characterizes normal prostate. Most importantly, in situ zinc staining of prostate tissue sections have identified that zinc is markedly decreased in the malignant cells as compared to the higher zinc levels in the normal acinar epithelium (Figure 3(a)). The decreased zinc is evident in the premalignant prostate intraepithelial neoplastic lesions and in early and progressing malignancy [2]. In contrast, there has been no confirmed or corroborated prostate cancer case in which the malignant prostate cells retained the higher zinc levels that typify the normal epithelium. Consequently, decreased zinc is a ‘hallmark’ condition that characterizes all or most PrCa cases.
Figure 2.
(a) The comparative tissue zinc levels in the peripheral zone of human prostate cancer cases versus normal prostate. Data taken from [15]. (b) The comparative tissue zinc levels in liver cancer cases versus normal liver. Data taken from [16]. Values are mean(SEM).
Figure 3.
In situ zinc dithizone staining (black) of normal and cancer tissue sections. (a). Prostate tissue sections show normal acini epithelium with high zinc versus decreased zinc in malignancy. (b). Pancreatic tissue sections show dithizone higher zinc in ductal and acinar epithelium versus decreased zinc in malignancy [17]. (c). Liver tissue sections show normal hepatocytes with high zinc versus decreased zinc in hepatoma cells. Reproduced from [18]. (d). Breast tissue sections show high zinc in normal ductal epithelium versus decreased zinc in malignancy. Reproduced from [19].
3.2. The decreased zinc status in hepatocellular carcinoma
The second most studied and established zinc status is for HCC, which we have described and reviewed in Costello and Franklin [5]. Beginning with Danielsen and Steinnis in 1970 [20], 10 reported population studies have consistently demonstrated a marked decrease (~55–75%) in zinc levels in HCC tissue compared with normal liver tissue. The composite of those studies reveal that virtually all HCC cases exhibited deceased tissue zinc levels. This is well represented in Figure 2 based on the report of [16]. Our studies [5,18] with in situ zinc staining of liver tissues identified and established that the hepatoma cells exhibited marked decreased zinc compared to the normal hepatocytes (Figure 3(c)). Moreover, the marked decrease in zinc is evident in well-differentiated malignancy, and persists in advancing malignancy.
3.3. The decreased zinc status in pancreatic ductal adenocarcinoma (PanCa)
Our publications in 2011 and 2012 [17,22] are the only reports that provide determinations of zinc levels in the human pancreatic exocrine tissue; that is, the ductal and acinar components. As represented in Figure 3(b), in situ zinc staining shows the marked decrease in zinc in malignancy as compared to the normal ductal and acinar epithelium. Our study involved human tissue sections from ~50 PanCa and ~15 normal cases, and showed the marked and consistent decreased zinc in well-differentiated grade1 and advancing malignancy. The premalignant pancreatic intraepithelial neoplasia (PanIN) epithelium also exhibits low zinc. No PanCa case exhibited increased zinc; and the consistency of the results with the ~50 PanCa cases indicates that decreased zinc might be a signature characteristic for all or most PanCa cases, along with PrCa and HCC.
Li et al. in 2009 [23] proposed the clinically unfounded relationship that increased zinc is associated with the development of PanCa, although they provided no determinations of zinc levels in human pancreatic tissue that would confirm such a conclusion. Instead, they employed experimental studies with malignant cell lines and animal studies, which is an inappropriate substitute for zinc determination in human pancreatic tissue to establish the clinical status of zinc in PanCa (discussed later).
3.4. The evidence for decreased zinc in breast invasive ductal adenocarcinoma (BrCa)
The most notable exception to the decreased tissue zinc levels in carcinomas had been the consistent reports of increased tissue zinc levels in BrCa (described in Costello et al. [19]). While various methodology approaches were employed in those studies, none included in situ zinc staining to establish the cellular zinc levels in the breast malignant cells compared to the normal ductal epithelium. This prompted us to apply the in situ zinc staining of breast tissue sections [19]. As shown in Figure 3(d), the zinc levels are markedly decreased in the malignant cells compared to the higher zinc levels in the normal ductal epithelial cells. The decrease in zinc exists in early malignancy and persists in progressing malignancy. Of the 25 BrCa cases and 12 normal cases included in this study, none of the BrCa cases exhibited an increased zinc level compared to the normal ductal epithelium.
The conundrum is why the reported studies of breast cancer tissue consistently resulted in increased levels of zinc. A possible explanation was revealed by the in situ zinc staining of the breast tissue sections, which showed the impact of the relative zinc levels of the different histological tissue/cellular components that exist in breast; more-so than in prostate, liver, pancreas, and other organs. Consistent with this are the reports that have identified increases and decreases in more than twenty elements along with the changes in zinc in the breast tissue samples. It is highly unlikely that any cell type would survive and/or be functionally capable with combinations of such element changes. Such considerations dictate that additional clinical studies, preferably employing in situ zinc staining, are required in order to corroborate and establish the consistency of the zinc status in BrCa cases.
3.5. Summary of the zinc status in carcinomas
It is well established by the in situ zinc determination of malignant versus normal epithelial cells in human tissue sections and consistent with zinc measurements in tissue preparations, that decreased zinc in malignancy is the clinical status for PrCa and HCC. In addition, the determination of malignant versus normal epithelial cells in situ in tissue sections has established that decreased zinc in malignancy is the clinical status for PanCa.
Compelling evidence from the determination of malignant versus normal epithelial cells in situ in tissue sections has revealed that decreased zinc in malignancy is the likely clinical status for breast invasive ductal adenocarcinoma. Additional studies are required to establish the clinical zinc status specifically in the malignant cells versus normal epithelium.
There have been no reported studies that employed the determination of malignant versus normal epithelial cells in situ in tissue sections in other cancers. Reports of measurements of zinc in tissue preparations have trended toward showing that zinc is decreased in those carcinomas that have been studied. However, any conclusions of the zinc status in malignant versus normal cells should be established by in situ determination of zinc levels in malignant versus normal cells in human tissue sections.
4. The clinical and functional status of ZIP family zinc uptake transporters in carcinomas
There are many reports that purport to identify the in situ functional ZIP transporter in various cells, especially in normal versus corresponding malignant cells. Most often, ZIP gene expression microarray analysis is employed to establish relative differences between normal and malignant tissues. However, the level of gene expression does not represent the transporter protein abundance or any functional relationship of the transporter. The common employment of Western blot analysis provides the level of transporter abundance; but does not establish the functionality of the ZIP transporter. In situ tissue analysis with ZIP immunohistochemistry is required to establish the abundance and localization of the transporter at the plasma membrane, which is required for the transporter function of zinc uptake from the extracellular fluid. In addition, for the uptake of zinc from the interstitial fluid derived from plasma, the transporter must exhibit localization at the basilar or basolateral membrane. In addition, the in situ changes in the plasma membrane-localized transporter should correlate with the concurrent in situ changes in the cellular zinc levels, which requires that in situ zinc levels must also be determined specifically in the normal versus malignant cells.
The above should be followed by kinetic studies of zinc uptake by the ZIP transporter, which requires in vitro experiments with appropriate cell lines or isolated primary cell preparations. Then the cell should be modified to increase and decrease the ZIP expression and abundance to establish its functional capability, and to establish that zinc uptake is dependent upon the specific ZIP transporter. When all of these conditions are achieved, the functional and clinical relevance of the ZIP transporter will be established. Aside from our PrCa, HCC, and PanCa studies, we are not aware of any reports in which the functional ZIP/Zn relationship has been established as described above.
Unfortunately, in the absence of identifying the status and function of the ZIP transporter and zinc levels in situ in human tissue sections, some reports have employed malignant cell lines and experimental animal models to determine the status of ZIP and zinc, which they then translated into the in situ clinical status that exists in human cancer. This is inappropriate; and more-so since malignant cell lines often exhibit constitutive ZIP transporter status that is not representative of the in vivo malignant cell status. As a result, such reports have misrepresented the normal and clinical status that exists in vivo in the human tissue.
4.1. ZIP1 downregulation in prostate cancer
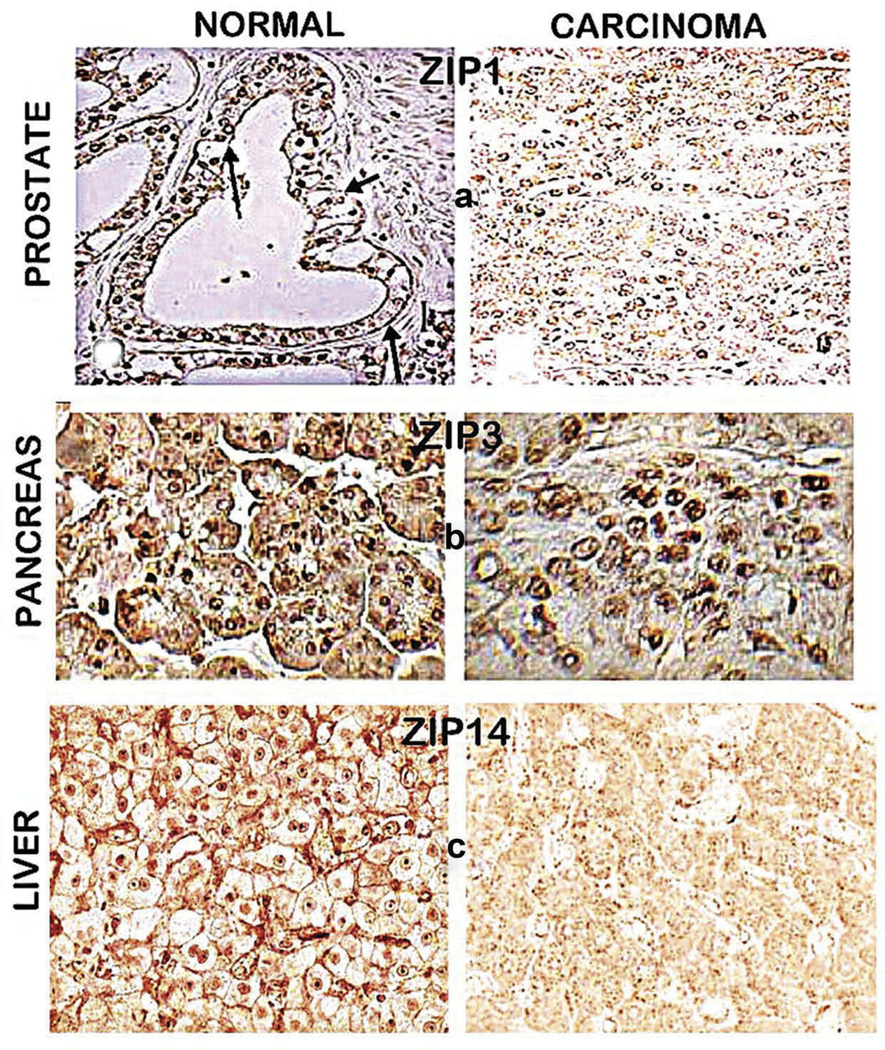
In accordance with the criteria described earlier, we identified the in situ status of ZIP1in the normal peripheral zone acinar epithelium and its downregulation in malignancy [21]. Figure 4(a) clearly shows the localization of ZIP1 at the basolateral and apical plasma membrane of the normal acinar epithelium; whereas detectable ZIP1 transporter is absent in the malignant cells. The loss of ZIP1 transporter is evident in well-differentiated and progressing malignancy and in PIN. ZIP1 in situ RT-PCR shows the gene expression of ZIP1 in the normal acinar epithelium, and the downregulation of expression in malignancy. In all cases, the downregulation of ZIP1 in the malignant cells is accompanied by the decrease in zinc. The downregulation of ZIP1 and the decrease in zinc in prostate cancer has been confirmed by others [24]. Although reports exist that suggest the involvement of other ZIP or ZnT transporters in the decrease of zinc in prostate cancer, none has provided the functionality and in situ identification that are required to establish such relationships.
Figure 4.
ZIP transporter immunohistochemistry of normal versus cancer tissue sections. (a) Normal prostate acinar epithelium shows ZIP1 transporter localized at the basolateral and apical membrane; and absence of detectable membrane-localized transporter in malignant cells. Reproduced from [21]. (b) Normal pancreas shows ductal and acinar epithelium with ZIP3 localized at the basilar membrane; and the malignant cells with the absence of detectable plasma membrane localized ZIP3. Reproduced from [17]. (c) Normal liver shows hepatocytes with plasma membrane localized ZIP14; and hepatoma cells with absence of detectable plasma membrane ZIP14. Reproduced from [18].
4.2. ZIP14 downregulation in hepatocellular carcinoma
Lichten et al. [25] had provided evidence that ZIP14 is the hepatocyte functional zinc uptake transporter in rat studies. We subsequently established that ZIP14 is the functional zinc uptake transporter in human hepatocytes [5,18]. Figure 4(c) shows the prominent plasma membrane localization of ZIP14 in the normal hepatocytes; and in HCC, the absence of detectable plasma membrane ZIP14 in the hepatoma cells. In addition, in situ RT-PCR demonstrated the downregulation of ZIP14 expression in the hepatoma cells. The decrease in ZIP14 transporter is evident in early malignancy and is sustained in progressing malignancy, concurrently with the decrease in zinc.
4.3. ZIP3 downregulation in pancreatic ductal adenocarcinoma
When we identified the decrease in zinc in PanCa, it became important to identify the functional ZIP-family transporter in the normal ductal epithelium that would be downregulated in the malignant cells. Our reported studies in 2011 and 2012 [17,22] provided the clinical and experimental evidence that ZIP3 is the functional zinc uptake transporter in the pancreatic normal epithelial cells. As shown in Figure 4(b), ZIP3 transporter is localized at the basolateral membrane of the normal ductal and acinar epithelium; whereas the plasma membrane localized ZIP3 does not exist in the malignant cells. The absence of ZIP3 is evident in early and progressing malignancy, and is also in PanIN epithelium. In situ RT-PCR revealed ZIP3 gene expression in the normal ductal/acinar epithelium; and its downregulation in malignancy. The loss of ZIP3 transporter in PanCa occurs concurrently with the decrease in zinc.
In contrast, Li et al. [23] purports to provide evidence that the upregulation of ZIP4 is the important zinc uptake transporter in PanCa. Since they do not provide any zinc measurements in human pancreatic tissues, no supporting clinical evidence exists to support increased ZIP4/Zn as the major zinc transporter in PanCa. Consequently, their conclusion is questionable; and more-so in recognition of our reports that clinically established ZIP3/Zn downregulation in early and progressing malignancy and in PanIN.
4.4. The status of ZIP transporters in other carcinomas
Aside from the studies described above, we are not aware of any other reports that have employed the criteria described earlier to reasonably establish the functional zinc uptake transporters associated with zinc changes in human cancers.
4.5. Summary of the ZIP status in carcinomas
It is well established that the respective functional plasma membrane localized ZIP transporters (ZIP1, ZIP14, ZIP3) are similarly downregulated in PrCa, HCC, and PanCa. The downregulation is evident in grade1 well-differentiated malignancy and persists in progressing malignancy; and is evident in premalignant lesions (PIN and PanIN). The ZIP transporter downregulation occurs concurrently with the decrease in zinc. Therefore, in these carcinomas, the ZIP/Zn downregulation is an essential event in the early development of malignancy and in the progression of malignancy; which is required to prevent the uptake and accumulation of cytotoxic levels of zinc in the malignant cells. This provides the explanation for the clinical absence or rarity of cancer cases that exhibit the high zinc levels that exist in the normal cells.
It is now necessary to determine the functional ZIP transporter and its downregulation in other carcinomas that exhibit decreased zinc. This should be achieved by employing the conditions and criteria as described above.
5. The ZIP/Zn transformation and zinc toxicity in malignant cells
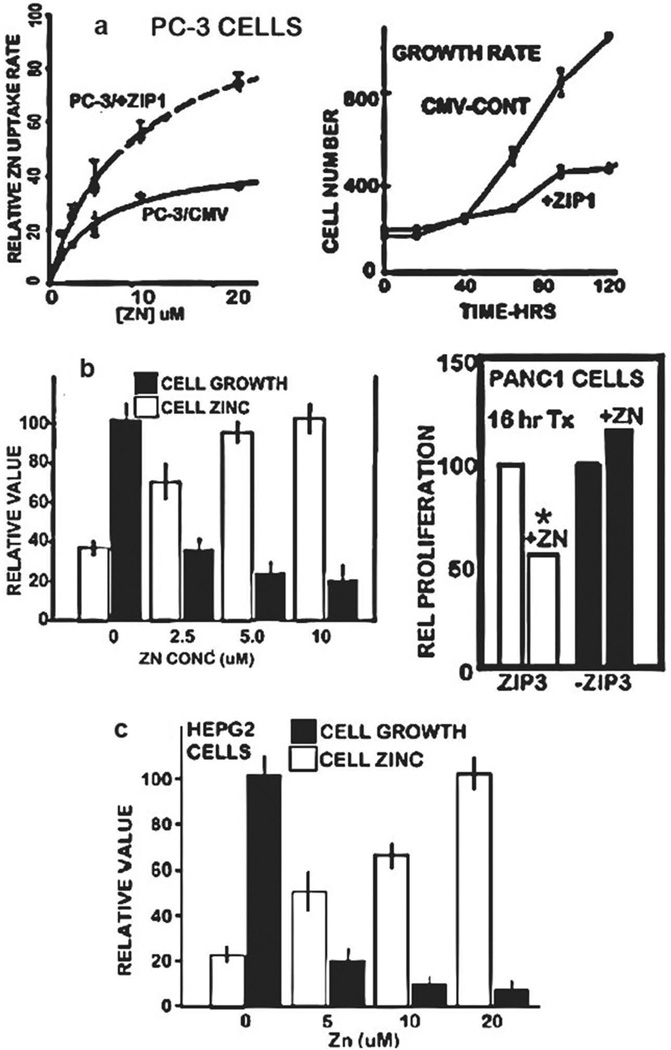
The ZIP/Zn transformation in malignancy is an important adaptive capability to prevent the cellular uptake and accumulation of cytotoxic levels of zinc in the malignant cells. Several reports have well established that the treatment of prostate, HCC, and PanCa malignant cells with physiological levels of zinc under conditions that result in zinc uptake and accumulation results in cytotoxic effects. Figure 5(a) shows that zinc uptake by PC-3 cells is dependent upon ZIP1 transporter, which increases zinc uptake and accumulation that inhibits cell proliferation [26]. Similarly, Figure 5(b) shows that physiological zinc treatment of Panc1 cells results in increased zinc and its inhibition of cell growth; and the dependency of zinc uptake on ZIP3 transporter [27]. Figure 5(c) shows that physiological zinc treatment of HepG2 cells results in zinc accumulation and its inhibition of cell growth [18]. These and other cytotoxic effects of zinc in malignant cells have been described in several reports (reviewed in [3,4]).
Figure 5.
ZIP transporter status and zinc uptake and cytotoxicity in malignant cells. (a) Effects of physiological zinc treatment of PC-3 cells with upregulated ZIP1 transporter show increased zinc uptake and inhibition of proliferation. Reproduced from [26]. (b) Effects of physiological zinc treatment of Panc1 cells showing increased accumulation of zinc and its inhibition of cell growth. Reproduced from [27]. Also shows the dependency on ZIP3 transporter. (c) Effects of physiological zinc treatment of HepG2 cells showing increased accumulation of zinc and its inhibition of cell growth. Reproduced from [18].
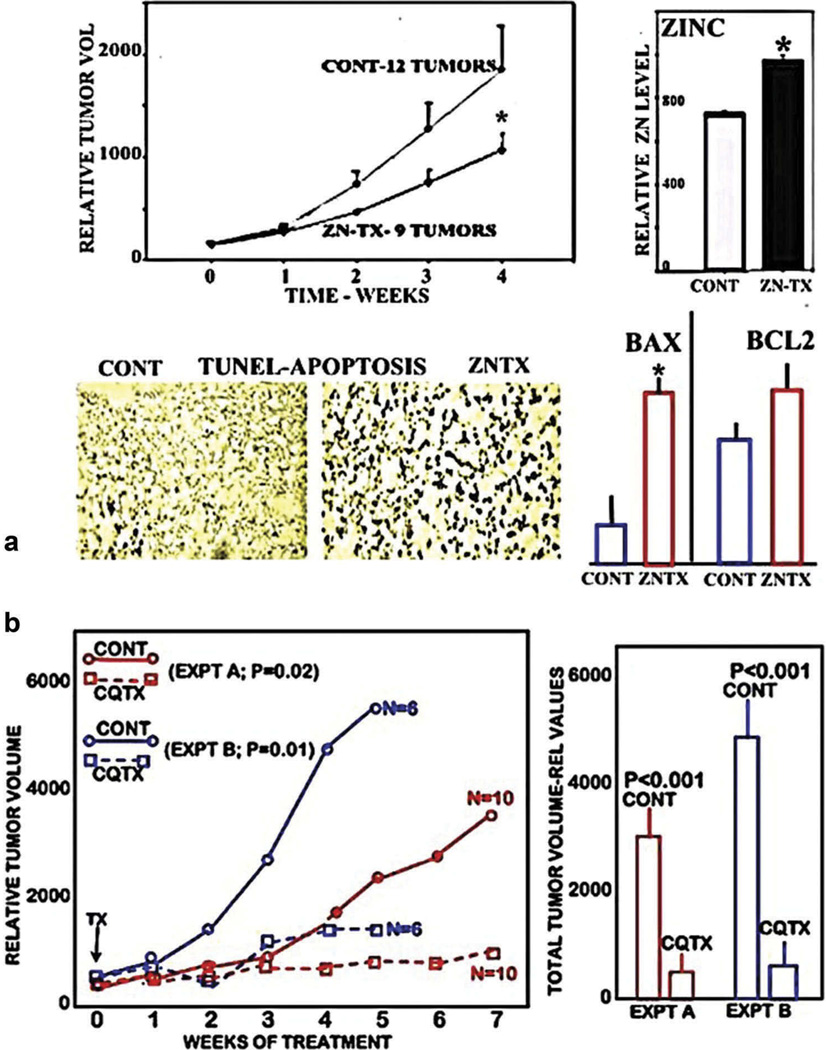
However, the complexity of the in vivo conditions that affect the bioavailability and process of circulating plasma zinc for cellular uptake make it imperative to establish that the in vitro cytotoxicity of zinc can be achieved when employed in animals and ultimately in humans. In 2003 [28], we showed that supplemental zinc treatment of mice with ectopic PC3/ZIP1 tumors resulted in increased tumor zinc and zinc-induced apoptosis; and significantly inhibited tumor growth (Figure 6(a)). Since then, other studies (such as [29–40]) have shown that zinc treatment regimens exhibit effective suppression of xenograft tumor growth representing several different cancers. Thus, conditions that will increase the uptake and accumulation of zinc in vivo in tumor cells will exhibit the cytotoxic effects of zinc.However, until recently, none of the animal studies employed malignant cell lines in which the ZIP transporter status had been identified to represent the clinical in vivo status of the malignant cells in the human malignancy. It is now recognized that prostate, HCC, and pancreatic malignant cell lines exhibit constitutive ZIP transporter although the ZIP-deficient status exists in situ in the human cancer. As such, some or all of the above xenograft tumors were likely to be ZIP-expressing tumors rather than ZIP-deficient tumors as exist in the human cancer. So, the issue becomes the delivery of zinc into the ZIP-deficient malignant cells, so as to induce the cytotoxic effects of zinc. One approach is to employ a zinc ionophore, which will facilitate the uptake of zinc in a mobile reactive form that will exhibit cytotoxic/tumor suppressor effects. Our recent studies [26,41] have shown that such an ionophore (clioquinol) was highly effective in suppressing ZIP1-deficient PC-3 tumor growth (~90% suppression) in mice (Figure 6(b)). Notably, Ding et al. [39] had also shown that clioquinol treatment of mice inhibited growth of ectopic human ovarian (A2780 cells) and B-cell lymphoma (Raji cells) tumors; and they established that this was a zinc ionophore effect; although the ZIP status was not established. Nevertheless, it supports the concept of zinc cytotoxicity for treatment and prevention of malignancy in cancers. In addition, in our study and in Ding et al.’s study, the animals treated with clioquinol did not exhibit any apparent adverse effects on their behavior (such as activity, water, and food consumption).
Figure 6.
The in vivo effects of zinc and clioquinol treatment of ectopic xenograft PC-3 tumor growth in mice. (a) The effects of zinc treatment on the growth of tumors developed from wildtype PC-3 cells that exhibit ZIP1 transporter (PC3/ZIP tumors) [28]. Shows the significant inhibition of tumor growth in zinc treated animals; along with increased tumor zinc concentration, increased apoptosis, and increased Bax and Bax/Bcl2 ratio. (b) The effects of clioquinol treatment on the growth of ZIP1-deficient tumors developed from PC-3 cells with ZIP1 knockdown (PC3/-ZIP1). Results of two experiments that show marked growth of ZIP1-deficient tumors in the untreated animals, and significant inhibition of ZIP1-deficent tumors in the clioquinol treated animals. Reproduced from [26,41].
6. The clinical application of the ZIP/Zn transformation for the prevention of development of malignancy and for the termination of established malignancy in these carcinomas
Collectively, the clinical and experimental evidence as described above provide a compelling basis for the potential zinc treatment approach for preventing the development and/or its early progression of malignancy in carcinomas that exhibit deceased zinc. The ZIP/Zn downregulation is evident in early Grade1 well-differentiated malignancy in prostate, HCC, and pancreatic carcinomas, which implies that this transformation occurs in premalignancy. This is confirmed by its identification in both PIN and PanIN lesions, and will likely apply to premalignancy in other carcinomas. Therefore, it is reasonable to expect that the successful zinc treatment of developing human tumors in animal models and in the zinc ionophore treatment of ZIP-deficient prostate tumor will also be applicable to ZIP-deficient premalignancy for prevention of the development of malignancy, as well as for the termination of established malignancy.
Based on the current evidence, this relationship will likely apply to pancreatic, prostate, and liver cancers, and collectively could prevent up to ~290,000 new cases and ~100,000 deaths/year [1]. This is especially relevant to PanCa, in which ~50,000 new cases and ~40,000 deaths/year occurs in the United States. PanCa has the highest mortality rate of all major cancers with ~94% of PanCa patients dying within five years and ~74% dying within the first year of diagnosis. Similarly, ~28,000 cases of HCC/year occur in the United States, of which ~75% results in death, generally within 1 year, and the incidence of HCC continues to increase in the United States and worldwide. Both PanCa and HCC have remained largely untreatable. PrCa accounts for ~220,000 new cases and ~30,000 deaths occur annually in the United States [1], and is the second leading cause of cancer deaths in males. The major problem is the absence of effective treatment of advanced stage and androgen-resistant malignancy and metastasis. Thus, the morbidity and mortality of PrCa continues to constitute a major health issue. Decades of research and unsuccessful development of an efficacious chemotherapy and/or prevention for these cancers makes it more imperative to pursue the zinc treatment approach, especially with clinical trials. Other carcinomas that exhibit decreased zinc could also benefit from the preventive and treatment potential of a zinc approach. It is important to determine if the ZIP/ZN relationship exists in any or all of those carcinomas.
7. The issues that have deterred progress in the development of a zinc regimen for the prevention and/or treatment of any cancers
For this discussion, we highlight the dominant consideration that a zinc approach could be efficacious for the treatment or prevention of terminal cancers for which no effective chemotherapy or chemoprevention presently exists. This is exemplified in HCC and PanCa and in advanced PrCa in which progressing morbidity and death is the outcome. With this perspective, the urgency for the development of efficacious agents for such cancers should be given the highest priority.
However, despite the overwhelming clinical and experimental evidence as presented above, the development of a zinc regimen for treatment or prevention of any cancer has not reached fruition. So, the question is ‘Why?’ Presumably, one would expect that important clinical and experimental evidence must exist, which have raised appropriate concerns of the validity and/or advisability of a zinc treatment for these cancers. The following section presents some of these issues and their credibility, which have deterred progress in the development of a zinc treatment approach for these cancers.
7.1. Epidemiology reports that purport to show that dietary/Supplemental zinc increases prostate cancer
This has been a major deterrent factor, which has been based on a 2003 epidemiology report by Leitzmann et al. [42]. The study purported to show that the consumption of high zinc levels is a contributing factor in promoting advanced prostate cancer. Because the report emanated from an NIH/Harvard collaborative study, it was heralded in the medical and public community as represented by HealthDay News (2 July 2003) reported, ‘Men who overdose on zinc supplements more than double their risk of prostate cancer, a government study finds’. The Washington Post (1 July 2003), ‘Study Links Zinc, Prostate Cancer-Men who take too much zinc may be raising their prostate cancer risk, U.S. researchers said yesterday’. The Mayo Clinic Health Letter (May, 2004) cites ‘Large doses of zinc may increase risk of prostate cancer’. This immediately became the dominant view among urologists, oncologists, and biomedical researchers; despite the fact that other epidemiology reports found that dietary/supplemental zinc protects against prostate cancer. As such, reviewers of our grant proposals have consistently commented that ‘epidemiology studies have identified zinc as a contributing factor in prostate cancer, which is contrary to the concept of a zinc treatment for prostate cancer’.
Because of this adverse impact, we published several reviews [43–45] of that report and other epidemiology reports, in which we provided the credible clinical, scientific and statistical evidence that the purported conclusion that ‘zinc promotes prostate cancer’ is unfounded. Moreover, the most recent epidemiology reports in 2009 [46] and 2011 [47] along with an earlier report [48] have shown that dietary and supplemental zinc protects against development and progression of advanced prostate cancer; which also negate the conclusions of Leitzmann et al. Despite the current epidemiology evidence and the overwhelming clinical and experimental evidence that zinc protects against the development and progression of PrCa, the unfounded implication of zinc promotion of prostate cancer remains a dominant view that continues to impede the support and funding for the development of a zinc treatment approach for prostate cancer.
The consequences of this serious deterrent is the best example of the admonitions of eminent epidemiologists, such as Dr. Trichopoulos, Dr. Walker, and Dr. MacMahon (in [43]): ‘Epidemiology studies will inevitably generate false positive and false negative results with disturbing frequency… when (people) do take us seriously, we may unintentionally do more harm than good’. ‘The first one or two papers about a suspected association spring into the general public consciousness in a way that does not happen in any other field of scientific endeavor. And once a possible link is in the public eye, it can be virtually impossible to discredit’. ‘There would be few drawbacks to publishing weak, uncertain associations if epidemiologists operated in a vacuum, but they do not. By the time the information reaches the public mind, the tentative suggestion is likely to be interpreted as a fact’. It is past the time for the clinical and biomedical community to recognize the correct prevailing status of epidemiology reports, and to abandon the selective adverse and unfounded conclusion of zinc promotion of prostate cancer.
7.2. Concerns regarding the potential toxic effects of zinc treatment in humans
The view of many, and probably most, clinical and biomedical investigators regarding zinc toxicity is not in accord with the physiological and pathophysiological relationships of zinc. It is a mistaken presumption and expectation that a treatment regimen based on increasing the cellular zinc levels that are cytotoxic to malignant cells would also be cytotoxic in normal cells; and thereby would have pathophysiological consequences. The eminent zinc biochemist/physiologist, Dr. Vallee, in the hallmark comprehensive reviews of the physiology and biochemistry of zinc [9] states ‘Clearly, a metal that is known to be essential to the inheritance of the genetic endowment and the induction of development, growth, and differentiation could not easily be intended to be deleterious to the perpetuation and evolution of the species. Instead, one would expect zinc to be regulated carefully to ensure the preservation and continuity of life. In fact, zinc is the only pre-, post-, and transitional element that has proven to be essentially nontoxic’. Consequently, zinc toxicity in humans rarely occurs, and is only evident under extreme conditions when accompanied by other exacerbating systemic pathophysiology. Notably, clioquinol has been employed in humans with minimal contraindications at effective treatment dosage [49,50]. There exists no confirmed substantial evidence in support of zinc toxicity being a committed outcome from zinc treatment. As described earlier, normal cells and malignant cells have homeostatic mechanisms that maintain their respective zinc status within the range that provides their optimal activities and protects against cytotoxic levels of zinc.
Even if potential systemic toxicity and contraindications were a concern, it should not deter the research and progress necessary for the development of an efficacious treatment for these cancers. More appropriately that issue should be addressed at the level of clinical trials; and with due considerations of the consequences of any adverse pathophysiological effects relative to the consequences of the outcome of no treatment for the tens of thousands of individuals each year that are afflicted with terminal carcinomas.
7.3. The requirement for establishing the molecular mechanisms associated with the cytotoxic/tumor suppressor actions of zinc
‘More data are necessary to support this idea’. We find such comment in every grant proposal that deals with the development of zinc treatment for these carcinomas. It has been well established that the cytotoxic effects of zinc include: the inhibition of cell proliferation by its inhibition of the cell cycle and its induction of apoptosis; the inhibition of cell migration; the inhibition of cell invasion; the alteration of cell metabolism; the inhibition of angiogenesis; and other effects. However, the molecular mechanisms for many of these actions remain poorly understood despite years of research. Nevertheless, the pragmatic clinical issue is the effectiveness of a zinc treatment approach for prevention and/or termination of malignancy in these deadly cancers. The prerequisite resolution of the molecular biology events is of no necessity relative to the urgency of the efficacy of the treatment. For the subject with the untreatable cancer, ‘Who cares what the molecular pathway is, if it works!’ Unfortunately, the molecular biology interest is a dominant influence that too often has been impeding the development of cancer treatment for the past two decades [51].
7.4. Zinc for the treatment of cancer is not an ‘innovative’ concept
‘The concept of zinc for the treatment of cancer is not new’. This is a common comment that appears in the recent reviews of our grant proposals since NIH and other granting agencies adopted ‘innovation’ as one of the major criteria for evaluation and scoring of research grant proposals. It also applies to the approach and experimental methods as is evident from reviewer comments such as ‘the approaches and techniques are not innovative’; and ‘the methodologies and experimental approaches that will be used for the investigations are state-of-the-art, but they cannot be considered especially innovative’. As will be described later, the recent inclusion of ‘innovation’ as one of the major criteria in the review of research grant proposal has been ill-advised and detrimental to the evaluation of the ‘best science’ that should be funded [52]. Of course, since we first offered this concept ~30 years ago, studies from our group and others have continually strengthened and supported the plausibility of the concept. To paraphrase the statement in the preceding section: for thousands of individuals with untreatable cancer, ‘Who cares if this zinc treatment is an old idea, if it works!’
8. Conclusions
In this review, we have presented compelling clinical and experimental evidence for the following conclusions.
8.1. ZIP/Zn downregulation is a clinically established common relationship in PrCa, HCC, and PanCa
The relationship that ZIP1 is downregulated and zinc is decreased in PrCa was established around 2008 from evolving consistent clinical and supporting experimental evidence that accumulated over an ~50-year period. Similarly, the ZIP14/Zn relationship in HCC was established in 2012 from accumulating clinical and experimental evidence over an ~ 50-year period. The ZIP3/Zn relationship in pancreatic ductal carcinoma has been established by our clinical and experimental reports over the period of 2009–2014. Notably, the reported studies of these three carcinomas collectively included several hundred or more cancer cases in which the corresponding ZIP/Zn relationship has been consistently identified. In contrast, there exists no corroborated report of cases that provide clinical evidence that is contradictory to this ZIP/Zn relationship. Until such significant and corroborated contradictory clinical evidence becomes available, the ZIP/Zn downregulation is a clinically established common relationship for prostate, liver, and pancreatic carcinomas. The issue now is to conduct the appropriate clinical and experimental studies to establish which of the other carcinomas exhibit this common ZIP/Zn relationship, perhaps all, or some, or none.
8.2. Compelling clinical and experimental evidence supports the concept of a zinc approach for prevention and/or treatment of these carcinomas
Experimental evidence has established that zinc accumulation in the malignant cells results in cytotoxicity and tumor suppression. An effective zinc treatment approach requires an agent or process (such as a zinc ionophore) that will facilitate the uptake and accumulation of zinc in the ZIP-deficient malignant/tumor cells.
8.3. Progress in zinc treatment for these cancers has been deterred by opposition and skepticism that lack credible clinical and experimental evidence
In the absence of direct compelling clinical and experimental evidence, critics have relied on tangential studies with extrapolated interpretations; on ill-informed and misrepresentation of zinc relationships; on subjective and unscientific criteria; and on results of inappropriate experimental studies and conclusions.
8.4. Support for clinical and experimental research is essential for progress leading to zinc for prevention and treatment of these untreatable ZIP/Zn-depleted cancers
The critical issue is the development of efficacious agents for treatment and prevention of these largely untreatable cancers. Decades of research have been largely unsuccessful in achieving this goal. Of all of the approaches and targets that have been employed as the basis for the development of effective anti-cancer agents and regimens, none is more rational than the clinical and experimental evidence for the zinc treatment approach. As such, the interest of the medical community and the public-at-large dictates that support for the development of a zinc approach for these cancers be given a high priority.
9. Expert opinion
9.1. The ZIP/Zn transformation is an established scientifically credible clinical relationship in PrCa, HCC, and PanCa
The consistent and extensive clinical and experimental evidence overwhelmingly demonstrates that decreased zinc and the downregulation of the functional ZIP-family zinc uptake transporter (i.e. ZIP/Zn transformation) is a common event in the development and progression of PrCa, HCC, and PanCa. When such evidence has been developed in accord with the principles of the scientific method, we refer to this as ‘scientifically credible’ evidence. Moreover, no comparable scientifically credible evidence exists that opposes or contradicts this concept. These criteria establish the ZIP/Zn relationship as the clinical status that exists in these carcinomas, until comparable scientific-credible evidence is presented that contradicts this currently established relationship.
9.2. The ZIP/Zn transformation is required for the development and progression of malignancy
In combination with Section 9.1, the scientifically credible evidence for zinc toxicity in the malignant cells provides the basis for the occurrence of the ZIP/Zn transformation in premalignancy and progressing malignancy. This explains the clinical observation that all or most of these cancer cases exhibit the ZIP/Zn status, and cases that retain the higher normal zinc status rarely, if ever, exist.
9.3. A zinc approach for prevention and/or treatment of these ZIP/Zn-deficient cancers is clinically and scientifically credible
Scientifically credible experimental evidence has demonstrated that zinc treatment of malignant cells and animals with experimental tumors results in cytotoxic/tumor suppressor effects. Treatment that employs an agent or process (such as a zinc ionophore) that facilitates zinc uptake and accumulation in ZIP-deficient malignancy has been shown to exhibit efficacious suppression of tumor growth.
9.4. The target for a zinc approach is the most rational compared to other targets that are being supported for research and development
Decades of researchsupport for the development of approaches based on the identification of potential targets for efficacious chemotherapy for these deadly cancers have been largely unsuccessful. There has been and continues to be major emphasis and research grant funding from NIH and other public and private agencies in search of an efficacious treatment or prevention of these cancers. Such funding has included targets such as hormone receptors, signaling pathways, microRNA, and immunotherapy. None of those targets exhibit the established clinical and experimental evidence that supports the highly likely successful target for an efficacious zinc treatment approach. This also includes the supporting evidence that a zinc treatment approach would be relatively innocuous with minimal contraindications. It is also advantageous since the ZIP/Zn transformation occurs during premalignancy and persists through advancing malignancy, so that the zinc treatment should be effective for prevention of malignancy as well as for termination of progressing malignancy. Despite these advantages, the bias against the zinc treatment approach has deterred its support in favor of other proposed targets.
9.5. Deterrence to progress in the development of a zinc approach for these cancers is not based on direct and scientifically credible evidence
The fact that an appropriate zinc approach has not been developed for the treatment and/or prevention of any cancer is the result of lack of support for the necessary research leading to clinical trials. This is especially apparent from the lack of progress in development of zinc treatment for PrCa, despite the overwhelming supporting clinical and experimental scientifically credible evidence. This should be the dominant criterion, above all other criteria, in assessing and determining the support and funding of research and clinical trials leading to a potential zinc treatment approach for prevention and treatment of specific cancers. Instead, the deterrent to the needed support and progress in the development of zinc treatment for cancer has been based on subjective views and impressions; on empirical information; on inadequate evaluation and assessment of existing information, such as published reports; on results and conclusions of inappropriate experimental designs; and other considerations; all of which have introduced and exacerbated the bias against zinc treatment for cancer, despite the fact that they are untenable substitutes for scientifically credible evidence.
9.6. Achievement of a zinc approach requires support for research and supporting information leading to clinical trials
We believe that the currently available clinical and experimental evidence should be sufficient to support the initiation of clinical trials for an ionophore approach (such as clioquinol) for PrCa. However, additional evidence with animal models that exhibit the ZIP1/Zn transformation during the development and progression to malignancy would provide strong supporting evidence. We identified [53] the ZIP1/Zn transformation occurs in TRAMP (transgenic adenocarcinoma of the mouse prostate) prostate cancer model, but we have not been successful in obtaining support for studies of zinc treatment in these animals. As such, the bias against zinc treatment studies will likely extend to other cancer animal models that exist or need to be developed.
9.7. The future
We will project the following views: (1) what we hope will happen; (2) what we expect will happen; and (3) why we have this expectation.
9.7.1. What we hope will happen
The progress in the development of a zinc approach for prevention and treatment of these cancers will require major change in the obvious bias that exists within the clinical and biomedical community. We hope that the considerations for the support and funding of the potential treatment approaches will be assessed on the scientific credibility of the clinical and experimental evidence relative to the advantages and disadvantages of the proposed targets and the treatment regimen. Under such conditions, future research should be supported for identifying the ZIP/Zn relationship in other cancers that exhibit decreased zinc; and ultimately leading to clinical trials to determine the potential efficacy for treatment and prevention of the untreatable conditions that exist in these cancers. This is in the best interest of the medical community and the general public.
9.7.2. What we expect will happen
Most likely, there will be little progress in the development of a zinc approach for these cancers. Notwithstanding continued evolving and expanding scientifically credible evidence, the bias that exists will continue to dominate the decisions against the support and funding for zinc treatment of cancers. This has been apparent for PrCa despite 50 years of accumulated overwhelmingly supporting scientifically credible clinical and experimental evidence of the potential for a zinc approach to prevent the development of malignancy and for the treatment of advanced malignancy and metastasis. This will apply to HCC and PanCa; and other cancers that might exhibit the ZIP/Zn relationship. So, the potential for efficacious zinc approaches for these cancers will likely ‘wither on the vine’; while support and funding will be dedicated for approaches and targets of less scientifically credible potential. This would not be the outcome if the support and funding is based on the best science; which had been the goal of NIH and other agencies ‘to fund the best science by the best scientists’ [54].
9.7.3. Why do we expect this outcome
This issue is addressed in Costello et al. [51], to which we refer the reader. A major factor is related to the focus on molecular biology/molecular genetics that has evolved over the past 30 years and now dominates the interests and decisions regarding the direction and funding of clinical and biomedical research. Along with this, the graduate and post-graduate training has focused on molecular biology/molecular genetics, to the detriment that such areas of cell physiology/cell bio-chemistry/cell metabolism and organ systems physiology/pathophysiology are seriously diminished or absent. The result is the training of highly specialized molecular biology/molecular genetics researchers; many with minimal understanding or interest in the appropriate translational application of the molecular events to the physiological/pathophysiological relationships and consequences. This has replaced the traditional and more relevant broad training and production of ‘scientists’ with a dominant holistic understanding of the cellular and organ system physiological/pathophysiologic relationships. This especially applies to the biochemical and physiological relationships of zinc and its pathophysiological implications; about which many, perhaps most, contemporary clinicians and investigators lack the knowledge and/or the interest. As such, a bias in favor of the dominant interest and knowledge in the molecular biology/molecular genetics issues is inherent in the contemporary clinical and biomedical research community. Until NIH leads, and other agencies follow, with a balanced emphasis between the molecular and cellular/organ systems interrelationships, the current basis for decisions of direction and funding will continue to the detriment of pursuing the ‘best science’, and this applies to the zinc approach for treatment and prevention of these currently untreatable and deadly cancers. This is not what the medical community should provide or what the public should expect nor should continue. The overwhelming scientifically credible evidence dictates that it is in the best interest of the medical community and the public-at-large to support the development of the zinc approach for the prevention and treatment of these currently untreatable carcinomas.
9.7.4. A challenge to the clinical and biomedical community and the general public
We expect that, following this presentation and the information herein, there will be detractors that continue to oppose the support and funding for the development of a zinc approach for prevention and treatment of these cancers. We welcome a debate that presents the basis for such opposition versus the scientifically credible evidence that supports the development of a zinc treatment approach for these cancers. Because this issue impacts the health and welfare of tens of thousands of current and future cancer patients, its importance dictates the advisability and necessity for a forum that is accessible to the medical community and the general public. We welcome any ideas and interest that will achieve this in the near future.
Article highlights.
Scientifically credible clinical and experimental evidence has established that PrCa, HCC, and PanCa are carcinomas that exhibit decreased zinc and downregulation of their respective functional ZIP-family zinc uptake transporter (i.e. ZIP/Zn transformation) during the development and progression of malignancy.
The ZIP/Zn transformation is essential during oncogenesis to prevent the accumulation of cytotoxic levels of zinc in the malignant cells.
This provides a most viable target for the development of an efficacious zinc approach for the prevention and treatment of these cancers; and for other cancers that exhibit the ZIP/Zn relationship.
Progress has been deterred by detracting issues that lack compelling scientific credibility; but have imposed bias within the clinical and biomedical community against the support and funding of the research and clinical trials that are required to achieve the development of a zinc treatment approach for these carcinomas.
The medical community and the public-at-large should expect that the assessment and decisions regarding the support and funding for the development of a zinc approach and/or other approaches for treatment of these cancers will be based on the scientific credibility of the clinical and experimental evidence, in accord with the funding of the ‘best science’. Under such criteria, the zinc approach would be the most, or among the most, viable efficacious treatment that warrants support and funding.
This box summarizes the key points contained in the article.
Acknowledgments
Funding
The studies of LC Costello and RB Franklin cited herein were supported by NIH grants CA79903, DK076783 and DK42839.
This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2. Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006 May 15;5:17. doi: 10.1186/1476-4598-5-17. •• This study brings together the implications of zinc and zinc transporters in prostate cancer, which will relate to other cancers.
- 3.Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Rev Anticancer Ther. 2012;12:121–128. doi: 10.1586/era.11.190. • This study expands on the issues described in this paper.
- 5.Costello LC, Franklin RB. The status of zinc in the development of hepatocellular cancer: an important, but neglected, clinically established relationship. Cancer Biol Ther. 2014;15:353–360. doi: 10.4161/cbt.27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costello LC, Franklin RB. A review of the current status and concept of the emerging implications of Zinc and Zinc transporters in the development of pancreatic cancer. Panc Disord Ther. 2013;(Suppl 4) doi: 10.4172/2165-7092.S4-002. pii: 002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gumulec J, Masarik M, Adam V, et al. Serum and tissue zinc in epithelial malignancies: a meta-analysis. PLoS One. 2014;9(6):e99790. doi: 10.1371/journal.pone.0099790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Costello LC, Fenselau CC, Franklin RB. Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J Inorg Biochem. 2011;105:589–599. doi: 10.1016/j.jinorgbio.2011.02.002. •• A major review of the implications and status of zinc in mammalian cells.
- 9. Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. •• Should be a read for all investigators involved in zinc relationships.
- 10.Maret W, Li Y. Coordination dynamics of zinc in proteins. Chem Rev. 2009;109:4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 11.Liang JY, Liu YY, Zou J, et al. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franklin RB, Milon B, Feng P, et al. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costello LC, Franklin RB. The genetic/metabolic transformation concept of carcinogenesis. Cancer Metastasis Rev. 2012;31:123–130. doi: 10.1007/s10555-011-9334-8. • Important understanding of metabolic implications in cancers.
- 14.Mawson CA, Fischer MI. The occurrence of zinc in the human prostate gland. Can J Med Sci. 1952;30:336–339. doi: 10.1139/cjms52-043. [DOI] [PubMed] [Google Scholar]
- 15.Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 16.Kew MC, Mallett RC. Hepatic zinc concentrations in primary cancer of the liver. Br J Cancer. 1974;29:80–83. doi: 10.1038/bjc.1974.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costello LC, Levy BA, Desouki MM, et al. Decreased zinc and down regulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol Ther. 2011;12:297–303. doi: 10.4161/cbt.12.4.16356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franklin RB, Levy BA, Zou J, et al. ZIP14 zinc transporter down-regulation and zinc depletion in the development and progression of hepatocellular cancer. J Gastrointest Cancer. 2012;43:249–257. doi: 10.1007/s12029-011-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costello LC, Zou J, Franklin RB. In situ clinical evidence that zinc levels are decreased in breast invasive ductal carcinoma. Cancer Causes Control. 2016;27:729–735. doi: 10.1007/s10552-016-0746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danielsen A, Steinnes E. A study of some selected trace elements in normal and cancerous tissue by neutron activation analysis. J Nucl Med. 1970;11:260–264. [PubMed] [Google Scholar]
- 21.Franklin RB, Feng P, Milon B, et al. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costello LC, Zou J, Desouki MM, et al. Evidence for changes in RREB-1, ZIP3, and zinc in the early development of pancreatic adenocarcinoma. J Gastrointest Cancer. 2012;43:570–578. doi: 10.1007/s12029-012-9378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Zhang Y, Liu Z, et al. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson LA, Kanak MA, Kajdacsy-Balla A, et al. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52:316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Lichten LA, Cousins RJ. Interleukin-1beta contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Annu Rev Nutr. 2009;29:153–176. doi: 10.1152/ajpgi.90676.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costello LC, Franklin RB, Zou J, et al. Evidence that human prostate cancer is a ZIP1-Deficient malignancy that could be effectively treated with a zinc Ionophore (Clioquinol) approach. Chemotherapy. 2015;4(2) doi: 10.4172/2167-7700.1000152. pii: 152. •• This study describes the conditions and results of clioquinol treatment.
- 27.Costello LC, Franklin RB. The cytotoxic role of RREB1, ZIP3 zinc transporter, and zinc in human pancreatic adenocarcinoma. Cancer Biol Ther. 2014;15:1431–1437. doi: 10.4161/cbt.29927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng P, Li TL, Guan ZX, et al. Effect of zinc on prostatic tumorigenicity in nude mice. Ann NY Acad Sci. 2003;1010:316–320. doi: 10.1196/annals.1299.056. [DOI] [PubMed] [Google Scholar]
- 29.Tailler M, Senovilla L, Lainey E, et al. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene. 2012;31:3536–3546. doi: 10.1038/onc.2011.521. [DOI] [PubMed] [Google Scholar]
- 30.Garufi A, Ubertini V, Mancini F, et al. The beneficial effect of Zinc(II) on low-dose chemotherapeutic sensitivity involves p53 activation in wild-type p53-carrying colorectal cancer cells. J Exp Clin Cancer Res. 2015;34:87. doi: 10.1186/s13046-015-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sztalmachova M, Gumulec J, Raudenska M, et al. Molecular response of 4T1-induced mouse mammary tumours and healthy tissues to zinc treatment. Int J Oncol. 2015;46:1810–1818. doi: 10.3892/ijo.2015.2883. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Xu P, Chen J, et al. Zinc phthalocyanine conjugated with the amino-terminal fragment of urokinase for tumor-targeting photodynamic therapy. Acta Biomater. 2014;10:4257–4268. doi: 10.1016/j.actbio.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 33.Liu YS, Li HS, Qi DF, et al. Zinc protoporphyrin IX enhances chemotherapeutic response of hepatoma cells to cisplatin. World J Gastroenterol. 2014;20:8572–8582. doi: 10.3748/wjg.v20.i26.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arriaga JM, Greco A, Mordoh J, et al. Metallothionein 1G and zinc sensitize human colorectal cancer cells to chemotherapy. Mol Cancer Ther. 2014;13:1369–1381. doi: 10.1158/1535-7163.MCT-13-0944. [DOI] [PubMed] [Google Scholar]
- 35.Ozawa H, Asahina T, Murakami H, et al. Zinc coproporphyrin I derived from meconium has an antitumor effect associated with singlet oxygen generation. Fetal Diagn Ther. 2013;33:90–97. doi: 10.1159/000342419. [DOI] [PubMed] [Google Scholar]
- 36.Tailler M, Senovilla L, Lainey E, et al. Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene. 2012;31:3536–3546. doi: 10.1038/onc.2011.521. [DOI] [PubMed] [Google Scholar]
- 37.Shah MR, Kriedt CL, Lents NH, et al. Direct intra-tumoral injection of zinc-acetate halts tumor growth in a xenograft model of prostate cancer. J Exp Clin Cancer Res. 2009;28:84. doi: 10.1186/1756-9966-28-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magda D, Lecane P, Wang Z, et al. Synthesis and anticancer properties of water-soluble zinc ionophores. Cancer Res. 2008;68:5318–5325. doi: 10.1158/0008-5472.CAN-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding WQ, Liu B, Vaught JL, et al. Anticancer activity of the antibiotic clioquinol. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- 40.Ding WQ, Lind SE. Metal ionophores - an emerging class of anticancer drugs. IUBMB Life. 2009;61:1013–1018. doi: 10.1002/iub.253. [DOI] [PubMed] [Google Scholar]
- 41.Franklin RB, Zou J, Zheng Y, et al. Zinc ionophore (clioquinol) inhibition of human zip1-deficient prostate tumor growth in the mouse ectopic xenograft model: a zinc approach for the Efficacious treatment of prostate cancer. Int J Cancer Clin Res. 2016;3(1) doi: 10.23937/2378-3419/3/1/1037. pii: 037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leitzmann MF, Stampfer MJ, Wu K, et al. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1004–1007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 43. Costello LC, Franklin RB, Tan MT. A critical assessment of epidemiology studies regarding dietary/supplemental Zinc and prostate cancer risk. Open Urol Nephrol J. 2008;1:26–35. doi: 10.2174/1874303X00801010026. •• An important review of the issues of epidemiology reports involving zinc and prostate cancer that applies to all such epidemiology studies.
- 44.Costello LC, Franklin RB, Feng P, et al. Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States) Cancer Causes Control. 2005;16:901–915. doi: 10.1007/s10552-005-2367-y. [DOI] [PubMed] [Google Scholar]
- 45.Costello LC, Feng P, Milon B, et al. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7:111–117. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez A, Peters U, Lampe JW, et al. Zinc intake from supplements and diet and prostate cancer. Nutrition Cancer. 2009;61:206–215. doi: 10.1080/01635580802419749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Epstein MM, Kasperzyk JL, Andrén O, et al. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr. 2011;93:586–593. doi: 10.3945/ajcn.110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristal AR, Stanford JL, Cohen JH, et al. Vitamin and mineral supplement use is associatedwith reduced risk of prostate cancer. Cancer Epidemiol Biomark Prev. 1999;8:887–892. [PubMed] [Google Scholar]
- 49.Bareggi SR, Cornelli U. Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci Ther. 2012;18:41–46. doi: 10.1111/j.1755-5949.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao X, Schimmer AD. The toxicology of Clioquinol. Toxicol Lett. 2008;182:1–6. doi: 10.1016/j.toxlet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Costello LC. The effect of contemporary education and training of biomedical scientists on present and future medical research. Acad Med. 2009;84:459–463. doi: 10.1097/ACM.0b013e31819a7c6b. [DOI] [PubMed] [Google Scholar]
- 52.Costello LC. ‘Innovation’; a misguided emphasis for clinical, biomedical, and life sciences education, research, and its funding. J Biosci. 2012;2:1–3. [Google Scholar]
- 53.Costello LC, Franklin RB, Zou J, et al. Human prostate cancer ZIP1/zinc/citrate genetic/metabolic relationship in the TRAMP prostate cancer animal model. Cancer Biol Ther. 2011;12:1078–1084. doi: 10.4161/cbt.12.12.18367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costello LC. Perspective: is NIH funding the "best science by the best scientists”? A critique of the NIH R01 research grant review policies. Acad Med. 2010;85:775–779. doi: 10.1097/ACM.0b013e3181d74256. [DOI] [PubMed] [Google Scholar]