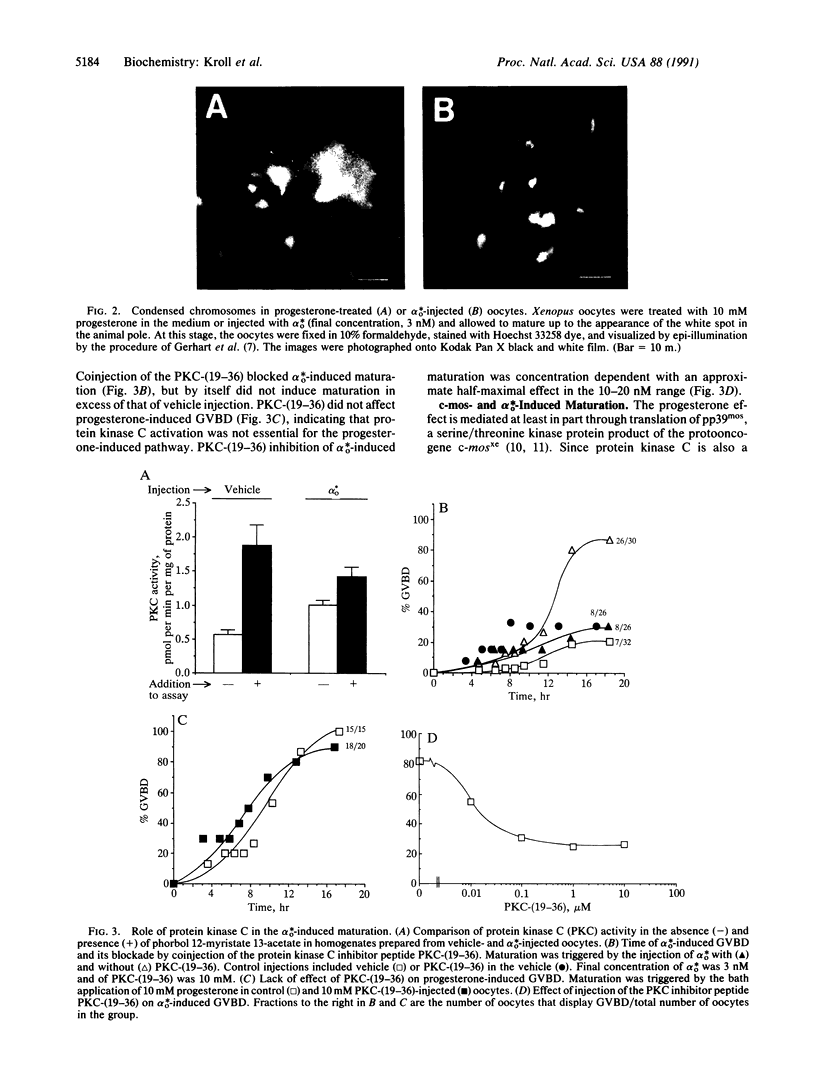
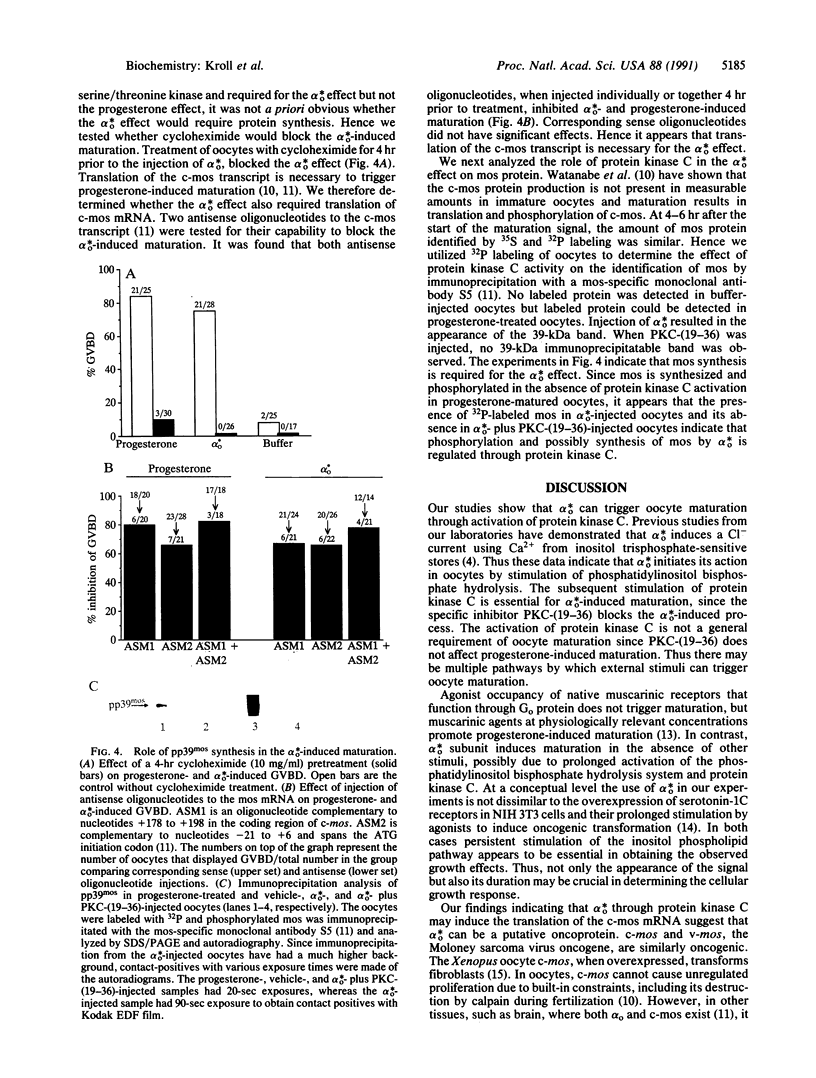
Abstract
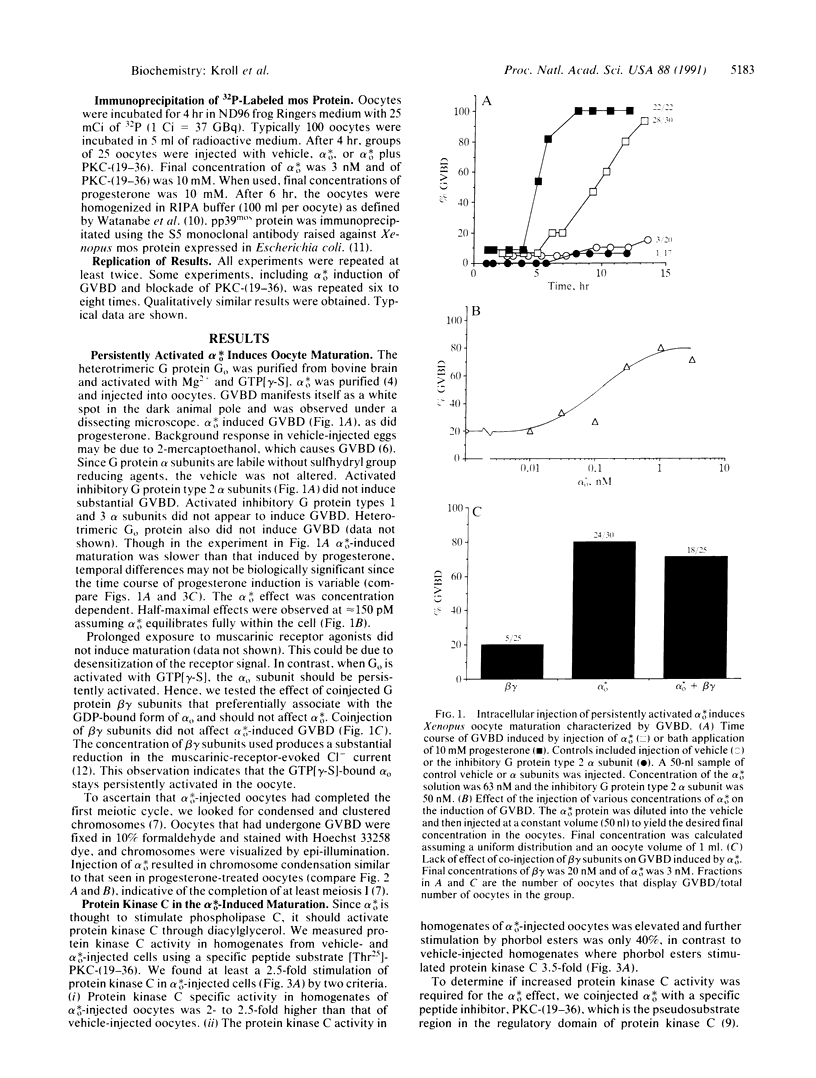
The capability of various activated guanine nucleotide binding regulatory protein (G protein) alpha subunits to induce meiotic maturation was studied. Activated Go protein alpha subunit (alpha o*) but not the three inhibitory G protein alpha subunits triggered meiotic maturation in Xenopus oocytes. The effect was concentration dependent with a half-maximal effect in the 100-200 pM range. Injection of alpha o* stimulated protein kinase C activity. Coinjection of the peptide containing residues 19-36 of protein kinase C [PKC-(19-36)], a specific protein kinase C inhibitor, blocked the alpha o*- but not progesterone-induced maturation. Cycloheximide and the injection of antisense oligonucleotides specific to the c-mos transcript blocked alpha o-induced maturation. Immunoprecipitation with a mos protein-specific monoclonal antibody showed that alpha o-injected oocytes had phosphorylated mos protein. When PKC-(19-36) was coinjected with alpha o*, phosphorylated mos protein was not observed. These observations indicate that alpha o*, through protein kinase C and the translation of c-mos, can trigger meiotic division of Xenopus oocytes. Our results raise the possibility that persistently activated G proteins through cellular protooncogenes may regulate cell-cycle resumption.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dascal N., Yekuel R., Oron Y. Acetylcholine promotes progesterone-induced maturation of Xenopus oocytes. J Exp Zool. 1984 Apr;230(1):131–135. doi: 10.1002/jez.1402300117. [DOI] [PubMed] [Google Scholar]
- Gerhart J., Wu M., Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984 Apr;98(4):1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C., Kemp B. E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987 Dec 18;238(4834):1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- Julius D., Livelli T. J., Jessell T. M., Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989 Jun 2;244(4908):1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Moriarty T. M., Gillo B., Carty D. J., Premont R. T., Landau E. M., Iyengar R. Beta gamma subunits of GTP-binding proteins inhibit muscarinic receptor stimulation of phospholipase C. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8865–8869. doi: 10.1073/pnas.85.23.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T. M., Padrell E., Carty D. J., Omri G., Landau E. M., Iyengar R. Go protein as signal transducer in the pertussis toxin-sensitive phosphatidylinositol pathway. Nature. 1990 Jan 4;343(6253):79–82. doi: 10.1038/343079a0. [DOI] [PubMed] [Google Scholar]
- Moriarty T. M., Sealfon S. C., Carty D. J., Roberts J. L., Iyengar R., Landau E. M. Coupling of exogenous receptors to phospholipase C in Xenopus oocytes through pertussis toxin-sensitive and -insensitive pathways. Cross-talk through heterotrimeric G-proteins. J Biol Chem. 1989 Aug 15;264(23):13524–13530. [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Mutter G. L., Wolgemuth D. J. Distinct developmental patterns of c-mos protooncogene expression in female and male mouse germ cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5301–5305. doi: 10.1073/pnas.84.15.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989 Nov 30;342(6249):505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]